Abstract
The evolutionarily conserved mRNA export receptor Mex67/NXF1 associates with mRNAs through its adaptor, Yra1/REF, allowing mRNA ribonucleoprotein (mRNP) exit through nuclear pores. However, alternate adaptors should exist, since Yra1 is dispensable for mRNA export in Drosophila and Caenorhabditis elegans. Here we report that Mex67 interacts directly with Nab2, an essential shuttling mRNA-binding protein required for export. We further show that Yra1 enhances the interaction between Nab2 and Mex67, and becomes dispensable in cells overexpressing Nab2 or Mex67. These observations appoint Nab2 as a potential adaptor for Mex67, and define Yra1/REF as a cofactor stabilizing the adaptor–receptor interaction. Importantly, Yra1 ubiquitination by the E3 ligase Tom1 promotes its dissociation from mRNP before export. Finally, loss of perinuclear Mlp proteins suppresses the growth defects of Tom1 and Yra1 ubiquitination mutants, suggesting that Tom1-mediated dissociation of Yra1 from Nab2-bound mRNAs is part of a surveillance mechanism at the pore, ensuring export of mature mRNPs only.
Keywords: Mex67/NXF1, Nab2, Yra1/REF, Tom1, mRNA export, ubiquitination
The cellular separation of transcription and translation in eukaryotic cells necessitates the transport of mRNAs through the nuclear pores (NPCs) by a machinery conserved from yeast to humans. Factors mediating mRNA maturation and export are loaded cotranscriptionally on nascent transcripts, resulting in the formation of mRNA ribonucleoprotein complexes (mRNPs) competent for export (Iglesias and Stutz 2008). Some heterogeneous nuclear RNPs (hnRNPs) associated with mRNPs are removed from the mRNA prior to export, whereas others shuttle between the nucleus and cytoplasm and accompany the mRNA through the NPC (Mili et al. 2001).
In Saccharomyces cerevisiae, Mex67, in concert with its cofactor, Mtr2, promotes the translocation of mature mRNPs into the cytoplasm by direct interaction with nucleoporins lining the pore. Despite its major role in mRNA export, Mex67/NXF1 binds RNA with low affinity and requires adaptor protein(s) to interact stably with mRNPs (Segref et al. 1997; Gruter et al. 1998; Santos-Rosa et al. 1998; Katahira et al. 1999). Yra1 in yeast and its metazoan counterpart, Aly/REF, were, until recently, the only known and alleged adaptors for Mex67/NXF1 (Strasser and Hurt 2000; Stutz et al. 2000; Zhou et al. 2000; Rodrigues et al. 2001; Zenklusen et al. 2001). However, other adaptors are likely to mediate the association of Mex67/NXF1 with mRNPs, since Yra1 does not associate with all yeast transcripts, and Aly/REF is not essential for mRNA export in Drosophila or Caenorhabditis elegans (Gatfield and Izaurralde 2002; Hieronymus and Silver 2003; Longman et al. 2003; MacMorris et al. 2003). Indeed, two shuttling SR (serine/arginine-rich) proteins—SRp20 and 9G8 in mammals (Huang and Steitz 2001; Huang et al. 2003)—and the SR-like protein Npl3 in yeast (Gilbert and Guthrie 2004) have been identified as proteins that act as other adaptors for Mex67/NXF1, as they bind directly to mRNAs and Mex67/NXF1.
Nab2—a shuttling hnRNP protein that strongly and specifically associates with nuclear poly(A)+ RNA in vivo—is required for both poly(A)+ tail length control and nuclear export of mRNA (Anderson et al. 1993; Green et al. 2002; Hector et al. 2002; Kelly et al. 2007). Previous models predicted the existence of at least two different mRNA export pathways defined by Npl3 or Nab2. This view is based on the observation that the ubiquitin E3 ligase Tom1 is required for the export of Nab2 and Nab2-bound mRNAs, but not the export of Npl3 (Duncan et al. 2000), and an early genome-wide analysis indicated that Npl3 and Nab2 interact with distinct but overlapping sets of transcripts (Kim Guisbert et al. 2005). However, a recent analysis of Nab2-bound transcripts through RNA sequencing, a more sensitive and exhaustive approach, showed that Nab2 associates with most transcripts and is therefore likely to represent a global mRNA export factor (Batisse et al. 2009).
mRNA biogenesis and packaging into export-competent mRNP complexes is subject to a variety of quality control steps (Jensen et al. 2003; Vinciguerra and Stutz 2004). More specifically, the Mlp1 and Mlp2 proteins associated with the inner basket of the NPC have been implicated in a late step of mRNP surveillance by retaining incorrectly processed or misassembled mRNP complexes (Green et al. 2003; Galy et al. 2004; Vinciguerra et al. 2005). We reported previously a common role of Nab2 and Yra1 in the docking of mRNP complexes to Mlp proteins, an event probably associated with mRNP surveillance at the pore. In addition, the ability of Nab2 overexpression to suppress yra1 mutant phenotypes indicated overlapping functions for Nab2 and Yra1 (Vinciguerra et al. 2005).
Here we show not only that Nab2 interacts directly with both Mex67 and Yra1, but that Yra1 also stimulates the interaction between Mex67 and Nab2. These observations, together with the dispensable nature of Yra1 in the presence of an excess of Mex67 or Nab2, suggest that Nab2 may act as an adaptor for Mex67, and that Yra1 chaperones this interaction. Importantly, we show that Yra1 is ubiquitinated by Tom1, identifying the first substrate of this E3 ligase with a role in mRNA export, and demonstrate that ubiquitination of Yra1 by Tom1 promotes its dissociation from mRNP complexes. Accordingly, Yra1 lysine mutants defective in ubiquitination lead to increased Yra1 bound to nuclear mRNPs and a defect in mRNA export. Finally, loss of perinuclear Mlp proteins suppresses the growth defects of Δtom1 as well as Yra1 lysine mutants. These data are consistent with the view that Yra1 ubiquitination by Tom1 is part of a surveillance mechanism that promotes the dissociation of Yra1 from the Nab2-bound mRNP complex, thereby facilitating the export of mature and properly assembled mRNPs into the cytoplasm.
Results
Nab2 interacts directly with Mex67 and Yra1
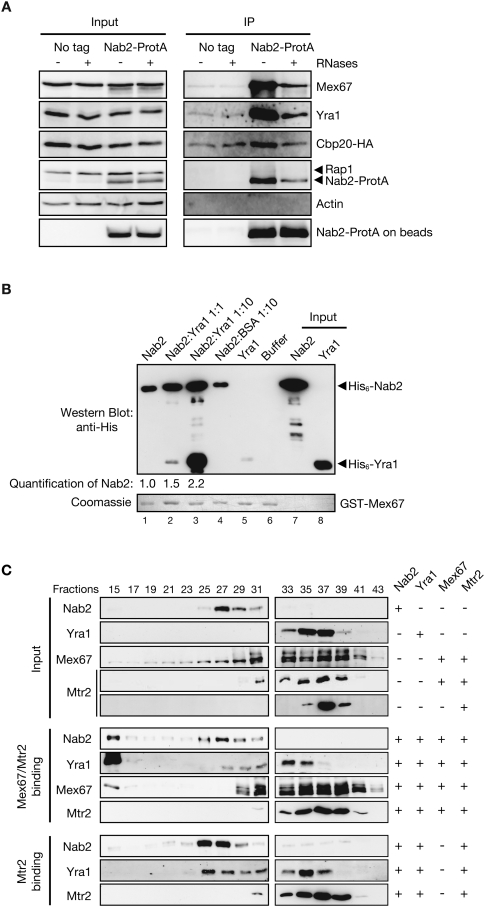
Since Nab2 plays a critical role in mRNA export (Green et al. 2002) and binds directly to poly(A)+ RNA (Anderson et al. 1993; Marfatia et al. 2003), we hypothesized that Nab2 could function as a factor linking Mex67 to mRNA. Consistent with this possibility, Mex67 copurifies efficiently with Nab2-ProtA but not with the untagged control strain, indicating that the interaction observed is specific (Fig. 1A). Yra1 also copurifies with Nab2, in agreement with earlier studies (Kashyap et al. 2005; Oeffinger et al. 2007). The interaction between Nab2 and Mex67 or Yra1 was also observed when cells were cross-linked for 5 min with 1.2% formaldehyde prior to coimmunoprecipitation, confirming that these proteins interact in vivo (Supplemental Fig. 1A). No interaction was detected between Nab2 and Actin or Rap1, two unrelated proteins (Fig. 1A; Supplemental Fig. 1A), indicating that the interaction observed between Nab2 and Mex67 or Yra1 is specific. Moreover, treating the extracts with RNases prior to purification strongly affected the association of Nab2 with Cbp20, indicating that the RNA digestion was efficient. RNase treatment, however, did not abolish the interaction of Mex67 or Yra1 with Nab2 (Fig. 1A), indicating that a fraction of Mex67 and Yra1 may interact directly with Nab2.
