Introduction
The Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) is a multicenter, randomized clinical trial designed to compare the safety and efficacy of carotid artery stenting (CAS) and carotid endarterectomy (CEA) in patients with severe carotid stenosis. The primary endpoint is the composite of death, stroke, and myocardial infarction (MI) during the first 30 days after the procedure and ipsilateral stroke up to 4 years. The trial enrolled 2,522 patients with symptomatic and asymptomatic carotid stenosis.
A trial like CREST in which two procedures are being compared with regard to immediate and long-term results requires establishing the expertise of investigators before their participation. The process of credentialing surgeons to perform CEA has been well established in 2 National Institutes of Health (NIH)–sponsored clinical trials (1,2). A similar rigorous credentialing process for surgeons was used in CREST (3,4,5). CAS is a comparatively new approach to carotid revascularization, with growing experience in patient selection and technique. The CREST investigators undertook a comprehensive and rigorous credentialing process that included a lead-in CAS registry. In this article, we describe the process of credentialing of operators to perform CAS in the randomized trial phase of CREST. We do not report results of the randomized trial phase.
Methods
Credentialing of CAS Operators in the CREST Lead-in Study
The first step in the credentialing process was selecting interventionalists for the lead-in phase of CREST. To document their experience and technical skills, candidates had to submit records of their CAS cases, including admission notes, indications for CAS, procedure reports, and discharge summaries, to a multidisciplinary Interventional Management Committee (IMC). The number of cases before the lead-in reflected cases over the preceding months to several years. A physician-reviewer on the IMC reported the data to the IMC at biweekly meetings. The criteria used to approve candidates for the lead-in were a low complication rate, experience with ≥ 15 procedures, use of proper standard carotid stenting technique, and avoidance of erroneous techniques (eg, improper device use, inappropriate balloon sizing, use of 0.0035” wires, use of general anesthesia). When deficiencies in knowledge base or technical skills were identified, interventionalists were counseled and offered the option to perform additional CAS cases and resubmit their data. Numerical cutpoints for complication rates were not applied because of the wide confidence intervals that would have resulted for individual operators.
Candidates who were approved for participation in the lead-in phase underwent training in the use of the CREST study devices, which included use of animal models and participation in the Carotid Stent Operators Certification Program. Interventionalists who had been proctored through participation in other trials or as part of device manufacturers’ training programs were permitted to transfer these credentials to CREST (6,7). After completing training, these interventionalists proceeded with enrollment in the lead-in phase of CREST. Those with more experience (≥ 30 cases) performed 5 to 10 procedures in the lead-in phase, and those with less experience (<30 cases) performed 10 to 20 procedures in the lead-in phase. (6,7).
The CREST Lead-in Study
The ethics review committee at each site approved the study protocol, and all subjects provided written informed consent. Eligibility criteria have been reported previously.8 Symptomatic subjects had to have >50% stenosis by angiography in accordance with North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria.9 Asymptomatic subjects had to have >70% stenosis by angiography. Subjects anticipated to be at high risk for CEA were also eligible, as were subjects with extensive anatomic and medical comorbid conditions. Interim analysis identified an excessive combined stroke and death rate among octogenarians.3 Beginning in March 2004, the CREST Executive Committee and the National Institute of Neurological Disorders and Stroke Data Safety Monitoring Board (DSMB) excluded these high-risk subjects from further lead-in enrollment.
The IMC initiated a review of lead-in case data for each interventionalist, after experienced interventionalists had completed up to 5 CAS cases and the less-experienced interventionalists had completed up to 10 CAS cases. Interventionalists who demonstrated appropriate technical skills and low morbidity and mortality with these cases were approved for the randomization phase. Those who failed to meet this standard were required to continue in the lead-in phase.
