Abstract
Background/Aims
The efficacy and safety of pemetrexed, gefitinib, and erlotinib administration in previously treated patients with non-small cell lung cancer (NSCLC) were compared.
Methods
The study patients met the following criteria: histologically confirmed, previously treated advanced (stage IIIB or IV) or recurrent NSCLC; a measurable lesion; ≥ 18 years of age; Eastern Cooperative Oncology Group Performance status 0 to 2; and no prior exposure to the three study drugs. Patients received 500 mg/m2 of pemetrexed intravenously every 3 weeks with vitamin supplementation, gefitinib (250 mg/day per os), or erlotinib (150 mg/day per os).
Results
Of 57 patients (pemetrexed, 20; gefitinib, 20; and erlotinib, 17), 55 were evaluated for a response. The numbers of males, smokers, and squamous histology were increased in the pemetrexed group compared to the other groups. The objective response rates were 5.3%, 25.0%, and 12.5% (p = 0.22), and the disease control rates (DCR) were 5.3%, 40.0%, and 50.0%, respectively (p < 0.01). The median progression-free survival (PFS) was 1.7, 3.5, and 4.4 months (p < 0.01) and the median overall survival (OS) was 5.6, 21.8, and 21.5 months (p = 0.04), respectively. In subgroup analyses, patients with non-squamous histology, males, and a smoking history had a higher DCR and longer PFS with gefitinib and erlotinib than with pemetrexed. All three chemotherapeutic agents had manageable toxicities.
Conclusions
Both oral epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) had comparable efficacy and safety. The superior PFS and OS of EGFR TKIs with more favorable baseline clinical characteristics than those of pemetrexed suggest the impact of baseline clinicopathological factors.
Keywords: Pemetrexed, efitinib, Erlotinib, Lung neoplasms
INTRODUCTION
Lung cancer represents the leading cause of cancer deaths in Korea [1] and Western countries [2]. Non-small cell lung cancer (NSCLC) accounts for approximately 75% of lung cancers [3]. About 50% of NSCLC patients are initially diagnosed with advanced or metastatic disease, and the treatment of choice is palliative chemotherapy. Platinum doublets have superior treatment outcomes over single-agent chemotherapy, and are regarded as standard first-line treatment in advanced NSCLC [4-6]. Although lung cancer has a dismal prognosis, a substantial percentage of patients progress after first-line treatment with good performance status (PS) and adequate organ function. Further salvage treatment should be considered [3].
After docetaxel was approved as a second-line therapy in patients with advanced NSCLC [7], several other drugs were evaluated for their efficacy and safety as potential substitutes. Pemetrexed (Alimta®; Eli Lilly and Company, Indianapolis, IN, USA) is a multi-targeted anti-folate compound. A randomized phase III trial demonstrated non-inferiority of pemetrexed to docetaxel with fewer grade 3 or 4 toxicities [8]. Erlotinib (Tarceva®; Genentech, San Francisco, CA, USA and OSI Pharmaceuticals, Melville, NY, USA) is an oral epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI). A phase III BR.21 study [9] compared erlotinib with best supportive care (BSC) in 731 advanced NSCLC patients. A higher progression-free survival (PFS) and overall survival (OS) was shown in patients treated with erlotinib compared to BSC. Gefitinib (Iressa®; AstraZeneca, Wilmington, DE, USA) is another EGFR TKI that was safe and efficacious at a dose of 250 mg/day in a large-scale phase II trial in patients with advanced NSCLC who had undergone a previous treatment regimen [10]. Although a phase III study by Thatcher et al. [11] failed to demonstrate the superiority of gefitinib over BSC, the subsequent phase III IRESSA NSCLC Trial Evaluating Response and Survival against Taxotere (INTEREST) study [12], which compared gefitinib with docetaxel, reported that gefitinib had similar clinical outcomes, with better tolerability and more convenient administration than docetaxel in previously treated patients with advanced or metastatic NSCLC who had failed platinum-based chemotherapy. Although these drugs were approved and have been commonly used as second-line or salvage therapy in patients with NSCLC, data directly comparing pemetrexed and EGFR TKIs are limited [13]. Therefore, this study compared the efficacy and safety of three agents in previously treated patients with NSCLC.
METHODS
Patients
Previously treated patients with advanced NSCLC at a single institution (Gachon University Gil Hospital, Incheon, Korea) were analyzed retrospectively. Eligibility for the study included the following: histologically or cytologically confirmed NSCLC; ≥ 18 years of age with advanced (stage IIIB or IV) or recurrence at initial diagnosis; Eastern Cooperative Oncology Group (ECOG) PS 0 to 2; at least one measurable lesion; previous chemotherapy without exposure to the three study drugs; and adequate marrow and organ function. This study was reviewed and approved by the Institutional Review Board of Gachon University Gil Hospital.
