Abstract
As a part of our continuing study of colchicinoids as therapeutically useful antitumor drugs, thiocolchicine derivatives, including their phosphate and other water soluble salts, were synthesized and evaluated for inhibition of tubulin polymerization and for in vitro cytotoxicity. Three compounds, 7, 10, and 11, showed potent inhibition of tubulin assembly (IC50 = 0.88 – 1.1 μM). In addition, compound 7, a water soluble succinic acid salt of N-deacetylthiocolchicine (4), showed potent cytotoxicity against a panel of tumor cell lines, suggesting it might be a potential lead to be developed as a therapeutic antitumor agent. Compound 8, a water soluble succinic acid salt of N,N-dimethyl-N-deacetylthiocolchicine (5), showed selective activities against HCT-8 and SK-BR-3 cells. N,N-Diethyl-N-deacetylthiocolchicine (6) seemed not to be a substrate for the P-gp efflux pump, based on the similar ED50 values obtained against P-gp over-expressing KBvin (0.0146 μg/mL) cells and the parent KB (0.0200 μg/mL) cell line.
Keywords: N-Alkylthiocolchinoids, Antitumor agents, Tubulin polymerization
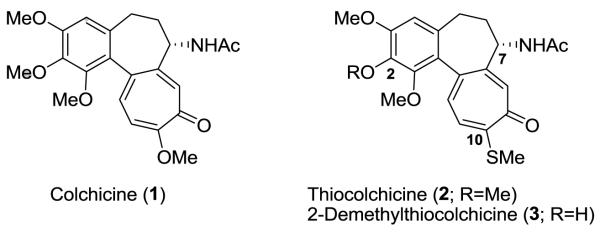
Colchicine (1), an alkaloid isolated from Colchicum autumnale and Gloriosa superba, is known to possess notable anti-inflammatory, anti-mitotic, and anti-fibrotic effects.2 Although colchicine has significant in vitro antitumor effects, its medicinal uses, as well as use of its derivatives, has been limited because of high toxicity, low bioavailability, and poor water solubility.3 Thiocolchicine (2), originally derived from colchicine by semi-synthesis, possesses comparable or greater biological activity than colchicine (1) and has been studied for many years.2 Although 2 is structurally more stable than 1, its substantial toxicity and poor water solubility limit interest in it as a drug candidate. Many efforts have been made to develop thiocolchicine analogs with the goal of reducing toxicity and increasing watersolubility.3, 4 2-Demethylthiocolchicine (3) was found to be slightly less active but also less toxic than 2 and 1.5 N-Deacetylthiocolchicine (4) was previously synthesized and was similar to 2 in its inhibitory effect on tubulin assembly.6 These findings suggested that further modifications could lead to more active compounds with reduced toxicity or greater water solubility and thus have greater potential as drug candidates.
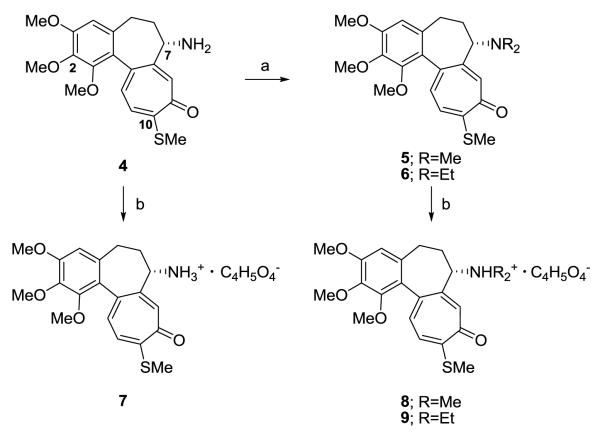
The preparation of di-alkyl-substituted amines of N-deacetylthiocolchicine (4) and related pharmaceutically and clinically accepted salts and phosphate derivatives has not been described. In this paper, we report such modifications of thiocolchicine and its derivatives at the amino group at C7 and at the hydroxy group at C2 of 3 and 4.
Treatment of 46 with formaldehyde and acetaldehyde in the presence of NaBH3CN under acidic conditions yielded N,N-dimethyl- (5) and N,N-diethyl-N-deacetylthiocolchicine (6) in 80% and 82% yields, respectively, without obtaining N-mono-alkyl-substituted compounds (Scheme 1). Amines 4–6 were converted to corresponding succinic acid salts 7–9 in quantitative yields.