Figure 1.
Nab2, Yra1, and Mex67 form a trimeric complex. (A) Nab2 coimmunoprecipitates Mex67 and Yra1 in vivo in an RNA-independent manner. Yeast extracts from Nab2-ProtA or nontagged (No tag) cells and expressing HA-tagged Cbp20 were treated (+) or not (−) with RNases and purified with IgG-Sepharose beads. Input and eluted fractions (IP) were analyzed by Western blotting with anti-Mex67, anti-Yra1, anti-HA, anti-Rap1, anti-Actin, and anti-ProtA antibodies. (B) Yra1 promotes binding of Nab2 and Mex67. GST-Mex67 on beads was incubated with purified His6-Nab2 (lane 1), His6-Nab2 plus equimolar (lane 2) or 10× molar (lane 3) amounts of purified His6-Yra1, BSA (lane 4), purified His6-Yra1 alone (lane 5), or buffer alone (lane 6). His6-Nab2 and His6-Yra1 input are in lanes 7 and 8, respectively. Western blot (top panel) with anti-His antibodies and Coomassie staining for GST-Mex67 (bottom panel) are shown. Quantification of the signal observed for His6-Nab2 association with GST-Mex67 is indicated below. (C) Size fractionation of purified Mex67/Mtr2, Nab2, and Yra1 alone or in complex. His6-Nab2-, His6-Yra1-, His6-Mtr2/Mex67-, or His6-Mtr2-purified proteins were loaded on S200 sizing columns alone or after complex assembly of His6-Mtr2/Mex67 or His6-Mtr2 with His6-Nab2 and His6-Yra1 as indicated on the right. (Top numbers) Collected fractions were analyzed by Western blotting with antibodies specific for Nab2, Yra1, Mex67, and Histidine to detect Mtr2.
Accordingly, in vitro binding experiments show that recombinant histidine-tagged Nab2 specifically copurifies with both GST-Mex67 and GST-Yra1 (Supplemental Fig. 1B,C). To define the region(s) of Nab2, Mex67, and Yra1 implicated in these interactions, the association between various portions of these proteins was either tested in vitro (Supplemental Fig. 2) or analyzed in a two-hybrid assay (Supplemental Fig. 3). The data indicate that the direct interaction of Mex67 or Yra1 with Nab2 involves both the RGG and CCCH domains of Nab2 (Supplemental Fig. 2B [lane 5], C [lane 6]). The N + LRR domain required for binding of both Mex67 and its human ortholog, NXF1, to Yra1/REF (Stutz et al. 2000) was sufficient for Nab2 binding (Supplemental Figs. 2A, 3). Finally, the association between Yra1 and Nab2 involves the Yra1-RBD, as the interaction was observed using a GST-Yra1-RBD fusion (Supplemental Fig. 2D, lane 7). Collectively, these data indicate that Mex67 and Yra1 interact directly with Nab2, and support the view that Nab2 could represent an alternate adaptor for Mex67.
Nab2, Mex67, and Yra1 form a tripartite complex in vitro
To directly test whether Nab2 and Yra1 form either distinct complexes with Mex67 or a tripartite complex, we first incubated GST-Mex67 on beads (Fig. 1B, bottom panel) with purified His6-Nab2 alone, or together with equimolar amounts or a 10-fold molar excess of purified His6-Yra1 (Fig. 1B, top panel, lanes 1–3). We found that increasing the amount of His6-Yra1 improved Nab2 association with GST-Mex67, while the addition of BSA had no effect (Fig. 1B, top panel, lane 4). Notably, the binding of His6-Yra1 to GST-Mex67 was also enhanced in the presence of His6-Nab2 (Fig. 1B, top panel, cf. lanes 5 and 2). These results show that Nab2 and Yra1 interact cooperatively with Mex67 in vitro rather than compete, strongly suggesting the formation of a Nab2–Mex67–Yra1 ternary complex.
The existence of such a ternary complex formation was further examined by size fractionation on an S200 column of the three recombinant proteins alone or after complex assembly (Fig. 1C). The binding reactions included His6-Yra1, His6-Nab2, and Mex67, purified from Escherichia coli as a heterodimer with His6-Mtr2 as described (Santos-Rosa et al. 1998). Western blot analysis of eluted samples showed that a fraction of each protein shifted into a fast-migrating high-molecular-weight complex when combined with the two other partners, clearly distinct from the slower-migrating individual proteins, confirming the ability of these three proteins to interact simultaneously (Fig. 1C, see Nab2, Mex67, or Yra1 in fraction 15). Interestingly, assembly of Mex67–Mtr2 with Nab2 and Yra1 induced a high-molecular-weight complex that excluded Mtr2, suggesting that Mex67 cannot interact with Mtr2 while in complex with both Nab2 and Yra1. Together, these results show that Mex67, Yra1, and Nab2 can form a tripartite complex, with Yra1 facilitating or stabilizing the interaction between Nab2 and Mex67.
Mex67, Nab2, and Yra1 function in the same mRNA export pathway
These physical in vitro interactions establish a strong functional link among these three proteins. Consistently, all nab2 domain deletion mutants examined exacerbate the growth defect of the formerly described GFP-yra1-8 temperature-sensitive mutant (Supplemental Fig. 4A; Vinciguerra et al. 2005), confirming a functional interaction between Yra1 and Nab2. In addition, the nab2ΔRGG mutation exhibits synthetic lethality with the temperature-sensitive mex67-5 and mex67-6 mutants (Segref et al. 1997; Strasser and Hurt 2000) at 30°C, while Nab2 overexpression partially suppresses the growth defect of these two mutants at 34°C (Supplemental Fig. 4B,C). Altogether, these genetic interactions functionally link Nab2 to the mRNA export factors Yra1 and Mex67.
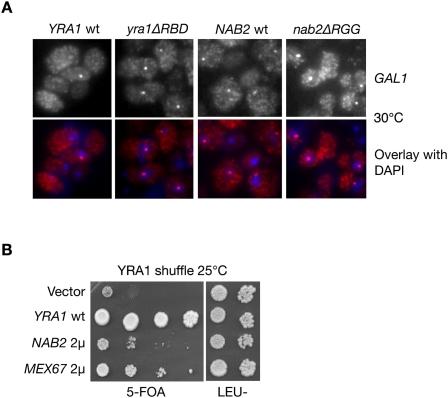
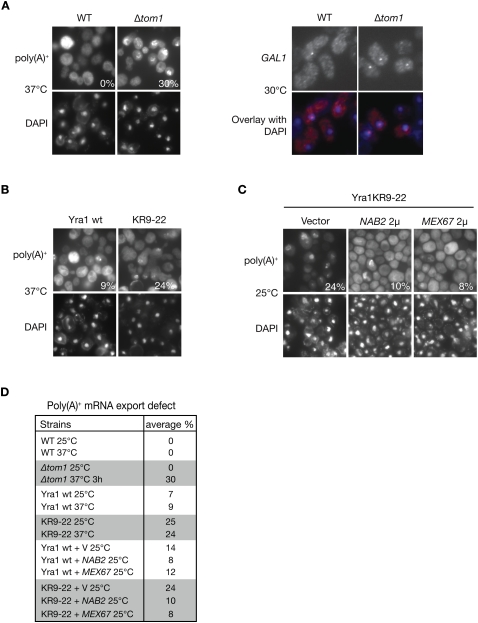
To define whether Nab2 and Yra1 mediate the export of the same transcripts, we examined the nucleo–cytoplasmic distribution of the specific GAL1 mRNA in the nab2ΔRGG and yra1ΔRBD mutants, shown previously to be defective in poly(A)+ mRNA export (Zenklusen et al. 2001; Green et al. 2002). GAL1 transcription was induced by growing wild-type and mutant cells for 30 min in galactose, and fixed cells were subsequently analyzed by fluorescent in situ hybridization (FISH) with GAL1-specific fluorescent probes (Fig. 2A). In wild-type cells, GAL1 mRNAs were detected mainly in the cytoplasm and also slightly in the nucleus, where they accumulated within a dot close to the nuclear periphery (see overlay of FISH and DAPI staining). This distribution is consistent with earlier studies in wild-type cells showing that the activated GAL1 gene relocates to the nuclear periphery, where GAL1 transcripts accumulate as an RNA dot in the vicinity of the NPC (Abruzzi et al. 2006). Importantly, brighter GAL1 mRNA nuclear dots were detected in the majority of nab2ΔRRG and yra1ΔRBD mutants cells, indicating that GAL1 mRNA stayed close to their transcription site longer, thus revealing a decreased efficiency of release and export (Fig. 2A). Thus, GAL1 mRNA nuclear export depends on both Yra1 and Nab2, indicating that these factors act in the same pathway. Finally, we found that overexpression of Mex67 or Nab2 can bypass the lethal phenotype of a YRA1 knockout strain (Fig. 2B), consistent with the view that Yra1 is not absolutely required for export, but acts as a cofactor favoring the interaction between Nab2 and Mex67.
