Abstract
Background/Aims
This study was conducted to examine the relationship between family history of type 2 diabetes (T2DM) and risk of developing gestational diabetes mellitus (GDM) in Korean women.
Methods
We performed a 100-g oral glucose tolerance test in 858 pregnant women who had abnormal glucose tolerance in 50-g oral glucose challenge. In addition, we reviewed the incidence of T2DM in the parents and siblings and analyzed the association between the familial history of T2DM and the risk of GDM.
Results
Of the 858 subjects, 427 were normal, and 431 were diagnosed with GDM. Compared with women with no family history of T2DM, women with first degree family history of T2DM displayed higher risk of T2DM (odd ratio: parent only 1.91, sibling only 6.24, any 2.27).
Conclusions
The risk of developing GDM was significantly increased in Korean women with a family history of T2DM in first-degree relatives.
Keywords: Diabetes, gestational; Diabetes mellitus, type 2; Cluster analysis
INTRODUCTION
The pathophysiology of type 2 diabetes mellitus (T2DM) is closely related to that of gestational diabetes mellitus (GDM) [1]. It is well known that a family history of T2DM is an important risk factor for the development of GDM. Even after delivery, women with a history of GDM are at increased risk of developing T2DM later in life [2-4]. In a study of a Western population, familial aggregation of both T2DM and GDM was reported [5]. Family history of T2DM is an important risk factor for GDM, as has been previously reported in Koreans [6]. However, familial clustering of T2DM has not been previously reported in Korean women with GDM.
This study was conducted in Korean subjects deemed to be at high risk of developing GDM based on the incidence of T2DM in parents and siblings.
METHODS
Subjects
Between January 2004 and August 2006, 858 pregnant women who had plasma glucose levels > 140 mg/dL were recruited for this study. All subjects were 24 to 28 weeks into gestation and had no previous history of T2DM. The study protocol was approved by the Institutional Review Board of Cheil General Hospital.
Methods
Baseline anthropometric measurements including pre-pregnancy body weight, parity, and history of T2DM among first-degree relatives were recorded. A 100-g oral glucose tolerance test (OGTT) was performed to diagnose GDM using the Carpenter-Coustan criteria [7]. Subjects were categorized as having normal glucose tolerance (NGT) or GDM.
Statistical analysis
Statistical analysis and data management were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Student's t test and chi-square test were used to identify significant differences in clinical variables between the two test groups. The risk of developing GDM according to the presence or absence of a family history of T2DM was assessed by calculating odds ratios (ORs) through logistic regression analysis (with adjustments for age, parity, and pre-pregnancy body mass index [BMI]).
RESULTS
Clinical characteristics
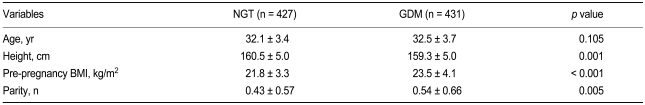
Of the 858 test subjects, 427 were classified as having NGT, and 431 were diagnosed with GDM based on the results of a 100-g OGTT. Mean age was comparable between the two study groups. However, significant differences in height, pre-pregnancy BMI, and parity were found (Table 1).
Table 1.
Clinical characteristics of the study subjects
NGT, normal glucose tolerance; GDM, gestational diabetes mellitus; BMI, body mass index.
Familial clustering
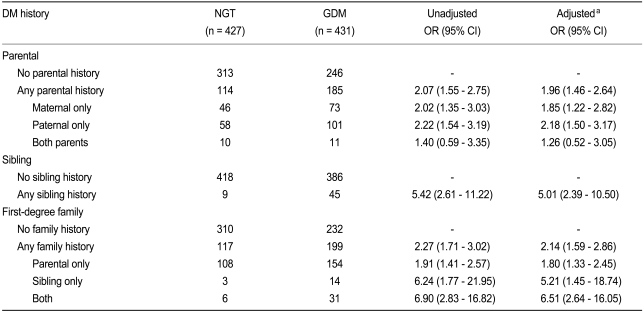
In patients with one parent having a history of T2DM, the unadjusted OR for developing GDM was 2.07. No significant difference between paternal or maternal history of diabetes was found (Table 2). Interestingly, when both parents had a history of T2DM, the OR for developing GDM was not significantly increased. When a sibling had a history of T2DM, the OR was 5.42. When any first-degree relative had a history of T2DM, the unadjusted OR for developing GDM was 2.27. When the first-degree relatives were subclassified to parents or siblings, the OR was 1.91 for cases in which only parents of T2DM. However, in cases in which only siblings had T2DM, the risk increased to 6.24. When both parents and siblings had a history of T2DM, the OR increased up to 6.90. All of the above results remained unchanged after adjustment for age, parity, and pre-pregnancy BMI using logistic regression analysis.
Table 2.
Odd ratios and 95% confidence intervals for the development of GDM based on parental, sibling, and firstdegree family history of type 2 diabetes mellitus
GDM, gestational diabetes mellitus; DM, diabetes mellitus; NGT, normal glucose tolerance; OR, odds ratio; CI, confiidence interval.
aAdjusted for age, parity, and pre-pregnancy body mass index.
