Abstract
Background/Aims
Based on the results of well designed clinical studies, intensive insulin therapy has been established to improve glycemic control in newly diagnosed diabetes. However, discrepancies exist between the findings of clinical trials and experiences in general practice. Furthermore, the efficacy of an early insulin therapy (EIT) - commonly used in general practice - on long-term glycemic control has not been established. Therefore, we evaluated the effects of EIT on pancreatic β-cell function and glycemic control using insulin-based methods widely employed in general practice.
Methods
We performed a retrospective cohort study that initially involved reviewing patients' medical records. Following a thorough review, 61 patients who received either biphasic or prandial EIT at the time of diagnosis were enrolled. We then evaluated changes in β-cell function and glycemic control during a 48-month follow-up period.
Results
Mean HbA1c decreased significantly as a result of EIT from 10.7 ± 1.8% to 6.2 ± 1.1% (p < 0.001). On average, 2.6 months was required to achieve an HbA1c value < 7%. EIT significantly improved the insulinogenic index. Glycemic control was well maintained for 48 months. More than 70% of patients were able to maintain glycemic control following lifestyle modifications or treatment with oral antidiabetic drugs. No significant differences were identified between patients receiving biphasic EIT and prandial EIT in terms of glycemic control or pancreatic β-cell function.
Conclusions
Our results suggest that regardless of the method of delivery, EIT significantly improves β-cell function and facilitates long-term glycemic control in patients with newly diagnosed type 2 diabetes mellitus.
Keywords: Insulin; Diabetes mellitus, type 2; Insulin-secreting cells
INTRODUCTION
Despite the development of new drugs and therapeutic strategies for treating type 2 diabetes mellitus (T2DM), achieving long-term glycemic control remains a challenge [1-3]. Results from the United Kingdom Prospective Diabetes Study (UKPDS) suggest that deterioration of glycemic control can be largely attributed to progressive β-cell loss, irrespective of the nature of pharmacological intervention [1,2]. Therefore, treatments that can preserve or improve β-cell function are of great interest in the field of T2DM therapeutics [3-5].
Recent studies have shown that short-term intensive insulin therapy (IIT) in patients newly diagnosed with T2DM produces beneficial effects on β-cell function, glycemic control, and rate of remission (euglycemic maintenance without antidiabetic therapy) within 1 year [4,6-8]. However, these studies applied the IIT by means of continuous subcutaneous insulin infusion (CSII), and the study patients were hospitalized or required to make daily clinic visits for strict, frequent monitoring of glucose levels and for insulin dose titration. Generally, such treatment protocols can be employed only in well controlled clinical trials that are funded and supported by numerous investigators, health care providers, and sponsors. Such protocols usually cannot be applied to general practice due to economic and treatment compliance constraints. To date, no studies have been conducted to examine the effects of more convenient methods for delivering early insulin therapy (EIT) on long-term glycemic control and improvement of β-cell function in patients newly diagnosed with T2DM. Our study was designed to determine the generalizability of results that have been reported in previous clinical trials. Therefore, we retrospectively evaluated the effect of EIT on pancreatic β-cell function and long-term glycemic control in newly diagnosed patients who had T2DM undergoing treatment in a general outpatient clinic. Insulin was applied in biphasic or prandial fashion with or without basal insulin, approaches that are commonly employed in general practice.
METHODS
Subjects
We retrospectively reviewed the medical records of 378 patients newly diagnosed with T2DM who were treated between January 2003 and December 2006 in the Department of Endocrinology and Metabolism at Kyung Hee Medical Center in the Republic of Korea. Of these patients, 52 were initially treated with diet and exercise therapy, 260 with oral antidiabetic drugs (OAD), and 66 with insulin (from the time of diagnosis). Of the 66 patients who received insulin therapy, 61 were treated with insulin to control severe hyperglycemic symptoms. The remaining five patients were treated with insulin due to the presence of comorbid disease: systemic tuberculosis (n = 2), asthma (being treated by steroid therapy) (n = 1), chronic renal failure (n = 1), and cancer (n = 1). We conducted statistical analyses of the data gathered from the medical records of the 61 patients who were treated with EIT from diagnosis to control hyperglycemia. During the follow-up period, we measured glycemic levels, tested insulin secretory function and insulin resistance, and examined the medication regimens employed.
