Abstract
Background
Women once treated for high grade cervical dysplasia have a high long term risk for developing new dysplasia or cancer.
Objectives
To investigate if human papilloma virus (HPV)-negativity after treatment of cervical dysplasia reduces the need for frequent long term follow up.
Design
Case/control study based on archival smears.
Methods
Women with cervical intraepithelial neoplasi (CIN)2–3, treated for dysplasia and with recurrence of CIN2+ more than 2 years after treatment were compared with controls without recurrence, matched for age and date of treatment. High risk-HPV-DNA were analysed with PCR from two archival smears per woman. Mean follow up time was 14.6 years.
Results
24% (45/189) of cases and 11% (43/378) of controls were HPV-positive in any of two smears. Odds ratio (OR) = 2.5 (1.6–3.8).
Conclusion
HPV-status 6–12 months after treatment of high grade dysplasia is of limited value for the design of long term follow up.
Keywords: HPV, Cervical intraepithelial neoplasia, Follow-up, Recurrence, Treatment, Case–control studies
1. Introduction
Women who have been treated for high grade dysplasia still have an increased risk of acquiring invasive cancer compared with the general female population.3–5
Follow up after treatment for cervical dysplasia fulfils two purposes. The first is to identify inadequate treatment which has been reported to occur in 4–17%.6,7 Several studies have assessed risk factors and evaluated tests for high risk papilloma virus to find short term residual disease.1,2,8–10 The other purpose of surveillance is protecting the women at risk from developing invasive cancer by finding new dysplastic lesions, recurrent disease. This is a long term task since the increased risk for recurrent disease and cancer does not seem to decrease with time.3 Recommendations differ from extra surveillance for not more than 5 years from treatment to yearly smears, possibly combined with human papilloma virus (HPV)-tests for the rest of the woman’s life.11 Swedish contemporary guidelines recommend bi-annual testing with cytology for at least 20 years.12 This policy, however, is expensive since it involves many women for a very long time, and the effectiveness of these recommendations has not been studied.
Testing for HPV in conjunction with cytological smear has been suggested for short term follow up and a double negative test could possibly extend the time to the next post-surgery control13 or just add to the existing follow up.14 To our knowledge, the role of HPV-testing in long term follow up has not been studied. The aim of this study was to investigate if long term follow up could be restricted to those patients who are HPV-positive after treatment and analyse how long term this could be a safe procedure. Since the time lag for recurrent disease can be very long we addressed the issue in a case–control study based on HPV-testing with PCR on archival smears.15
2. Patients and methods
2.1. Selection of cases
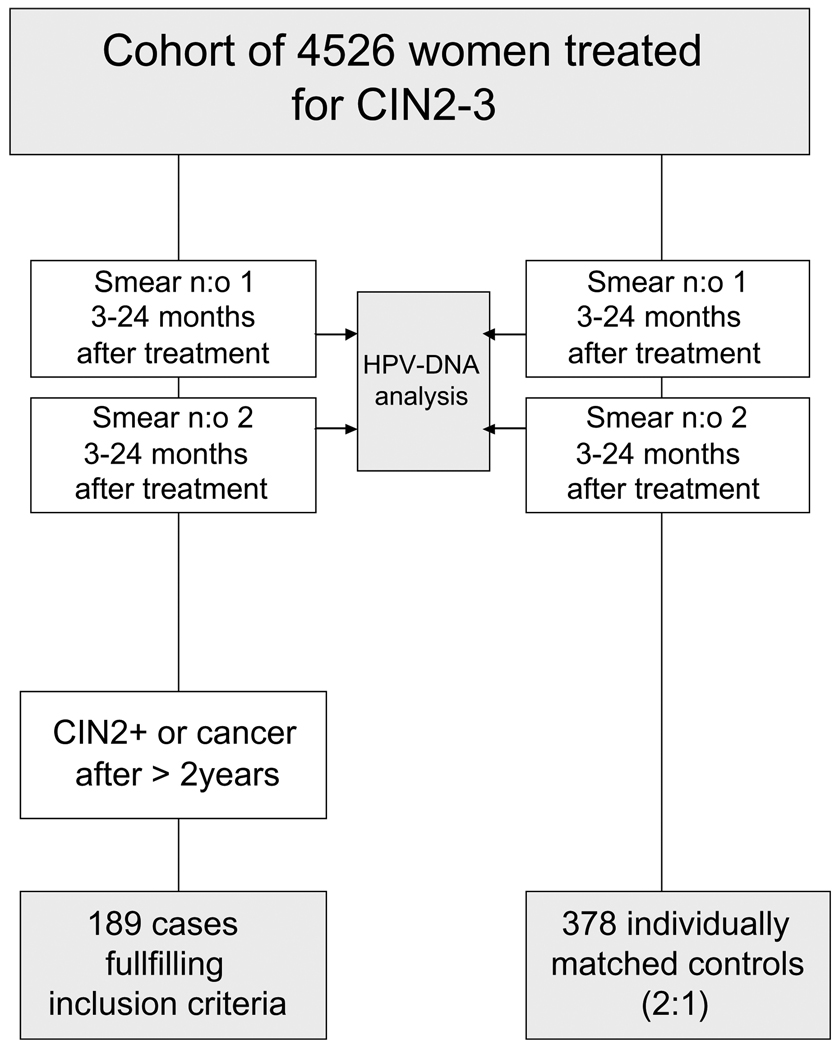
