Abstract
AIM: To perform a meta-analysis to derive a more precise estimation of imatinib treatment for different genotypes of gastrointestinal stromal tumors (GIST).
METHODS: Studies were identified by searching PubMed and Embase. Inclusive criteria were patients with exon 9-mutant, exon 11-mutant or wide type (WT) GIST, receiving chemotherapy of imatinib for clinical trial, and efficacy evaluation was cumulative response (CR) including complete response and partial response. The odds ratios (OR) for CR in stem cell factor receptor (KIT) mutation patients vs WT genotype patients, KIT exon 11-mutant genotype patients vs KIT exon 9-mutant genotype patients and KIT exon 9-mutant genotype patients vs WT genotype patients were calculated with 95% confidence interval (CI) for each study as an estimation of the efficacy of imatinib.
RESULTS: Five studies including 927 patients were involved in this meta-analysis. The overall OR (KIT group vs WT group) was 3.34 (95% CI: 2.30-4.86, P < 0.00001, Pheterogeneity = 0.04). The overall OR in KIT exon 11 group vs KIT exon 9 group was 3.29 (95% CI: 2.17-5.00, P < 0.00001, Pheterogeneity = 0.33). The overall OR in KIT exon 9 group vs WT group was 1.23 (95% CI: 0.73-2.10, P = 0.44, Pheterogeneity = 0.42).
CONCLUSION: Most patients with different genotypes of GIST and KIT exon 11-mutant will benefit from the individualized treatment of imatinib.
Keywords: Gastrointestinal stromal tumors, Gene, Imatinib, Efficacy, Meta-analysis
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is a rare tumor, but the most common mesenchymal malignancy of the gastrointestinal tract[1]. GIST expresses the tyrosine kinase receptor KIT, which is the protein product of the KIT proto-oncogene. GIST is generally characterized by gain-of-function mutations of KIT[2]. These mutations result in the constitutive activation of KIT signaling and are the likely causal molecular events of GIST[3,4]. No effective systemic treatment is available. Imatinib (STI571) inhibits a similar tyrosine kinase, BCR-ABL, leading to responses in chronic myeloid leukemia, and has also been shown to inhibit KIT.
Imatinib, an active tyrosine kinase inhibitor against KIT and platelet-derived growth factor receptor, has been shown to be highly effective in the treatment of advanced GIST. Clinical benefit was demonstrated in more than 80% of patients, resulting in a substantial improvement in the 2-year survival rate from 26% to 76%[5,6]. Imatinib has, therefore, become the standard of care in patients with advanced GIST. However, secondary resistance to imatinib often occurs within the first or second year of treatment[7], which indicated the need for differential treatment of patients with GIST. According to the previous reports, laboratory studies revealed significant molecular heterogeneity among GIST. Notably, 75%-85% of GIST had an activating mutation of KIT, 5%-7% had an activating mutation of the homologous PDGFRA kinase, and approximately 12%-15% of GIST did not have a detectable mutation of either Kinase[8-10]. And several studies have been designed to test the sensitivity of imatinib to different genotypes of GIST. Therefore, we made a meta-analysis of response to different genotypes to identify which one is more sensitive to imatinib.
MATERIALS AND METHODS
Publication search
Two electronic databases (PubMed and Embase) were searched (the last search was done on January 1, 2010, using the terms: “gastrointestinal stromal tumor” and “imatinib”). All eligible studies were retrieved, and their bibliographies were checked for other relevant publications. Only published studies with full-text articles were included. When more than one of the same patient population was included in several publications, only the most recent or complete study was used in this meta-analysis.
Inclusion criteria
The inclusion criteria were as follows: (1) assessing the efficacy of imatinib in treatment of patients with different genotypes of GIST; (2) clinical trial studies; and (3) sufficient data for estimating an odds ratio (OR) with 95% confidence interval (CI).
