Abstract
Islet transplantation has great potential as an effective means of treating type 1 diabetes. However, its successful application greatly depends on the rapid revascularization of islets and prevention from their apoptotic cell death. We co-expressed human vascular endothelial growth factor (hVEGF) and human interleukin-1 receptor antagonist (hIL-1Ra) after transduction of human islets with Adv-hVEGF-hIL-1Ra. Since hepatocyte growth factor (HGF) increases β-cell proliferation and promotes revascularization of islets, we also constructed Adv-hHGF-hIL-1Ra. There was dose and time dependent expression of hVEGF and hIL-1Ra or hHGF and hIL-1Ra by islets, which led to decrease in caspase-3 activity and apoptosis induced by a cocktail of TNF-α, IL-1β and IFN-γ. Compared to non-treated islets, transduction of islets with these bipartite Adv vectors prior to transplantation under the kidney capsules of diabetic NOD-SCID mice reduced the blood glucose levels, and increased serum insulin and c-peptide levels. Immunohistochemical staining of the islet bearing kidney sections was positive for human insulin, growth factor (hVEGF or hHGF) and von Willebrand factor. Transduction with Adv-caspase-3-shRNA also prevented islets from cytokine induced apoptosis and improved islet transplantation. In conclusion, bipartite Adv vector efficiently co-expressed both growth factor and antiapoptotic genes or shRNA targeting pro-apoptotic genes, decreases apoptosis and improves the outcome of islet transplantation.
Keywords: Human islets, adenoviral vectors, hVEGF, IL-1Ra, hepatocyte growth factor, caspase-3, siRNA, islet transplantation
Introduction
Type 1 diabetes is an autoimmune disease resulting from destruction of insulin producing pancreatic β-cells, which necessitates a lifelong insulin replacement therapy. Successful transplantation requires a large number of islets, requiring islets from two to four donor pancreases. A major obstacle in islet transplantation is the high rate of primary nonfunction and early islet destruction. An acute blood-mediated inflammatory injury is largely responsible for islet destruction and may well amplify subsequent immune reactions. The number of islets required to achieve normoglycemia can be significantly decreased if apoptosis can be prevented in the first week post transplantation by overcoming several obstacles related to the loss of islet viability during isolation, purification, and culture; and due to prolonged hypoxia and oxidative stress during revascularization process; and inflammation and immune mediated graft destruction [1]. Upon transplantation, islets are continuously exposed to immunosuppressant drugs including tacrolimus and sirolimus which adversely impact β-cell survival and function [2]. In addition progressive decline in graft function is commonly observed in islet transplant recipients treated with these steroids.
Islet destruction due to hypoxia, oxidative stress, immune- and inflammation-mediated destruction still remains one of the major obstacles for successful islet transplantation. Ex vivo genetic modification of islets can effectively address these problems. For successful human islet transplantation, we need to overcome several obstacles including (1) loss of islet viability during isolation; (2) lack of islet revascularization, (3) inflammatory or immune rejection causing islet destruction, (4) inadequate islet mass at the time of transplantation, and the site of transplants [1]. Strategies to overcome these barriers are discussed in this article (Table 1), with special emphasis on our work on gene therapy for improving the outcome of islet transplantation.
Table1.
Obstacles to islet transplantation and proposed strategies for a successful islet transplantation.
| Obstacles to islet transplantation | Strategies for Successful Islet Transplantation |
|---|---|
|
|
Gene Therapy for Preventing Islet Graft Rejection
Gene therapy has the potential to replace frequent injections of insulin to type I diabetic patients. One conceptually simple approach is to transfer an exogenous insulin gene into non-β cells, such as hepatocytes, muscles or other cell types. However, it is difficult to induce non-β-cells to produce and secrete appropriate amounts of insulin in response to changes in blood glucose concentration. Therefore, attempts are made to replace damaged β-cell mass by islet transplantation. Since islet is a compact cluster of about 1000 non-dividing cells, most nonviral approaches including cationic liposomes and polymer-based systems are ineffective in transfecting intact islets and are toxic at high doses [3].
Replication deficient (E1-, E3- deleted) adenoviral (Adv) vectors are known to transduce both dividing and non-dividing cells including islets. These vectors can be produced in high titers and there is no risk of insertional mutagenesis as their genomes are not integrated into the host chromosomes [4]. While host immune response against Adv vectors is well known at high MOI involving direct administration of Adv for in vivo applications, this may not be significant for ex vivo gene therapy, where islets are transfected in petri-dishes and washed of viral particles prior to transplantation. Most of the Adv genomes would, therefore, be inside the cells at the time of infusion of transfected islets into the subjects. Moreover, in recent studies, the local delivery of low and intermediate dose of Adv vectors in humans indicated that these vectors are well tolerated even up to the dose of 1011 particles or below [5].