Figure 2.
Nab2 and Yra1 are part of the same mRNA export pathway. (A) FISH analysis of GAL1 mRNA in the indicated cells induced with galactose for 30 min at 30°C. DAPI staining or FISH/DAPI overlays are shown below each panel to localize the RNA signal with respect to the nucleus. (B) Δyra1 is bypassed by overexpressing NAB2 or MEX67. The Yra1 shuffle strain transformed with the indicated plasmids was spotted as 10-fold serial dilutions onto 5-FOA to select against the wild-type pURA3-YRA1 plasmid, or onto a selective plate to control for cell number (last two dilutions only). (2μ) High-copy plasmids.
Yra1 is ubiquitinated by the E3 ligase Tom1
While Nab2 and Mex67 are shuttling mRNA-binding proteins, Yra1 remains nuclear, and was proposed to dissociate from the mRNP prior to accessing the NPC (Strasser and Hurt 2000; Stutz et al. 2000; Lund and Guthrie 2005). Importantly, the nuclear ubiquitin E3 ligase Tom1 is required for the export of Nab2-bound mRNP complexes. Ubiquitination by Tom1 of a yet unidentified partner of Nab2 has thus been proposed to control mRNA export (Duncan et al. 2000). Our finding that Yra1, Nab2, and Mex67 form a ternary complex operating in the same export pathway raised the question of whether one of these proteins was ubiquitinated by Tom1, and whether the dynamics of this trimeric complex might be regulated by ubiquitin.
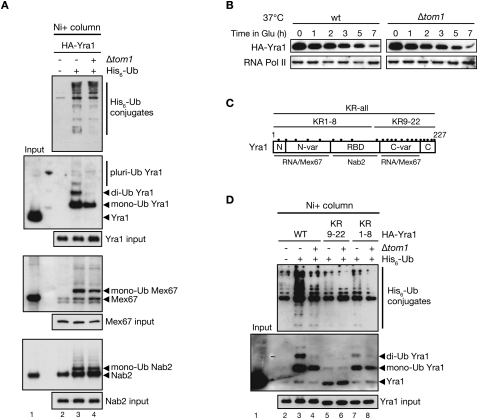
To answer this question, ubiquitinated proteins from a wild-type or a Δtom1 strain overexpressing copper-inducible His6-tagged ubiquitin were purified on nickel columns and analyzed by immunoblotting for the presence of ubiquitinated Yra1, Mex67, or Nab2 (Fig. 3A). Interestingly, Yra1 as well as Mex67 and Nab2 are ubiquitinated in a wild-type strain (Fig. 3A, lane 3). Both Mex67 and Nab2 were detected mainly as monoubiquitinated species, while monoubiquitinated and diubiquitinated as well as low amounts of pluriubiquitinated forms of Yra1 were observed. Importantly, loss of the E3 ligase Tom1 did not affect ubiquitination of Nab2 and Mex67, but substantially reduced the monoubiquitinated and eliminated the diubiquitinated forms of Yra1, indicating that Yra1 is monoubiquitinated and diubiquitinated in a Tom1-dependent manner (Fig. 3A, cf. lanes 3 and 4). This conclusion is further supported by the fact that Tom1 interacts with Yra1 in vivo (Supplemental Fig. 5A; Oeffinger et al. 2007). The remaining monoubiquitinated form of Yra1 in Δtom1 indicates that an additional, yet unidentified, E3 ligase monoubiquitinates Yra1, probably on another lysine.
Figure 3.
Yra1 is ubiquitinated by Tom1. (A) HA-YRA1 cDNA-shuffled (lanes 2,3) and Δtom1 HA-YRA1 cDNA-shuffled (lane 4) strains containing a PCUP1∷HIS6-ubiquitin plasmid (+) or an empty vector (−). His6-Ub-conjugated forms purified on nickel column (Ni+ column) were examined with their corresponding total input extracts by Western blotting with anti-His, anti-HA (Yra1), anti-Nab2, and anti-Mex67 antibodies. (Lane 2) The empty vector allowed us to control for background binding to the nickel column. (B) Tom1 does not affect Yra1 turnover. Wild-type (wt) and Δtom1 cells were transformed with a plasmid expressing HA-Yra1 from a galactose (GAL)-inducible promoter. Cells were exponentially grown in galactose. At time 0, cultures were shifted to 37°C to exacerbate the potential instability, and HA-Yra1 expression was shut off by the addition of 2% glucose. Yra1 turnover was followed by collecting protein samples at the indicated time points in glucose and Western blotting with antibodies against HA or the C-terminal domain of RNA polymerase II (RNA Pol II) to confirm equal loading. (C) Yra1 domain organization and binding partners, and position of the 22 lysines (black dots). (D) Yra1 ubiquitination in Yra1 wild-type or KR mutant strains. His6-Ub-conjugated forms of HA-Yra1 from YRA1-shuffled (lanes 2,3,5,7) or Δtom1 YRA1-shuffled (lanes 4,6,8) strains expressing the indicated wild-type and Yra1 KR mutant plasmids were purified and analyzed as in A.
Polyubiquitin chains can be formed by different linkages of lysine residues in the ubiquitin molecule, the most common ones being K48- and K63-linked polyubiquitination. K48-linked polyubiquitination often leads to proteasome-mediated degradation, while K63 linkages are used for other functions (Pickart 2000). To define whether the Tom1-dependent Yra1 diubiquitination might regulate Yra1 amount (K48) or function (K63), the ubiquitination assay was performed in wild-type cells expressing wild-type His6-Ub or the lysine-to-arginine ubiquitin mutants His6-Ub-K48R and His6-Ub-K63R. The diubiquitinated form of Yra1 was lost in the presence of the His6-Ub-K48R mutant, but not with the His6-Ub-K63R mutant, indicating that the diubiquitin is branched at Lys 48, a linkage often associated with protein targeting to the proteasome (Supplemental Fig. 5B). To determine whether Tom1-dependent Yra1 ubiquitination targets Yra1 for degradation, Yra1 protein turnover was examined in wild-type and Δtom1 cells transformed with a plasmid expressing HA-tagged Yra1 from a galactose (GAL1)-inducible promoter. The steady-state level of Yra1 was not increased in Δtom1 compared with wild-type cells when Yra1 transcription was shut off by shifting the cells to glucose-containing media (Fig. 3B). Therefore, monoubiquitination and diubiquitination of Yra1 by Tom1 appears to regulate primarily Yra1 function rather than its degradation by the proteasome.