Data extraction
Information was carefully extracted from all eligible studies. The following data were collected from each study: first author’s surname, publication date, treatment protocols and total number of KIT mutation cases, KIT exon 11 cases, KIT exon 9 cases and WT cases, and numbers of KIT mutation cases, KIT exon 11 cases, KIT exon 9 cases and wild type (WT) cases, with the clinical CR after the treatment of imatinib, respectively. We did not define any minimum number limit of patients to include a study in our meta-analysis.
Statistical analysis
Odd ratios with 95% CI were used to assess the efficacy of imatinib in treatment of patients with different genotypes of GIST according to the method of Woolf. Heterogeneity assumption was checked by the χ2-based Q test. P > 0.10 for the Q test indicates a lack of heterogeneity among studies, so the OR estimate of each study was calculated by the fixed-effects model (the Mantel-Haenszel method). Otherwise, the random-effects model (the DerSimonian and Laird method) was used. The significance of the pooled OR was determined by the Z test and P > 0.05 was considered as statistically significant. Sensitivity analyses were carried out to check if modification of the inclusion criteria of this meta-analysis affected the final results. Potential publication bias was estimated by the funnel plot, in which the OR of each study was plotted against its log. An asymmetric plot suggests a possible publication bias. Funnel plot asymmetry was assessed by the method of Egger’s linear regression test, and funnel plot asymmetry on the natural logarithm scale of the OR was measured by a linear regression approach. The significance of the intercept was determined by t test (P < 0.05 was representative of statistically significant publication bias). All the statistical tests were performed with Review Manager Version 4.2 (The Cochrane Collaboration, Oxford, England) and STATA version 9.2 (Stata Corporation, College Station, TX, USA).
RESULTS
Study characteristics
Five publications met the inclusion criteria. The study by Blanke et al [11] was excluded due to the fact that it only revealed the prognostic factor and so did the study by Tzen et al [12]. Likewise, the study by Andersson et al[13] was excluded because the study was designed for a random, double-blind, 400 mg vs 600 mg imatinib controlled trial only used to prove the effective dosage to treat GIST. Hence, five groups including 927 patients were used in the pooled analyses. Table 1 lists the studies identified and their main characteristics. Of the five groups, sample sizes ranged from 32 to 392. Almost all of the patients with GIST were confirmed by histology and immunohistochemistry, and DNA sequence was identified by polymerase chain reaction technique. No significant differences were found in the age distributions and sex difference among all the studies.
Table 1.
Main characteristics of all studies included in the meta-analysis
| Author | Dose distribution | Cumulative response (%) | Genotype |
n |
|||
| KIT | Exon 11 | Exon 9 | WT | ||||
| Debiec-Rychter et al[14], 2006 | 400 mg/800 mg | 56 | Exon 11, 9, 13, 17 | 315 | 248 | 58 | 52 |
| WT | |||||||
| Wardelmann et al[15], 2006 | NA | 50 | Exon 11, 9 | 29 | 22 | 7 | 3 |
| WT | |||||||
| Yeh et al[16], 2007 | 400 mg | 52 | Exon 11, 9 | 49 | 40 | 9 | 5 |
| WT | |||||||
| Rutkowski et al[17], 2007 | 400 mg/800 mg | 63 | Exon 11, 9, 13, 17 | 63 | 52 | 9 | 19 |
| WT | |||||||
| Heinrich et al[18], 2008 | 400 mg/800 mg | 56 | Exon 11, 9, 8, 13, 17 | 325 | 283 | 32 | 67 |
| WT | |||||||
WT: Wide type; NA: Not available.