DISCUSSION
In the present study, a history of T2DM in first-degree relatives was associated with an increased risk of developing GDM. The risk of developing GDM was increased approximately two-fold in cases with a parental history of T2DM, approximately five-fold in cases with sibling history of T2DM, and approximately 6.5-fold in cases with both sibling and parental histories of T2DM. These findings indicate the familial aggregation of T2DM in Korean women with GDM, consistent with the findings of other studies performed in different ethnic groups [3,5,8].
We found no difference in the risk of developing GDM between cases with a maternal history of T2DM and those with a paternal history of T2DM. In several previous studies, maternal influence on the development GDM has been reported, as shown a high prevalence of T2DM in the mothers of women with GDM. These findings suggest that maternal genetic background and environmental factors are important in the pathophysiology of GDM [9-12]. However, other studies have not confirmed a strong influence of maternal history of T2DM [5]. Indeed, the prevalence of T2DM in a Korean population were found to be not higher in subjects whose mothers had a history of T2DM than in those whose mothers had no history of T2DM [13].
Our finding that the risk of developing GDM was not increased in cases in which both parents had a history of T2DM differed from both our own expectations and the results of a previous study [5]. Further studies using larger numbers of subjects are required to address this unexpected finding.
Our study had several limitations. First, it was conducted at a single hospital and thus does not represent the entire Korean GDM patient population. Second, our case-control study was designed such that women with GDM were definitively diagnosed, but the control group included women that had abnormal glucose tolerance in 50-g glucose tolerance test and thus did not represent normal controls. Third, analyses of disease histories were based solely on patient report and may have been affected by recall bias. Finally, the presence or absence of familial disease history was assessed purely based on the results of a questionnaire; the influence of other genetic and/or environmental influences was not evaluated. Despite these limitations, the results of our study suggest the familial aggregation of T2DM in Korean women with GDM.
Acknowledgements
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (Grant No. A050463).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341:1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–1083. [PubMed] [Google Scholar]
- 4.Retnakaran R, Connelly PW, Sermer M, Zinman B, Hanley AJ. The impact of family history of diabetes on risk factors for gestational diabetes. Clin Endocrinol (Oxf) 2007;67:754–760. doi: 10.1111/j.1365-2265.2007.02958.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams MA, Qiu C, Dempsey JC, Luthy DA. Familial aggregation of type 2 diabetes and chronic hypertension in women with gestational diabetes mellitus. J Reprod Med. 2003;48:955–962. [PubMed] [Google Scholar]
- 6.Jang HC, Cho NH, Jung KB, Oh KS, Dooley SL, Metzger BE. Screening for gestational diabetes mellitus in Korea. Int J Gynaecol Obstet. 1995;51:115–122. doi: 10.1016/0020-7292(95)02524-g. [DOI] [PubMed] [Google Scholar]
- 7.Coustan DR, Carpenter MW. The diagnosis of gestational diabetes. Diabetes Care. 1998;21(Suppl 2):B5–B8. [PubMed] [Google Scholar]
- 8.McLellan JA, Barrow BA, Levy JC, et al. Prevalence of diabetes mellitus and impaired glucose tolerance in parents of women with gestational diabetes. Diabetologia. 1995;38:693–698. doi: 10.1007/BF00401841. [DOI] [PubMed] [Google Scholar]
- 9.Martin AO, Simpson JL, Ober C, Freinkel N. Frequency of diabetes mellitus in mothers of probands with gestational diabetes: possible maternal influence on the predisposition to gestational diabetes. Am J Obstet Gynecol. 1985;151:471–475. doi: 10.1016/0002-9378(85)90272-8. [DOI] [PubMed] [Google Scholar]
- 10.Moses R, Rodda M, Griffiths R. Predominance of a maternal history of diabetes for patients with non-insulin-dependent diabetes mellitus. Implications for the intrauterine transmission of diabetes. Aust N Z J Obstet Gynaecol. 1997;37:279–281. doi: 10.1111/j.1479-828x.1997.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 11.Harder T, Franke K, Kohlhoff R, Plagemann A. Maternal and paternal family history of diabetes in women with gestational diabetes or insulin-dependent diabetes mellitus type I. Gynecol Obstet Invest. 2001;51:160–164. doi: 10.1159/000052916. [DOI] [PubMed] [Google Scholar]
- 12.Kelly LA, Lane CJ, Weigensberg MJ, et al. Parental history and risk of type 2 diabetes in overweight Latino adolescents: a longitudinal analysis. Diabetes Care. 2007;30:2700–2705. doi: 10.2337/dc07-0050. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Cho NH, Noh JH, Lee MS, Lee MK, Kim KW. Lack of excess maternal transmission of type 2 diabetes in a Korean population. Diabetes Res Clin Pract. 2004;65:117–124. doi: 10.1016/j.diabres.2003.11.020. [DOI] [PubMed] [Google Scholar]