To analyze the role of ubiquitination in Yra1 function, identification of the lysines subjected to modification was carried out. For this purpose, all 22 lysines of Yra1 were mutated one by one into arginine using site-directed mutagenesis. Unfortunately, none of these individual lysine mutants of Yra1 had an effect on ubiquitination (data not shown), indicating that Tom1 and the other E3 ligase were able to ubiquitinate an adjacent lysine when their normal target site was mutated. We therefore took a more drastic approach by synthesizing three Yra1 mutant cDNA sequences in which all lysine codons (KR-all), Lys 1–Lys 8 (KR1–8), or Lys 9–Lys 22 (KR9–22) were converted into arginine codons (Fig. 3C). Similar to the wild-type HA-Yra1-expressing construct used above, mutant sequences were expressed with an N-terminal HA tag and controlled by the natural YRA1 promoter and terminator sequences. All three Yra1 KR mutants are viable but temperature-sensitive, the strongest growth defect being observed in the presence of Yra1 KR-all (Supplemental Fig. 5C). Importantly, when GFP-tagged, all three Yra1 KR mutant proteins localized in the nucleus as efficiently as wild-type GFP-tagged Yra1 (Supplemental Fig. 5D), indicating that the lysine-to-arginine mutations did not perturb their localization.
The three Yra1 KR mutant strains were then subjected to a ubiquitination assay to identify which part of the Yra1 protein is ubiquitinated by Tom1 and the other E3 ligase. As anticipated, no ubiquitinated Yra1 species were detected in cells expressing the Yra1 KR-all mutant (Supplemental Fig. 6A, cf. lanes 3 and 4). Importantly, the Yra1 KR1–8 exhibited the same ubiquitination profile as the Yra1 wild type in the presence or absence of Δtom1 (Fig. 3D, cf. lanes 3 and 7, and lanes 4 and 8), indicating that Yra1 ubiquitination does not occur on the eight lysines located within its N-terminal and RBD domains. In contrast, both monoubiquitinated and diubiquitinated Yra1 species were lost in the Yra1 KR9–22 mutant whether in the presence or absence of Tom1 (Fig. 3D, cf. lanes 3–6), indicating that the ubiquitinated lysines are located either within the C-terminal variable (C-var) or highly conserved (C) region of Yra1 (Fig. 3C). Because no substantial amounts of triubiquitinated Yra1 could be detected, it is likely that Tom1-dependent and Tom1-independent ubiquitinations (among Lys 9–Lys 22) take place on different pools of Yra1, potentially implicated in different steps of mRNA export or distinct Yra1 functions.
Tom1-dependent ubiquitination releases Yra1 from Nab2–Mex67–mRNP complexes
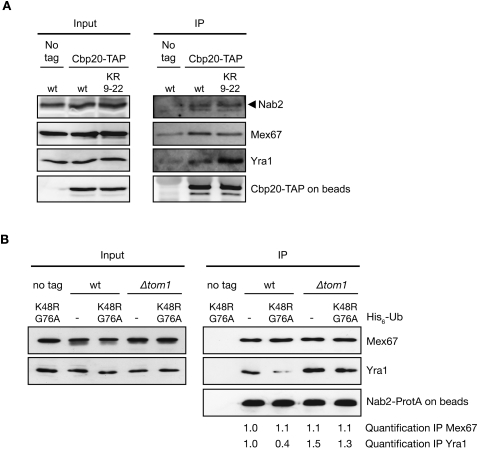
To determine whether Yra1 ubiquitination controls the levels of Yra1 associated with export-competent mRNPs, we affinity-purified nuclear mRNPs from strains expressing wild type or the Yra1 KR9–22 mutant using a genomically TAP-tagged version of the nuclear cap-binding protein Cbp20. Cbp20 binds to the 5′ cap of all transcripts in the nucleus, and is replaced by eIF4E after export into the cytoplasm (Fortes et al. 1999). TAP-tagged Cbp20 should therefore specifically select nuclear mRNPs during affinity purification. Cbp20-associated mRNP complexes were eluted from the beads with high salt and analyzed for the presence of HA-Yra1 wild type, HA-Yra1 KR9–22, Mex67, and Nab2. Using this approach, Mex67, Nab2, and HA-Yra1 could be coprecipitated specifically with Cbp20-TAP and not with untagged Cbp20 (Fig. 4A). In addition, these interactions were inhibited by pretreating the extracts with RNase A, indicating that association of Mex67, Nab2, and HA-Yra1 with Cbp20 is bridged by RNA, and that nuclear mRNP complexes were indeed selected under these conditions (Supplemental Fig. 6B). As shown in Figure 4A, the levels of Mex67 and Nab2 bound to nuclear mRNPs remained unchanged in both wild-type and Yra1 KR9–22 mutant backgrounds, confirming that similar amounts of mRNP complexes had been selected from wild-type and mutant extracts. In contrast, higher amounts of HA-Yra1 protein were reproducibly purified in complex with Cbp20-TAP from cells expressing Yra1 KR9–22 mutant protein, although total extracts contain comparable amounts of wild-type Yra1 and Yra1 KR9–22 mutant proteins (Fig. 4A). These data support the view that lack of Yra1 ubiquitination by Tom1 results in increased levels of Yra1 associated with nuclear mRNPs.
Figure 4.
Yra1 ubiquitination reduces interaction with mRNP components. (A) The Yra1 KR9–22 lysine mutant increases the association of Yra1 with nuclear mRNPs. Total extracts (Input) or IgG-Sepharose-purified extracts (IP) from Cbp20-TAP-tagged or nontagged (No tag) strains expressing wild-type or KR9–22 mutant Yra1 were analyzed by Western blotting with anti-Nab2, anti-Mex67, and anti-HA antibodies to detect Yra1. Bead-associated Cbp20-TAP was revealed with an anti-ProtA antibody, and served as control for equal amounts of mRNP pulled down. (B) Tom1-mediated ubiquitination of Yra1 induces Yra1 release from Nab2–Mex67 complexes. Total extracts prepared from Nab2 ProtA-tagged or nontagged (no tag) strains transformed with UbK48R,G76A or empty vector (−) were purified on IgG beads. Western blotting of total extract (Input) or high-salt eluted proteins (IP) were examined with antibodies against Mex67, Yra1, and ProtA. Quantification of the signal observed in the Nab2-ProtA immunoprecipitate for Mex67 and Yra1 (normalized to the signal in wild-type cells transformed with empty vector) is indicated below.
To confirm this result, we took advantage of the ubiquitin mutant UbK48R,G76A to increase and stabilize the Yra1 ubiquitinated pool. Indeed, transient expression of UbK48R,G76A is expected to result in the accumulation of monoubiquitinated Yra1, since K48 is required for ubiquitin chain elongation by Tom1, and G76 is essential for cleavage by ubiquitin isopeptidases (Finley et al. 1994; Wilkinson et al. 1995). As anticipated, only monoubiquitinated Yra1 was indeed detected in cells expressing His6-tagged UbK48R,G76A (Supplemental Fig. 6C). Moreover, purification of TAP-tagged Cbp20 from cells expressing mutant UbK48R,G76A revealed a modest decrease in the amount of Yra1 associated with nuclear mRNPs compared with those expressing wild-type ubiquitin (Supplemental Fig. 6D). Together, these experiments indicate that ubiquitination favors Yra1 dissociation from nuclear mRNPs.
To more precisely address whether Tom1-mediated ubiquitination controls the integrity of the Yra1–Nab2–Mex67 complex, wild-type or Δtom1 strains expressing ProtA-tagged Nab2 were transformed with the plasmid expressing UbK48R,G76A or an empty vector. Lysates were purified on IgG-Sepharose beads, and eluted proteins copurifying with Nab2-ProtA were analyzed for the presence of Mex67 or Yra1 by Western blotting (Fig. 4B). Loss of Tom1 did not affect or even increased the amount of Yra1 associated with Nab2, indicating that Tom1-dependent ubiquitination of Yra1 is not required for complex formation in vivo. In contrast, in the presence of Tom1, Yra1 binding to Nab2-ProtA was substantially reduced when UbK48R,G76A was expressed in comparison with empty vector. Altogether, these observations support the view that ubiquitination of Yra1 by Tom1 promotes its dissociation from Nab2–Mex67–mRNP complexes.