Meta-analysis results
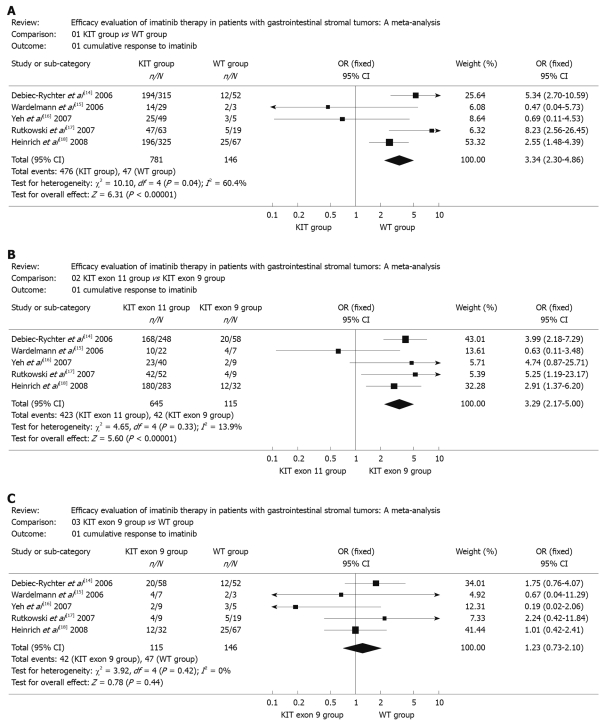
Overall meta-analysis indicated that the cumulative response of KIT mutation group to imatinib was significantly different compared with that of WT group (OR 3.34, 95% CI: 2.30-4.86; P < 0.00001, Pheterogeneity = 0.04) (Figure 1A). A significant heterogeneity was found by simply comparing those five combined samples (P < 0.10). The overall OR for KIT exon 11 group vs KIT exon 9 group and KIT exon 9 group vs WT group were 3.29 (95% CI: 2.17-5.00, P < 0.00001, Pheterogeneity = 0.33) and 1.23 (95% CI: 0.73-2.10, P = 0.44 Pheterogeneity = 0.42), respectively (Figure 1B and C). Although the CR in the study of Wardelmann et al[15] and Yeh et al[16] did not follow the tendency of other studies, the corresponding pooled OR was not materially altered with or without these two studies. No other single study influenced the pooled OR qualitatively as indicated by sensitivity analyses (data not shown).
Figure 1.
Meta-analysis. A: KIT group vs wide type (WT) group; B: KIT exon 11 group vs KIT exon 9 group; C: KIT exon 9 group vs WT group. OR: Odds ratios.
Publication bias
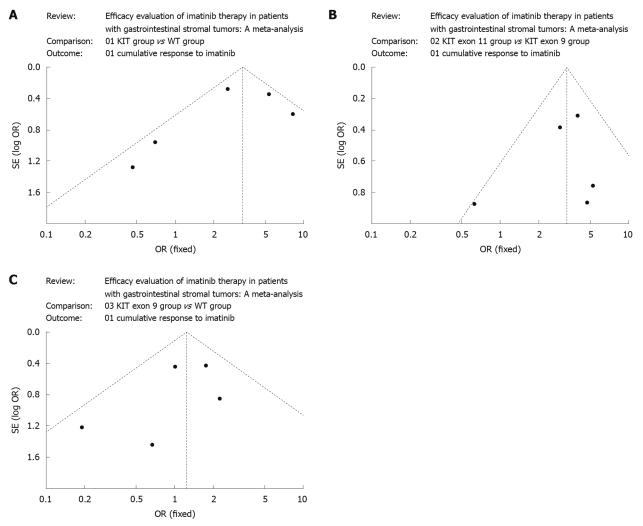
Begg’s funnel plot was performed to access the publication bias of literatures. The shapes of the funnel plots did not reveal any evidence of obvious asymmetry (Figure 2A-C).
Figure 2.
Begg’s funnel plot for publication bias test. A: KIT group vs wide type (WT) group; B: KIT exon 11 group vs KIT exon 9 group; C: KIT exon 9 group vs WT group. OR: Odds ratios.
DISCUSSION
Before the introduction of imatinib mesylate (formerly known as STI571), poor responses to radiotherapy and chemotherapy made surgery the only realistic treatment to cure GIST[19-21].
Molecularly targeted therapy with imatinib can inhibit the etiologic aberrant cell signaling mechanisms in GIST, leading to major objective responses and prolonged disease control. Patients experienced a dramatic response, supporting the rational use of imatinib in this disease.