Need for Revascularization
Islets have extensive intra-islet vasculature formed of fenestrated capillary endothelial lining, which gets disrupted during islet isolation and purification process leading to the collapse of vasculature, accumulation of endothelial fragments and compromised perfusion in the core of islets. Revascularization to the transplanted islets is known to improve the delivery of nutrients and oxygen to the inner core of islets leading to their survival. Angiogenesis helps establish the adequate microvascular blood supply of islet grafts soon after transplantation. Isolated islets are reported to revascularize within 10∼14 days after transplantation [6].
One of the approaches to improve islet engraftment is to induce neovascularization and matrix formation around the transplanted islets. VEGF is a family of at least four polypeptides (with 121, 165, 189 and 206 amino acids) that result from alternative mRNA splicing and these isoforms vary in permeability and heparin binding. These isoforms specifically bind to receptors on endothelial cells, resulting in their growth, proliferation and migration. VEGF165 is a 45 kDa endothelial cell mitogen that is secreted and binds to heparin. hVEGF165 is known to increase vascular permeability, which is important for the maintenance of normal endocrine function in highly vascularized organs. Also, in the devascularized islet, VEGF may play a role in the revascularization process after transplantation [7, 8].
Since hVEGF induces angiogenesis and regulates vessel permeability [9], we and others have shown that hVEGF expression promotes new blood vessel formation and improves the outcome of islet transplantation [10, 11]. We transfected islets with hVEGF expression plasmid/lipid complexes and then transplanted these islets under the kidney capsules of NOD-SCID mice. We demonstrated that hVEGF gene expression promotes new blood vessel formation after islet transplantation, with the involvement of endothelial cells of both the host (mouse) and the donor (human). Even though the occurrence of revascularization of the transplanted islets could be seen by immunohistochemical staining, transfection efficiency was very low [10].
To enhance hVEGF gene expression, we transduced human islets with bipartite Adv vectors encoding both green fluorescent protein (GFP) and hVEGF (Adv-GFP-hVEGF). We have further demonstrated a linear relationship between hVEGF gene expression and the dose of this viral vector in human islets. More importantly, we showed that Adv-GFP-hVEGF causes little apoptosis to human islets and there was no adverse effect on insulin release in response to glucose challenge at the MOI when high levels of hVEGF gene expression can be achieved [12]. Zhang et al. [11] reported that ex vivo transduction of murine islets with Adv-hVEGF followed by transplantation under the kidney capsules resulted in elevated VEGF expression and enhanced islet revascularization. Linn et al. [7] also have demonstrated that the addition of recombinant VEGF to the culture medium increases proliferation of islet endothelial cells and causes substantial cord formation in a fibrin gel model.
Preventing Apoptosis for Minimizing Graft Rejection
Effective prevention of islet destruction after transplantation requires not only revascularization of islets, but also abrogation of cytokine-mediated islet cell death and dysfunction triggered by immune and inflammatory reactions [13]. Injury due to islet isolation, purification, culture, transfection and transplantation will activate resident islet macrophages and passenger leukocytes to release proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interferon γ (IFN-γ) and interleukin-1β (IL-1β), which in turn can lead to activation of inducible nitric oxide synthase (iNOS) and release cytotoxic nitric oxide (NO) [14]. Inhibition of IL-1β binding to the type I IL-1 signaling receptor, using the naturally occurring interleukin-1 receptor antagonist protein (IL-Ra) has been shown to prevent IL-1β induced suppression of glucose-stimulated insulin release and NO production in mouse and rat islets [15, 16]. Transduction with Adv-hIL-1Ra has been shown to result in protection of human islets against IL-1β-induced NO formation, impairment of glucose-stimulated insulin production, and Fas-triggered apoptosis activation [17]. Moreover, continuous infusion of IL-1Ra into NOD mice has been shown to prevent insulitis and diabetes onset and prolongs syngeneic islet graft survival [16].
We co-expressed genes that target different insults to transplanted islets to improve the outcome of islet transplantation better than either gene alone. Transduction of human islets with two Adv vectors, one encoding hVEGF and the other encoding hIL-1Ra, synergistically suppressed islet dysfunction and NO production in human islets. It also reduced the blood glucose levels and increased the level of blood insulin and c-peptide of mice upon transplantation of human islets transduced with a mixture of Adv encoding hVEGF and hIL-1Ra [18].