Tom1-dependent ubiquitination of Yra1 is part of an mRNP surveillance step prior to nuclear export
To define whether the impaired dissociation of Yra1 from the mRNP results in an mRNA export block, the Δtom1 or Yra1 KR9–22 mutant strains were analyzed for RNA export defects by FISH. Consistent with earlier data, the Δtom1 strain showed no defect at 25°C, but nuclear poly(A)+ accumulation was detected in 2% and 30% of cells heated to 37°C for 1 h and 3 h, respectively (Fig. 5A [left panel], D; Duncan et al. 2000). Notably, analysis of GAL1 mRNA distribution revealed an increased RNA nuclear dot in Δtom1 versus wild-type cells, consistent with a role of the Tom1 E3 ligase in the export efficiency of this specific transcript (Fig. 5A, right panel). In agreement with a role of Tom1-dependent ubiquitination of Yra1 in mRNA export, the Yra1 KR9–22 mutant strain also showed a poly(A)+ RNA export defect in 25% of cells at both 25°C and 37°C (Fig. 5B,D). The observation that the export defect in Yra1 KR9–22 at 25°C is not rate-limiting for growth (Supplemental Fig. 5C) suggests that Tom1-induced ubiquitination and dissociation of Yra1 become essential for mRNA export and growth only at 37°C, when mRNA export rates are enhanced in response to increased metabolic activity. Together, these data indicate that Yra1 is the proposed Nab2-interacting partner that controls Nab2-bound mRNP export via Tom1. Consistent with this view, Nab2 overexpression suppresses the growth defects associated with both Δtom1 and the Yra1 KR-all mutant (Supplemental Fig. 6E,F) and the export defect of the Yra1 KR9–22 mutant was suppressed at both 25°C and 37°C by overexpression of Nab2 or Mex67 from a high-copy plasmid (Fig. 5C,D; data not shown).
Figure 5.
Yra1 ubiquitination is required for efficient mRNA export. (A) Loss of Tom1 interferes with mRNA export. (Left panel) Indicated strains were grown at 25°C, shifted for 3 h to 37°C, and analyzed for poly(A)+ RNA distribution with an oligo (dT)50 probe. (Right panel) Cells were induced with galactose for 30 min at 30°C, and GAL1 mRNA distribution was analyzed by FISH using specific probes. DAPI staining or FISH/DAPI overlays are shown below each panel to localize the RNA signal with respect to the nucleus. (B) Loss of Yra1 ubiquitination interferes with mRNA export. Yra1 wild type and Yra1 KR9–22 cells were grown at 25°C, shifted for 1 h to 37°C, and analyzed for poly(A)+ RNA distribution with an oligo (dT)50 probe. (C) Overexpression of Nab2 and Mex67 suppresses the Yra1 ubiquitination defect. Yra1 KR9–22 cells transformed with high-copy NAB2, MEX67 plasmids, or empty vector were grown at 25°C and analyzed for poly(A)+ RNA distribution as in B. (D) Quantification of FISH analyses: The average percent of cells with an mRNA export defect was determined by examining at least 100 DAPI-stained cells from three independent FISH experiments (except for wild type and Δtom1, the values of which correspond to one experiment). Note that wild type is W303, the corresponding wild type for Δtom1, while Yra1 wild type is YRA1-shuffled + YRA1 wild-type cDNA, the corresponding wild-type for KR9–22.
These data confirm that Yra1, Nab2, and Mex67 function in the same pathway, and that Yra1 ubiquitination, rather than being essential for mRNA export, is more likely to act as a facilitator of this process.
Yra1 ubiquitination by Tom1 is linked to the NPC
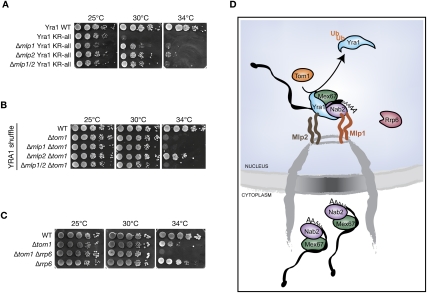
Our earlier work proposed that the docking of mRNP-bound Nab2 and Yra1 at the NPC-associated Mlp proteins is part of an mRNP surveillance step preceding mRNP translocation through the NPC (Vinciguerra et al. 2005). We therefore tested whether the Mlp proteins are part of the mRNP retention mechanism controlled by Tom1-dependent Yra1 ubiquitination. Consistent with this view, loss of Mlp proteins substantially rescues the growth defect of the Yra1 KR-all mutant (Fig. 6A). Moreover, deletion of Mlp2 suppresses the temperature-sensitive growth of Δtom1 (Fig. 6B). In all, these data suggest that Mlps are involved in the retention of Nab2-bound mRNP containing nonubiquitinated Yra1. Finally, we observed a synthetic interaction between Tom1 and Rrp6 (Fig. 6C), a nuclear 3′-to-5′ exonuclease involved in the degradation of malformed mRNP complexes (Vinciguerra and Stutz 2004), suggesting that this exosome component participates in the degradation of nonexportable and potentially toxic mRNP complexes formed in Δtom1. These genetic observations taken together indicate that Tom1-induced ubiquitination of Yra1 may occur in association with the Mlp protein platform, and may be part of an mRNP surveillance step taking place at the nuclear periphery, ensuring that only well-formed and mature mRNPs can reach the cytoplasm (Fig. 6D).
Figure 6.
Yra1 ubiquitination may be part of an mRNA export surveillance mechanism associated with NPCs. (A) Loss of Mlp2 suppresses the Yra1 KR mutant temperature-sensitive phenotypes. Tenfold serial dilutions of the indicated YRA1-shuffled strains were spotted onto YEPD plates and grown at the indicated temperatures. (B) Loss of Mlp2 suppresses the Δtom1 growth defect. Indicated strains were analyzed as in A. (C) Loss of Rrp6 enhances the growth defect of Δtom1. Indicated strains were analyzed as in A. (D) Model showing the ubiquitin-dependent release of Yra1 from Mlp-retained mRNP complexes, allowing their nuclear exit. Ubiquitination is mediated by Tom1 and another still unidentified E3 ligase (not shown).
Discussion
In the present study, we identify the essential shuttling mRNA-binding protein Nab2 as a new cofactor for Mex67, and provide evidence that Yra1 acts as a stimulator for Nab2–Mex67 interaction. We further demonstrate that the ubiquitin ligase Tom1 regulates the dissociation of Yra1 from Nab2–Mex67, and that lack of Yra1 ubiquitination results in accumulation of Yra1 on nuclear mRNPs, a phenotype that correlates with mRNA export defects. These observations together lead us to propose that Tom1-mediated ubiquitination of Yra1 participates in the dissociation of the nonshuttling mRNA adaptor Yra1 from the mRNP before its export into the cytoplasm (Fig. 6D)
Yra1 facilitates the interaction between Nab2 and Mex67
In agreement with the previously reported genetic and biochemical relationship between Yra1 and Nab2 (Kashyap et al. 2005; Vinciguerra et al. 2005; Batisse et al. 2009), we now demonstrate that Nab2 and Yra1 interact directly and act in the same mRNA export pathway (Figs. 2, 5C; Supplemental Figs. 1C, 2C,D). Both Yra1 and Nab2 interact directly with the N-terminal domain of Mex67 (Supplemental Figs. 1A, 2A, 3). Yet the findings that Yra1, Nab2, and Mex67 can form a trimeric complex, and that an excess of Nab2 bypasses Δyra1, identify the essential shuttling mRNA-binding protein Nab2 as a potential new adaptor for Mex67, and suggest that Yra1 is chaperoning the association of Nab2 with Mex67 rather than acting as a bona fide adaptor for Mex67 (Figs. 1B,C, 2B).
This view is also consistent with the finding that, in C. elegans and Drosophila, the Yra1 homolog Aly/REF is not essential for mRNA export. Yra1/REF has been implicated in several cellular processes independently from its role in mRNA export, including transcription and DNA replication (Bruhn et al. 1997; Swaminathan et al. 2007). Identification of multiple synthetic lethal interactions between Yra1 and factors involved in all steps of mRNA biogenesis, from transcription down to the pore (N Iglesias and D Zenklusen, unpubl.), as well as with the finding of a great variety of Yra1 interaction partners in a large-scale protein complex analysis (Krogan et al. 2006), suggest that Yra1 could function as a cofactor chaperoning a diverse set of protein–protein interactions.