Figure 1.
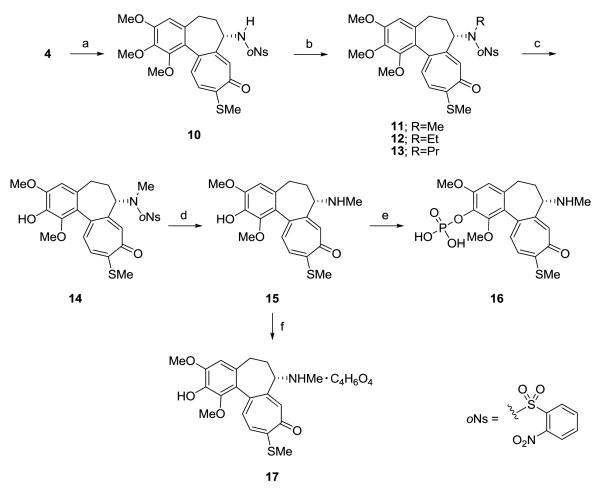
A series of N-mono-alkylated analogs of 4 was synthesized through ready addition and removal of the o-nitrobenzenesulfonamide (o-Ns) group, which is widely tolerant of basic and acidic conditions (Scheme 2). According to Fukuyama’s protocol,7 4 was converted to N-[o-Ns]-N-deacetylthiocolchicine (10) with o-NsCl in the presence of 2,6-lutidine in CH2Cl2, followed by alkylation with RI (R = Me, Et or Pr) in the presence of K2CO3 in MeCN to afford 11, 12 and 13 in 72%, 97% and 53% overall yields, respectively. Treatment of 11 with BBr3 in CH2Cl2 at −78°C succeeded in the regioselective demethylation to afford 14 in 75% yield, while the reported method8 by heating with H2SO4 gave a lower yield of the desired compound. The o-Ns group was removed from 14 with thioglycolic acid and Et3N in CH2Cl2 to obtain the secondary amine 15 in 59% yield. Compound 16, a phosphate of 15, was synthesized by using POCl3 in MeCN and purified with reverse phase column chromatography (MeOH–H2O). Treatment of 15 with succinic acid in acetone gave 17. The water solubility at room temperature of analogs 7–9, 16, and 17 were 180 mg/mL, 103 mg/mL, 107 mg/mL, 15 mg/mL and 95 mg/mL, respectively.
Scheme 1.
Reagents and conditions; a) RCHO (D=H or Me), NaBH3CN, HOAc,CH2Cl2, 5; 80% (R’=H), 6; 82% (R’=Me); b) succinic acid, acetone, 7, 8, 9; quant.
All newly synthesized compounds were evaluated for effects on tubulin assembly and in vitro antitumor activities against several tumor cell lines. Studies were also performed with colchicine (1), thiocolchicine (2), and N-deacetylthiocolchicine (4). The cell line panel included KB (human epidermoid carcinoma of the nasopharynx), HCT-8 (human ileocecal carcinoma), A549 (human lung adenocarcinoma), DU145 (human prostate carcinoma), SK-BR-3 (human breast cancer) and KBvin (vincristine-resistant line derived from the KB cell) cells. In addition, the most active inhibitors of tubulin assembly were also evaluated as inhibitors of the binding of [3H]colchicine to tubulin. The tubulin data are summarized in Table 1. Relative to the activity shown by 2 and 3, N,N-dimethyl- (5) and N,N-diethyl-N-deacetylthiocolchicine (6), derived from 4 by alkylation, exhibited reduced activity as inhibitors of both tubulin assembly and colchicine binding Compounds 7, 8, and 9 are three succinic acid salts of 4, 5, and 6. Compound 7, derived from N-deacetylthiocolchicine (4), had activity comparable with that of its parent compound 4, while compounds 8 and 9, salts of 5 and 6, were somewhat less potent than their parent compounds. N-o-Ns substituted compounds 10 and 11, with no other N-alkyl (10) or a methyl substitutuent (11) in addition to the o-Ns group, also displayed potent anti-tubulin activities. However, replacing the methyl group with an ethyl (12) or propyl (13) moiety led to reduced or lost activity relative to compound 11 in the tubulin assays. This suggests that the size of alkyl groups at the C7–amino position of N-deacetylthiocolchicines may play a role in the interaction of these compounds with the binding site of the target protein, while the o-Ns group may not significantly affect antitubulin activity.