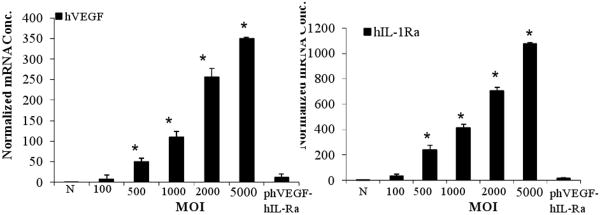
Considering the favorable uses of hIL-1Ra and hVEGF, we constructed a plasmid vector encoding these two genes (phVEGFhIL-1Ra) by cloning hIL-1Ra under the cytomegalovirus (CMV) promoter and hVEGF under the elongation factor-1R (EF-1R) promoter in pBudCE4.1 vector, and demonstrated dose and time dependent expression of hVEGF and hIL-1Ra after transfection into human islets [19]. Because the transfection efficiency of this bipartite plasmid into human islets was very low, we then constructed a bipartite Adv vector encoding hVEGF and hIL-1Ra driven by separate CMV promoters [20]. The use of bipartite vector encoding these genes not only simplifies the amplification and purification process of Adv vectors but also decreases the use of total Adv backbone for transduction, which is expected to minimize the immunogenic and toxic effect of Adv vectors. There was a dose and time dependent expression of hVEGF and hIL-1Ra after transduction of human islets with Adv-hVEGF-hIL1Ra (Fig 1). Transduced islets were viable as evidenced by insulin release upon glucose challenge. Co-expression of hVEGF and hIL-1Ra showed decrease in caspase-3 activity and apoptosis induced by the inflammatory cytokine cocktail. Compared to non-treated islets, transduction of islets with Adv-hVEGF-hIL-1Ra prior to transplantation under the kidney capsules of NOD-SCID mice reduced blood glucose and increased the level of serum insulin and c-peptide levels upon glucose challenge.
Fig. 1.
Real-time PCR of islets at day 3 post transduction with AdvhVEGF-IL-1Ra and phVEGF-hIL-1Ra. Reproduced from Panakanti and Mahato (2009) Mol Pharm 6: 274-284.
A balance of hVEGF expression is important, as an attempt to overexpress this cytokine may result in damaging islets and surrounding tissues. Over-expression of hVEGF is known to cause tumorigenesis [9, 21], it does not induce expansion of β-cells and has no antiapoptotic property. In contrast, human Hepatocyte Growth Factor (hHGF) is a potent mitogen for human islets and is known to increase the proliferation and expansion of β-cells and promotes revascularization of islets [22]. Over-expression of HGF in the β-cells of transgenic mice directed by the rat insulin type II promoter (RIP) has shown to increase β-cell mass and proliferation, and improve islet function and islet transplant outcome [23]. Ex vivo transduction of islets with an Adv vector encoding HGF has been reported to improve the outcome of islet transplantation [24]. Intravenous injection of Adv-HGF in diabetic mice has been shown to ameliorate hyperglycemia and prolong survival period in the diabetic mice [25]. Moreover, HGF gene transfer has also shown to increase the expression of three key β-cell genes, glucokinase, Glut-2 (the β-cell glucose transporter), and insulin [26].
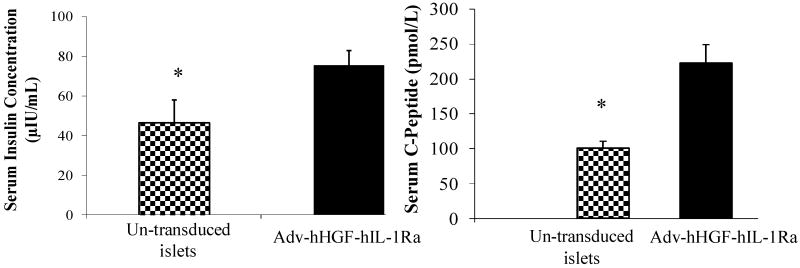
Considering the favorable uses of hHGF and hIL-1Ra, we constructed a bipartite Adv vector encoding these two genes (Adv-hHGF-hIL-1Ra) by cloning hIL-1Ra and hHGF under a separate CMV promoter [27]. HGF and hIL-1Ra gene expression increased with increase in MOI. There was gradual increase in caspase-3 with increase in Adv-hHGF-hIL-1Ra in the presence of inflammatory cytokines, but caspase-3 levels were much lower compared to the un-transduced islets. Transduction of islets with Adv-hHGF-hIL-1Ra greatly enhanced the level of Bcl-2 protein while inhibited Bax protein level, demonstrating the protective effect of hHGF and hIL-1Ra co-expression, since Bcl-2 has been shown to inhibit cytochrome C release and protect against oxidative stress-induced apoptosis. We observed a reduction in blood glucose levels of mice transplanted with Adv-HGF-hIL-1Ra transduced islets than that observed with un-transduced islets, but increase in blood glucose levels for the mice transplanted with un-transduced islets. This decrease in blood glucose levels by Adv-hHGF-hIL-1Ra-transduced mice correlated with the higher amount of serum insulin and C-peptide secreted by the islets at day 28 post-transplantation (Fig. 2). Immunohistochemical staining in the islet bearing kidney sections revealed stronger positive staining for human insulin, hHGF and vWF, suggesting blood vessel formation in the transplanted islets more efficient when islets were transduced with Adv-hHGF-hIL-1Ra.