Ubiquitination regulates Yra1 function
The ubiquitin pathway and, more specifically, the E3 ligases Rsp5 and Tom1 are involved in the regulation of mRNA export (Utsugi et al. 1999; Duncan et al. 2000; Neumann et al. 2003; Rodriguez et al. 2003). Our studies identify Yra1 as the first export-relevant substrate of Tom1. Ubiquitination of Yra1 by Tom1 is consistent with the physical interactions between these two proteins (Supplemental Fig. 5A; Oeffinger et al. 2007). Our results show that Yra1 is ubiquitinated on lysines lying within the C-terminal domain of Yra1, one of which is monoubitquitinated and diubiquitinated by Tom1, and another monoubiquitinated by a yet unidentified E3 ligase (Fig. 3A,D). Our analyses suggest that Tom1-dependent and Tom1-independent ubiquitination may occur on different pools of Yra1 potentially implicated in different steps of mRNA export or distinct Yra1 functions, as no substantial amounts of triubiquitinated Yra1 could be detected. While Tom1 catalyzes K48-branched diubiquitin on Yra1 (Supplemental Fig. 5B), it does not promote Yra1 degradation (Fig. 3B), but rather regulates its interaction with Nab2 (Fig. 4B). Similarly, ubiquitination of the SAGA component Spt7, the other known substrate of Tom1, does not result in Spt7 degradation, but modulates protein interactions within the SAGA complex (Saleh et al. 1998).
Previous studies indicated that Yra1 is not a shuttling protein (Strasser and Hurt 2000; Stutz et al. 2000; Lund and Guthrie 2005), suggesting that it might dissociate from the mRNPs prior to their export. We observed that stabilizing the monoubiquitinated form of Yra1 (by preventing its polyubitquitination and deubiquitination) decreased its association with Nab2 and, more slightly, with the mRNP (Fig. 4B; Supplemental Fig. 6D), while lack of ubiquitination (Yra1 KR9–22 mutant) increased Yra1 binding to mRNPs (Fig. 4A). These data, together with the export defects observed in the Yra1 KR9–22 mutant (Fig. 5B,D), are consistent with the view that ubiquitination of the Yra1 C-terminal domain by Tom1 promotes the dissociation of Yra1 from nuclear Nab2-bound mRNPs, thereby allowing their export. The ability of Nab2 overexpression to suppress both the Δtom1 and the Yra1 KR mutant slow-growth phenotypes (Supplemental Fig. 6E,F), as well as the Yra1 KR9–22 poly(A)+ mRNA export defect (Fig. 5C), further indicates that this post-translational modification is linked to Nab2-mediated mRNA export, consistent with the early hypothesis by Duncan et al. (2000) that the ubiquitination of a Nab2-interacting protein by Tom1 is required for efficient poly(A)+ RNA export. Both Nab2 and Tom1 have a homolog in higher eukaryotes (Hall et al. 2007; Kelly et al. 2007), raising the possibility that this mechanism of mRNA export might be evolutionarily conserved.
While earlier studies suggested that Nab2 and Npl3 specify overlapping but distinct mRNA export pathways (Duncan et al. 2000; Kim Guisbert et al. 2005), more recent data show that Nab2 is bound to most transcripts in a complex also containing Mex67 and Yra1, indicating that Nab2 is likely to function in global mRNA export (Batisse et al. 2009). The observation that Δtom1 induces a poly(A)+ mRNA export defect only at 37°C could suggest that Yra1 ubiquitination is required for the export of transcripts specifically expressed under these conditions. However, heat-shock mRNAs did not accumulate in Δtom1 cells heated for 30 min to 42°C (data not shown). Thus, Tom1-induced ubiquitination of Yra1 may rather be required to increase bulk poly(A)+ export efficiency under conditions of increased metabolic activity. The effect of Tom1 on GAL1 mRNA export at 30°C, however, indicates that ubiquitination by Tom1 may facilitate the export of highly induced transcripts also at permissive temperatures (Fig. 5A).
Overall, the partial mRNA export defects observed in the Δtom1 and Yra1 KR9–22 mutants (Fig. 5) indicate that Yra1 post-translational modification is not essential for export, but more likely facilitates translocation into the cytoplasm. Alternatively, the limited fraction of cells with a poly(A)+ RNA export defect in these mutants could indicate a preferential requirement for Tom1-induced Yra1 ubiquitination at a specific stage of the cell cycle. Future studies will address the possibility that Yra1 ubiquitination is cell cycle-dependent.
mRNP surveillance and dynamics during export
The functional interaction between Yra1, Tom1, and Mlp proteins (Fig. 6A,B) suggests that Yra1 ubiquitination by Tom1 occurs at a late step of mRNA export, possibly in association with the NPC. In particular, we observed that loss of Mlp2 rescues the growth defect of both Δtom1 and the KR-all mutant, suggesting an interconnection between ubiquitination by Tom1 and the surveillance system located at the nuclear periphery. Consistently, both Yra1 and Nab2 interact physically with Mlp proteins (Green et al. 2003; Vinciguerra et al. 2005; Fasken et al. 2008), raising the possibility that these interactions are part of a checkpoint used by the surveillance system to rearrange and export properly processed mRNPs only. The synthetic interaction between Δtom1 and Δrrp6 (Fig. 6C) further suggests a connection between Tom1 and mRNP surveillance. Ubiquitination by Tom1 may occur preferentially on well-formed mRNP complexes, allowing their efficient export, while lack of modification would result in retention and degradation by the exosome (Fig. 6D). Finally, Yra1 ubiquitination may favor a conformational change or the association with ubiquitin-binding proteins facilitating Yra1 dissociation and mRNP release into the cytoplasm.
mRNA biogenesis and export rely on multiple highly coordinated steps and the sequential ordered associations and dissociations of factors implicated in mRNA processing, translocation, and release from the NPC, as well as translation and stability in the cytoplasm (Iglesias and Stutz 2008). So far, two HECT domain E3 ligases—Rsp5 and Tom1—have been implicated in mRNA export (Duncan et al. 2000; Neumann et al. 2003; Rodriguez et al. 2003). Rsp5 was shown to polyubiquitinate Hpr1, a component of the THO complex associated with the transcription machinery and involved in cotranscriptional recruitment of mRNA export factors (Gwizdek et al. 2005, 2006). Polyubiquitination of Hpr1 by Rsp5 is transcription-dependent and, in contrast to modification of Yra1 by Tom1, targets Hpr1 for proteasomal degradation, a mechanism proposed to coordinate transcription elongation with the release of mature mRNPs from transcription sites (Gwizdek et al. 2006). In addition to Hpr1, a proteome-wide approach also identified Npl3 as an additional export-relevant substrate of Rsp5 (Gupta et al. 2007). We found that Yra1 is ubiquitinated by Tom1, and that Nab2 and Mex67 are ubiquitinated as well, albeit by some other yet unidentified E3 ligase(s). Thus, post-translational modification by ubiquitin may regulate the dynamics of mRNP packaging and remodeling at multiple steps along the export pathway. Ubiquitin emerges, therefore, as a major regulator of mRNA export, and many more components of the ubiquitin pathway participating in this process may be identified in the future.
Materials and methods
Yeast strains, plasmid constructions, and recombinant proteins
Yeast strains and plasmids used in this study are listed in Supplemental Tables 1 and 2. Expression and purification of recombinant proteins are described in the Supplemental Material.
In vitro binding assay
Five micrograms of GST, GST-Mex67, or GST-Yra1 fusion proteins immobilized on 20 μL of packed glutathione agarose beads was incubated with purified full-length or truncated His6-Nab2. Binding was performed for 1 h at 4°C on a rotating wheel; beads were washed three times with 500 μL of lysis buffer plus 0.1% Triton X-100. Bound proteins were eluted with sample buffer (SB), and were analyzed by SDS-PAGE followed by Coomassie staining or Western blot analysis.