Method of insulin therapy
The insulin treatment protocols used in this study are commonly employed in general practice. Specifically, we performed biphasic and prandial insulin injections with or without basal insulin. Patients in the biphasic group were injected twice daily with commercially available premixed biphasic human insulin or insulin analog (NovoLet® 30/70 or NovoMix® 30, Novo Nordisk A/S, Copenhagen, Denmark; or Humulin® 70/30 or Humalog® Mix75/25™, Eli Lilly and Co., Indianapolis, IN, USA). Patients in the prandial group were injected three times daily with a rapid-acting insulin analog (Humalog®), with or without a daily injection of a long-acting insulin (intermediate insulin neutral protamine hagedorn [NPH]) at bedtime or long-acting insulin glargine (Lantus®, Aventis Pharmaceuticals Inc., Tucson, AZ, USA) in the morning or at bedtime. Patients were given instructions regarding diet and the self-monitoring of blood glucose, and shown the proper techniques for injecting insulin and managing hypoglycemia.
The ultimate goal of glucose control was to achieve a glycosylated hemoglobin (HbA1c) level of less than 7%. Physicians adjusted their patients' insulin doses to achieve this target. Patients visited the clinic every 1 to 3 months, and glycemic status was assessed during each visit by measuring HbA1c levels. Some patients were treated with relatively intensive insulin therapy (RIIT). In RIIT, the method of insulin therapy was the same as for biphasic or prandial insulin, but clinic visits took place every 1 to 2 weeks during the first month of treatment, and self-monitoring of blood glucose was emphasized to a greater degree.
After the target HbA1c level was reached, insulin therapy was replaced by diet therapy alone or OAD therapy. Where possible, patients underwent a 75 g standard oral glucose tolerance test (OGTT) at baseline and after reaching the target HbA1c level. Insulin treatment was halted the day before posttreatment OGTT. We retrospectively reviewed changes in treatment regimens during the follow-up period and classified treatment modalities into four groups: lifestyle modification (LSM), OAD only, OAD in conjunction with basal insulin, and biphasic or prandial insulin with or without basal insulin. We defined the absolute insulin retreatment group (AIRT) as patients who received insulin injections after completing EIT. Patients who received no insulin after completing EIT were defined as the non-AIRT group.
Biochemical analysis
Clinical and biochemical parameters were tested at the same time the OGTT was performed. Blood samples were collected 10 to 12 hours after overnight fasting to determine baseline levels of total serum cholesterol, triglycerides, high density lipoprotein-cholesterol (HDL-C), insulin, and C-peptide. A solution containing 75 g of glucose was then immediately administered (orally) over a 5-minutes period. Blood glucose levels were measured at 30, 60, 90, and 120 minutes, and insulin and C-peptide levels at 30 minutes.
Plasma glucose levels were determined by the hexokinase method using an automatic glucose analyzer (Hitachi, Tokyo, Japan). Total serum cholesterol and triglyceride levels were determined by an enzymatic assay and serum HDL-C levels by a precipitation assay. Low density lipoprotein-cholesterol (LDL-C) levels were calculated using the Friedewald equation. Serum insulin and C-peptide levels were measured using immunoradiometric assays: insulin - Insulin RIA Bead II; C-peptide - Daiichi III (both SRL, Tokyo, Japan). HbA1c was measured by high-performance liquid chromatography using a G7 HPLC analyzer (Tosoh Corporation, Tokyo, Japan).
