Abstract
Background & Aims
The revised Bethesda guidelines for Lynch syndrome recommend microsatellite instability (MSI) testing all colorectal cancers in patients diagnosed before age 50 years and colorectal cancers diagnosed in patients between ages 50 and 59 years with particular pathology features. Our aim was to identify pathology and other features that independently predict high MSI (MSI-H).
Methods
Archival tissue from 1098 population-based colorectal cancers diagnosed before age 60 years was tested for MSI. Pathology features, site, and age at diagnosis were obtained. Multiple logistic regression was performed to determine the predictive value of each feature, as measured by an odds ratio (OR), from which a scoring system (MsPath) was developed to estimate the probability a colorectal cancer is MSI-H.
Results
Fifteen percent of tumors (162) were MSI-H. Independent predictors were tumor-infiltrating lymphocytes (OR, 9.1; 95% confidence interval [CI], 5.9 –14.1), proximal subsite (OR, 4.7; 95% CI, 3.1–7.3), mucinous histology (OR, 2.8; 95% CI, 1.7– 4.8), poor differentiation (OR, 1.9; 95% CI, 1.2–3.1), Crohn’s-like reaction (OR, 1.9; 95% CI, 1.2–2.9), and diagnosis before age 50 years (OR, 1.9; 95% CI, 1.3–2.9). MsPath score ≥ 1.0 had a sensitivity of 93% and a specificity of 55% for MSI-H.
Conclusions
The probability an individual colorectal cancer is MSI-H is predicted well by the MsPath score. There is little value in testing for DNA mismatch repair loss in tumors, or for germline mismatch repair mutations, for colorectal cancers diagnosed in patients before age 60 years with an MSPath score <1 (approximately 50%). Pathology can identify almost all MSI-H colorectal cancers diagnosed before age 60 years.
Lynch syndrome (often termed hereditary nonpolyposis colorectal cancer) is caused by germline mutation of a DNA mismatch repair (MMR) gene, most notably MLH1 or MSH2.1,2 Although only around 2% of all colorectal cancers are attributable to germline MMR mutations, the early onset of malignancy in carriers is responsible for a major loss of years of life. In addition, the identification of a case of Lynch syndrome has clinical implications for approximately 50% of their first-degree relatives. It is, therefore, potentially important to be able to identify subjects with Lynch syndrome because they and their relatives can be offered colonoscopic surveillance, which has been shown to prevent cancers and reduce mortality for carriers of pathogenic germline mutations. 3 High frequency of DNA microsatellite instability (MSI-H) and loss of MMR protein expression by immunohistochemistry (IHC) are very sensitive markers for identifying tumors occurring in Lynch syndrome.4 However, testing for loss of DNA MMR in all cases of colorectal cancer that present within the community would be a very nonspecific and expensive way to identify cases of Lynch syndrome because most colorectal cancers with MSI do not occur as a result of an inherited germ-line mutation in an MMR gene (frequently described as “sporadic” MSI-H colorectal cancers) and therefore are not cases of Lynch syndrome.5
The Amsterdam criteria were established to identify Lynch syndrome for multiple case families presenting to high-risk clinics for research purposes and were based on family cancer history.6 The original and revised Bethesda guidelines were developed to help identify Lynch syndrome families by categorizing colorectal cancer cases, presenting in a community setting, by molecular evaluation using MSI testing or IHC analysis (Table 1).7–9 The incorporation in the Bethesda guidelines of testing for DNA MMR status in colorectal cancers diagnosed at an early age and/or in association with a suggestive personal or family history was designed to provide a sensitive test for Lynch syndrome while excluding colorectal cancers not due to germline mutations in MMR genes.
Table 1.
Bethesda Guidelines (Revised)
| 1. | Colorectal cancer diagnosed before age 50 years |
| 2. | Multiple colorectal cancer or HNPCC-related cancersa |
| 3. | Colorectal cancer with MSI-related histologyb diagnosed before age 60 years |
| 4. | Colorectal cancer or HNPCC-related cancer diagnosed in at least one first-degree relative before age 50 years |
| 5. | Colorectal cancer or HNPCC-related cancer diagnosed in at least 2 first- or second-degree relatives at any age |
NOTE. Any criterion (1–5) can be met.
HNPCC, hereditary nonpolyposis colorectal cancer.
Includes cancer of endometrium, small bowel, pelviureter, biliary tract, stomach, ovary, pancreas, or brain (mainly glioblastoma multiforme).
Tumor-infiltrating lymphocytes, Crohn’s-like lymphocytic reaction, mucin/signet ring cell differentiation, medullary growth pattern.
The revised Bethesda guidelines include the presence of one or more pathology features shown to be associated with MSI status,10–19 namely the presence of tumor-infiltrating lymphocytes, a Crohn’s-like lymphocytic reaction, mucinous or signet ring differentiation, and a medullary or undifferentiated and solid growth pattern.9 The revised Bethesda guidelines do not provide details on the predictive value of these features, either alone or in combination, but recommend MSI testing and/or IHC testing for DNA MMR proteins for all colorectal cancers diagnosed in patients before age 50 years and for those colorectal cancers diagnosed in patients between the ages of 50 and 59 years that have one or more of these pathology features. Tumor pathology may therefore be useful for triaging tumors for testing for loss of MMR function and to facilitate the diagnosis of some later-onset colorectal cancers with “likely Lynch syndrome” with little or no other suggestive personal or family history. It is not known what proportion of a population-based series of colorectal cancer meet the revised Bethesda criteria for MSI testing. Primarily this is because there have been no studies testing any series of cases for all 5 of the guidelines listed in Table 1. The guideline most underused is pathology, hence the need for this study.
The aim of this study was to identify the pathology and other features that independently predict DNA MMR deficiency, as defined by having MSI-H, using a large population-based study of colorectal cancers diagnosed in patients before the age of 60 years and to develop a scoring system to assist clinical decision making.