Gel filtration analyses of the Mex67–Nab2–Yra1 complex
Gel filtrations were performed at 4°C on a 16/60 Superdex 200 column equilibrated in binding buffer (20 mM Hepes at pH 7.9, 200 mM NaCl, 0.2 mM DTT, 2% [w/v] CHAPS at pH 7.5) and run at a flow rate of 1 mL/min. The column was calibrated with gel filtration standard proteins giving the following elution volumes: Dextran Blue, 2000 kDa, 38 mL; ferritin, 460 kDa, 52 mL; catalase, 206 kDa, 60 mL; aldolase, 167 kDa, 64 mL; BSA, 67 kDa, 70 mL; lysosyme, 17 kDa, 106 mL. His6-Mtr2, His6-Mtr2/Mex67, His6-Nab2, and His6-Yra1 were all diluted to 100 μg/500 μL in binding buffer prior to their individual loadings as 500 μL of sample and elution from the gel filtration column. For the detection of complex, the following reaction mixtures were prepared (500-μL final volume): 1 μM His6-Mtr2/Mex67, 1 μM His6-Nab2, and 1 μM His6-Yra1 in binding buffer; or 1 μM His6-Mtr2, 1 μM His6-Nab2, and 1 μM His6-Yra1 in binding buffer; the reactions were incubated on a rotating wheel for 1 h and 30 min at 4°C prior to loading and elution from the gel filtration column. Fractions of 2 mL were collected, monitored at 280 nm, and analyzed by Western blotting after TCA precipitation using the suitable antibodies.
Purification of ubiquitinated proteins
Cells expressing wild-type or mutant His6-Ub under a copper-inducible promoter (PCUP1) were grown overnight in selective medium containing 0.1 mM CuSO4, and 200 OD600 units were collected and resuspended in 1.5 mL of A-buffer (100 mM sodium phosphate at pH 8, 10 mM Tris-HCl, 6 M guanidinium, 10 mM imidazole, 0.2% Triton X-100, 10 mM NEM, complete protease inhibitor mix [Roche]). The cell suspension was disrupted with glass beads at 4°C in a magnalyser (Roche) and spun at 13,000 rpm for 20 min. The supernatant was incubated with 100 μL of Ni-NTA acid-agarose (Qiagen) for 2 h at room temperature with mild rotation. Agarose beads were washed three times with B buffer (100 mM sodium phosphate at pH 6.8, 10 mM Tris-HCl, 8 M urea, 20 mM imidazole, 0.2% Triton X-100, complete protease inhibitor mix [Roche]). His6-ubiquitinated proteins were eluted by resuspending the beads in 100 μL of 2× SB and boiling for 3 min at 95°C. Twenty microliters of samples was analyzed by Western blot with the relevant antibodies.
Affinity purification of proteins/mRNPs in vivo
ProtA/TAP-tagged strains and a nontagged control strain were grown in selective or yeast extract–peptone–dextrose (YEPD) at 25°C to an OD600 = 0.8–1.0. For mRNP pull-down from cells expressing mutant UbK48RG76A, transformed cells were grown overnight in 0.05 mM CuSO4 to induce wild-type or mutant His6-Ub. Cells were collected at OD600 = 0.8–1, resuspended as 160 OD600 per milliliter in ice-cold lysis buffer (20 mM Hepes–KOH at pH 7.5, 150 mM NaCl, 5 mM MgCl2, 10% glycerol, 0.5% Triton X-100, complete protease inhibitor mix [Roche]), lysed at 4°C with glass beads, and centrifuged at 13,000 rpm for 20 min at 4°C. For Nab2-ProtA immunoprecipitate with cross-linked extracts, cells were grown until OD600 = 0.8–1, cross-linked for 5 min with 1.2% formaldehyde, and quenched with 360 mM glycine, and the extracts were sonicated 10 times for 30 sec. Protein extracts corresponding to 100–150 OD600 were precleared for 1 h or overnight in the presence of empty Sepharose beads, and were then incubated with 40 μL of IgG-Sepharose beads (Pharmacia) for 1.5 h at 4°C. For RNase treatment, protein extracts corresponding to 100–150 OD600 were incubated for 15 min at 25°C with or without 200 ng of DNase-free RNases (Qiagen). Beads were washed three times with ice-cold lysis buffer, and associated proteins were eluted from the beads by addition of 200 μL of 2 M KCl and 20 mM HEPES-KOH (pH 7.5) for 20 min at 4°C with mild rotation. IgG-Sepharose beads were washed once with cold 1× PBS and resuspended in 40 μL of 2× SB, and one-tenth was analyzed by Western blot; the supernatant was precipitated with cold trichloroacetic acid (20% final) at 13,000 rpm for 50 min at 4°C, the protein pellets were resuspended in 20 μL of 1× SDS SB and Tris Base, and one-fifth was analyzed by Western blot.
Antibodies and Western blot analysis
The anti-HA antibody and anti-CTD (Covance) were used at 1:2000, anti-His (Sigma) was used at 1:3000, anti-Nab2 was used at 1:10,000, anti-Mex67 was used at 1:10,000, anti-ProtA was used at 1:2500, and anti-Rap1 (Santa Cruz Biotechnology) and anti-Actin (Millipore) were used at 1:1000. The protein signals were revealed with ECL Western Blotting Detection Reagents (Amersham).
FISH experiments
Cells were grown in YEPD to mid-log phase at 25°C and shifted for the indicated time to 37°C before fixation with paraformaldehyde. Poly(A)+ mRNA in situ hybridization was performed with a Cy3-labeled oligo-dT(50) probe essentially as described (Zenklusen et al. 2001). GAL1 mRNA in situ hybridization was performed with a set of five Cy3-labeled oligonucleotide probes (50 mers) as described (Jensen et al. 2001; Gwizdek et al. 2006).
Acknowledgments
We thank E. Hurt and D. Finley for plasmids. We are grateful to V. Panse for discussions, and to J. Lingner for his support. We also thank D. Gatfield and S. Montessuit for technical advice, and members of the laboratory for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation (SNF grant no. 116541 to F.S.) and the NCCR Frontiers in Genetics, and by grants from the Agence Nationale pour la Recherche (grant BLAN06-1_134099 to C.D.) and the Ligue contre le Cancer (C.D. is an “Equipe labellisée”).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.583310.
Supplemental material is available at http://www.genesdev.org.