Prior studies have noted that imatinib can be effectively and safely administered. Imatinib has, therefore, become the standard of care in patients with advanced GIST. However, the secondary resistance to imatinib often occurs within the first or second year of treatment, which indicated the need for differential treatment protocol for patients with GIST. According to previous reports, laboratory studies revealed significant molecular heterogeneity among GIST. Notably, 75%-85% of GIST had an activating mutation of KIT, 5%-7% had an activating mutation of the homologous PDGFRA kinase, and approximately 12%-15% of GIST did not have a detectable mutation of either kinase. Mutations in KIT exon 11 were the most common imatinib-target mutation found among the confirmed and unconfirmed CD117-positive GISTs (71.3%), followed by mutations in KIT exon 9 (8.2%), KIT exon 13 (1.2%), PDGFRA exon 18 (1.2%), and KIT exon 17 (approximately 1%)[18]. Several studies have been designed to test the sensitivity of different genotypes of GIST to imatinib. Therefore, we made a meta-analysis of response to imatinib at different genotypes to identify which one is more sensitive to imatinib.
Objective tumor response was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST)[22]. The best clinical response to imatinib was classified as cumulative response (CR) including complete response and partial response, stable disease, progressive disease, or not assessable. The conclusion shows that KIT mutation genotype correlates with improved treatment outcome when compared with WT genotype for cumulative response. Furthermore, patients whose tumor had a KIT exon 11 mutation were significantly more likely to achieve a CR than patients with tumors having a KIT exon 9 mutation, or WT genotype. There was no statistically significant difference in the likelihood of achieving a CR for patients with KIT exon 9-mutant GIST compared with WT GIST. Our findings confirmed that KIT exon 11 mutation is a positive predictive factor for cumulative response.
According to some phase III trials that were designed to compare 400 mg and 800 mg daily doses of imatinib, 400 mg remains the standard starting dose[23,24]. The survival of the patients with exon 9-mutant, exon 11-mutant or WT GIST was not affected by imatinib dosage. However, there was evidence of improved response rates for patients with exon 9-mutant tumors treated with imatinib 800 mg vs 400 mg (complete response/partial response, 67% vs 17%, P = 0.02)[16]. Remarkably, patients with tumors expressing an exon 9 mutant KIT protein show significant imatinib dose dependency for CR as compared with patients with tumors harbouring mutant exon 11 or wild-type KIT isoforms. These results suggest that 400 mg imatinib should be administered twice a day to patients with tumors bearing KIT exon 9 mutations. Other patients could safely start at an initial imatinib dose of 400 mg once daily, and increase to 800 mg when there is evidence of disease progression.
It appears that the WT expression of KIT is not sufficient to confer the antitumor activity of imatinib mesylate. Thus, inhibiting a normal target may not have antitumor activity if the target does not provide an essential function to the tumor cell. Therefore, identification of molecular abnormalities that are essential for tumorigenesis will help develop new anticancer therapies.
COMMENTS
Background
Gastrointestinal stromal tumor (GIST) commonly shows oncogenic activating mutations of the KIT tyrosine kinase. Imatinib mesylate, a small-molecule inhibitor of BCR-ABL, KIT and PDGFR tyrosine kinases, targets the aberrant signaling pathways that are critical for tumor cell proliferation and survival. Recent advances in understanding the molecular pathogenesis of GIST has led to the remarkably successful use of imatinib in the treatment of advanced tumors, inducing high response rates resulting in unprecedented improvement in the overall survival of the patients. Although several studies reported clinical response to imatinib with the mutational status to explore if the response to imatinib is linked to tumor genotype, a small sample can not provide persuasive evidence.
Research frontiers
Several studies with limited samples have concluded that clinical response to imatinib may correlate with mutational status. It is essential to give a personalized treatment by genotypes so as to improve the effectiveness in clinical treatment of GIST.