Table 1.
Inhibition of tubulin assembly of 2–17
| Compound | Inhibition of tubulin assembly IC50 (μM) ± SD |
Inhibition of colchicine binding % Inhibition ± SD |
|
|---|---|---|---|
| 5 μM | 50 μM | ||
| 2 | 0.77 ± 0.2 | 74 ± 2 | NDa |
| 4 | 0.91 ± 0.05 | 63 ± 1 | ND |
| 5 | 2.5 ± 0.3 | 42 ± 4 | 88 ± 0.6 |
| 6 | 5.4 ± 0.2 | 31 ± 3 | 84 ± 3 |
| 7 | 0.88 ± 0.03 | 64 ± 2 | ND a |
| 8 | 3.6 ± 0.04 | 27 ± 4 | 85 ± 0.1 |
| 9 | 16 ± 1 | 17 ± 6 | 65 ± 1 |
| 10 | 1.1 ± 0.06 | 58 ± 2 | ND |
| 11 | 0.92 ± 0.03 | 43 ± 3 | 60 ± 2 |
| 12 | 4.2 ± 0.7 | 22 ± 5 | 26 ± 5 |
| 13 | > 40 | ND a | ND |
| 14 | 3.6 ± 0.2 | 20 ± 7 | 34 ± 6 |
| 15 | > 40 | ND | ND |
| 16 | > 40 | ND | ND |
| 17 | 23 ± 1 | ND | ND |
| CAb | 1.2 ± 0.2 | 99 ± 1 | ND |
Not Done
Combretastin A-4: positive control
2-Demethylthiocolchicine (3) was previously reported as an active inhibitor of tubulin assembly and colchicine binding. 3 However, in our recent study, a series of 2-demethyl-N-deacetylthiocolchicine derivatives (14–17) showed reduced activity against tubulin assembly. Compared with the parent compound 11, compound 14, the 2-demethyl derivative of 11, was about four times less active against tubulin assembly (IC50 = 3.6 μM) and half as potent in inhibition of colchicine binding (20% inhibition of colchicine binding). Compound 15, the N-de-o-Ns analog of compound 14, showed remarkably reduced activity as an antitubulin agent, since it was essentially inactive as an inhibitor of tubulin assembly (IC50 > 40 μM). These findings suggest that a trimethoxyphenyl moiety with its lipophilic molecular feature may be required for N-deacetylthiocolchicines to fit into a hydrophobic pocket in the binding site. The acetamido group at C7 of compound 3 may play a role in maintaining the drug molecule in a suitable conformation to interact with the target protein. Although the hydroxy group at C2 of compound 16 was masked with a phosphoryl moiety, the highly polar and H-bonding properties of the phosphate moiety may prevent the molecule from interacting optimally with the colchicinebinding domain and thereby eliminating its anti-tubulin activity (IC50 > 40 μM).
In vitro antitumor activities of the newly synthesized compounds were evaluated, and the results are summarized in Table 2. Consistent with the tubulin data discussed above, 5 and 6, N-deacetylthiocolchicine derivatives with N,N-di-alkyl substituents at C7, were on average ten times more active than the non-alkyl substituted compound 4. The N,N-diethyl compound 6 generally had antiproliferative activity similar to that of the N,N-dimethyl compound 5 and colchicine (1). Compound 6 was the only highly active agent tested that was equally active against the vincristine resistant, MDR nasopharyngeal cancer cell line (KBvin) as the parental non-MDR KB cell line. This result suggests that the size of N-alkyl substituent at C7 may affect recognition of a colchicinoid by P-glycoprotein and/or the mechanism of action of 6 may be other than inhibition of tubulin activity. Although, in general, the succinic acid salts, 7–9, showed decreased activities relative to their parent compounds 4–6 N,N-dimethyl compound 8 was selectively more potent against HCT-8 (ED50 = 0.0220 μg/mL) and SK-BR-3 (ED50 = 0.0129 μg/mL) than its parent compound 5, and 8 was 10-fold more cytotoxic against other tested cell lines. The cytotoxicity of N-o-Ns-N-deacetylthiocolchicine derivatives, 10–13, revealed the same tendency as described above. In summary, the smaller alkyl substituents on the amino group at the C7–position tended to have stronger activities against all cell lines (10, R = H > 11, R = Me > 12, R = Et > 13, R = Pr). N-non-alkyl-substituted N-[o-Ns] compound 10 showed the highest activity among 10–13 against all tested cell lines (ED50 = 0.0100–0.0270 μg/mL). Although 2-demethylthiocolchicine (3) showed similar activity to thiocolchicine (2) (ED50 = 0.01 μg/mL against A549, 0.01 μg/mL against KB),3 the cytotoxic potency decreased for the 2-demethyl-N-[o-Ns]-N-deacethylthiocolchicine series (e.g., 11 vs. 14), as occurred in the tubulin assay. Differing from the tubulin assay results, compound 15, derived from 14 by removal of the o-Ns protection group, did not show significant loss in inhibition of tumor cell growth. In addition, the inhibitory activity toward DU145 and SK-BR-3 cells slightly increased. Compound 16, a phosphate of 15, showed two-three times more potent cytotoxicity than 15 against A549, DU145, SK-BR-3, and KB cell growth, suggesting that the phosphate group may increase selectivity to the target tumor cells. Notably, the new compounds, N,N-dialkyl-N-deacetylthiocolchicines, 5 and 6, as well as the succinates, 7–9, showed comparable or better cytotoxicity against P-gp over-expressing KBvin cells than colchicine.