Materials and Methods
Subjects
A population-based series of men and women diagnosed with colorectal cancer before the age of 60 years was recruited into the Colon Cancer Family Registry, a National Cancer Institute–supported consortium established in 1997 to create a comprehensive collaborative infrastructure for interdisciplinary studies of the genetic and molecular epidemiology of colorectal cancer (detailed information about the registry is available at http://epi.grants.cancer.gov/CFR/). Briefly, colorectal cancer cases were recruited from 1997 to 2003 from local state or regional cancer registries in the following locations: Fred Hutchinson Cancer Research Center, Seattle, Washington (Seattle); University of Melbourne, Melbourne, Victoria, Australia (Melbourne); Cancer Care Ontario, Toronto, Ontario, Canada (Ontario); Mayo Clinic, Rochester, Minnesota (Mayo); and University of Hawaii Cancer Research Center, Honolulu, Hawaii (Hawaii). Eligibility was all cases (Seattle), all cases diagnosed before age 45 years and one half of cases diagnosed between ages 45 and 59 years (Melbourne), all cases with ≥1 first- or second-degree relatives with colorectal cancer and one fourth of all other cases (Ontario), all cases diagnosed before age 50 years or with ≥1 first- or second-degree relative with colorectal cancer and one third of all other cases (Mayo), and all cases with ≥1 first-degree relatives with colorectal cancer (Hawaii). Subjects were asked to provide consent to access their tumor tissue and to donate a blood sample.
Measurement of MSI
All colorectal cancer tumors were tested for MSI status using 10 DNA microsatellite markers (BAT25, BAT26, BAT40, BAT34C4, D5S346, D17S250, ACTC, D18S55, D10S197, and MYCL) as described previously.20 Immunostaining for the DNA MMR proteins MLH1, MSH2, MSH6, and PMS2 was undertaken as described previously.21 Only cases with 5 or more evaluable microsatellite markers were included. Those showing instability in at least 30% of those tested were classed as MSI-H. Cases with no evaluable markers showing instability were classed as microsatellite stable (MSS), and the remainder was classed as MSI-low (MSI-L).
Measurement of Pathology and Other Features
Eight pathologists at the locations participated in the scoring of features while blinded to MSI status. The features included histology subtype (adenocarcinoma, mucinous carcinoma, signet ring cell carcinoma, and medullary carcinoma), degree of differentiation (poor vs other), presence of tumor-infiltrating lymphocytes (yes or no), and presence of Crohn’s-like lymphocytic reaction (yes or no). Mucinous carcinoma was defined as at least 50% of the tumor area comprising secretory mucin. Signet ring cell carcinoma was defined as at least 50% of the tumor composed of signet ring cells. Medullary carcinoma was defined as a tumor that was poorly differentiated or undifferentiated and composed of masses of cells circumscribed with a well-circumscribed margin and a marked lymphocytic infiltrate that was both peritumoral and intratumoral. Poor differentiation was defined as a tumor with at least some glandular structures and/or mucin production but the glands were highly irregular and difficult to discern. The grade of differentiation was based on the least differentiated area and not the predominant appearance but excluded dedifferentiation or tumor budding at the invasive margin. Tumor-infiltrating lymphocytes were scored as present when there were at least 5 intra-epithelial lymphocytes in at least one high-power field (40×) and at least 10 high-power fields had been thoroughly searched. A Crohn’s-like lymphocytic reaction was scored as present when at least 4 nodular lymphoid aggregates were counted in a low-power field (4×) beyond the advancing edge of the tumor and generally within the subserosa or mesenteric fat.
At least one pathologist from each location participated in discussions regarding definition of the histologic entities. Typing and grading of colorectal cancers was based on the World Health Organization tumor classification. 22 Despite the standardization of pathology scoring, which applied strictly to classifying the presence of tumor-infiltrating lymphocytes and Crohn’s-like lymphocytic reaction, there were small differences in approaches across the 5 centers. In the 2 largest recruiting locations (Seattle and Melbourne), only a single representative tumor block was available for analysis and the more well-established pathology features (type and grade) were therefore extracted from original pathology reports, while in other locations these features were reassessed according to the previous definitions. Anatomic site and age at diagnosis were abstracted from cancer registry forms.
Eligibility
Only tumors for which at least 5 DNA microsatellite markers were evaluable, and for which anatomic site and all relevant pathology features could be determined, were included.
Statistical Methods
Proportions were compared using Pearson χ2 test statistics or Fisher exact test when the smallest cell had <10 observations. All nominal P values were 2-tailed. Sensitivity and specificity for MSI-H (vs MSI-L and MSS combined) were estimated, with 95% confidence intervals, for each feature using standard definitions.23 Using all data, unconditional multiple logistic regression was used to measure the association between the tumor being MSI-H and each of the pathology features and age at diagnosis while adjusting for all features and age at diagnosis.
Development of the MsPath model to predict MSI-H
We developed the model using the data of North American participants (Seattle, Ontario, Mayo, and Hawaii recruitment) and assessed the model on the independent Australian data (Melbourne recruitment). Using the data from the North American cases, unconditional multiple logistic regression was used to model the probability that the tumor was MSI-H as a function of the pathology features and age at diagnosis. Model selection was based on forward selection and confirmed by backward elimination. Interactions between pathology features and age at diagnosis were tested.
Based on the results of the logistic regression analysis, we developed a model to estimate MSI-H probability called “MsPath” (Microsatellite instability by Pathology). The development of the MsPath model is described in detail in Results.
Validation of the MsPath model to predict MSI-H
The fit of the model was assessed using the Australian data, which are independent of the North American data that were used to develop the model. Validation was based on tests for underestimation or overestimation or dispersion as described by Cox and Snell24 and applied by Apicella et al25 and by estimating the area under the curve of the receiver operating characteristic curve and its 95% confidence interval. An area under the curve value >0.8 is considered a good test in terms of ranking the tumors according to their likelihood of being MSI-H. All statistical computations were performed using Stata (Stata Corp, College Station, TX).
Results
Of the 1098 eligible colorectal cancer tumors (Seattle, 386; Melbourne, 361; Ontario, 157; Mayo, 149; Hawaii, 45), 15% were MSI-H, 12% MSI-L, and 73% MSS. The distribution of MSI status did not differ across recruitment sites (P = .4).
The proportion of tumors that were MSI-H decreased from 24% for those diagnosed before age 40 years to 12% for those diagnosed between ages 55 and 59 years.
Table 2 shows that 36% of tumors were right sided, 11% were mucinous or of other nonadenocarcinoma histology (10 were signet ring and one was medullary), 21% were poorly differentiated, 28% had a Crohn’s-like lymphocytic reaction, and 26% had tumor-infiltrating lymphocytes. Forty-three percent were diagnosed in patients before age 50 years.
Table 2.