Fig. 2.
Effect of Adv-hHGF-hIL-1Ra transduction on serum insulin and c-peptide levels at 28 days post-islet transplantion under the kidney capsule of streptozotocin-induced diabetic NOD-SCID mice. The mice transplanted with un-transduced islets were used as controls. Reproduced from Panakanti and Mahato (2009) Pharm Res 26: 587-596.
Role of Pro-Apoptotic Genes in Islet Destruction
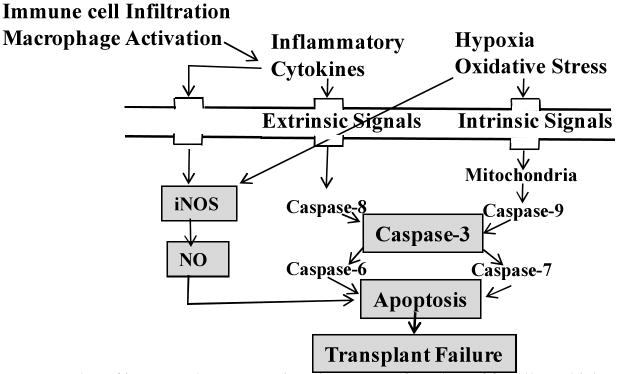
Upon transplantation, inflammatory cytokines will be released due to immune cell infiltration and macrophage activation. This will upregulate iNOS that will eventually increase the cellular level of NO, which eventually impair in insulin secretion due to islet apoptosis, which eventually impair in insulin secretion due to islet apoptosis (Fig. 3) [28]. iNOS inhibition prevented β-cell death. iNOS inhibitors, such as NG-monomethyl-L-arginine (NMMA), aminoguanidine, and nitro-L-arginine methyl ester, prevented the inhibitory effects of cytokines on insulin secretion as well as cytokine-induced NO production by rat, mouse, and human islets. iNOS inhibition using synthetic isothiourea derivatives reduced the requirement of corticosteroid immunosuppreser in vivo [29]. Since the low concentration of NO has antiapoptotic effect in islets, optimal iNOS silencing would have the added advantage of additional antiapoptotic effect. Programmed cell death is a cascade of events leading to upregulation of procaspase-9→caspase-9→procaspase-3→caspase-3→apoptosis. Caspase-8 and Caspase-9 are the upstream caspases involved in the extrinsic and intrinsic pathways, respectively. Hypoxia and oxidative stress are involved in apoptosis via intrinsic mitochondrial pathway [30]. Similarly, cytokines and/or elevated glucose level upregulate Fas, which is known to activate caspase-8 through the extrinsic apoptotic pathway [31]. Bcl-2 family proteins can induce or inhibit the release of cytochrome C into the cytosol, which activates caspase-9 and caspase-3. They include anti-apoptotic members (Bcl-2, Bcl-XL, and Bcl-w among others) which are critical for islet survival, whereas pro-apoptotic members (Bax, Bak, Bad, and Bok among others) promote apoptosis. Heterodimerization of Bcl-2 with Bax or Bak modulates apoptosis, and the ratio of Bcl-2 to Bax and/or Bak determines survival or death following an apoptotic stimulus. Similarly, cytokines and/or elevated glucose level upregulates Fas, which is known to activate caspase-8 through the extrinsic apoptotic pathway. Caspase-3 is a converging point of apoptosis for intrinsic and extrinsic pathways and caspase-6 and 7 are generated from caspase-3 [32]. It is the major effector caspase involved in apoptotic pathway in β-cells. Caspase-3 inhibitors prevented islet apoptosis and improved graft function.
Fig.3.
Roles of iNOS and caspase-3 in primary non-function of β-cells and islet graft failure.