Event monitoring of the lead-in phase was initiated through a Clinical Events Committee (CEC) at the Harvard Clinical Research Institute. Serious adverse events, including stroke and death, were reported to the CREST Statistical and Data Management Center. The center notified the principal investigator whenever a stroke or death occurred at an institution, and that institution was placed on “watch” status. A second event triggered an audit; the nature of the audit was determined by the IMC and depended on the type and severity of the event. At the conclusion of the lead-in phase, 21 sites were placed on watch status, and 15 sites were on audit status. An independent DSMB appointed by NIH met about twice yearly for additional oversight of the lead-in study.
The primary endpoint for the lead-in was the composite of stroke, MI, or death during the 30-day periprocedural period. Secondary endpoints were death and any stroke, death and major stroke, and death and major and minor stroke rates. Stroke severity was categorized as either major or minor by a single physician (D.E.C.), who reviewed both objective outcomes (eg, Barthel Index, NIH Stroke Scale scores) and the general clinical record. This single physician served as the medical monitor throughout the entire nine-year CREST trial, ensuring consistency over the entire study period. A fatal stroke was defined as death attributed to ischemic stroke or intracerebral hemorrhage. Diagnosis of MI was based on evidence of myocardial ischemia and elevation of cardiac enzymes (creatine kinase-MB or troponin) to twice the upper limit of normal in the individual clinical center. Myocardial ischemia was defined as clinical history of ischemic chest pain or electrocardiographic (ECG) evidence of ischemia, including new ST-segment depression or elevation more than 1 mm in two or more contiguous leads (10). MI also could be diagnosed by the presence of new pathologic Q waves. (10). All endpoints were evaluated by the CEC.
Statistical Analysis
Survival estimates at 30 days were made using Kaplan– Meier survival functions,11 with the standard error of the survival estimate determined using the Greenwood formula (SAS Institute, Cary, NC). Differences in event rates between strata of subjects were determined using simple linear contrast of the 2 strata-specific survival estimates, with the variance of the difference being the sum of individual variances.
Because there was little censoring (<3%) in the 30-day event rate estimates, generalized linear models could be used to model the proportion of participants with events, account for the joint or multivariate effect of multiple predictors, and account for correlation of outcomes among subjects with procedures performed by the same operator. Multivariate models were conducted in stages.
Models that included all patient characteristics were included in an overall model. Backward stepwise regression was then performed until only significant patient characteristics remained in the model (most parsimonious patient model).
Operator characteristics were added to the most parsimonious patient model, and backward regression was repeated until only significant operator characteristics and the previously identified patient characteristics remained (most parsimonious patient plus operator model).
Results
Credentialing Process
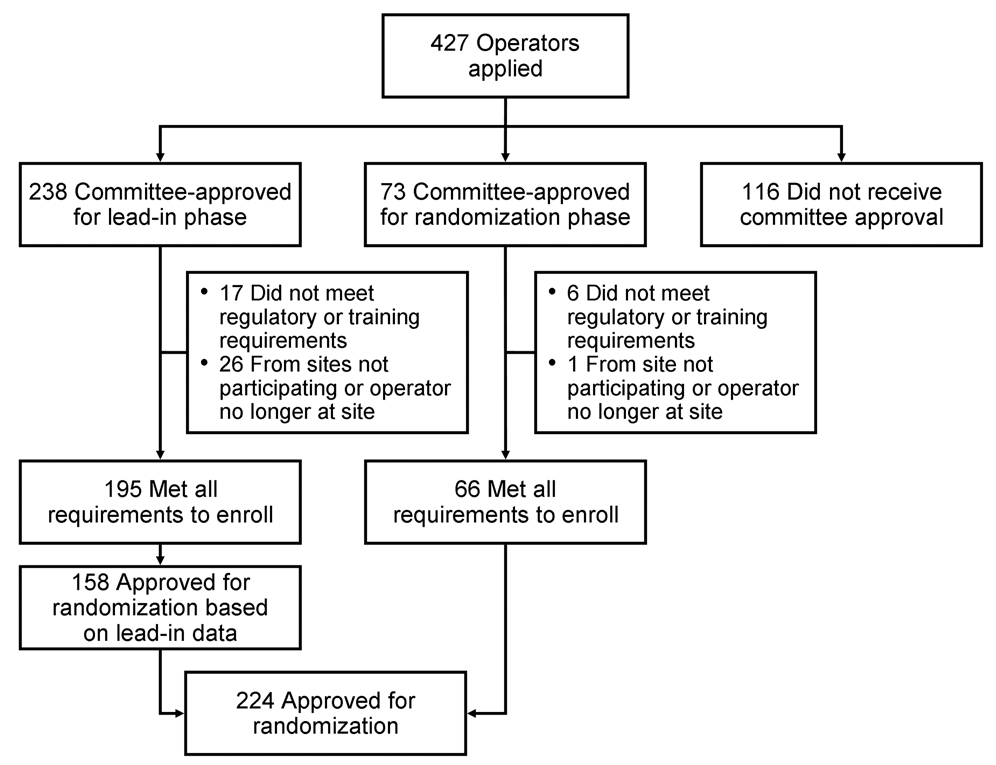