Treatment
Patients in the pemetrexed group were administered 500 mg/m2 of pemetrexed mixed with 100 mL of normal saline as a 10-minute intravenous infusion on day 1 every 3 weeks. Patients in the gefitinib group received gefitinib (250 mg per os [PO] daily), and patients in the erlotinib group received erlotinib (150 mg PO daily). Cycles were repeated until disease progression, unacceptable toxicity, or the patient declined further treatment. Patients in the pemetrexed group were instructed to take folic acid (1 mg orally daily) from day 7 of the first cycle to the end of pemetrexed treatment and a vitamin B12 (1,000 µg) was injected intramuscularly 1 week before the first dose of pemetrexed in the first cycle, and then every three chemotherapy cycles. A delay of the next cycle for up to 21 days was permitted. For erlotinib, one dose reduction per patient from 150 to 100 mg was permitted in the case of grade 3 or 4 diarrhea or skin reactions.
Evaluation of the tumor response and toxicity
Response Evaluation Criteria in Solid Tumor (RECIST) version 1.0 [14] was used to evaluate the response. Chest computed tomography (CT) and other modalities to evaluate measurable or evaluable lesions were performed within 2 weeks before treatment initiation and every two cycles of pemetrexed or every 2 months of EGFR TKI therapy. Common Terminology Criteria for Adverse Event (CTCAE) version 3.0 [15] was used to identify adverse events.
Statistical consideration
We analyzed the PFS, response rates (RRs), disease control rates (DCRs; the sum of complete response, partial response, and stable disease, as defined by RECIST), safety profiles of each group, and PFS according to clinical characteristics.
Fisher's exact test was applied to compare response rates and toxic effects between treatments. The Kaplan-Meier method was used for survival analysis. The log-rank test was performed for univariate analysis of survival, and variables showing an association with survival on univariate analysis with p < 0.1 were included in multivariate analysis using Cox proportional hazard regression models.
RESULTS
Patient characteristics
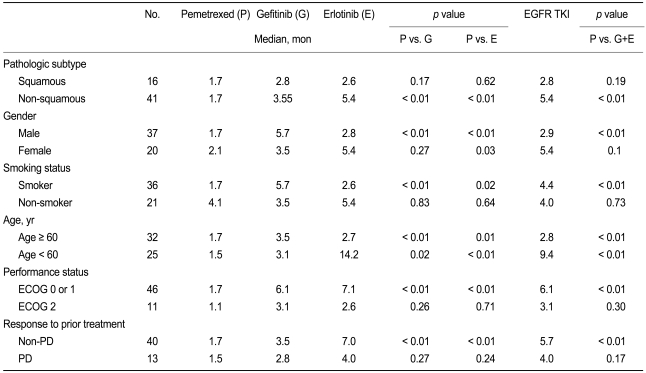
Between September 2005 and August 2008, 57 patients received pemetrexed (n = 20), gefitinib (n = 20), or erlotinib (n = 17). The patient characteristics were balanced, except for the number of cigarette smokers, which was more common in the pemetrexed group (Table 1). The median number of chemotherapy cycles administered was 2 (range, 1 to 4) in the pemetrexed group. The median duration of treatment was 3.2 months (range, 0.8 to 18.3) and 4.4 months (range, 0.5 to 17.5) for patients receiving gefitinib and erlotinib, respectively.
Table 1.
Patient characteristics
Evaluation of tumor response and survival
Two of 57 patients (one each for pemetrexed and gefitinib) were not evaluated for treatment response. The objective RR was 5.3% for pemetrexed, 25% for gefitinib, and 12.5% for erlotinib (p = 0.22). The DCR of pemetrexed, gefitinib, and erlotinib was 5.3%, 40.0%, and 50.0%, respectively (p < 0.01). No patient in the pemetrexed group maintained a partial response (PR) or stable disease defined by the RECIST criteria for at least 1 month, except for one patient with a PR.
The median duration of follow-up was 12.1 months. Fifty-two patients had disease progression (20 in the pemetrexed, 19 in the gefitinib, and 13 in the erlotinib groups) and 20 patients died (8 in the pemetrexed, 7 in the gefitinib, and 5 in the erlotinib groups). The median PFS in the pemetrexed, gefitinib, and erlotinib groups was 1.7, 3.5, and 4.4 months, respectively (p < 0.01). The median OS was 5.6, 21.8, and 21.5 months in the respective groups (p = 0.04). Fig. 1 shows the Kaplan-Meier curves for PFS and OS.
Figure 1.
Kaplan-Meier curves of the (A) progression-free and (B) overall survival of patients treated with pemetrexed, gefitinib, and erlotinib.