Distribution of Anatomic Site and Pathology Feature by MSI, Association Between Feature and MSI-H (Odds Ratio and 95% CI), and Sensitivity and Specificity (95% CI) of Each Feature for MSI-H
| Pathology | MSI-Ha (row %) |
MSI-La (row %) |
MSSa (row %) |
TOTAL (column %) |
Odds ratiob (95% CI) |
Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
|---|---|---|---|---|---|---|---|
| Age at diagnosis | |||||||
| Younger than 50 years | 84 (17) | 61 (13) | 324 (69) | 469 (43) | 1.9 | 52 | 59 |
| 50–59 years | 78 (12) | 71 (11) | 480 (76) | 629 (57) | (1.3–2.9) | (44–60) | (56–62) |
| Anatomic site | |||||||
| Right-sided | 120 (30) | 46 (12) | 233 (58) | 399 (36) | 4.7 | 74 | 70 |
| Left-sided | 42 (6) | 86 (12) | 571 (82) | 699 (64) | (3.1–7.3) | (67–81) | (67–73) |
| Histology | |||||||
| Mucinous or other | 45 (36) | 12 (10) | 69 (55) | 126 (11) | 2.8 | 28 | 91 |
| Adenocarcinoma | 117 (12) | 120 (12) | 735 (76) | 972 (89) | (1.7–4.8) | (21–35) | (89–93) |
| Grade | |||||||
| Poorly differentiated | 62 (27) | 25 (11) | 143 (62) | 230 (21) | 1.9 | 38 | 82 |
| Other | 100 (12) | 107 (12) | 661 (76) | 868 (79) | (1.2–3.1) | (31–46) | (79–84) |
| Crohn’s-like lymphocytic reaction | |||||||
| Yes | 90 (30) | 35 (12) | 180 (59) | 305 (28) | 1.9 | 56 | 77 |
| No | 72 (9) | 97 (12) | 624 (79) | 793 (72) | (1.2–2.9) | (48–63) | (74–80) |
| Tumor-infiltrating lymphocytes | |||||||
| Yes | 116 (41) | 30 (11) | 136 (48) | 282 (26) | 9.1 | 72 | 82 |
| No | 46 (6) | 102 (13) | 668 (82) | 816 (74) | (5.9–14.1) | (64–78) | (80–85) |
| Any one feature (including age at diagnosis) | |||||||
| Yes | 160 (18) | 110 (12) | 627 (70) | 897 (82) | 21.6c | 99 | 21 |
| No (no features) | 2 (1) | 22 (11) | 177 (88) | 201 (18) | (5.3–87.9) | (96–100) | (18–24) |
| Total | 162 (15) | 132 (12) | 804 (73) | 1098 (100) |
CI, confidence interval.
Cases with 5 or more evaluable microsatellite markers were included; those showing instability in at least 30% of these were classed as MSI-H. Cases with no evaluable markers showing instability were classed as MSS and the remainder classed as MSI-L.
Adjusted for all other features.
Not adjusted for any features.
Table 2 shows that tumors were more likely to be MSI-H if they were right sided, mucinous or other, poorly differentiated, having Crohn’s-like lymphocytic reaction, having tumor-infiltrating lymphocytes, or diagnosed in patients before age 50 years. After adjusting for all features, the odds of a tumor being MSI-H were approximately 9 times higher if the tumor had infiltrating lymphocytes and approximately twice as high if it was poorly differentiated, with odds ratios for other features between 3 and 5. Tumors having at least one of the predictive features were about 20 times more likely to be MSI-H than tumors with none of the predictive features.
Table 2 also shows that the most sensitive features for MSI-H were tumor-infiltrating lymphocytes or right-sided location, with about three fourths of MSI-H tumors having each of these characteristics. All features were specific for MSI-H, particularly histologic type, with approximately 90% of adenocarcinomas (nonmucinous) being either MSI-L or MSS.
Of the 201 tumors with none of the predictive features, 199 (99%) were MSI-L or MSS. Only 2 tumors (0.2% of all tested) had none of the tested features yet were MSI-H according to the MSI definition used. One of these tumors, diagnosed in a patient at age 55 years, was unstable for 30% of markers (D17S250, ACTC, D10S197), and one diagnosed in a patient at age 52 years was unstable for 60% of markers (D5S346, D17S250, ACTC, MYCL, D18S55, D10S197). Neither showed instability in mononucleotide markers (more specific for DNA MMR deficiency) or loss of MMR protein expression by IHC (MLH1, MSH2, MSH6, PMS2).
Development of the MsPath Model
Table 3 shows that, using the North American data, when all predictive features were included in a multivariate model, all remained statistically significant predictors.
Table 3.
Regression Coefficients (β) (and SE), Odds Ratios (and 95% Confidence Intervals), and Nominal Significance Levels for Potentially Predictive Factors for MSI-H, Derived From Multiple Linear Logistic Regression Using the North American Data
| Pathology | β (SE) | Odds ratio (95% confidence interval)a |
P value |
|---|---|---|---|
| Baseline | −4.31 (0.32) | < .001 | |
| Diagnosis before age 50 years | 0.67 (0.25) | 2.0 (1.2–3.2) | .007 |
| Right-sided | 1.64 (0.26) | 5.2 (3.1–8.6) | < .001 |
| Mucinous or other | 1.06 (0.30) | 2.9 (1.6–5.2) | < .001 |
| Poorly differentiated | 0.61 (0.26) | 1.8 (1.1–3.1) | .02 |
| Crohn’s-like lymphocytic reaction | 0.54 (0.26) | 1.7 (1.0–2.8) | .04 |
| Tumor-infiltrating lymphocytes | 2.09 (0.26) | 8.1 (9.9–13.4) | < .001 |
Adjusted for all other features.