Measurement of insulin resistance and β-cell function
-
1) Insulin resistance: the homeostasis model assessment of insulin resistance (HOMAIR) [9,10] was calculated as follows:
HOMAIR = (FPG × FPI) / 405
(FPG: fasting plasma glucose, mg/dL; FPI: fasting plasma insulin, µU/mL)
-
2a) β-cell function: the insulinogenic index (IGI), which is commonly used as an indicator of β-cell function (10), was calculated from the OGTT data as follows:
IGI = (Ins30 - Ins0) / (Glc30 - Glc0)
(Glc0: fasting plasma glucose, mg/dL; Glc30: plasma glucose at 30 minutes during OGTT, mg/dL; Ins0: fasting plasma insulin, µU/mL; Ins30: plasma insulin at 30 minutes during OGTT, µU/mL)
-
2b) β-cell function: the acute C-peptide response (ACR) was calculated as follows:
ACR = (C-pep30 - C-pep0) × 1000 / (Glc30 - Glc0)
(Glc0: fasting plasma glucose, mg/dL; Glc30: plasma glucose at 30 minutes during OGTT, mg/dL; C-pep0: fasting plasma C-peptide, ng/mL; C-pep30: plasma C-peptide at 30 minutes during OGTT, ng/mL)
-
2c) β-cell function: the homeostasis model assessment of β-cell function (HOMAβ) [9] was calculated as follows:
HOMAβ = 20 × FPI / (FPG - 3.5)
(FPG: fasting plasma glucose, mmol/L; FPI: fasting plasma insulin, mU/L)
Statistical analysis
Statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Normally distributed data (i.e., relating to continuous variables) are presented as means ± standard deviations, and non-normally distributed data (i.e., those relating to triglyceride, IGI, ACR, HOMAβ, and HOMAIR) as median (interquartile range, IQR). Student's t tests and Mann-Whitney U tests were respectively used to compare normally distributed and non-normally distributed data from the biphasic and prandial groups. Chi-square tests were used to analyze dichotomous variables. Paired Student's t tests were used to compare data relating to pre- and post-EIT continuous variables, and Wilcoxon signed-rank tests data relating to pre- and post-EIT noncontinuous variables. In cases in which the treatment method was not changed, missing HbA1c data were replicated from data collected 1 month before or after the missing time point, since HbA1c levels represent the average glycemic status over a period of 2 to 3 months. Glycemic control during the 48-month follow-up period was analyzed by two methods: a trend analysis of glycemic control performed using patients' HbA1c values (recorded trimonthly) and a repeated measures analysis of variance. A p value < 0.05 was considered to be statistically significant.
RESULTS
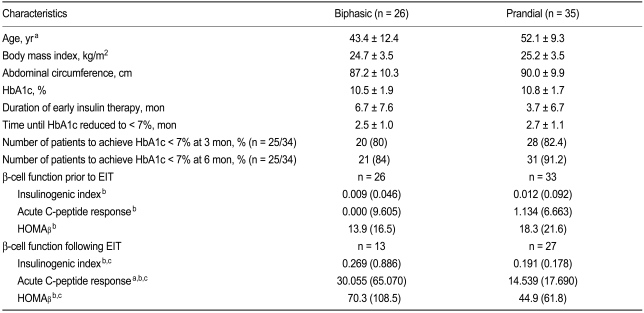
Baseline demographics and clinical characteristics of the 61 patients (biphasic group, n = 26; prandial group, n = 35) are shown in Table 1. The majority of the patients were men (n = 37), and the mean patient age was 48.3 ± 11.5 years. Mean patient body mass index (BMI) was 25.9 ± 3.5 kg/m2, which falls in the obese range according to the criteria proposed for the Asia-Pacific region [11]. Mean abdominal circumference was 88.8 ± 10.1 cm. Despite being newly diagnosed with T2DM, the patients were severely hyperglycemic, with a mean FPG of 213 mg/dL, a mean 2 hours post-load plasma glucose level of 387.7 ± 74.2 mg/dL, and a mean initial HbA1c of 10.7 ± 1.8%. In addition, the patients' pancreatic β-cell function was markedly reduced (IGI 0.010 [0.065]; ACR 0.000 [7.393]; HOMAβ 15.3 [21.3]), and insulin resistance was elevated (HOMAIR 2.8 [3.8]). Patient blood pressure was normal and lipid profiles dyslipidemic.
Table 1.
Baseline demographics and clinical characteristics (across all treatment groups)
Values are presented as mean ± SD unless otherwise indicated.
HOMA, homeostasis model assessment; IR, insulin resistance; LDL, low density lipoprotein; HDL, high density lipoprotein.
aValues are presented as sample size.
bValues are presented as median (interquartile range).