Based on univariate analysis, patients administered an EGFR TKI, female, and no cigarette smoking had a significantly better PFS. Multivariate analysis revealed that only the use of an EGFR TKI contributed independently to prolonging the PFS (Table 2). There was no significant difference in the RR (p = 0.43), DCR (p = 0.74), and PFS (p = 0.43) between patients treated with gefitinib and erlotinib.
Table 2.
Univariate and multivariate analyses of the progression-free survival
HR, hazard ratio; CI, confidence interval; EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; Sq., squamous cell carcinoma; ECOG PS, Eastern Cooperative Oncology Group performance status; PD, progressive disease.
The DCRs of patients treated with gefitinib or erlotinib were also higher among patients with non-squamous carcinoma (p < 0.01), male gender (p = 0.02), cigarette smokers (p = 0.02), patients with a good performance status (0 or 1; p < 0.01), patients with a good prior response (p < 0.01), and patients ≥ 60 years of age (p = 0.03) than patients treated with pemetrexed. Analysis of the PFS according to clinical factors is summarized in Table 3.
Table 3.
Analysis of the progression-free survival according to clinical factors
EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; ECOG, Eastern Cooperative Oncology Group; PD, progressive disease.
Twelve of 20 patients in the gefitinib group and 10 of 17 patients in the erlotinib group received subsequent pemetrexed as a salvage treatment after progression. Three of these 22 patients achieved a partial response (PR); however, the response lasted for less than 1 month. In the pemetrexed group, 16 of 20 patients received gefitinib (2 of 16) or erlotinib (14 of 16) as salvage therapy after progression; only one patient had a PR with a duration of response of 4.5 months.
Toxicity
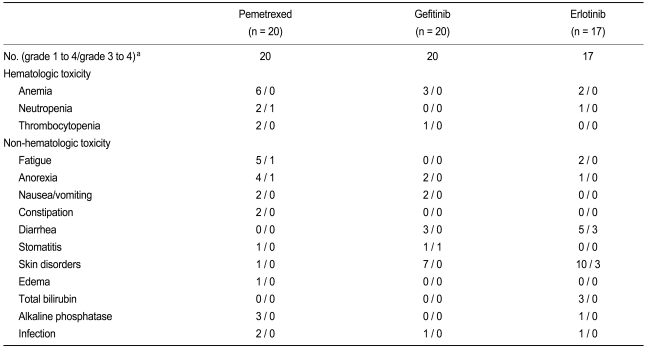
All treated patients (n = 57) were assessable for toxicity. Of the erlotinib group, 11.7% required a dose reduction because of drug-related toxic effects. The median number of chemotherapy cycles administered was 2 (range, 1 to 9) in the pemetrexed group. The median duration of treatment was 3.2 months for the pemetrexed group, and 4.4 months for the patients receiving gefitinib and erlotinib. One patient in the gefitinib group and three patients in the erlotinib group discontinued EGFR TKI because of severe or prolonged non-hematologic toxicity (one patient for diarrhea in the gefitinib group and three patients for skin rashes in the erlotinib group). The frequently reported toxicities in the gefitinib and erlotinib groups were skin disorders (rash, dry skin, pruritus, and acne), diarrhea, and anorexia. The toxicity profiles are shown in Table 4.
Table 4.
Toxicity profiles
aAccording to the National Cancer Institute-Common Toxicity Criteria, version 3.0.
DISCUSSION
In contrast to pemetrexed [8] and gefitinib [12], which were not inferior to docetaxel in a large phase III trial, there are no reported clinical trials that directly compare erlotinib with docetaxel. Instead, erlotinib was approved as a second- or third-line agent based on the result of the BR21 study [9] comparing erlotinib with placebo. In BR21, the median OS and median PFS improved significantly with erlotinib (6.7 vs. 4.7 months for OS; p = 0.002 and 2.2 vs. 1.8 months for PFS; p < 0.001). In our study, erlotinib was efficacious and safe, comparable to the other EGFR TKI, gefitinib. There was no difference in the baseline characteristics, RRs, DCRs, or survivals between the gefitinib and erlotinib arms.