Gene Silencing for Prolonged Islet Graft Survival
Up-regulation of pro-apoptotic genes upon islet transplantation may lead to primary non-function of islet graft. Therefore, gene silencing of these genes may improve the outcome of islet transplantation. This can be achieved using short interfering RNAs (siRNAs), which are short RNA duplexes that mediate sequence-specific degradation of targeted mRNA transcripts [33, 34]. RNA interference (RNAi) in isolated rat and mouse islets and β-cell lines have been reported using synthetic siRNA [35], Adv vectors encoding small hairpin RNA (Adv-shRNA) [36], and long chain double stranded RNA [37]. Silencing of harmful genes from the islets by RNAi offers a great promise for improving the outcome of islet transplantation. Since an islet is a compact cluster of about 1000 non-dividing cells, most plasmid-based approaches are ineffective in transfecting islets. Further, long dsRNA often causes off target effects. Therefore, chemically synthesized siRNA are most commonly being investigated. However, most studies were performed using β-cell lines which are rapidly dividing single cells and do not represent the cluster characteristic of relatively slowly dividing islets which are difficult to transfect.
iNOS Gene Silencing in β-Cells and Human Islets
We and others have demonstrated that transfection of insulin-producing β-cells with siRNA against iNOS significantly decreases iNOS expression and NO production, and protects these cells from apoptosis [38-40]. Effect of siRNAs on iNOS silencing and NO production was proportional to their concentration. To determine whether iNOS gene silencing inhibit β-cell apoptosis induced by inflammatory cytokines, INS-1E cells were transfected with control and iNOS-siRNAs followed by incubation with cytokine cocktail, and visualization under microscopy. Cells in control siRNA group were aggregated and round up, whereas only a small cell fraction in iNOS-siRNA group was aggregated suggesting antiapoptic effect of iNOS gene silencing [38]. Flow cytometric terminal deoxynucleotidyltransferase dUTP nick end labeling (TUNEL) assay showed 0.8%, 42.3% and 12.3% apoptotic cells in no cytokine treatment group, control siRNA-cytokines treatment group, and iNOS-siRNA-cytokines treatment group, respectively, suggesting the protective effect of iNOS gene silencing on β-cells death from inflammatory cytokine induced apoptosis. siRNA against human iNOS reduced the percentage of apoptotic cells to 28.5% compared to the control siRNA group, which had 36.2% apoptotic cells. Our results suggested that iNOS gene silencing partly protected human islets from inflammatory cytokine induced apoptosis. The prevention of apoptosis in human islets was not as high as shown in rat β-cell lines because unlike human islets which are a cluster of 200-1000 cells and are difficult to transfect using lipoplexes, rat β-cells are single cells and are easy to transfect.
Caspase-3 Gene Silencing
Since caspase-3 inhibitors have been reported to prevent apoptosis of islets and improve graft function [41], siRNA mediated caspase-3 gene silencing is also an attractive approach. We have demonstrated that caspase-3 gene silencing has beneficial effect on protecting islets from apoptosis. Although caspase-3 has been inhibited using peptides, peptide inhibitors are likely to cause severe side-effects at the required therapeutic dose. We determined the effect of siRNA sequences on rat caspase-3 gene silencing by transfecting rat insulinoma (INS-1E) cells with three different siRNA duplexes (s1, s2 and s3), followed by incubation with cytokine cocktail [42]. Unlike irrelevant siRNA, all caspase-3-siRNAs silenced caspase-3 gene expression by 72% to 53%. We further examined RNAi effect on caspase-3 protein level using the polyclonal antibody to detect the precursor caspase-3 by Western blotting. In agreement with real time RT-PCR results, band thickness for caspase-3 protein was much thinner for s1 and s2 siRNA groups than that of irrelevant siRNA, suggesting a significant decrease in caspase 3 protein and confirming the effectiveness of these capspase-3-siRNAs. As determined using TUNEL assay, there was significant decrease in the number of apoptotic cells when transfected with caspase-3 siRNAs compared to that with the control siRNA treated group.
After demonstrating a significant inhibition of caspase-3 in rat β-cells using rat caspase-3 siRNA, we determined whether validated siRNA against human caspase-3 can suppress caspase 3 in human islets. Caspase-3-siRNA effectively silenced caspase-3 at transcript and protein levels after transfection into human islets and subsequent incubation with cytokine cocktail for additional 24h.
Since human islets are hard to transfect with siRNA/lipid complexes and gene silencing did not last beyond two days, we constructed replication deficient (E1 and E3 deleted) serotype 5 Adv encoding caspase-3-shRNA. There was significant reduction in caspase-3 mRNA ranging from 46% to 68% at days 3 and 5 post transduction of islets with Adv-caspase-3-shRNA. Similarly, infection of Adv-caspase-3 shRNA markedly decreased caspase 3/7 activity at days 3 and 5 (Fig. 4) as determined by Caspase-Glo™ 3/7 Assay. Similarly, there was significant decrease in Caspase-3 protein with Adv-caspase-3-shRNA infected islet group as determined by Western blot analysis.