Effects of early insulin therapy on glycemic control and pancreatic β-cell function
Three months following the initiation of EIT, mean HbA1c had decreased significantly from 10.7 ± 1.8% to 6.2 ± 1.1% (p < 0.001). The proportion of patients who achieved the target HbA1c (< 7%) at 3 months post-EIT was 78.7%, and the proportion that achieved the target HbA1c within 6 months was 85.2%. The mean duration of EIT necessary to reach the target HbA1c was 2.6 ± 1.1 months. Mean total duration of EIT was 4.9 ± 7.1 months. Prior to treatment with EIT, no significant differences in HbA1c or pancreatic β-cell function (IGI, ACR) were observed between patients who achieved the HbA1c target and those who did not. However, following treatment with EIT, the mean IGI of the patients who reached the HbA1c target tended to be higher than that of those who did, although the difference was not significant (p = 0.126).
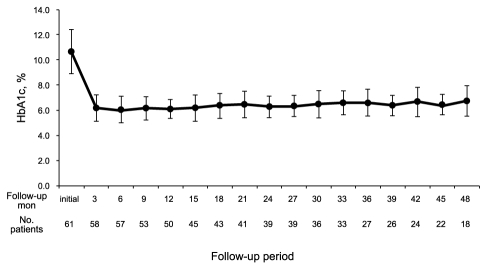
With the exception of two patients who dropped out during the 48-month follow-up period and five patients who persisted with EIT during the follow-up period on the advice of their physician, the remaining patients (n = 54) changed their treatment modality to LSM or OAD. The mean follow-up duration was 36.6 ± 18.6 months. After this change in treatment modality, the significant improvement in glycemic control observed in most patients was maintained for the duration of the 48-month follow-up period (Fig. 1). Specifically, a repeated measures analysis of variance indicated that glycemic control at each follow-up time point (50 patients at year 1, 38 at year 2, 24 at year 3, and 15 at year 4) was well maintained (p < 0.001).
Figure 1.
Changes in HbA1c following early insulin therapy (EIT). Immediately following EIT, glycemic control improved significantly (p < 0.001); the improvement was maintained during the follow-up period.
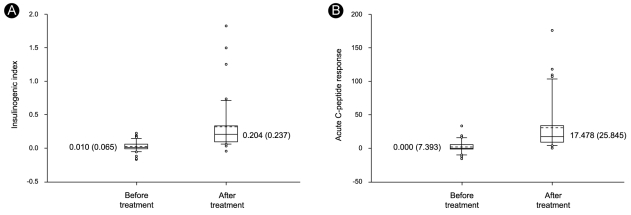
Plasma glucose concentrations during the OGTT were significantly improved following EIT (p < 0.001). In addition, pancreatic β-cell function significantly improved. Notably, significant improvements in IGI and ACR were observed (Fig. 2), and the HOMAβ index improved from 15.3% (21.3) to 47.2% (68.54) (p < 0.001). Finally, the HOMAIR index improved from 2.9 (IQR 3.8) to 2.2 (2.5) (p < 0.001).
Figure 2.
Changes in β-cell function following early insulin therapy (EIT). Insulinogenic index (A) and acute C-peptide response (B) increased significantly following EIT (p < 0.001). Solid lines indicate median values, and dashed lines denote mean values. Data are presented as the median (interquartile range).
Comparison of glycemic control and pancreatic β-cell function between patients in the biphasic and prandial groups
Table 2 shows the demographics and clinical characteristics of the patients in the biphasic and prandial groups. With the exception of mean age and duration of EIT, no significant differences were observed in the baseline characteristics between the two groups. While the mean patient age was significantly higher in the prandial group than in the biphasic group (p = 0.003), overall duration of EIT for patients in the biphasic group tended to be longer than that for patients in the prandial group, although the difference was not significant (p = 0.111). No significant differences were observed in the duration of EIT required to achieve the target HbA1c. In both the biphasic and prandial groups, pancreatic β-cell function improved significantly following EIT. No significant differences in pancreatic β-cell function (baseline or post-EIT) were detected between the biphasic and prandial groups. However, significant differences were seen between the two groups with respect to ACR, which was significantly higher in the biphasic group than the prandial group (p = 0.029). Percentages of patients reaching the target HbA1c were comparable. No significant differences were detected between the two groups with respect to glycemic control during the follow-up period (data was not shown).
Table 2.
Comparison of biphasic and prandial group characteristics
Values are presented as mean ± SD unless otherwise indicated.