No prospective trials have reported the results of a comparison between pemetrexed and EGFR TKIs after the failure of first-line chemotherapy. Although treatment with gefitinib or erlotinib was associated with longer survival in our study, the result should be interpreted cautiously because our study has several limitations. The numbers of patients in each group were not large enough for a conclusive analysis and the baseline patient characteristics were not stratified homogeneously in this study. The numbers of male patients, smokers, and squamous cell carcinoma were higher in the pemetrexed arm than in the other groups, although the differences were not significant, except for smoking. Many previous studies reported good efficacy and improved survival of both EGFR TKIs for ethnic Asians, women, non-smokers, and non-squamous histology [9-11,16,17]. Based on these results, the Korean Health Insurance Review & Assessment Service accepted the use of EGFR TKIs as second-line treatment if at least two of the following three are satisfied: female gender, adenocarcinoma, and non-smoker [18]. As this was a retrospective study that analyzed previously treated NSCLC patients who underwent salvage chemotherapy in clinical practice without specific standards for the use of one of the three drugs, the criteria of the Korean Health Insurance Service for EGFR TKIs probably affected the heterogeneous patient characteristics because TKIs are more convenient as they do not need intravenous administration, unlike pemetrexed.
The larger number of patients with squamous histology might also be a disadvantage for the pemetrexed arm. Pemetrexed shows higher efficacy in advanced NSCLC with non-squamous histology. In a prospective phase III study [19], cisplatin with pemetrexed conferred similar efficacy with better tolerability than cisplatin with gemcitabine as first-line chemotherapy in advanced NSCLC. In this large-scale (1,725 patients) study, the OS of the patients with adenocarcinoma or large cell carcinoma histology on cisplatin in the pemetrexed arm was significantly longer than the OS of patients with the same histology on cisplatin with gemcitabine (for adenocarcinoma, median 12.6 vs. 10.9 months, p = 0.03; for large cell carcinoma, median 10.4 vs. 6.7 months, p = 0.03). By contrast, for patients with squamous cell carcinoma, cisplatin with pemetrexed had a slightly shorter OS than cisplatin with gemcitabine (median 9.4 vs. 10.8 months, respectively, p = 0.05). This difference in the clinical characteristics probably contributed to the substantially poorer tumor response and disease control in this study (Eighteen of 19 evaluable patients had progressive disease as their best response after pemetrexed), compared to the previous study [8]. A hypothesis generated by this study is "baseline clinicopathological characteristics have substantial effects on clinical outcomes" rather than "EGFR TKIs are superior to pemetrexed."
Of the 41 patients with non-squamous carcinoma, a longer PFS in patients treated with gefitinib or erlotinib than pemetrexed (p < 0.01) was observed. This may reflect 4 of 11 (36.4%) 'non-small cell carcinomas' in which further detailed pathology could not be determined. In the patients with squamous carcinoma, the outcomes were universally poor, and no superiority of TKIs was shown. Superiority of TKIs was also observed in patients with a good PS, a good prior response, male gender, or cigarette smokers, although the interpretation should be made with caution because the results of univariate or multivariate analysis have limitations with a small sample size.
Since no head-to-head comparison with docetaxel exists, second-line treatment of advanced NSCLC with erlotinib remains controversial in the UK [13]. The results are pending for a randomized trial with erlotinib as second-line treatment versus docetaxel or pemetrexed (Tarceva in Treatment of Advanced NSCLC [TITAN study]). The accumulation of clinical data and the application of genetic mutational analysis will enable more efficacious and tailored therapy in patients with advanced NSCLC.
In summary, this retrospective study showed that both oral EGFR TKIs had comparable efficacy with manageable toxicities. The comparison between pemetrexed and the EGFR TKIs was limited by the heterogeneous baseline patient characteristics. The superior PFS and OS of the patients with EGFR TKIs with more favorable clinical factors compared to those of the patients with pemetrexed reflects the effect of baseline characteristics and underlines the necessity of patient selection according to baseline clinicopathological factors for optimal treatment in advanced NSCLC.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol. 2008;38:327–333. doi: 10.1093/jjco/hyn026. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Stinchcombe TE, Socinski MA. Considerations for second-line therapy of non-small cell lung cancer. Oncologist. 2008;13(Suppl 1):28–36. doi: 10.1634/theoncologist.13-S1-28. [DOI] [PubMed] [Google Scholar]
- 4.Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–130. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 6.Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol. 1998;16:2459–2465. doi: 10.1200/JCO.1998.16.7.2459. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 8.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 10.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 11.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 13.Boysen M, Longson C, Stevens A. Erlotinib for the treatment of non-small-cell lung cancer. Lancet Oncol. 2009;10:15–16. doi: 10.1016/s1470-2045(08)70283-3. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Bethesda (MD): National Cancer Institute - Cancer Therapy Evaluation Program; 2006. [cited at 2010 Jul 2]. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [Internet] Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30. [Google Scholar]
- 16.Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Korean Health Insurance Review and Assessment Service [Internet] Seoul: Korean Health Insurance Review and Assessment Service; c2009. [cited 2009 Jan 01]. Korean Health Insurance Review and Assessment Service Announcement No. 2009-1. Available from: http://www.hira.or.kr/common/dummy.jsp?pgmid=HIRAA030103000000. [Google Scholar]
- 19.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]