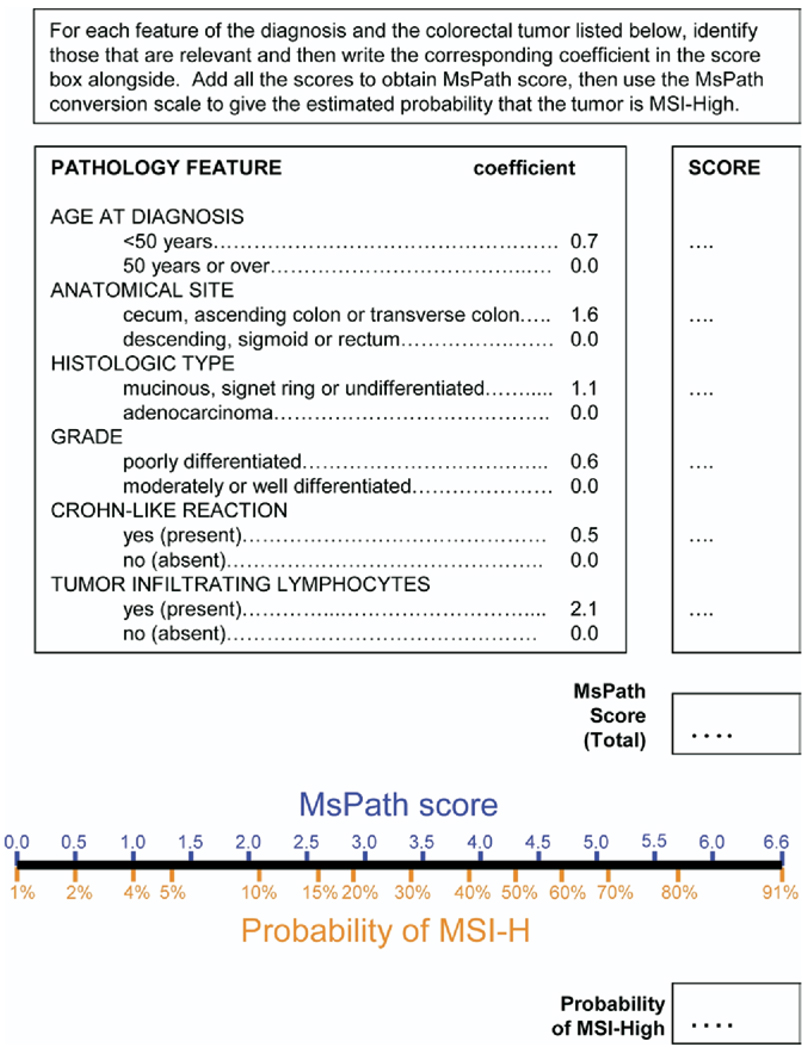
To simplify the logistic model displayed in Table 3, we rounded the β coefficients. The resulting model is shown in Figure 1. The total MsPath score was calculated on the log odds scale and was converted to a probability by the formula 1/(1+e−(MsPath score−4.31)). This is readily done for categories of scores using the MsPath conversion scale at the bottom of Figure 1 and is also presented in Table 4.
Figure 1.
Estimation of the probability that a colorectal tumor from a case diagnosed before age 60 years is MSI-H based on age at diagnosis and the site and pathology features of the tumor.
Table 4.
The Distribution of Australian Cases by MsPath Score Categories, and the Probability of Tumor Being MSI-H, as Predicted by the MsPath Model
| Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|
| MsPath score |
Probability tumor being MSI-H (%) |
Distribution (%) of cases younger than 50 years (n) |
Distribution (%) of cases 50–59 years (n) |
(if MSI test all tumors exceeding MsPath score) |
|
| 0.0a | 1 | 0 (0) | 47 (91) | 98 | 28 |
| 0.1–0.9 | 1–3 | 41 (69) | 10 (20) | 93 | 55 |
| 1.0–1.9 | 4–8 | 13 (22) | 19 (37) | 88 | 73 |
| 2.0–2.9 | 9–20 | 22 (38) | 13 (24) | 71 | 91 |
| 3.0–3.9 | 21–40 | 10 (17) | 4 (7) | 57 | 96 |
| 4.0–4.9 | 41–64 | 8 (13) | 6 (11) | 21 | 99 |
| 5.0–5.9 | 65–83 | 4 (7) | 1 (2) | 7 | 100 |
| 6.0–6.6b | 84–91 | 2 (3) | 0 (0) | 0 | 100 |
| Total | 100 (169) | 100 (192) | |||
Minimum score.
Maximum score.
For example, a tumor with none of the features diagnosed between the ages of 50 and 59 years would have an MsPath score of 0, which corresponds to a probability of MSI-H of 1%. That is, MsPath predicts that only one in 100 cases with none of these features will be MSI-H. A right-sided, poorly differentiated, mucinous tumor diagnosed in a patient before age 50 years would have an MsPath score of 4.0 (would be higher if it also exhibited Crohn’s-like reaction of tumor-infiltrating lymphocytes), which corresponds to a 41% probability of MSI-H.
Validation of the MsPath Model
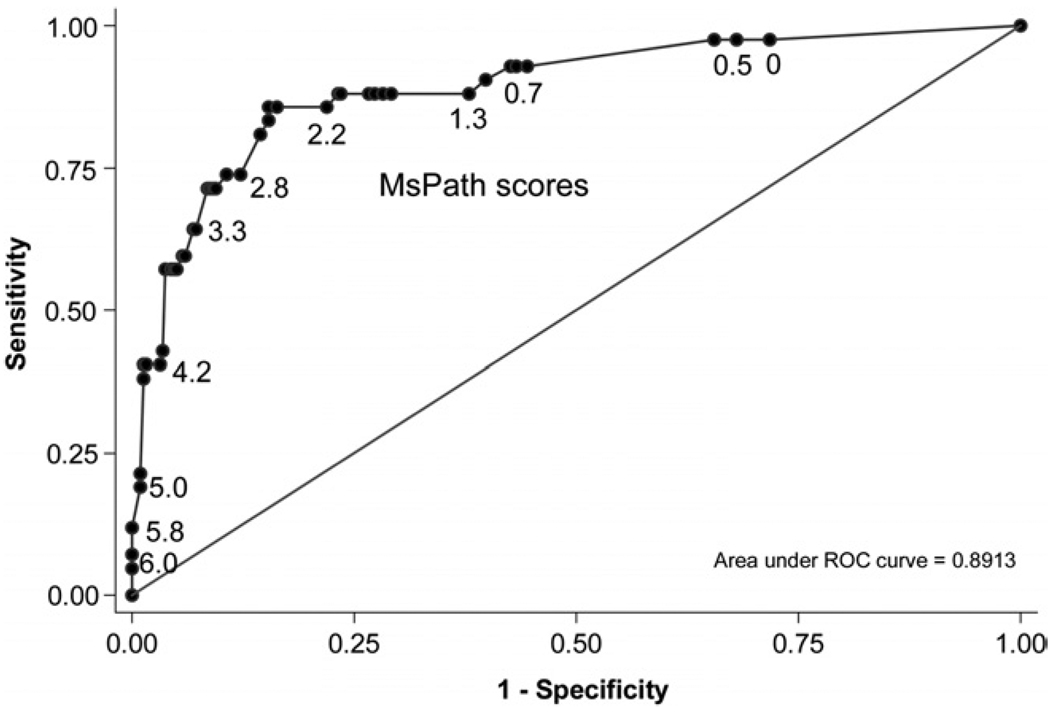
A predictive model developed on the North American tumors was shown to be valid when tested against the Australian tumors. It predicted 45 MSI-H cases overall compared with the observed 42, and within each of 6 risk categories (probability of MSI-H <3%, 3%–9%, 10%–24%, 25%–49%, 50%–74%, and >75%) the predicted versus observed numbers of MSI-H cases were 3 versus 3, 3 versus 2, 9 versus 7, 11 versus 12, 11 versus 10, and 9 versus 8, respectively. There was no evidence for overestimation or underestimation (P = .5) or dispersion (P = .4), and Figure 2 shows the area under the curve was 89% (95% confidence interval, 83%–94%).