EIT, early insulin therapy; HOMA, homeostasis model assessment.
aComparison between the biphasic and prandial groups, p < 0.05.
bValues are presented as the median (interquartile range).
cComparison between values pre- and post-EIT treatment, p < 0.001.
Changes in treatment modality during the 48-month follow-up period
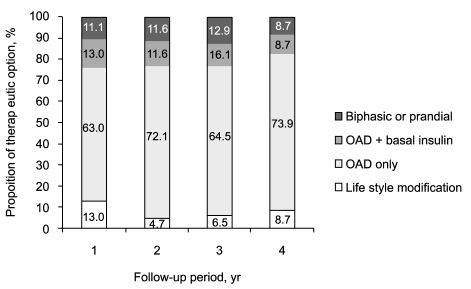
After attaining the target HbA1c, 54 patients (88.5%) were treated with LSM or OAD only. The remaining five patients did not undergo changes in their treatment regimen but instead continued with insulin therapy. More than 70% of patients continued with their LSM or OAD treatment for the entire 4-year follow-up period (Fig. 3). No significant differences in treatment method (LSM/OAD) existed between the biphasic and prandial groups.
Figure 3.
Changes in treatment modality during the 4-year follow-up period. OAD, oral antidiabetic drugs.
Of the 43 patients who completed the entire follow-up period, those receiving AIRT (n = 20) displayed no differences in HbA1c at the 3- and 6-month follow-up time points from non-AIRT patients. However, HbA1c was significantly higher in the AIRT group than in the non-AIRT group at the 9-, 12-, 15-, 21-, 24-, and 30-month follow-up time points (p < 0.05). No significant differences were observed in baseline pancreatic β-cell function between AIRT and non-AIRT patients. However, following treatment with EIT, the non-AIRT group patients displayed significantly better β-cell function than AIRT patients (IGI: AIRT 0.144 [0.201], non-AIRT 0.226 [0.341], p = 0.098; ACR: AIRT 11.904 [21.210], non-AIRT 24.077 [55.109], p = 0.040).
Comparisons between the RIIT and non-RIIT groups
No significant differences were noted in the proportion of biphasic or prandial insulin-treated patients or baseline HbA1c levels between the RIIT (n = 30) and non-RIIT (n = 23) groups. Moreover, no significant differences were observed in the length of time taken by the RIIT and non-RIIT patients to reach the target HbA1c (2.4 ± 0.8 months and 2.9 ± 1.4 months, respectively). However, the overall duration of EIT was significantly shorter in the RIIT group than in the non-RIIT group (2.8 ± 2.5 months vs. 8.5 ± 10.4 months, p = 0.017). Furthermore, a significantly higher proportion of patients in the RIIT group had reached the target HbA1c by the third month of follow-up than patients in the non-RIIT group (89.5% vs. 66.7%, p = 0.042). Also, significantly fewer RIIT patients fell into the AIRT group than non-RIIT patients (35.7% vs. 64.3%, p < 0.001). During the 4-year follow-up period, more than 80% of the patients in the RIIT group were able to maintain glucose control with LSM or OAD only, compared to fewer than 40% of the non-RIIT patients (p < 0.001). However, no significant differences existed between the RIIT and non-RIIT groups with respect to pre- and post-EIT pancreatic β-cell function.
DISCUSSION
The primary objective when treating T2DM is the prevention or delay of diabetic complications through optimal glycemic control, which ultimately improves patients' quality of life. Despite such clear objectives, T2DM is a progressive, complicated disease, and maintaining long-term glycemic control and preventing complications are very difficult.
Many institutions have developed treatment guidelines for T2DM. A consensus meeting of the American Diabetes Association and European Association for the Study of Diabetes suggested lifestyle intervention and metformin therapy at diagnosis and the early application of insulin therapy if a patient does not meet the primary target (HbA1c < 7%) [12]. According to these guidelines, early intervention with insulin therapy and lifestyle modification is recommended in cases of severe, uncontrolled hyperglycemia. Although many clinical studies [1,3,4] have reported the short- and long-term effects of various drugs on glycemic levels in newly diagnosed patients with T2DM, studies performed in general practice have been limited.