The Department of Pathology and Cytology at Sahlgrenska University Hospital, Göteborg, Sweden, has stored histological samples for several decades and all cytological smears since 1983. A computerised register contains data from this year onwards and covers practically all inhabitants in the Göteborg area. There are also limited data in the register from the period 1978 to 1983. The database was searched for all patients who had a histological sample of cervical intraepithelial neoplasia (CIN) 2 or 3, adenocarcinoma in situ or equivalent diagnoses up to May 2000. Thus a cohort of 4526 women with high grade dysplasia was established. Cases were selected from this cohort if they had a second histopathological diagnosis of CIN 2 or 3, adenocarcinoma in situ or invasive cervical cancer more than 2 years later than the original one. Two cytological smears, taken within 3–24 months (90–730 days) after treatment, had to be identified in the register. Records were retrieved to confirm that the patients had also been treated for the initial cervical dysplasias.
2.2. Selection of controls
Two controls, from the original cohort of 4526 women with high grade dysplasia, were matched for each case. Matching was made for age and time for treatment of dysplasia in the following way: Age at the time for base line treatment should be within ±21/2 years. Two cytological smears should be in the registry during the period 3–24 months after treatment. The controls should not have had a hysterectomy before the time for second diagnosis of CIN2+ in the matched cases, nor a second diagnosis of high grade dysplasia during the observation time that ended in May 7, 2002. As proof of follow up a cytological smear should be in the registry later than 1 year before the date of the recurrence of the matching case. The controls were finally chosen as the two patients fulfilling these criteria, treated for high grade dysplasia closest in time to the treatment of the corresponding case. Fig. 1 shows a flow-chart of the selection of cases and controls.
Fig. 1.
Flow chart for case–control study. Smear n:o 1 is referred to as 6 month smear and smear n:o 2 as 12 month smear according to mean time after treatment. Matching was made for age and time for diagnosis and treatment of CIN2–3.
2.3. Exposure and sub-group analysis
In the overall analysis, a woman was defined as HPV-positive if she had one or two HPV-positive smears. The material was also divided into two groups according to time from date of treatment to cytological smear. In these calculations HPV-positive was defined as a single HPV-positive smear. The data set was also divided into three groups (equally large sample in each group) according to time between treatment and new diagnosis of dysplasia for the cases. Period 1 = 2–3.60 years, Period 2 = 3.60–6.66 years and Period 3 = after 6.66 years.
2.4. Selection of smears for the study base
Three smears belonging to cases could not be found in the archive as well as two of the control smears. 1129 smears went to HPV DNA-analysis. Before analysis and removal of the material from the slides, documentation of the morphological picture was made by PAPNET-imaging16 for future reference.
Among the 1129 smears analysed 12 (1.06%) were S14 negative, five cases and seven control smears, and were excluded from further analysis. The remaining 1117 smears constituted the base of the study and belonged to 189 cases with two controls from each case. Eight of the cases and nine of the controls lacked a second smear in the final analysis.
2.5. Validation of original histopathological diagnosis
One hundred samples were randomly selected for review by an expert cervical pathologist (WR) from the 567 histopathological samples that constituted the inclusion criteria for the cases and the controls in the final study base and the 189 samples that made up the endpoints for the cases.