Figure 2.
Receiver operating characteristic curve displaying the sensitivity and specificity for each MsPath score (given in body of plot) used for a cutoff for testing for MSI-H. Plot is for the Australian validation data set.
If we tested for MSI-H only the Australian tumors with at least one predictive feature (ie, MsPath score >0), we would have identified 98% of tumors with loss of MMR function (sensitivity). This would avoid the unnecessary testing of 28% of all tumors with no loss of MMR function (specificity) (see Figure 2).
If MSI testing were conducted for all tumors with an MsPath score cutoff of 1.0 (ie, right sided, or mucinous tumors, or tumors with infiltrating lymphocytes, and all tumors at least 2 features present), then 93% of the Australian MSI-H tumors would be tested (sensitivity) without having to test 55 of tumors not MSI-H (specificity). Among the 181 Australian colorectal cancers not meeting this cutoff (50% of all tumors), only 3 were MSI-H (7% of all MSI-H tumors). IHC testing of these 3 tumors showed no loss of expression of a DNA MMR protein. Given the distribution of MSI-H and pathology features among the Australian tumors, we would detect all MSI-H colorectal cancers with unequivocal deficiency of DNA MMR by testing only 50% of tumors. Table 4 shows that for patients with colorectal cancer aged younger than 50 years at diagnosis, we estimate that 59% of tumors would have an MsPath score of ≥1.0, meaning that 41% of such tumors would not need to be screened for MSI. For cases aged between 50 and 59 years at diagnosis, 43% exceeded the MsPath cutoff and 57% would not require MSI testing.
Discussion
The identification of patients likely to be carrying a germline mutation in a DNA MMR gene (Lynch syndrome) is important; however, universal molecular testing of all colorectal cancers would be expensive and time consuming. Use of family history of cancer as a predictive marker is insensitive and can be difficult to assess accurately in a clinical setting. Particular pathology features of colorectal cancers have previously been associated with MSI-H status in the setting of Lynch syndrome10,26 and in series unselected for family history and likely to comprise mainly MSI-H colorectal cancers not caused by germline MMR mutations (often referred to as “sporadic”).12–16,18,19 We have developed the MsPath score that uses easily assessable clinicopathologic characteristics to capture all MSI-H colorectal cancers presenting in patients younger than 60 years, the age group most likely to be associated with Lynch syndrome, while ruling out colorectal cancers that are highly unlikely to be MSI-H. We show that the probability of a tumor being MSI-H can be estimated at pathology review at no additional cost and indicate how a simple scoring system can then be used to triage tumors for MSI or IHC testing.
For example, for cases diagnosed in patients younger than 50 years (all of which should be tested for MSI or by IHC according to the Bethesda guidelines), we found, in the independent validation data, that only approximately 60% equaled or exceeded our recommended MsPath score of 1.0 (Figure 2), leaving 40% that we suggest would not require MSI testing. For cases diagnosed in patients between 50 and 59 years, we estimate approximately 60% would not need to be tested. Importantly, the few MSI-H colorectal cancers missed by a cutoff MsPath score of 1.0 were unlikely to be due to MMR deficiency. They showed instability in only 30%–40% of markers, and none of the 8 markers mutated in the 3 tumors were a highly specific mononucleotide marker. Furthermore, none of these tumors showed loss of expression of a DNA MMR protein. These findings validate the inclusion of pathology features in the Bethesda guidelines.9 We have recommended a cutoff MsPath score of 1.0 to maximize the specificity while maintaining a high sensitivity, because it is important not to miss MSI-H cases.
The principal advantage of our multivariate predictive model is its ability to exceed the sensitivity and specificity of models based on individual items taken alone. Some of the features in the MsPath model, such as mucinous differentiation, are more specific for MSI-H status, while others, such as presence of tumor-infiltrating lymphocytes and anatomic subsite, are more sensitive.
The biological explanations for the distinctive morphology of MSI-H colorectal cancer are not well understood. Colorectal cancers secreting abundant mucin have been associated with precursor lesions that also show abundant mucin secretion,27 such as villous adenomas in the case of Lynch syndrome28 and serrated polyps in the case of MSI-H colorectal cancers outside Lynch syndrome. 29 The increased lymphocytic reaction in MSI-H colorectal cancer may be caused by the enhanced immunogenicity associated with the generation of mutant proteins transcribed from genes with frameshift mutations. 30,31 Alternatively, MSI-H colorectal cancers may fail to elaborate Fas ligand implicated in the counterattack by malignant cells on contact with lymphoid cells.32 Loss of CDX2 expression has been linked with poor differentiation and particularly with the undifferentiated variant described as medullary carcinoma.33 Only one medullary carcinoma was diagnosed in this study, but it is likely that examples with focal glandular differentiation would have been included in the poorly differentiated subset.