References
- Abruzzi KC, Belostotsky DA, Chekanova JA, Dower K, Rosbash M 2006. 3′-end formation signals modulate the association of genes with the nuclear periphery as well as mRNP dot formation. EMBO J 25: 4253–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT, Wilson SM, Datar KV, Swanson MS 1993. NAB2: A yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol 13: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batisse J, Batisse C, Budd A, Boettcher B, Hurt E 2009. Purification of nuclear poly(A)-binding protein Nab2 reveals association with the yeast transcriptome and a messenger ribonucleoprotein core structure. J Biol Chem 284: 34911–34917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn L, Munnerlyn A, Grosschedl R 1997. ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRα enhancer function. Genes Dev 11: 640–653 [DOI] [PubMed] [Google Scholar]
- Duncan K, Umen JG, Guthrie C 2000. A putative ubiquitin ligase required for efficient mRNA export differentially affects hnRNP transport. Curr Biol 10: 687–696 [DOI] [PubMed] [Google Scholar]
- Fasken MB, Stewart M, Corbett AH 2008. Functional significance of the interaction between the mRNA-binding protein, Nab2, and the nuclear pore-associated protein, Mlp1, in mRNA export. J Biol Chem 283: 27130–27143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Sadis S, Monia BP, Boucher P, Ecker DJ, Crooke ST, Chau V 1994. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol Cell Biol 14: 5501–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes P, Kufel J, Fornerod M, Polycarpou-Schwarz M, Lafontaine D, Tollervey D, Mattaj IW 1999. Genetic and physical interactions involving the yeast nuclear cap-binding complex. Mol Cell Biol 19: 6543–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V, Gadal O, Fromont-Racine M, Romano A, Jacquier A, Nehrbass U 2004. Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell 116: 63–73 [DOI] [PubMed] [Google Scholar]
- Gatfield D, Izaurralde E 2002. REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J Cell Biol 159: 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W, Guthrie C 2004. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell 13: 201–212 [DOI] [PubMed] [Google Scholar]
- Green DM, Marfatia KA, Crafton EB, Zhang X, Cheng X, Corbett AH 2002. Nab2p is required for poly(A) RNA export in Saccharomyces cerevisiae and is regulated by arginine methylation via Hmt1p. J Biol Chem 277: 7752–7760 [DOI] [PubMed] [Google Scholar]
- Green DM, Johnson CP, Hagan H, Corbett AH 2003. The C-terminal domain of myosin-like protein 1 (Mlp1p) is a docking site for heterogeneous nuclear ribonucleoproteins that are required for mRNA export. Proc Natl Acad Sci 100: 1010–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izaurralde E 1998. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell 1: 649–659 [DOI] [PubMed] [Google Scholar]
- Gupta R, Kus B, Fladd C, Wasmuth J, Tonikian R, Sidhu S, Krogan NJ, Parkinson J, Rotin D 2007. Ubiquitination screen using protein microarrays for comprehensive identification of Rsp5 substrates in yeast. Mol Syst Biol 3: 116 doi: 10.1038/msb4100159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwizdek C, Hobeika M, Kus B, Ossareh-Nazari B, Dargemont C, Rodriguez MS 2005. The mRNA nuclear export factor Hpr1 is regulated by Rsp5-mediated ubiquitylation. J Biol Chem 280: 13401–13405 [DOI] [PubMed] [Google Scholar]
- Gwizdek C, Iglesias N, Rodriguez MS, Ossareh-Nazari B, Hobeika M, Divita G, Stutz F, Dargemont C 2006. Ubiquitin-associated domain of Mex67 synchronizes recruitment of the mRNA export machinery with transcription. Proc Natl Acad Sci 103: 16376–16381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR, Kow E, Nevis KR, Lu CK, Luce KS, Zhong Q, Cook JG 2007. Cdc6 stability is regulated by the Huwe1 ubiquitin ligase after DNA damage. Mol Biol Cell 18: 3340–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector RE, Nykamp KR, Dheur S, Anderson JT, Non PJ, Urbinati CR, Wilson SM, Minvielle-Sebastia L, Swanson MS 2002. Dual requirement for yeast hnRNP Nab2p in mRNA poly(A) tail length control and nuclear export. EMBO J 21: 1800–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieronymus H, Silver PA 2003. Genome-wide analysis of RNA-protein interactions illustrates specificity of the mRNA export machinery. Nat Genet 33: 155–161 [DOI] [PubMed] [Google Scholar]
- Huang Y, Steitz JA 2001. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Cell 7: 899–905 [DOI] [PubMed] [Google Scholar]
- Huang Y, Gattoni R, Stevenin J, Steitz JA 2003. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol Cell 11: 837–843 [DOI] [PubMed] [Google Scholar]
- Iglesias N, Stutz F 2008. Regulation of mRNP dynamics along the export pathway. FEBS Lett 582: 1987–1996 [DOI] [PubMed] [Google Scholar]
- Jensen TH, Patricio K, McCarthy T, Rosbash M 2001. A block to mRNA nuclear export in S. cerevisiae leads to hyperadenylation of transcripts that accumulate at the site of transcription. Mol Cell 7: 887–898 [DOI] [PubMed] [Google Scholar]
- Jensen TH, Dower K, Libri D, Rosbash M 2003. Early formation of mRNP: License for export or quality control? Mol Cell 11: 1129–1138 [DOI] [PubMed] [Google Scholar]
- Kashyap AK, Schieltz D, Yates J 3rd, Kellogg DR 2005. Biochemical and genetic characterization of Yra1p in budding yeast. Yeast 22: 43–56 [DOI] [PubMed] [Google Scholar]
- Katahira J, Strasser K, Podtelejnikov A, Mann M, Jung JU, Hurt E 1999. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J 18: 2593–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SM, Pabit SA, Kitchen CM, Guo P, Marfatia KA, Murphy TJ, Corbett AH, Berland KM 2007. Recognition of polyadenosine RNA by zinc finger proteins. Proc Natl Acad Sci 104: 12306–12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Guisbert K, Duncan K, Li H, Guthrie C 2005. Functional specificity of shuttling hnRNPs revealed by genome-wide analysis of their RNA binding profiles. RNA 11: 383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, et al. 2006. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440: 637–643 [DOI] [PubMed] [Google Scholar]
- Longman D, Johnstone IL, Caceres JF 2003. The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans. RNA 9: 881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MK, Guthrie C 2005. The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol Cell 20: 645–651 [DOI] [PubMed] [Google Scholar]
- MacMorris M, Brocker C, Blumenthal T 2003. UAP56 levels affect viability and mRNA export in Caenorhabditis elegans. RNA 9: 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfatia KA, Crafton EB, Green DM, Corbett AH 2003. Domain analysis of the Saccharomyces cerevisiae heterogeneous nuclear ribonucleoprotein, Nab2p. Dissecting the requirements for Nab2p-facilitated poly(A) RNA export. J Biol Chem 278: 6731–6740 [DOI] [PubMed] [Google Scholar]
- Mili S, Shu HJ, Zhao Y, Pinol-Roma S 2001. Distinct RNP complexes of shuttling hnRNP proteins with pre-mRNA and mRNA: Candidate intermediates in formation and export of mRNA. Mol Cell Biol 21: 7307–7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Petfalski E, Brugger B, Grosshans H, Wieland F, Tollervey D, Hurt E 2003. Formation and nuclear export of tRNA, rRNA and mRNA is regulated by the ubiquitin ligase Rsp5p. EMBO Rep 4: 1156–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger M, Wei KE, Rogers R, DeGrasse JA, Chait BT, Aitchison JD, Rout MP 2007. Comprehensive analysis of diverse ribonucleoprotein complexes. Nat Methods 4: 951–956 [DOI] [PubMed] [Google Scholar]
- Pickart CM 2000. Ubiquitin in chains. Trends Biochem Sci 25: 544–548 [DOI] [PubMed] [Google Scholar]
- Rodrigues JP, Rode M, Gatfield D, Blencowe BJ, Carmo-Fonseca M, Izaurralde E 2001. REF proteins mediate the export of spliced and unspliced mRNAs from the nucleus. Proc Natl Acad Sci 98: 1030–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MS, Gwizdek C, Haguenauer-Tsapis R, Dargemont C 2003. The HECT ubiquitin ligase Rsp5p is required for proper nuclear export of mRNA in Saccharomyces cerevisiae. Traffic 4: 566–575 [DOI] [PubMed] [Google Scholar]
- Saleh A, Collart M, Martens JA, Genereaux J, Allard S, Cote J, Brandl CJ 1998. TOM1p, a yeast hect-domain protein which mediates transcriptional regulation through the ADA/SAGA coactivator complexes. J Mol Biol 282: 933–946 [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, Hurt E 1998. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol 18: 6826–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, Hurt E 1997. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J 16: 3256–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K, Hurt E 2000. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J 19: 410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E 2000. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6: 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Kile AC, MacDonald EM, Koepp DM 2007. Yra1 is required for S phase entry and affects Dia2 binding to replication origins. Mol Cell Biol 27: 4674–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsugi T, Hirata A, Sekiguchi Y, Sasaki T, Toh-e A, Kikuchi Y 1999. Yeast tom1 mutant exhibits pleiotropic defects in nuclear division, maintenance of nuclear structure and nucleocytoplasmic transport at high temperatures. Gene 234: 285–295 [DOI] [PubMed] [Google Scholar]
- Vinciguerra P, Stutz F 2004. mRNA export: An assembly line from genes to nuclear pores. Curr Opin Cell Biol 16: 285–292 [DOI] [PubMed] [Google Scholar]
- Vinciguerra P, Iglesias N, Camblong J, Zenklusen D, Stutz F 2005. Perinuclear Mlp proteins downregulate gene expression in response to a defect in mRNA export. EMBO J 24: 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KD, Tashayev VL, O'Connor LB, Larsen CN, Kasperek E, Pickart CM 1995. Metabolism of the polyubiquitin degradation signal: Structure, mechanism, and role of isopeptidase T. Biochemistry 34: 14535–14546 [DOI] [PubMed] [Google Scholar]
- Zenklusen D, Vinciguerra P, Strahm Y, Stutz F 2001. The yeast hnRNP-like proteins Yra1p and Yra2p participate in mRNA export through interaction with Mex67p. Mol Cell Biol 21: 4219–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R 2000. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 407: 401–405 [DOI] [PubMed] [Google Scholar]