CREST sent invitations to 140 candidate sites, requesting data from potential interventionalists and surgeons (12). During 137 teleconferences, the IMC reviewed 10,164 CAS cases from 427 applicants (Figure 1). Of 427 stent operators who applied for the trial, 224 (52.4%) were ultimately approved for the randomization phase.
Figure 1.
Credentialing process used to identify stent operators approved for the lead-in (n=238) and randomized (n=224) phases of CREST.
The IMC did not approve 116 applicants for participation in the lead-in study. The primary reason that approval was not granted was insufficient experience—applicants either did not submit an adequate number of cases or were not the primary operator in the cases submitted. The number of cases submitted by these applicants ranged from one to 56 (median, 12 cases). Other reasons for nonapproval included a high periprocedural event rate, improper use of devices, inappropriate sizing of balloons, using 0.035” wires, and the use of general anesthesia; 44 applicants were not approved because of periprocedural events (stroke, transient ischemic attack or death).
The IMC judged that 73 of the 427 applicants could be exempted from the lead-in study. Approval for these operators to begin CAS in the randomization phase was based on greater experience (>30 carotid stenting cases submitted for review with low event rates) and previous experience (median of 30 cases) with the CREST study devices (ACCULINK™ or RX ACCULINK® Carotid Stent System and the ACCUNET™ or RX ACCUNET® Embolic Protection Device) from previous participation in other trials, such as the ARCHeR and CAPTURE registries (6, 7).This group included 36 operators who were initially approved for the lead-in phase, but through subsequent submission of data demonstrated sufficient experience and skill to warrant approval directly to the randomization phase. Of these 73 operators, six (8.2%) were unable to meet the regulatory or training requirements, and one (1.4%) was from a site that ultimately did not receive approval to participate.
A total of 238 applicants were approved to participate in the the lead-in phase. Approval was based on greater experience (>30 carotid stenting cases submitted for review with low event rates), use of proper standard carotid stenting technique, or an adequate number of submitted procedures (15–30 cases [median, 29], with low event rates), plus appropriate interventional skills using the correct standard technique. The median number of cases submitted by this group was 29 (range, 3–63 cases). Forty-three applicants did not meet the regulatory and training requirements, moved to another institution, or were from an institution that was not CREST approved. The remaining 195 applicants were approved by the IMC to enroll in the lead-in phase.
Of these 195 operators, 86 submitted 30 cases or more for review and were considered more experienced; 109 submitted fewer than 30 cases and were considered less experienced. Those in the more experienced group performed an average of 8.05 procedures in the lead-in phase, and those with less experience performed an average of 6.67 procedures to receive approval for the randomization phase. Overall, 158 of the 195 operators completed an average of nine cases (range, 1–35 cases) to gain approval for the randomization phase; the remaining 37 did not perform the required number of cases before trial enrollment ended. These 158 operators and the 66 expedited operators composed the 224 operators at 122 sites who were qualified as interventionalists in the randomized phase.