2.6. DNA extraction of archival smears
The method used for DNA extraction of the Pap smears has been established and validated in previous publications9,15,17 by the same researcher as in our study (Chua-Wallin). However, to further improve the method, the addition of saturated ammonium acetate to the lysate and procedure for DNA precipitation were not included. Instead, after extended digestion at 60 °C for a minimum of 2 h, the lysates were heated at 98 °C for 10 min to inactivate proteinase K. The samples were stored at 4 °C.
2.7. HPV PCR by general primers
The quality of the DNA extract was evaluated by PCR targeting the human S14 gene generating products of 150 bp.15 HPV was detected using a single GP5+/6+ primer set for PCR amplifying products of similar size to S14 PCR. The contents and conditions for the PCR were as described in previous work,9 with exceptions to 2mM MgCl2 and 5 µl of 2% bovine serum albumin (BSA) used in the PCR buffer reaction mix. The PCRs were performed in a 96-titre plate format consisting of CaSki cell DNA in dilution series of 10 ng to 10−7 ng as positive controls as well as a sensitivity panel simultaneously with patients’ samples in every run. The template volumes used were 1 µl, 2 µl and 3 µl, respectively, and several blanks containing no DNA were included. The limit of detection for the PCR system was 0.1 ng of CaSki DNA with 1 µl DNA template.
2.8. HPV typing by pyrosequencing
Single-stranded PCR product preparation was performed semi-automatically.18 Twelve type-specific sequencing primers for the high risk HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58 and -59 were designed.19 The minimum detection limit was found to be 0.154 pmol of PCR product. Sequencing by pyrosequencing technology as described earlier18 was performed on an automated plate-based bench-top PSQ™ HS96A System. The sequence results were obtained in pyrogram™ formats.
2.9. PCR cloning
PCR products generated by consensus primers GP5+/6+ that were out of the range of the above multiplex pyrosequencing method were cloned by TOPO TA cloning kit and ONE SHOT TOP 10 chemically competent cells (Invitrogen, Carlsbad, CA, USA) according to company instructions. On average, ten colonies from each cloned PCR amplicon were picked for further analysis. The cloned PCR fragments were amplified with the general GP5+/6+ PCR primers and were thereafter sequenced on the PSQ™ HS96A System (Biotage, Uppsala, Sweden) with GP5+ as the sequencing primer.
2.10. Statistics
To estimate an odds ratio (OR) between odds for being a case given exposure (HPV-positive test) and odds for being a case given non-exposure, conditional logistic regression was performed using the PHREG procedure in SAS (SAS Institute Inc. Cary, NC USA), stratifying on sample-id. One sample contains one case and its two controls, matched individually for age at time of (first) treatment and date for treatment. χ2 test and Fisher’s exact test were used for comparing groups shown in Table 3. Trends were calculated using Mantel–Haenszels extended test.
Table 3.
HPV-status after treatment including results from typing
| Cases | Controls | ||
|---|---|---|---|
| HPV-positive in any smear | 45/189 (23.8%) | 44/378 (11.6%) | p < 0.001 |
| HPV-positive in both smears | 13/181 (7.2%) | 10/369 (2.7%) | p < 0.01 |
| HPV-negative in first smear and HPV-positive in second smear | 14/181 (8.7%) | 10/369 (2.7%) | p < 0.01 |
| HPV-positive in first smear and HPV-negative in second smear | 17/181 (9.4%) | 23/369 (6.2%) | n.s. (p=0.09) |
| HPV-types identified in any smear | 34/189 (18%) | 31/378 (8.2%) | |
| As proportion of HPV positives | 34/45 (76%) | 31/44 (70%) | |
| Different HPV-types in both samples | 2/181 (1.1%) | 1/369 (0.3%) | n.s. (p = 0.26) |
| Same HPV-types in both samples | 8/181 (4.4%) | 4/369 (1.1%) | p = 0.02 |
| As proportion of HPV+ in both smears | 8/13 (61%) | 4/10 (40%) | n.s. |
Descriptive data and comparison between cases and controls. The denominator differs since not all women were represented by two samples.
n.s. = not significant.
2.11. Ethical approvement
The study was approved by the Ethics Committee of the Medical Faculty, University of Göteborg.