Our study was based on colorectal cancers selected in a population-based manner at locations in 3 countries. Multiple pathologists using a combination of data from pathology reports and pathology reviews reported different distributions for the pathology features used in the final model. Such differences are expected, given the spectrum of pathologist training and experience. Despite such differences, the model we have developed appears robust, even when comparing across continents. Therefore, our results are likely to be generalizable and indicate that the MsPath scoring should be widely reproducible as a triage procedure for prioritizing colorectal cancers for MSI testing. Our sample size was large, and we used a relatively limited set of variables in constructing our statistical model. We found strong evidence for validity by generating the model on the North American data and validating it against the Australian data. Nonetheless, independent validation of the model in other samples is warranted.
Of patients younger than 60 years diagnosed with an MSI-H colorectal cancer, up to 50% may be due to Lynch syndrome.20,34 In a recent population-based study, 16 colorectal cancer cases diagnosed before the age of 60 years were shown to have an MSI-H tumor; of these, 10 (63%) had a deleterious germline mutation in a DNA MMR gene, while 6 (37%) had methylation of the MLH1 promoter.35 MLH1 methylation is a highly age-related process,36 and most MSI-H colorectal cancers outside Lynch syndrome are diagnosed after the age of 60 years. Therefore, the use of the MsPath score for directing subsequent MSI analysis should provide a reasonably specific, as well as highly sensitive, screening test for Lynch syndrome.
The extent to which pathology features may identify examples of Lynch syndrome that are overlooked by other Bethesda guideline criteria cannot be determined in this study. However, when germline status of this study sample is known, it will be possible to compare the performance of the pathology component of the Bethesda guidelines with the other Bethesda guideline components for identifying MMR gene mutation carriers. It should be noted that the MsPath model cannot be used to detect the rare occurrence of a pathogenic MMR gene mutation that does not result in MMR deficiency.37
The distinction between early-onset MSI-H colorectal cancers not due to Lynch syndrome and those that are due to Lynch syndrome remains a problem for both clinicians and laboratory scientists. Although initially combined as an otherwise indistinguishable entity, these subsets of MSI-H colorectal cancer differ with respect to both pathogenesis and genotype.38–41 The subset of MSI-H colorectal cancers outside Lynch syndrome is characterized by frequent mutation of BRAF and wide-spread DNA methylation.39 Although the pathology features may be shared across the 2 subtypes, certain features such as lymphocytic infiltration may be more frequent in Lynch syndrome, while others, such as mucinous differentiation, may be more common in the subset outside Lynch syndrome.38,41 When information with respect to germline mutations, MLH1 methylation, and BRAF mutation status becomes available, it may be possible to identify subtle differences in pathology and modify the MsPath model accordingly. It may then function as a much more specific indicator of Lynch syndrome.
This study did not consider colorectal cancers diagnosed after the age of 60 years. To identify late-onset cases of Lynch syndrome, the Bethesda guidelines rely exclusively on personal or family history. However, just as the MsPath model may identify colorectal cancers diagnosed in patients before age 60 years that do not require MSI testing, it is possible that the MsPath model could also be applied to later-onset colorectal cancers but meeting other Bethesda guideline criteria. However, it would be inappropriate to exclude tumors from MSI testing on the basis of the MsPath model when the family history was strongly suggestive of Lynch syndrome. Refinements of the MsPath model that would allow distinction between Lynch syndrome versus “sporadic” MSI-H colorectal cancers might permit the recognition of later-onset Lynch syndrome colorectal cancers. This would be a useful contribution given the later age at onset of Lynch syndrome colorectal cancers in the population setting.42
Loss of MSH2 expression serves as strong evidence of an MSH2 germline mutation.20 The finding of BRAF mutation or methylation of MLH1 would argue against a diagnosis of Lynch syndrome40,43,44 but may occur in the context of the recently described “serrated pathway syndrome.” 45 Rarer mechanisms for MSI-H status not caused by germline MMR mutations should also be considered, for example, somatic 2-hit inactivation of a DNA MMR gene or germline hemiallelic methylation (epimutation) of MLH1.46–48
Although we used MSI testing as the primary measure of DNA MMR proficiency, we would also expect our model to predict IHC, because it is highly correlated with MSI.21 Most hospital-based laboratories would favor IHC as the first line of testing4 because this approach already has very wide diagnostic applications and IHC has the advantage of being routinely available in diagnostic laboratories as well as having the potential for pinpointing the causative gene. If the results are unequivocal, there is no need to undertake confirmatory MSI testing.49 This study has also highlighted a gray zone in the separation of MSI-L and MSI-H as indicated by the 3 MSI-H cases that would have been missed by the cutoff MsPath score of 1.0. The inclusion of nonmononucleotide markers in the MSI panel carries the risk of overdiagnosing bona fide DNA MMR repair deficiency; a fact that is now formally recognized in the revised Bethesda guidelines.9 With the recognition of such likely overdiagnosed MSI-H cases, the MsPath model has a sensitivity approaching 100%.
The Bethesda guidelines were developed specifically for identifying patients with possible Lynch syndrome. Therefore, those guidelines and our MsPath score are not intended for the identification of other forms of hereditary colorectal cancer.
In conclusion, this study adds to the growing literature demonstrating the feasibility of population-based screening for Lynch syndrome.4,50–52 The present study has validated the inclusion of pathology features within the Bethesda guidelines and shown that the new MsPath model can be used to effectively separate colorectal cancer tumors highly unlikely to be MSI-H from those with a possibility of being MSI-H in a community setting. The features utilized in MsPath may be obtained with a minimum of time, training, and expense, and the risk of MMR deficiency can be estimated without using complex calculations or a computer. By means of the MsPath model, expensive and unnecessary special investigations can be avoided. Finally, the MsPath model provides a screening instrument for identifying Lynch syndrome in subjects aged 50 – 60 years who lack a suggestive family history.
Acknowledgments
Supported by the National Cancer Institute, National Institutes of Health, under RFA #CA-95-011 and through cooperative agreements with the Australasian Colorectal Cancer Family Registry (U01 CA097735), Familial Colorectal Neoplasia Collaborative Group (U01 CA074799), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01 CA074800), Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783), Seattle Colorectal Cancer Family Registry (U01 CA074794), and University of Hawaii Colorectal Cancer Family Registry (U01 CA074806), as well as R01 CA74806, U01-CA74800, and CA74778.
The authors thank project managers Allyson Templeton, Darshana Daftary, Judi Maskiell, Sandy Nigon, and Terrilea Burnett, as well as all the patients and family members who participated in this study.