Fig. 4.
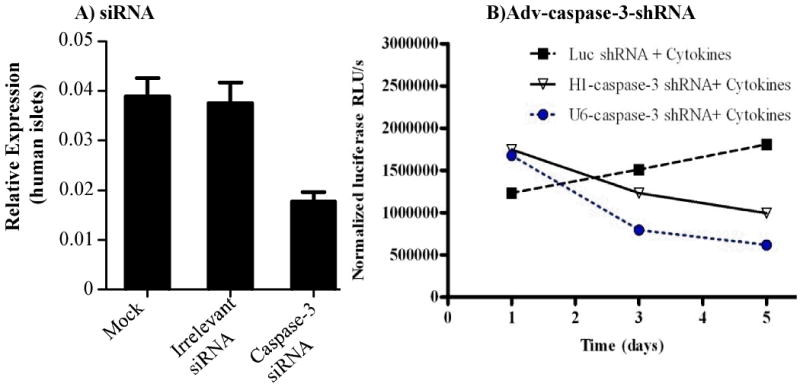
Casepase-3 gene silencing in human islets. A) caspase-3-siRNA, B) Adv-caspase-3-shRNA. Reproduced with modifications from Cheng et al. (2008) Mol Pharm 5: 1093-1102.
Effect of promoters was determined by transducing islets with Adv-shRNA driven by either H1 or U6 promoter followed by incubation with the cytokine cocktail. Transduction with both Adv-H1-caspase-3-shRNA and Adv-U6-caspase-3-shRNA resulted in significant reduction in caspase activity, with Adv-U6-caspase-3-shRNA being more effective (Fig. 4). Adv-mediated shRNA expression by either a H1 promoter or U6 promoter can counteract apoptosis induced by cytokines.
To determine the effect of caspase-3 gene silencing on the outcome of human islet transplantion, islets were transduced with Adv-caspase-3-shRNA at 1000MOI for 18h and 1200 transduced islets were transplanted under the kidney capsules of diabetic mice. Return to euglycemia was achieved in 100% of mice transplanted with Adv-caspase-3-shRNA transduced islets at 1 day posttransplantation and maintained up to 17 days (last days of measuring blood glucose). In contrast, normoglycemia was achieved in only 60% at day 1, 80% at 4 and 8% at day 100% of mice transplanted with untreated islets. In some animals, islet graft bearing kidneys were removed to confirm the function of islet grafts. Blood glucose levels returned to be ≥325mg/dl after nephrectomy, confirming that transplanted islets were functional. Our results are in good agreement with the work of Shapiro and associates who incubated islets with a peptide caspase inhibitor for 2h prior to transplantation and also administered this caspase inhibitor intraperitoneally for 5 consecutive day post-transplantation.
Concluding Remarks
Ex vivo gene therapy has great promise to improve the outcome of islet transplantation by co-expressing growth factor and anti-apoptotic genes or siRNA against proapoptotic genes prior to islet transplantation. Co-expression of growth factor (hVEGF or hHGF) and antiapoptotic (hIL-1Ra) genes promoted angiogenesis, protected islets from apoptosis and significantly improved the outcome of islet transplantation. iNOS and caspase-3 gene silencing also protected islets from apoptosis and improved their survival and function.
Acknowledgments
I would like to thank the National Institute of Health (NIH) for financial support (RO1 DK69968) and my following current and past students and postdoctoral fellows for technical assistance: Ajit S Narang, Kun Cheng, Xiangxu Jia, Guofeng Cheng, Ravikiran Panakanti and Feng Li for technical assistance.