3. Results
The mean age of the participants was 35 years (range 17–83 years) at the time of the initial treatment, with no difference between cases and controls. The mean time elapsed between treatment and first smear analysed was 165 days for cases and 164 days for controls. These smears were labelled 6 month samples. The mean time between treatment and second smear was 342 and 362 days, respectively, and these smears were labelled 12 month samples.
The cases had a new high grade dysplasia or cancer 5 years and 8 months (mean time, range 2.0–16.3 years) after the first treatment for high grade dysplasia. The mean observation time, that is the time between first treatment for dysplasia and time for closing the study base, was 14 years and 7 months (range 4–24 years) and no control patient had a new high-grade dysplasia or cervical cancer observed during that time. Twenty-eight women were diagnosed with invasive cervical cancer as recurrent disease. The mean time to cancer diagnosis from first biopsy was 8 years 2 months (range 2.1–16.3 years). Four of the 100 histological samples reviewed by an expert cervical pathologist showed less than high grade CIN. None of the initial diagnoses reviewed were cancer.
The OR for recurrent disease if one or two post-treatment smears were HPV-DNA positive was 2.5 (1.6–3.8). The numbers of women with a positive test in one or two of the samples were 45/189 (24%) of the cases and 43/378 (12%) of the controls (Table 1). The total number of samples containing HPV-DNA was 58/370 (14%) among the cases and 53/729 (7%) among the controls. ORs and proportions of HPV-DNA positivity for cases and controls at the time for the first and second test respectively and according to time between treatment and recurrent high grade disease for the cases are shown in Table 2. The relative protection of a negative HPV-status decreased with time.
Table 1.
HPV-status post-treatment related to time until recurrence was noted
| Time between treatment and recurrence (for cases) |
Cases HPVpos | Controls HPVpos | OR of HPVpos |
|---|---|---|---|
| 2–16.3 Years (whole population) | 45/189 (24%) | 44/378 (12%) | 2.5 (1.6–3.8) |
| 2–3.6 Years | 21/63 (33%) | 14/126 (11%) | 4.9 (2.2–11.0) |
| 3.6–6.7 Years | 14/63 (22%) | 12/126 (10%) | 2.4 (1.1–5.3) |
| >6.7 Years | 10/63 (16%) | 18/126 (14%) | 1.1 (0.5–2.5) |
| Test for trend | p < 0.05 |
HPV-positivity was defined as presence of HPV-DNA in any smear. Odds ratios (ORs) with 95% confidence intervals.
Table 2.
Result of HPV-testing separated at 6 and 12 month tests post-treatment
| Time between treatment and recurrence (for cases) |
Cases HPVpos at 6 months |
Controls HPVpos at 6 months |
OR of HPVpos at 6 months |
Cases HPVpos at 12 months |
Controls of HPVpos at 12 months |
OR of HPVpos at 12 months |
|---|---|---|---|---|---|---|
| 2–16.3 Years | 31/189 (16%) | 33/378 (9%) | 2.0 (1.2–3.4) | 27/181 (15%) | 20/369 (5%) | 2.8 (1.6–4.8) |
| (whole population) | ||||||
| 2–3.6 Years | 16/63 (25%) | 11/126 (9%) | 3.8 (1.5–9.4) | 13/59 (22%) | 4/123 (3%) | 6.3 (2.3–17.0) |
| 3.6–6.7 Years | 10/63 (16%) | 7/126 (6%) | 2.0 (1.1–7.5) | 8/59 (14%) | 8/121 (7%) | 2.1 (0.9–5.2) |
| >6.7 Years | 5/63 (8%) | 15/126 (12%) | 0.6 (0.2–1.9) | 6/63 (10%) | 8/125 (6%) | 1.4 (0.5–4.0) |
| Test for trend | p < 0.1 | p < 0.05 |
Total material and divided into three groups according to time interval for recurrence of CIN2+ for cases. Odds ratios (Ors) with 95% confidence intervals.
HPV-positivity in both 6 and 12 month samples was more common in cases than in controls and the difference in type specific persistence was also more common among the cases as shown in Table 3. A higher proportion of cases than controls picked up a new HPV-infection between 6 and 12 month sampling as shown by the number of HPV-negatives in first smears who were HPV-positive in second smears.