In 2003, we began using EIT for the treatment of T2DM in our outpatient practice. It was our belief that rapid amelioration of glucotoxicity would improve β-cell function and thus facilitate long-term glycemic control. In this study, we retrospectively analyzed the results we obtained during the 4-year period.
We found that an average of 3 months of EIT was needed to decrease HbA1c to the near-normal level of 6.2 ± 1.1%. Patients were treated according to our usual clinical care schedules. Although we titrated the insulin dosage every 2 to 4 weeks during the initial treatment period, which represents a relatively low frequency compared to the treatment intervals reported in previous clinical trials [4,6-8], glycemic control rapidly improved, with an average HbA1c decrease of 4 percentage points within 3 months. The proportion of patients who achieved the target HbA1c of < 7% within 6 months of EIT (85.2%) was higher than that reported in a previous study by Hirao et al. (32.1%) [13]. The two studies were different in terms of previous duration of diabetes. In the study by Hirao et al. [13], drug-naïve patients in the biphasic group had suffered from diabetes for an average of 6.58 ± 8.17 years and those in the multiple daily injection (MDI) group an average of 10.05 ± 13.59 years. In contrast, our patients had all been diagnosed within the past year. This suggests that the shorter time since diagnosis may have contributed to the recovery of β-cell function and amelioration of hyperglycemia in our study patients.
Following the discontinuation of EIT, most patients were able to maintain long-term glycemic control during the 4-year follow-up period. No significant differences were observed in short-term glycemic control between the biphasic and prandial groups, consistent with the results reported by Hirao et al. [13]. Similarly, no significant differences in long-term glycemic control were detected between the two treatment groups.
Recently, Weng et al. [4] reported a 40 to 50% remission rate at 1-year follow-up in patients given transient IIT (2 to 5 weeks). We ourselves found that improvements in glycemic control were well maintained after the withdrawal of EIT for the remainder of the 4-year follow-up period, despite the variety of treatment modalities employed following the cessation of EIT. Specifically, 70 to 80% of patients treated with LSM or OAD alone maintained a reasonable glycemic status. We presume that rapid improvement of glucotoxicity results in early restoration of pancreatic β-cell function, which in turn facilitates long-term glycemic control. Pancreatic β-cell function following the discon-tinuation of EIT was better in the non-AIRT group than in the AIRT group. This suggests that the degree of improvement in pancreatic β-cell function impacts upon the degree to which glycemic control is maintained.
Data from several studies indicate that EIT prolongs endogenous insulin secretion and promotes metabolic control in both type 1 and type 2 diabetes [4,14,15]. The shorter the period of prior glucotoxicity is, the more likely the restoration of β-cell function will be [16]. Many studies have reported that the rapid correction of hyperglycemia improves β-cell function and insulin resistance [17-19]. In previous studies [3,4], the elimination of glucotoxicity and improved insulin secretion were achieved not only through intensive insulin therapy, but also with oral antidiabetic therapy. However, the effect of the latter was highly transient and no oral antidiabetic agent has yet been shown to profoundly reverse the inexorable β-cell deterioration and worsened glycemia in type 2 diabetes [20]. Although few comparative studies have investigated the ability of oral antidiabetic agents and early insulin therapy to control glycemia, a study performed in a Chinese population [4] showed higher insulin-secreting capacity in subjects treated with insulin than in those who received oral antidiabetics. Therefore, as well as eliminating glucotoxicity, early insulin treatment may also help to restore pancreatic β-cell function. Insulin therapy itself would be expected to decrease the demand placed on pancreatic β-cells to secrete insulin. This is referred to as the "β-cell rest" effect and the results of previous studies seem to support this hypothesis. Insulin has been reported to possess antiinflammatory properties and to directly influence β-cell growth and survival [21]. The mechanisms by which it improves β-cell function remain unclear. However, a prospective study involving eight university medical centers is being conducted at our institute to determine the effects of intensive glycemic control, achieved through early IIT or early combined oral antidiabetic therapy, on the restoration of β-cell function and on long-term glycemic control (www.clinicaltrial.gov, NCT00474838). We believe that the results of this study will provide invaluable insights.