References
- 1.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58(2):194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 2.Hyder A, Laue C, Schrezenmeir J. Effect of the immunosuppressive regime of Edmonton protocol on the long-term in vitro insulin secretion from islets of two different species and age categories. Toxicol In Vitro. 2005;19(4):541–546. doi: 10.1016/j.tiv.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Mahato RI, Henry J, Narang AS, Sabek O, Fraga D, Kotb M, Gaber AO. Cationic lipid and polymer-based gene delivery to human pancreatic islets. Mol Ther. 2003;7(1):89–100. doi: 10.1016/s1525-0016(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 4.Muruve DA, Nicolson AG, Manfro RC, Strom TB, Sukhatme VP, Libermann TA. Adenovirus-mediated expression of Fas ligand induces hepatic apoptosis after Systemic administration and apoptosis of ex vivo-infected pancreatic islet allografts and isografts. Hum Gene Ther. 1997;8(8):955–963. doi: 10.1089/hum.1997.8.8-955. [DOI] [PubMed] [Google Scholar]
- 5.Harvey BG, Maroni J, O'Donoghue KA, Chu KW, Muscat JC, Pippo AL, Wright CE, Hollmann C, Wisnivesky JP, Kessler PD, Rasmussen HS, Rosengart TK, Crystal RG. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13(1):15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- 6.Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg. 2001;25(4):509–515. doi: 10.1007/s002680020345. [DOI] [PubMed] [Google Scholar]
- 7.Linn T, Erb D, Schneider D, Kidszun A, Elcin AE, Bretzel RG, Elcin YM. Polymers for induction of revascularization in the rat fascial flap: application of vascular endothelial growth factor and pancreatic islet cells. Cell Transplant. 2003;12(7):769–778. doi: 10.3727/000000003108747244. [DOI] [PubMed] [Google Scholar]
- 8.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Legeay G, Bellocq JP, Pinget M, Kessler L. Induction of angiogenesis in omentum with vascular endothelial growth factor: influence on the viability of encapsulated rat pancreatic islets during transplantation. J Vasc Res. 2003;40(4):359–367. doi: 10.1159/000072700. [DOI] [PubMed] [Google Scholar]
- 9.Christofori G, Naik P, Hanahan D. Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol. 1995;9(12):1760–1770. doi: 10.1210/mend.9.12.8614412. [DOI] [PubMed] [Google Scholar]
- 10.Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D, Kotb M, Gaber AO, Mahato RI. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21(1):15–25. doi: 10.1023/b:pham.0000012147.52900.b8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, Zhang H, Song K, Meseck M, Bromberg J, Dong H. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53(4):963–970. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 12.Cheng K, Fraga D, Zhang C, Kotb M, Gaber AO, Guntaka RV, Mahato RI. Adenovirus-based vascular endothelial growth factor gene delivery to human pancreatic islets. Gene Ther. 2004;11(14):1105–1116. doi: 10.1038/sj.gt.3302267. [DOI] [PubMed] [Google Scholar]
- 13.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46(2):255–266. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990;71(1):152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- 15.Welsh N, Eizirik DL, Bendtzen K, Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991;129(6):3167–3173. doi: 10.1210/endo-129-6-3167. [DOI] [PubMed] [Google Scholar]
- 16.Sandberg JO, Eizirik DL, Sandler S, Tracey DE, Andersson A. Treatment with an interleukin-1 receptor antagonist protein prolongs mouse islet allograft survival. Diabetes. 1993;42(12):1845–1851. doi: 10.2337/diab.42.12.1845. [DOI] [PubMed] [Google Scholar]
- 17.Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48(9):1730–1736. doi: 10.2337/diabetes.48.9.1730. [DOI] [PubMed] [Google Scholar]
- 18.Narang AS, Sabek O, Gaber AO, Mahato RI. Co-expression of vascular endothelial growth factor and interleukin-1 receptor antagonist improves human islet survival and function. Pharm Res. 2006;23(9):1970–1982. doi: 10.1007/s11095-006-9065-7. [DOI] [PubMed] [Google Scholar]
- 19.Jia X, Cheng K, Mahato RI. Coexpression of vascular endothelial growth factor and interleukin-1 receptor antagonist for improved human islet survival and function. Mol Pharm. 2007;4(2):199–207. doi: 10.1021/mp060091s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panakanti R, Mahato RI. Bipartite vector encoding hVEGF and hIL-1Ra for ex vivo transduction into human islets. Mol Pharm. 2009;6(1):274–284. doi: 10.1021/mp800183b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gannon G, Mandriota SJ, Cui L, Baetens D, Pepper MS, Christofori G. Overexpression of vascular endothelial growth factor-A165 enhances tumor angiogenesis but not metastasis during beta-cell carcinogenesis. Cancer Res. 2002;62(2):603–608. [PubMed] [Google Scholar]