The distribution of HPV-types from the smears taken 6 and 12 months after treatment is presented in Table 4. There were no significant differences in the prevalence of separate HPV-types in cases versus controls when compared to the total number of HPV-positives. 16/45 (35%) of HPV-positive samples among cases and 15/44 (34%) among controls showed multiple infections with two or more of the 12 high risk primers used.
Table 4.
Results of HPV-typing with pyrosequencing
| Cases – 370 smears |
Controls – 747 smears |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Multiple infections | ||||
| Total | 16 | 4.32 | 15 | 2.01 |
| Two types | 9 | 2.43 | 8 | 1.07 |
| Three types | 4 | 1.08 | 2 | 0.27 |
| Four types | 2 | 0.54 | 4 | 0.54 |
| Five types | 1 | 0.27 | 1 | 0.13 |
| HPV type | ||||
| 16 | 16 | 4.32 | 25 | 3.35 |
| 18 | 6 | 1.62 | 9 | 1.20 |
| 31 | 7 | 1.89 | 8 | 1.07 |
| 33 | 16 | 4.32 | 9 | 1.20 |
| 35 | 5 | 1.35 | 2 | 0.27 |
| 45 | 5 | 1.35 | 3 | 0.40 |
| 51 | 6 | 1.62 | 1 | 0.13 |
| 52 | 3 | 0.81 | 1 | 0.13 |
| 56 | 7 | 1.89 | 5 | 0.67 |
| 58 | 1 | 0.27 | 2 | 0.27 |
| 59 | 2 | 0.54 | 2 | 0.27 |
The total number of types exceeds the number of HPV-positive smears as some smears contain multiple infections. There are no significant differences between cases and controls when n for individual HPV-types were compared with the number of HPV-positives in each category.
Only 4 of the 28 women who developed invasive cancer were HPV positive in any of the two smears taken within 2 years after the treatment of the pre-cancer.
4. Discussion
Women who were free from HPV after treatment for high grade dysplasia were still at high risk for the development of high grade disease occurring more than 2 years after treatment and 76% of the women who subsequently developed high grade disease or cancer had negative HPV-status post-treatment. Consequently, our findings do not support differentiated long term follow up based upon the HPV-status after surgery. The relative protection offered by a negative test tended to be higher if the test was performed 12 instead of 6 months post-treatment and the predictive ability of post-treatment HPV-test diminished with time (p for trend <0.05). When cases got their second high grade lesion more than 6 years and 8 months after the first there was no significant difference in HPV-status between cases and controls, neither overall (Table 1) nor 6 or 12 months post-treatment (Table 2). It was more common among the cases that the first smear was HPV-negative while the second smear contained HPV-DNA compared with the controls. This we interpret as a higher proportion of re-infections. Among the women infected with HPV we found a high proportion of multiple infections (34%). This is in concordance with other results with the same basic techniques19 and studies with other sensitive HPV-typing methods.20
The strengths of this study are the large number of patients with recurrent disease, the long observation time and the design that allowed us to specifically study recurrent cases occurring more than 2 years after treatment while excluding the residual and presumably incompletely treated patients. The latter is important, as there is a great need for studies evaluating strategies for long term follow up. To our knowledge no such studies have been published previously.
The observational case–control design is prone to bias, although we tried to minimise these by selecting controls matched for the potential confounders age and time-period for treatment. We have not included specific data on treatment modality. The way to treat dysplasia has changed over time since the 1970s but for each period of time it has been quite uniform for different grades of CIN.21 The risk that cases and controls would have received different treatments should be minimal by these matching parameters.
The HPV-testing was made on archival smears, a method that is considered reliable22 and has become increasingly common in research of HPV-epidemiology.23 The somewhat surprisingly small difference in HPV-status between cases and controls could theoretically be caused by a lack of sensitivity in the analysis of HPV. However, the fraction of HPV positive smears among the controls was higher than that found in a Swedish population study with a smaller range in age but the same median age, and where fresh samples were used for analysis.24 Furthermore, two samples could be analysed in 97% (550/567) of the women in the study and a case or a control is considered positive if only one of the samples are HPV-positive, and this decreases the risk for lack of sensitivity in the laboratory analysis. There is, of course, a theoretical possibility of cross contamination that could decrease the ratio of HPV between controls and cases when using a highly sensitive method of DNA-detection.25 Such contamination can occur at the time of extraction and HPV-testing. This risk, however, we rule out as the laboratory work was meticulous on this point, with blanks and in very experienced hands. Cross contamination could also have occurred in the fixation bath during collection or when mixed with other samples during staining in the cytology laboratory. However, such hypothetical contamination that could have increased the rate of HPV-positivity among controls, is still not in accordance with the absence of HPV-DNA in 74% of the cases.