Table 2.
In Vitro anticancer activity data of 1–17
| compounds | ED50 [μg/mL] a / cell line |
|||||
|---|---|---|---|---|---|---|
| HCT-8 | A549 | DU145 | SK-BR-3 | KB | KBvin | |
| 1 | 0.0540 | 0.0199 | 0.0027 | 0.0027 | 0.0038 | 0.1578 |
| 2 | 0.0080 | 0.0019 | 0.0012 | 0.0015 | 0.0008 | 0.0147 |
| 4 | 0.0050 | 0.0077 | 0.0018 | 0.0016 | 0.0018 | 0.0139 |
| 5 | 0.0380 | 0.0476 | 0.0180 | 0.0179 | 0.0270 | 0.0413 |
| 6 | 0.0310 | 0.0193 | 0.0138 | 0.0141 | 0.0200 | 0.0146 |
| 7 | 0.0291 | 0.0256 | 0.0185 | 0.0115 | 0.0080 | 0.0330 |
| 8 | 0.0220 | 0.1902 | 0.1243 | 0.0129 | 0.1200 | 0.1250 |
| 9 | 0.1100 | 0.1808 | 0.1249 | 0.1361 | 0.0630 | 0.1512 |
| 10 | 0.0270 | 0.0209 | 0.0129 | 0.0126 | 0.0100 | 0.1120 |
| 11 | 0.1200 | 0.1288 | 0.0312 | 0.0285 | 0.0350 | 0.1526 |
| 12 | 0.5200 | 0.2116 | 0.1630 | 0.1482 | 0.1300 | 0.1737 |
| 13 | > 1.000 | 1.6330 | 0.3574 | 0.6155 | 0.9600 | 1.3626 |
| 14 | > 1.000 | 1.6791 | 1.1483 | 1.0118 | 0.7200 | 1.4892 |
| 15 | > 1.000 | 1.5474 | 0.5054 | 0.4874 | 0.5500 | 1.3798 |
| 16 | > 1.000 | 0.5332 | 0.1572 | 0.1368 | 0.2500 | 1.7062 |
| 17 | > 1.000 | 1.2599 | 0.3420 | 0.3899 | 0.3500 | 1.3456 |
Mean concentration giving 50% inhibition of cell growth following 2 days continual exposure (three replicates varied less than 5% between and within experiments)
HCT-8 = human ileocecal carcinoma cell line, A549 = human lung adenocarcinoma epithelial cell line, DU145 = human prostate carcinoma cell line, SK-BR-3 = human breast cancer cell line, KB = human epidermoid carcinoma of the nasopharynx, KBvin = vincristine-resistant nasopharyngeal cancer cell line derived from the KB line
In summary, modifications of the amino group at C7 and the methoxy group at C2 of N-deacetylthiocolchicine (4) were achieved. Two N,N-di-alkyl-N-deacetylthiocolchicine deerivatives, 5 and 6, corresponding succinates 7–9, N-[o-Ns]-N-deacetylthiocolchicines 10–13, and 2-demethyl-N-methyl-N-deacetylthiocolchicines 14 and 15, salt 17, and C2–phosphate 16 were generated. We analyzed this series of compounds for their anti-tubulin activity and in vitro cytotoxicity. Although none of the newly synthesized compounds showed more potent activity than their parent compound 4 or thiocolchicine (2), the succinic acid salts, especially compound 7, have potential as promising antitumor agents, because of both their excellent antiproliferative activity and their superior water solubility. The size of the N-substituted alkyl groups played a role in inhibition of tubulin assembly and colchicine binding and in vitro cytotoxicity. Generally, the larger alkyl substituents showed reduced activity against both tubulin and tumor cell growth. N,N-Diethyl analog 6, however, showed more potent cytotoxicity than its N,N-dimethyl counterpart, which differed somewhat from the tubulin results. This result may indicate an additional mechanism of action for 6 versus 5. In addition, compound 6 also showed higher potency against the KBvin cell line relative to the parental KB line. Thus, compound 6 may merit further investigation to understand its apparent resistance to the P-gp efflux pump. Finally, the succinic acid salt of N,N-dimethyl-N-deacetylthiocolchicine, compound 8, showed selective activities against HCT-8 and SK-BR-3 cells.