Innovations and breakthroughs
In the previous studies, it was found that the reason why a small sample size could not supply remarkable evidence to prove the response to imatinib may be linked to tumor genotype. The statistical analysis of a large collection of analysis results from individual studies for the purpose of integrating the findings. Meta-analysis is a statistical technique for assembling the results of several studies in a review into a single numerical estimate so as to provide a best evidence in making decisions about the treatment of individual patients.
Applications
The results indicate that most patients with different genotypes of GIST and KIT exon 11 mutant will benefit from the personalized treatment of imatinib.
Peer review
In this report, Chen et al performed a meta-analysis to confirm the prognostic importance of KIT mutation exon location with respect to imatinib sensitivity. Although this work does not add new information beyond what is already known, it is confirmatory and potentially useful for other investigators in this field.
Footnotes
Peer reviewer: Clifford S Cho, MD, Assistant Professor, Department of Surgery, Section of Surgical Oncology, University of Wisconsin School of Medicine and Public Health, H4/724 Clinical Sciences Center, 600 Highland Avenue, Madison, WI 53792-7375, United States
S- Editor Wang JL L- Editor Ma JY E- Editor Lin YP
References
- 1.George S, Desai J. Management of gastrointestinal stromal tumors in the era of tyrosine kinase inhibitors. Curr Treat Options Oncol. 2002;3:489–496. doi: 10.1007/s11864-002-0068-2. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 4.Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, Shiraga S, Bainbridge T, Morich J, Heinrich MC. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–5364. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 5.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 6.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 7.Chen LL, Trent JC, Wu EF, Fuller GN, Ramdas L, Zhang W, Raymond AK, Prieto VG, Oyedeji CO, Hunt KK, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 2004;64:5913–5919. doi: 10.1158/0008-5472.CAN-04-0085. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 10.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 11.Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 12.Tzen CY, Wang MN, Mau BL. Spectrum and prognostication of KIT and PDGFRA mutation in gastrointestinal stromal tumors. Eur J Surg Oncol. 2008;34:563–568. doi: 10.1016/j.ejso.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Andersson J, Bümming P, Meis-Kindblom JM, Sihto H, Nupponen N, Joensuu H, Odén A, Gustavsson B, Kindblom LG, Nilsson B. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology. 2006;130:1573–1581. doi: 10.1053/j.gastro.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S, Stul M, Casali PG, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Wardelmann E, Merkelbach-Bruse S, Pauls K, Thomas N, Schildhaus HU, Heinicke T, Speidel N, Pietsch T, Buettner R, Pink D, et al. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res. 2006;12:1743–1749. doi: 10.1158/1078-0432.CCR-05-1211. [DOI] [PubMed] [Google Scholar]
- 16.Yeh CN, Chen TW, Lee HL, Liu YY, Chao TC, Hwang TL, Jan YY, Chen MF. Kinase mutations and imatinib mesylate response for 64 Taiwanese with advanced GIST: preliminary experience from Chang Gung Memorial Hospital. Ann Surg Oncol. 2007;14:1123–1128. doi: 10.1245/s10434-006-9288-1. [DOI] [PubMed] [Google Scholar]
- 17.Rutkowski P, Nowecki ZI, Debiec-Rychter M, Grzesiakowska U, Michej W, Woźniak A, Siedlecki JA, Limon J, vel Dobosz AJ, Kakol M, et al. Predictive factors for long-term effects of imatinib therapy in patients with inoperable/metastatic CD117(+) gastrointestinal stromal tumors (GISTs) J Cancer Res Clin Oncol. 2007;133:589–597. doi: 10.1007/s00432-007-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer C, Gunawan B, Schüler P, Huber W, Füzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg. 2003;90:332–339. doi: 10.1002/bjs.4046. [DOI] [PubMed] [Google Scholar]
- 20.Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383–389. doi: 10.1001/archsurg.136.4.383. [DOI] [PubMed] [Google Scholar]
- 21.Plaat BE, Hollema H, Molenaar WM, Torn Broers GH, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper RJ, van der Graaf WT. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol. 2000;18:3211–3220. doi: 10.1200/JCO.2000.18.18.3211. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 24.Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]