Another limitation is that we have relied on the original histology diagnosis. However, the pathology expert re-analysis of 100 random samples showed that the original diagnosis were quite accurate at this university laboratory and did not indicate that a re-analysis of all the 756 histological samples would have had a decisive impact on our results.
At first glance the results can seem somewhat surprising and contrary to the present scientific opinion. However, to our knowledge, only one study has addressed the issue of HPV-testing for recurrent disease after trying to sort out the residual cases. A study by Bollen and colleagues 26 separated residual and recurrent disease (less or more than 1 year post-treatment) but the results are not comparable as HPV-status was checked shortly before biopsy. The case–control study of the co-author Chua/Wallin and Hjerpe9 showed much higher difference between cases and controls but the numbers were small and the great majority of the recurrences occurred within the first 2 years that were excluded in our study. Most other studies are prospective with fairly short follow up periods, a small number of patients and a minimal number of recurrences.13,27,28 A Dutch study29 reported high negative predictive value of post-treatment HPV-test, but only two women in this material developed high grade CIN later than 2 years after initial treatment. However, with a case–control design, Cruickshank and colleagues30 had results in close concordance with ours. They studied 107 cases of CIN3 with time to recurrence unaccounted for, and 101 matched controls. About half the women were positive for HPV 16/18 at baseline and HPV status at 6 months post-treatment revealed an OR of 3.1 compared with controls without recurrence.
In this study the fraction of HPV-positives was constant between 6 and 12 months post-surgery for the cases while it has been almost halved among the controls. 4.4% of the cases had the same HPV-types in the two cervical samples which is a sign of persistent infection. Successful treatment of dysplasia is most often followed by eradication of HPV.10 Thus we cannot rule out the possibility that these recurrent CIN2+ dysplasias in some cases can be attributed to unsuccessful treatment. However, the vast majority of the cases did not have HPV in either sample and most probably have been infected with high risk HPV infection at a later stage. This is in accordance with our finding that a higher proportion of cases became HPV-positive in the 12-month smear with 6-month smear negative, compared with the controls. The condition (host and possibly environmental) that once led to the cervical lesion treated, usually persists and these women have an increased risk for re-infection and persistence with high risk viruses.31,32 This is the probable cause for the limited protection we found with negative HPV-test when the long term effects are evaluated. It should be noted that our study is restricted to the predictive ability of high risk HPV- testing for long time recurrence when HPV is tested within 2 years after treatment and does not rule out a better use of HPV-testing after 2 years, a strategy we have not studied.
5. Conclusion
There was a significant difference in ORs based on post-treatment HPV status 6–12 months after treatment, between cases who had recurrent diagnosis of high grade cervical dysplasia more than 2 years after treatment and controls who had not. This difference is most pronounced in the earliest period studied 2–3.6 years and decreases when cases that had their recurrences later, were compared with their matched controls. However, 76% of the cases were HPV-negative in both samples taken 3–24 months post-treatment. This study does not support the notion of separate protocols for long term follow up based on HPV-status within 24 months after treatment for high grade cervical lesions.
Acknowledgements
Methodological and statistical aid and suggestions from Prof. Kjell Torén, Prof. Dimitrios Trichopoulos, Prof. Joakim Dillner and statistician Ingmarie Johanson are gratefully acknowledged. This study was conducted with grants from the Göteborg Medical Society, King Gustav V Jubilee Clinic Cancer Research Foundation, the Swedish Cancer Society, the Stockholm County Council, the Swedish Cancer Foundation, the Swedish Medical Research Council and the County of Halland. None of the funding sources have had any role in the analysis of the data or preparation and writing of the manuscript.
Footnotes
Conflict of interest statement
None declared.