Abbreviations used in this paper
- IHC
immunohistochemistry
- MMR
mismatch repair
- MSI-H
high microsatellite instability
- MSI-L
low microsatellite stability
- MSS
microsatellite stable
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Lynch HT, Smyrk T, Lynch JF. Overview of natural history, pathology, molecular genetics and mangement of HNPCC (Lynch syndrome) Int J Cancer. 1996;69:38–43. doi: 10.1002/(SICI)1097-0215(19960220)69:1<38::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Peltomäki P, Vasen HFA The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. Gastroenterology. 1997;113:1146–1158. doi: 10.1053/gast.1997.v113.pm9322509. [DOI] [PubMed] [Google Scholar]
- 3.Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomaki P, De La Chapelle A, Mecklin JP. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 4.Southey MC, Jenkins MA, Mead LJ, Whitty J, Trivett M, Tesoriero AA, Smith LD, Jennings K, Grubb G, Royce SG, Walsh MD, Barker MA, Young JP, Jass JR, St John DJ, Macrae FA, Giles GG, Hopper JL. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol. 2005;23:6524–6532. doi: 10.1200/JCO.2005.04.671. [DOI] [PubMed] [Google Scholar]
- 5.Samowitz WS, Slattery ML, Kerber RA. Microsatellite instability in human colonic cancer is not a useful clinical indicator of familial colorectal cancer. Gastroenterology. 1995;109:1765–1771. doi: 10.1016/0016-5085(95)90742-4. [DOI] [PubMed] [Google Scholar]
- 6.Vasen HFA, Mecklin J-P, Khan PM, Lynch HT. The international collaborative group on hereditary non-polyposis colorectal cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Bigas MA, Boland CR, Hamilton SR, Hamilton SR, Henson DE, Jass JR, Khan PM, Lynch H, Perucho M, Smyrk T, Sobin L, Srivastava S. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 8.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 9.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mecklin J-P, Sipponen P, Jarvinen HJ. Histopathology of colorectal carcinomas and adenomas in cancer family syndrome. Dis Colon Rectum. 1986;29:849–853. doi: 10.1007/BF02555362. [DOI] [PubMed] [Google Scholar]
- 11.Jass JR, Cottier DS, Jeevaratnam P, Pokos V, Browett P. Pathology of hereditary non-polyposis colorectal cancer with clinical and molecular genetic correlations. In: Baba S, editor. New strategies for treatment of hereditary colorectal cancer. Tokyo, Japan: Churchill Livingstone; 1996. [Google Scholar]
- 12.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145:148–156. [PMC free article] [PubMed] [Google Scholar]
- 13.Jass JR, Do K-A, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander J, Watanabe T, Tsung-Teh W, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gafa R, Maestri I, Matteuzzi M, Santini A, Ferretti S, Cavazzini L, Lanza G. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer. 2000;89:2025–2037. [PubMed] [Google Scholar]
- 16.Ward R, Meagher A, Tomlinson I, O’Connor T, Norrie M, Wu R, Hawkins N. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut. 2001;48:821–829. doi: 10.1136/gut.48.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham DM, Appelman HD. Crohn’s-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol. 1990;3:332–335. [PubMed] [Google Scholar]
- 18.Greenson JK, Bonner JD, Ben-Yzhak O, Cohen HI, Miselevich I, Resnick MB, Trougouboff P, Tomsho LD, Kim E, Low M, Almog R, Rennert G, Gruber SB. Phenotype of microsatellite unstable colorectal carcinomas. Am J Surg Pathol. 2003;27:563–570. doi: 10.1097/00000478-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal cancer. Cancer. 2001;91:2417–2422. [PubMed] [Google Scholar]
- 20.Casey G, Lindor NM, Papadopoulos N, Thibodeau SN, Moskow J, Steelman S, Buzin CH, Sommer SS, Collins CE, Butz M, Aronson M, Gallinger S, Barker MA, Young JP, Jass JR, Hopper JL, Diep A, Bapat B, Salem M, Seminara D, Haile R. Colon Cancer Family Registry. Conversion analysis for mutation detection in MLH1 and MSH2 patients with colorectal cancer. JAMA. 2005;293:799–809. doi: 10.1001/jama.293.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, Jass JR, Hopper J, Gallinger S, Bapat B, Redston M, Thibodeau SN. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton SR, Aaltonen LA. World Health Organization classification of tumours. Lyon: IARC; pathology and genetics. 2000
- 23.Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. Br Med J. 2002;322:477–480. doi: 10.1136/bmj.324.7335.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox DR, Snell EJ. Analysis of binary data. 2nd ed. London: Chapman and Hall; 1989. [Google Scholar]
- 25.Apicella C, Andrews L, Hodgson SV, Fisher SA, Lewis CM, Solomon E, Tucker K, Friedlander M, Bankier A, Southey MC, Venter DJ, Hopper JL. Log odds of the probability of carrying an ancestral mutation in BRCA1 or BRCA2 for a defined personal and family cancer history in an Ashkenazi Jewish woman (LAMBDA) Breast Cancer Res. 2003;5:R206–R216. doi: 10.1186/bcr644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jass JR, Smyrk TC, Stewart SM, Lane MR, Lanspa SJ, Lynch HT. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res. 1994;14:1631–1634. [PubMed] [Google Scholar]
- 27.Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer. 1976;37:1891–1900. doi: 10.1002/1097-0142(197604)37:4<1891::aid-cncr2820370439>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Jass JR, Stewart SM. Evolution of hereditary non-polyposis colorectal cancer. Gut. 1992;33:783–786. doi: 10.1136/gut.33.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biemer-Hüttmann A-E, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, Jass JR. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyper-plastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039–1047. doi: 10.1177/002215549904700808. [DOI] [PubMed] [Google Scholar]
- 30.Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93:6–11. doi: 10.1002/ijc.1298. [DOI] [PubMed] [Google Scholar]