Table 1 lists the specialties for the interventionalists participating in the lead-in phase and for those approved to proceed directly to the randomized phase. Interventionalists were board certified (or the Canadian equivalent) in cardiology (40%), vascular surgery (21%), interventional neuroradiology (15%), interventional radiology (15%), neurosurgery (8%), and neurology (2%).
Table 1.
Operators approved to enroll participants in the lead-in phase (n=195) and to enroll directly into the randomization phase (n=66)
| Specialty | Lead-in Phase, No. (%)a |
Randomization Phase, No. (%)b |
|---|---|---|
| Interventional cardiology | 77 (39.5) | 21 (31.8) |
| Vascular surgery | 42 (21.5) | 18 (27.3) |
| Interventional neuroradiology | 29 (14.9) | 9 (13.6) |
| Interventional radiology | 29 (14.9) | 12 (18.2) |
| Neurosurgery | 15 (7.7) | 6 (9.1) |
| Neurology | 3 (1.5) | 0 (0) |
An additional 43 operators received committee approval but never enrolled any participants in the trial.
An additional 7 operators received committee approval but never enrolled any participants in the trial.
Patient Characteristics
We report on 1,565 subjects enrolled in the CREST lead-in phase between November 2000 to April 2008 at 98 sites in the United States and Canada. The mean (standard error [SE]) subject age was 69.6 (0.2) years, 28% were older than 75 years, 37% were female, 84% were hypertensive, 83% were dyslipidemic, 33% were diabetic, and 18% were smokers. In terms of study groups, 73% were asymptomatic and 26% were symptomatic. Mean (standard deviation [SD]) carotid stenosis was 79.4% (21%), and 48% of subjects had a lesion length of 16 mm or more (Table 2).
Table 2.
Baseline demographic and clinical criteria (N=1,565)
| Characteristic | Value |
|---|---|
| Age, mean (SE), y | 69.6 (0.2) |
| Age >75 y, No. (%) | 432 (28) |
| Female, No. (%) | 580 (37) |
| White, No. (%) | 1,382 (88) |
| Percent carotid stenosis, mean (SD) | 79.4 (21) |
| Symptomatic carotid stenosis, No. (%) | 414 (26) |
| Hypertension, No. (%) | 1,307 (84) |
| Diabetes, No. (%) | 510 (33) |
| Dyslipidemia, No. (%) | 1,292 (83) |
| Current cigarette or cigar smoker, No. (%) | 286 (18) |
| Prior CABG, No. (%) | 376 (24) |
| Eccentric lesion, No. (%) | 820 (52) |
| Ulcerated lesion, No. (%) | 498 (32) |
| Lesion length ≥16 mm, No. (%) | 758 (48) |
Abbreviations: CABG, coronary artery bypass graft; SD, standard deviation; SE, standard error.