Unfortunately, the optimal period of euglycemia for the full amelioration of glucotoxicity and restoration of β-cell function remains unknown. In a study performed on rats suffering from streptozotocin-induced diabetes, 4 weeks of insulin intervention resulted in improved glycemic control and increased β-cell mass, islet insulin content, and proinsulin production [22]. Garvey et al. [23] showed that 24-hour integrated insulin secretion and second-phase insulin secretion, but not first-phase insulin secretion, improved in patients with T2DM following treatment with CSII. In clinical studies on IIT [4,6-8], the duration of IIT has typically been 2 to 3 weeks. The rationale behind the choice of treatment period has not been properly detailed in these studies. McFarlane et al. [14] reported that pancreatic β-cell function stably improved during 8 to 12 weeks of euglycemia following the initial restoration of euglycemia. It was not clear, however, that β-cell function would be sufficiently improved during the initial period of euglycemia to sustain the long-term maintenance of normal fasting and postprandial glucose levels. We also believe that the normalization of HbA1c levels, which paralleled the improvement in glycemic status over the course of 2 to 3 months, reflected the amelioration of glucotoxicity during the same time period.
The present study involved retrospective observational analyses. We took the decision to continue EIT until the target HbA1c of < 7.0% was reached. This took an average of 2.6 ± 1.1 months, similar to the findings of McFarlane et al. [14]. We cannot directly compare our findings with those of other studies because of differences in baseline characteristics and treatment protocols. Nevertheless, we tentatively propose that the optimal duration of EIT for long-term glycemic control is either 3 months or however long is required to achieve a normal HbA1c level.
In this study, RIIT patients had a significantly shorter duration of EIT than non-RIIT patients and more of them achieved the target HbA1c by the 3-month time point. Following EIT, a larger proportion of patients in the RIIT group were treated with LSM or OAD alone than in the non-RIIT group. Some investigators have suggested that active titration of insulin, which requires frequent contact with health care providers, facilitates the achievement of effective glycemic control [24,25]. In our study, care patterns in the RIIT group may have influenced patient compliance and induced other behavioral changes.
Research into the effects of biphasic insulin treatment on pancreatic β-cell function has been limited [26,27]. Moreover, a literature search revealed few studies that have compared the effects of biphasic and prandial EIT on pancreatic β-cell function. In the present study, biphasic and prandial EIT were equally effective for achieving glycemic control and restoration of pancreatic β-cell function in patients newly diagnosed with T2DM. Based upon the results of our study, we suggest that biphasic EIT is as effective as prandial EIT with or without basal insulin. We further found that insulin treatment protocols commonly employed in outpatient clinics, both biphasic or prandial, were as effective at achieving short- and long-term glycemic control and restoration of pancreatic β-cell function as intensive insulin therapy in well designed and well funded clinical studies requiring patient admission, frequent visits, and use of CSII or MDI.
Our study has some limitations. First, it was a retrospective, non-randomized, and uncontrolled study. In addition, some data were missing and losses occurred during follow-up. Despite these limitations, our findings may be more clinically useful than those of controlled clinical trials. The suggestion was made that the goals of clinical research trials are not always in line with clinical medicine's goal of optimal patient care [28]. The failure of physicians and patients to understand the difference between scientific research and personalized medical treatment has been termed "therapeutic misconception" [29]. Several reports have identified contributing factors and potential solutions [30-32]. We were able to avoid the problem of therapeutic misconception because our study employed a retrospective design and drew patients from general practice. Moreover, our patients were offered personalized insulin treatment. Our study therefore allowed us to assess the effects of early insulin therapy in a genuine clinical context.
In conclusion, our results suggest that EIT based on insulin treatment protocols that are commonly employed significantly improves β-cell function and facilitates long-term glycemic control in patients newly diagnosed with T2DM.