Scheme 2.
Reagents and conditions; a) o-NsCl, 2,6-lutidine, CH2Cl2; b) Rl (R=Me, Et or Pr), K2CO3, MeCN 11; 85% 12; 97% 13; 53%; c) BBr3, CH2Cl2, −78 °C, 75%; d) thioglycolic acid, Et3N, 59%; e) POCl3, Et3N, MeCN, 0 °C to rt; then H2O, pyridine,43%; f) succinic acid, acetone, quant.
Acknowledgments
This study was supported by NIH grant CA17625 from the National Cancer Institute awarded to K. H. Lee.
Footnotes
For part 272 of this series, see reference 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Dong Y, Shi Q, Pai HC, Peng CY, Pan SL, Teng CM, Nakagawa-Goto K, Yu D, Liu YN, Wu PC, Bastow KF, Morris-Natschke SL, Brossi A, Lang JY, Hsu JL, Hung MC, Lee EY, Lee KH. J. Med. Chem. 2010;53:2299. doi: 10.1021/jm1000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 (a).Capraro HG, Brossi A. In: The Alkaloids. Brossi A, editor. Vol. 23. Academic Press; New York: 1984. p. 1. and references therein. Boyé O, Brossi A. In: The Alkaloids. Brossi A, Cordell GA, editors. Vol. 41. Academic Press; New York: 1992. p. 125. and references therein. Brossi A. J. Med. Chem. 1990;33:2311. doi: 10.1021/jm00171a001. Zhang SH, Feng J, Kuo SC, Brossi A, Hamel E, Tropsha A, Lee KH. J. Med. Chem. 2000;43:167. doi: 10.1021/jm990333a.
- 3.Nakagawa-Goto K, Chen CX, Hamel E, Wu CC, Bastow KF, Brossi A, Lee KH. Bioorg. Med. Chem. Lett. 2005;15:235. doi: 10.1016/j.bmcl.2004.07.098. [DOI] [PubMed] [Google Scholar]
- 4.Muzaffar A, Brossi A, Lin CM, Hamel E. J. Med. Chem. 1990;33:567. doi: 10.1021/jm00164a015. [DOI] [PubMed] [Google Scholar]
- 5 (a).Velluz L, Muller G. Bull. Soc. Chim. Fr. 1954:1072. [Google Scholar]; (b) Huant E. Therapie. 1955;10:448. [PubMed] [Google Scholar]
- 6.Shi Q, Verdier-Pinard P, Brossi A, Hamel E, Wu CC, Lee KH. Bioorg. Med. Chem. Lett. 1997;5:2277. doi: 10.1016/s0968-0896(97)00171-5. [DOI] [PubMed] [Google Scholar]
- 7 (a).Fukuyama T, Jow CK, Cheung M. Tetrahedron Lett. 1995;36:6373. [Google Scholar]; (b) Cifuentes M, Schilling B, Ravindra R, Winter J, Janik ME. Bioorg. Med. Chem. Lett. 2006;16:2761. doi: 10.1016/j.bmcl.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Kerkes P, Sharma PN, Brossi A, Chignell CF, Quinn FR. J. Med. Chem. 1985;28:1204. doi: 10.1021/jm00147a014. [DOI] [PubMed] [Google Scholar]