Outcomes
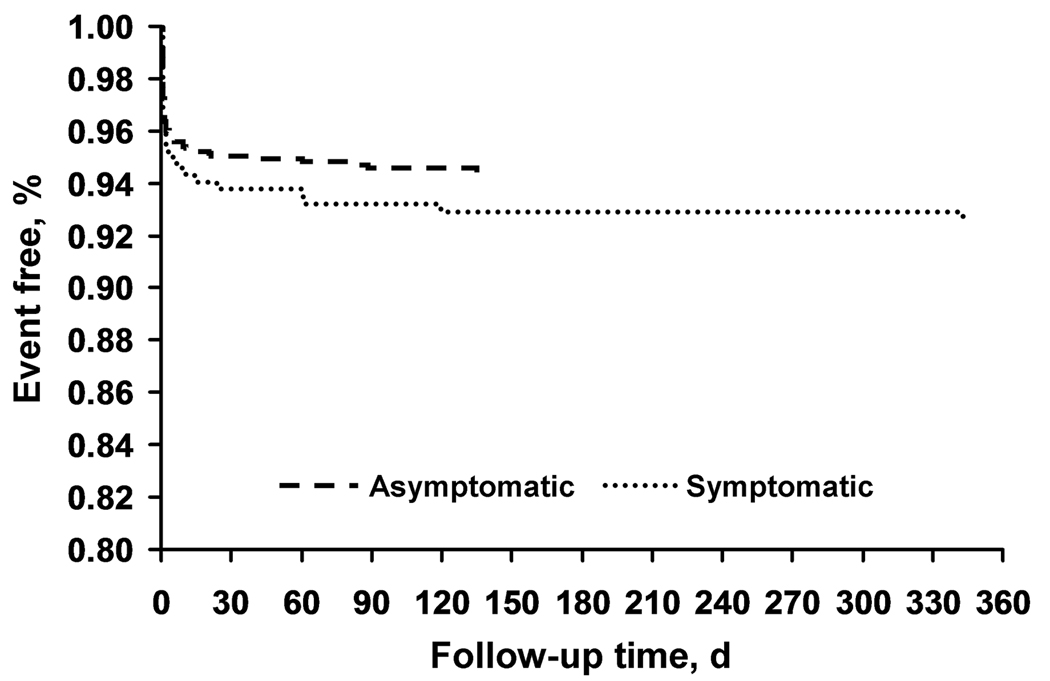
Among the 1,565 patients, 97% had one-month follow-up and 86% had one-year follow-up. Outcome events by symptomatic status at 30 days and one year are shown in Table 3 and Figure 2. The composite endpoint (SE) of death, stroke, and MI was 5.2% (0.6%) for the total population, 4.8% (0.6%) for the asymptomatic population, and 6.1% (1.2%) for the symptomatic population. The composite endpoint of stroke and death was 4.5% (0.5%) for the total population, 3.8% (0.6%) for the asymptomatic population, and 5.8% (1.2%) for the symptomatic population. The stroke and death rate was 2.9%, if subjects older than 75 years were excluded. Outcomes did not vary by symptomatic status, sex, common cardiac risk factors, prior coronary artery bypass graft surgery, or lesion features. The proportions of symptomatic and asymptomatic patients who were event free at one year are shown in Figure 2
Table 3.
Kaplan-Meier estimates (SE) for outcome events at 30 days and 1 year based on symptom Classa
| Symptomatic | 30 Days Asymptomatic |
Symptomatic | 1 Year Asymptomatic |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome Event | Event Rate |
SE | Event Rate |
SE | P Value | Event Rate |
SE | Event Rate |
SE | P Value |
| Death/stroke/MI | 6.1 | 1.2 | 4.8 | 0.6 | .37 | 7.2 | 1.3 | 5.4 | 0.7 | .22 |
| Death/any stroke | 5.8 | 1.2 | 3.8 | 0.6 | .14 | 7.0 | 1.3 | 4.3 | 0.6 | .06 |
| Death/major stroke | 3.2 | 0.9 | 1.8 | 0.4 | .16 | 3.4 | 0.9 | 1.9 | 0.4 | .13 |
| Death | 1.5 | 0.6 | 0.5 | 0.2 | .11 | … | … | … | … | … |
| Major stroke | 2.4 | 0.8 | 1.6 | 0.4 | .37 | 2.7 | 0.8 | 1.7 | 0.4 | .26 |
| Minor stroke | 2.7 | 0.8 | 2.0 | 0.4 | .43 | 3.5 | 0.9 | 2.3 | 0.5 | .24 |
Abbreviations: MI, myocardial infarction; SE, standard error.
Values are percentages unless indicated otherwise.
Figure 2.
Kaplan-Meier analyses showing the composite outcome of death, stroke, and myocardial infarction for asymptomatic (n=1,151) and symptomatic (n=414) patients. At 12 months, the event rates were 5.4% vs 7.2%, respectively (P=.22). Difference in event rates between symptomatic and asymptomatic patients and between age strata was tested assuming normality of the estimated even rates for both groups using a standard linear contrast.