Acknowledgements
This study was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. A050463).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 3.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 4.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 5.Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 6.Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Xu W, Liao Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27:2597–2602. doi: 10.2337/diacare.27.11.2597. [DOI] [PubMed] [Google Scholar]
- 8.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 11.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 12.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 13.Hirao K, Arai K, Yamauchi M, Takagi H, Kobayashi M Japan Diabetes Clinical Data Management Study Group. Six-month multicentric, open-label, randomized trial of twice-daily injections of biphasic insulin aspart 30 versus multiple daily injections of insulin aspart in Japanese type 2 diabetic patients (JDDM 11) Diabetes Res Clin Pract. 2008;79:171–176. doi: 10.1016/j.diabres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 14.McFarlane SI, Chaiken RL, Hirsch S, Harrington P, Lebovitz HE, Banerji MA. Near-normoglycaemic remission in African-Americans with Type 2 diabetes mellitus is associated with recovery of beta cell function. Diabet Med. 2001;18:10–16. doi: 10.1046/j.1464-5491.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- 15.Retnakaran R, Drucker DJ. Intensive insulin therapy in newly diagnosed type 2 diabetes. Lancet. 2008;371:1725–1726. doi: 10.1016/S0140-6736(08)60736-9. [DOI] [PubMed] [Google Scholar]
- 16.Gleason CE, Gonzalez M, Harmon JS, Robertson RP. Determinants of glucose toxicity and its reversibility in the pancreatic islet beta-cell line, HIT-T15. Am J Physiol Endocrinol Metab. 2000;279:E997–E1002. doi: 10.1152/ajpendo.2000.279.5.E997. [DOI] [PubMed] [Google Scholar]
- 17.Yki-Järvinen H, Esko N, Eero H, Marja-Riitta T. Clinical benefits and mechanisms of a sustained response to intermittent insulin therapy in type 2 diabetic patients with secondary drug failure. Am J Med. 1988;84:185–192. doi: 10.1016/0002-9343(88)90412-3. [DOI] [PubMed] [Google Scholar]
- 18.Samanta A, Burden AC, Jones GR, Clarkson L. The effect of short term intensive insulin therapy in non-insulin-dependent diabetics who had failed on sulphonylurea therapy. Diabetes Res. 1986;3:269–271. [PubMed] [Google Scholar]
- 19.Glaser B, Leibovich G, Nesher R, Hartling S, Binder C, Cerasi E. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh) 1988;118:365–373. doi: 10.1530/acta.0.1180365. [DOI] [PubMed] [Google Scholar]
- 20.Turner RC, Cull CA, Frighi V, Holman RR UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 21.Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–1088. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 22.He QH, Zhou YS, Wang Z, et al. Improvement of the function of islet beta and alpha cells by intervention against glucotoxicity: experiment with rats. Zhonghua Yi Xue Za Zhi. 2008;88:374–377. [PubMed] [Google Scholar]
- 23.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 24.Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31:240–250. doi: 10.1177/0145721705274927. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy L, Herman WH, Strange P, Harris A GOAL AIC Team. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care. 2006;29:1–8. doi: 10.2337/diacare.29.01.06.dc05-1058. [DOI] [PubMed] [Google Scholar]
- 26.Ovalle F, Bell DS. Effect of rosiglitazone versus insulin on the pancreatic beta-cell function of subjects with type 2 diabetes. Diabetes Care. 2004;27:2585–2589. doi: 10.2337/diacare.27.11.2585. [DOI] [PubMed] [Google Scholar]
- 27.Alvarsson M, Sundkvist G, Lager I, et al. Effects of insulin vs. glibenclamide in recently diagnosed patients with type 2 diabetes: a 4-year follow-up. Diabetes Obes Metab. 2008;10:421–429. doi: 10.1111/j.1463-1326.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 28.Miller FG, Rosenstein DL. The therapeutic orientation to clinical trials. N Engl J Med. 2003;348:1383–1386. doi: 10.1056/NEJMsb030228. [DOI] [PubMed] [Google Scholar]
- 29.Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: consent to research and the therapeutic misconception. Hastings Cent Rep. 1987;17:20–24. [PubMed] [Google Scholar]
- 30.Lidz CW, Appelbaum PS. The therapeutic misconception: problems and solutions. Med Care. 2002;40(9 Suppl):V55–V63. doi: 10.1097/01.MLR.0000023956.25813.18. [DOI] [PubMed] [Google Scholar]
- 31.Miller PB, Weijer C. Trust based obligations of the state and physician-researchers to patient-subjects. J Med Ethics. 2006;32:542–547. doi: 10.1136/jme.2005.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charuvastra A, Marder SR. Unconscious emotional reasoning and the therapeutic misconception. J Med Ethics. 2008;34:193–197. doi: 10.1136/jme.2006.018960. [DOI] [PubMed] [Google Scholar]