- 22.Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, Lakey JR, Hart ME, Hayek A. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes. 2002;51(12):3435–3439. doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Ocana A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275(2):1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Talavera JC, Garcia-Ocana A, Sipula I, Takane KK, Cozar-Castellano I, Stewart AF. Hepatocyte growth factor gene therapy for pancreatic islets in diabetes: reducing the minimal islet transplant mass required in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology. 2004;145(2):467–474. doi: 10.1210/en.2003-1070. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera JC, Vasavada RC, Stewart AF. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem. 2003;278(1):343–351. doi: 10.1074/jbc.M207848200. [DOI] [PubMed] [Google Scholar]
- 26.Dai C, Yang J, Liu Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol. 2002;13(2):411–422. doi: 10.1681/ASN.V132411. [DOI] [PubMed] [Google Scholar]
- 27.Panakanti R, Mahato RI. Bipartite Adenoviral Vector Encoding hHGF and hIL-1Ra for Improved Human Islet Transplantation. Pharm Res. 2009;26(3):587–596. doi: 10.1007/s11095-008-9777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98(19):10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandhorst D, Brandhorst H, Zwolinski A, Nahidi F, Bretzel RG. Prevention of early islet graft failure by selective inducible nitric oxide synthase inhibitors after pig to nude rat intraportal islet transplantation. Transplantation. 2001;71(2):179–184. doi: 10.1097/00007890-200101270-00002. [DOI] [PubMed] [Google Scholar]
- 30.Emamaullee JA, Shapiro AM. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes. 2006;55(7):1907–1914. doi: 10.2337/db05-1254. [DOI] [PubMed] [Google Scholar]
- 31.Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY. Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes. 2001;50(8):1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- 32.Liadis N, Murakami K, Eweida M, Elford AR, Sheu L, Gaisano HY, Hakem R, Ohashi PS, Woo M. Caspase-3-dependent beta-cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol Cell Biol. 2005;25(9):3620–3629. doi: 10.1128/MCB.25.9.3620-3629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Paula D, Bentley MV, Mahato RI. Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. Rna. 2007;13(4):431–456. doi: 10.1261/rna.459807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahato RI, Cheng K, Guntaka RV. Modulation of gene expression by antisense and antigene oligodeoxynucleotides and small interfering RNA. Expert Opin Drug Deliv. 2005;2(1):3–28. doi: 10.1517/17425247.2.1.3. [DOI] [PubMed] [Google Scholar]
- 35.Bradley SP, Rastellini C, da Costa MA, Kowalik TF, Bloomenthal AB, Brown M, Cicalese L, Basadonna GP, Uknis ME. Gene silencing in the endocrine pancreas mediated by short-interfering RNA. Pancreas. 2005;31(4):373–379. doi: 10.1097/01.mpa.0000179730.69081.64. [DOI] [PubMed] [Google Scholar]
- 36.Bain JR, Schisler JC, Takeuchi K, Newgard CB, Becker TC. An adenovirus vector for efficient RNA interference-mediated suppression of target genes in insulinoma cells and pancreatic islets of langerhans. Diabetes. 2004;53(9):2190–2194. doi: 10.2337/diabetes.53.9.2190. [DOI] [PubMed] [Google Scholar]
- 37.Hagerkvist R, Mokhtari D, Myers JW, Tengholm A, Welsh N. siRNA Produced by Recombinant Dicer Mediates Efficient Gene Silencing in Islet Cells. Ann N Y Acad Sci 1040. 2005:114–122. doi: 10.1196/annals.1327.014. [DOI] [PubMed] [Google Scholar]
- 38.Li F, Mahato RI. iNOS gene silencing prevents inflammatory cytokine-induced beta-cell apoptosis. Mol Pharm. 2008;5(3):407–417. doi: 10.1021/mp700145f. [DOI] [PubMed] [Google Scholar]
- 39.De Paula D, Bentley MV, Mahato RI. Effect of iNOS and NF-kappaB gene silencing on beta-cell survival and function. J Drug Target. 2007;15(5):358–369. doi: 10.1080/10611860701349695. [DOI] [PubMed] [Google Scholar]
- 40.McCabe C, O'Brien T. Beta cell cytoprotection using lentiviral vector-based iNOS-specific shRNA delivery. Biochem Biophys Res Commun. 2007;357(1):75–80. doi: 10.1016/j.bbrc.2007.03.115. [DOI] [PubMed] [Google Scholar]
- 41.Nakano M, Matsumoto I, Sawada T, Ansite J, Oberbroeckling J, Zhang HJ, Kirchhof N, Shearer J, Sutherland DE, Hering BJ. Caspase-3 inhibitor prevents apoptosis of human islets immediately after isolation and improves islet graft function. Pancreas. 2004;29(2):104–109. doi: 10.1097/00006676-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Cheng G, Zhu L, Mahato RI. Caspase-3 Gene Silencing for Inhibiting Apoptosis in Insulinoma Cells and Human Islets. Mol Pharm. 2008 doi: 10.1021/mp800093f. [DOI] [PubMed] [Google Scholar]