REFERENCES
- 1.Paraskevaidis E, Lolis ED, Koliopoulos G, Alamanos Y, Fotiou S, Kitchener HC. Cervical intraepithelial neoplasia outcomes after large loop excision with clear margins. Obstet Gynecol. 2000;95(6 Pt 1):828–831. doi: 10.1016/s0029-7844(00)00791-2. [DOI] [PubMed] [Google Scholar]
- 2.Zielinski GD, Bais AG, Helmerhorst TJ, et al. HPV testing and monitoring of women after treatment of CIN 3: review of the literature and meta-analysis. Obstet Gynecol Surv. 2004;59(7):543–553. doi: 10.1097/00006254-200407000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Pettersson F, Malker B. Invasive carcinoma of the uterine cervix following diagnosis and treatment of in situ carcinoma. Record linkage study within a National Cancer Registry. Radiother Oncol. 1989;16(2):115–120. doi: 10.1016/0167-8140(89)90028-5. [DOI] [PubMed] [Google Scholar]
- 4.Soutter WP, de Barros Lopes A, Fletcher A, et al. Invasive cervical cancer after conservative therapy for cervical intraepithelial neoplasia. Lancet. 1997;349(9057):978–980. doi: 10.1016/s0140-6736(96)08295-5. [DOI] [PubMed] [Google Scholar]
- 5.Andersson-Ellstrom A, Seidal T, Grannas M, Hagmar B. The pap-smear history of women with invasive cervical squamous carcinoma. A case–control study from Sweden. Acta Obstet Gynecol Scand. 2000;79(3):221–226. [PubMed] [Google Scholar]
- 6.Benedet JL, Miller DM, Nickerson KG. Results of conservative management of cervical intraepithelial neoplasia. Obstet Gynecol. 1992;79(1):105–110. [PubMed] [Google Scholar]
- 7.Bigrigg A, Haffenden DK, Sheehan AL, Codling BW, Read MD. Efficacy and safety of large-loop excision of the transformation zone. Lancet. 1994;343(8888):32–34. doi: 10.1016/s0140-6736(94)90881-8. [DOI] [PubMed] [Google Scholar]
- 8.Bekkers RL, Melchers WJ, Bakkers JM, et al. The role of genotype-specific human papillomavirus detection in diagnosing residual cervical intraepithelial neoplasia. Int J Cancer. 2002;102(2):148–151. doi: 10.1002/ijc.10691. [DOI] [PubMed] [Google Scholar]
- 9.Chua KL, Hjerpe A. Human papillomavirus analysis as a prognostic marker following conization of the cervix uteri. Gynecol Oncol. 1997;66(1):108–113. doi: 10.1006/gyno.1997.4753. [DOI] [PubMed] [Google Scholar]
- 10.Elfgren K, Bistoletti P, Dillner L, Walboomers JM, Meijer CJ, Dillner J. Conization for cervical intraepithelial neoplasia is followed by disappearance of human papillomavirus deoxyribonucleic acid and a decline in serum and cervical mucus antibodies against human papillomavirus antigens. Am J Obstet Gynecol. 1996;174(3):937–942. doi: 10.1016/s0002-9378(96)70330-7. [DOI] [PubMed] [Google Scholar]
- 11.Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189(1):295–304. doi: 10.1067/mob.2003.633. [DOI] [PubMed] [Google Scholar]
- 12.HARG. Att förebygga cervixcancer samt vaginal och vulvacancer. Riktlinjer för diagnos, behandling och kontroll av intraepitelial neoplasi och papillomvirusinfektioner i cervix, vagina och vulva [Protecting from cervical, vaginal and vulva cancer – Guidelines] Swedish Society for Obstetrics and Gynecology. 1997 [Google Scholar]
- 13.Zielinski GD, Rozendaal L, Voorhorst FJ, et al. HPV testing can reduce the number of follow-up visits in women treated for cervical intraepithelial neoplasia grade 3. Gynecol Oncol. 2003;91(1):67–73. doi: 10.1016/s0090-8258(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 14.Paraskevaidis E, Arbyn M, Sotiriadis A, et al. The role of HPV DNA testing in the follow-up period after treatment for CIN: a systematic review of the literature. Cancer Treat Rev. 2004;30(2):205–211. doi: 10.1016/j.ctrv.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Chua KL, Hjerpe A. Polymerase chain reaction analysis of human papillomavirus in archival cervical cytologic smears. Anal Quant Cytol Histol. 1995;17(4):221–229. [PubMed] [Google Scholar]
- 16.Koss LG, Sherman ME, Cohen MB. Significant reduction in the rate of false-negative cervical smears with neural network-based technology (PAPNET Testing System) Hum Pathol. 1997;28(10):1196–1203. doi: 10.1016/s0046-8177(97)90258-6. [DOI] [PubMed] [Google Scholar]