- 31.Banerjea A, Bustin SA, Dorudi S. The immunogenicity of colorectal cancer with high-degree microsatellite instability. World J Surg Oncol. 2005;3:26–35. doi: 10.1186/1477-7819-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael-Robinson JM, Pandeya N, Cummings MC, Walsh MD, Young JP, Leggett BA, Purdie DM, Jass JR, Radford-Smith GL. Fas ligand and tumour counter-attack in colorectal cancer stratified according to microsatellite instability status. J Pathol. 2003;201:46–54. doi: 10.1002/path.1406. [DOI] [PubMed] [Google Scholar]
- 33.Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol. 2001;159:2239–2248. doi: 10.1016/S0002-9440(10)63074-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrington SM, Lin-Goerke J, Ling J, Wang Y, Burczak JD, Robbins DJ, Dunlop MG. Systematic analysis of hMSH2 and hMLH1 in young colon cancer patients and controls. Am J Hum Genet. 1998;63:749–759. doi: 10.1086/301996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yearsley M, Hampel H, Lehman A, Nakagawa H, de la Chapelle A, Frankel WL. Histologic features distinguish microsatellite-high from microsatellite-low and microsatellite-stable colorectal carcinomas, but do not differentiate germline mutations from methylation of the MLH1 promoter. Hum Pathol. 2006;2006:831–838. doi: 10.1016/j.humpath.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Kakar S, Burgart LJ, Thibodeau SN, Rabe KG, Petersen GM, Goldberg RM, Lindor NM. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer. 2003;2002:1421–1427. doi: 10.1002/cncr.11206. [DOI] [PubMed] [Google Scholar]
- 37.Lipkin SM, Rozek LS, Rennert G, Yang W, Chen PC, Hacia J, Hunt N, Shin B, Fodor S, Kokoris M, Greenson JK, Fearon E, Lynch H, Collins F, Gruber SB. The MLH1 D132H variant is associated with susceptibility to sporadic colorectal cancer. Nat Genet. 2004;36:694–699. doi: 10.1038/ng1374. [DOI] [PubMed] [Google Scholar]
- 38.Young J, Simms LA, Biden KG, Wynter C, Whitehall V, Karamatic R, George J, Goldblatt J, Walpole I, Robin SA, Borten MM, Stitz R, Searle J, McKeone D, Fraser L, Purdie DR, Podger K, Price R, Buttenshaw R, Walsh MD, Barker M, Leggett BA, Jass JR. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001;159:2107–2116. doi: 10.1016/S0002-9440(10)63062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kambara T, Simms LA, Whitehall VLJ, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, Tanaka N, Higuchi T, Young J, Jass JR, Leggett BA. BRAF mutation and CpG island methylation: an alternative pathway to colorectal cancer. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGivern A, Wynter CVA, Whitehall VLJ, Kambara T, Spring KJ, Walsh MD, Barker MA, Arnold S, Simms LA, Leggett BA, Young J, Jass JR. Promoter hypermethylation frequency and BRAF mutations distinguish hereditary non-polyposis colon cancer from sporadic MSI-H colon cancer. Fam Cancer. 2004;3:101–107. doi: 10.1023/B:FAME.0000039861.30651.c8. [DOI] [PubMed] [Google Scholar]
- 41.Jass JR. HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Fam Cancer. 2004;3:93–100. doi: 10.1023/B:FAME.0000039849.86008.b7. [DOI] [PubMed] [Google Scholar]
- 42.Hampel H, Stephens JA, Pukkala E, Sankila R, Aaltonen LA, Mecklin JP, de la Chapelle A. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129:741–744. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Cunningham JM, Winters JL, Guenther JC, French AJ, Boardman LA, Burgart LJ, McDonnell SK, Schaid DJ, Thibodeau SN. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res. 2003;63:5209–5212. [PubMed] [Google Scholar]
- 44.Koinuma K, Shitoh K, Miyakura Y, Furukawa T, Yamashita Y, Ota J, Ohki R, Choi YL, Wada T, Konishi F, Nagai H, Mano H. Mutations of BRAF are associated with extensive hMLH1 promoter methylation in sporadic colorectal carcinomas. Int J Cancer. 2004;108:237–242. doi: 10.1002/ijc.11523. [DOI] [PubMed] [Google Scholar]
- 45.Young J, Barker MA, Simms LA, Walsh MD, Biden KG, Buchanan D, Buttenshaw R, Whitehall VL, Arnold S, Jackson L, Kambara T, Spring KJ, Jenkins MA, Walker GJ, Hopper JL, Leggett BA, Jass JR. BRAF mutation and variable levels of microsatellite instability characterize a syndrome of familial colorectal cancer. Clin Gastroenterol Hepatol. 2005;3:254–263. doi: 10.1016/s1542-3565(04)00673-1. [DOI] [PubMed] [Google Scholar]
- 46.Gazzoli I, Loda M, Garber J, Syngal S, Kolodner RD. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the hMLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 2002;62:3925–3928. [PubMed] [Google Scholar]
- 47.Miyakura Y, Sugano K, Akasu T, Yoshida T, Maekawa M, Saitoh S, Sasaki H, Nomizu T, Konishi F, Fujita S, Moriya Y, Nagai H. Extensive but hemiallelic methylation of the hMLH1 promoter region in early-onset sporadic colon cancers with microsatellite instability. Clin Gastroenterol Hepatol. 2004;2:147–156. doi: 10.1016/s1542-3565(03)00314-8. [DOI] [PubMed] [Google Scholar]
- 48.Suter CM, Martin DIK, Ward RL. Germline epimutation of MLH1 in individuals with multiple cancers. Nat Genet. 2004;36:497–501. doi: 10.1038/ng1342. [DOI] [PubMed] [Google Scholar]
- 49.Jass JR. Should colorectal cancers with loss of expression of DNA mismatch repair proteins be routinely tested for MSI status? J Pathol. 2006;208:590–591. [Google Scholar]
- 50.Gologan A, Krasinskas A, Hunt J, Thull DL, Farkas L, Sepulveda AR. Performance of the revised Bethesda guidelines for identification of colorectal carcinomas with high level microsatellite instability. Arch Pathol Lab Med. 2005;129:1390–1397. doi: 10.5858/2005-129-1390-POTRBG. [DOI] [PubMed] [Google Scholar]
- 51.Chai SM, Zeps N, Shearwood A-M, Grieu F, Charles A, Harvey J, Goldblatt J, Joseph D, Iacopetta B. Screening for defective DNA mismatch repair in stage II and III colorectal cancer patients. Clin Gastroenterol Hepatol. 2004;2:1017–1025. doi: 10.1016/s1542-3565(04)00451-3. [DOI] [PubMed] [Google Scholar]
- 52.Barnetson RA, Tenesa A, Farrington SM, Nicholl ID, Cetnarskyj R, Porteous ME, Campbell H, Dunlop MG. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354:2751–2763. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]