- 17.Wallin KL, Wiklund F, Angstrom T, et al. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer. N Engl J Med. 1999;341(22):1633–1638. doi: 10.1056/NEJM199911253412201. [DOI] [PubMed] [Google Scholar]
- 18.Gharizadeh B, Oggionni M, Zheng B, et al. Type-specific multiple sequencing primers: a novel strategy for reliable and rapid genotyping of human papillomaviruses by pyrosequencing technology. J Mol Diagn. 2005;7(2):198–205. doi: 10.1016/S1525-1578(10)60546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gharizadeh B, Zheng B, Akhras M, et al. Sentinel-base DNA genotyping using multiple sequencing primers for high-risk human papillomaviruses. Mol Cell Probes. 2006;20(3–4):230–238. doi: 10.1016/j.mcp.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Swan DC, Smith SJ, et al. Simultaneous amplification and identification of 25 human papillomavirus types with Templex technology. J Clin Microbiol. 2006;44(11):4157–4162. doi: 10.1128/JCM.01762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strander B. How cancer in the uterine cervix became a rare disease (Swe.Hur cervixcancer blev en ovanlig sjukdom) In: Lindberg B, editor. Swedish gynaecology during one century (Swe. Svensk gynekologi under ett sekel) Uppsala: SFOG; 2004. [Google Scholar]
- 22.Jacobs MV, Zielinski D, Meijer CJ, et al. A simplified and reliable HPV testing of archival Papanicolaou-stained cervical smears: application to cervical smears from cancer patients starting with cytologically normal smears. Br J Cancer. 2000;82(8):1421–1426. doi: 10.1054/bjoc.1999.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franceschi S, Herrero R, Clifford GM, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119(11):2677–2684. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 24.Forslund O, Antonsson A, Edlund K, et al. Population-based type-specific prevalence of high-risk human papillomavirus infection in middle-aged Swedish women. J Med Virol. 2002;66(4):535–541. doi: 10.1002/jmv.2178. [DOI] [PubMed] [Google Scholar]
- 25.Grainge MJ, Seth R, Coupland C, et al. Human papillomavirus infection in women who develop high-grade cervical intraepithelial neoplasia or cervical cancer: a case–control study in the UK. Br J Cancer. 2005;92(9):1794–1799. doi: 10.1038/sj.bjc.6602538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bollen LJ, Tjong AHSP, van der Velden J, et al. Prediction of recurrent and residual cervical dysplasia by human papillomavirus detection among patients with abnormal cytology. Gynecol Oncol. 1999;72(2):199–201. doi: 10.1006/gyno.1998.5250. [DOI] [PubMed] [Google Scholar]
- 27.Elfgren K, Jacobs M, Walboomers JM, Meijer CJ, Dillner J. Rate of human papillomavirus clearance after treatment of cervical intraepithelial neoplasia. Obstet Gynecol. 2002;100(5 Pt 1):965–971. doi: 10.1016/s0029-7844(02)02280-9. [DOI] [PubMed] [Google Scholar]
- 28.Kjellberg L, Wadell G, Bergman F, Isaksson M, Angstrom T, Dillner J. Regular disappearance of the human papillomavirus genome after conization of cervical dysplasia by carbon dioxide laser. Am J Obstet Gynecol. 2000;183(5):1238–1242. doi: 10.1067/mob.2000.107322. [DOI] [PubMed] [Google Scholar]
- 29.Nobbenhuis MA, Meijer CJ, van den Brule AJ, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84(6):796–801. doi: 10.1054/bjoc.2000.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruickshank ME, Sharp L, Chambers G, Smart L, Murray G. Persistent infection with human papillomavirus following the successful treatment of high grade cervical intraepithelial neoplasia. Bjog. 2002;109(5):579–581. doi: 10.1111/j.1471-0528.2002.01554.x. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson PK, Lichtenstein P, Gyllensten UB. Heritability of cervical tumours. Int J Cancer. 2000;88(5):698–701. doi: 10.1002/1097-0215(20001201)88:5<698::aid-ijc3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32 Suppl 1:S16–S24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]