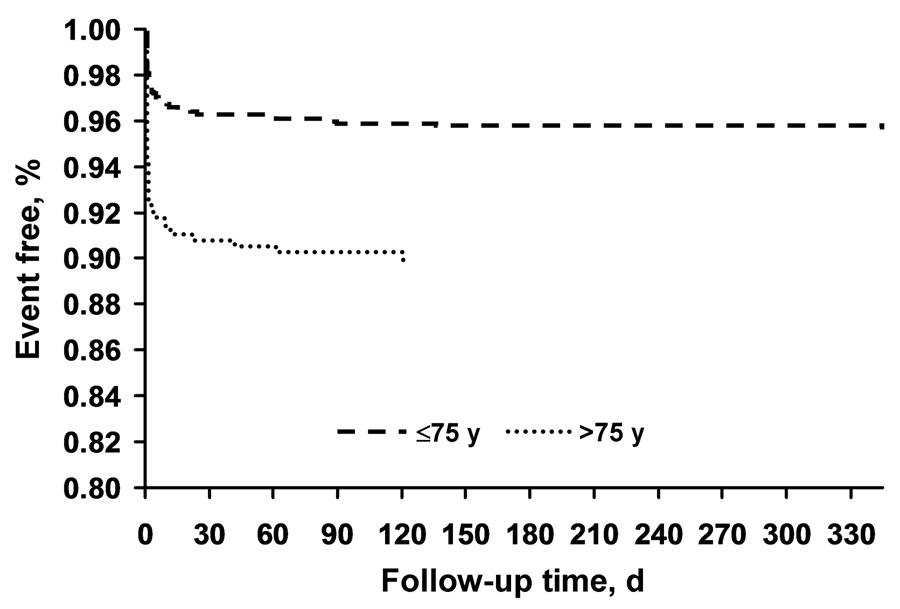
Advancing age was associated with worse outcomes. The outcomes in patients older than 75 years versus those aged 75 years or younger are shown in Figure 3 and in Table 4 and Table 5 by symptomatic status. A significant difference by age in stroke and death at 30 days was seen between the symptomatic subjects (9.1% vs 4.5%) and asymptomatic subjects (7.5% vs 2.4%). The multivariate model demonstrated that the effect of age on the primary outcome was independent of potential confounders, including symptomatic status, sex, vascular risk factors, and lesion characteristics. The odds ratio (OR) was 2.38 (95% confidence interval [CI], 1.58–3.58) for advancing age and the primary endpoint. Applying the most parsimonious model, the OR for age and occurrence of the primary endpoint was also constructed; that OR was 2.74 (95% CI, 1.16–4.51) at age older than 75 years.
Figure 3.
Kaplan-Meier analyses showing the composite outcome of death, stroke, and myocardial infarction for patients aged 75 years or younger (n=1,133) and older than 75 years (n=432). At 12 months, the event rates were 4.3% vs 10.0%, respectively (P=.001). Difference in event rates between symptomatic and asymptomatic patients and between age strata was tested assuming normality of the estimated even rates for both groups using a standard linear contrast.
Table 4.
Kaplan-Meier estimates for outcome events at 30 days and 1 year based on age strata: symptomatic patientsa
| 30 Days | 1 Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤75 y | >75 y | ≤75 y | >75 y | |||||||
| Outcome Event | Event Rate |
SE | Event Rate |
SE | P Value | Event Rate |
SE | Event Rate |
SE | P Value |
| Death/stroke/MI | 4.8 | 1.3 | 9.1 | 2.6 | .14 | 5.6 | 1.4 | 11.0 | 2.8 | .08 |
| Death/any stroke | 4.5 | 1.2 | 9.1 | 2.6 | .11 | 5.3 | 1.3 | 11.0 | 2.9 | .07 |
| Death/major stroke | 2.8 | 1.0 | 4.2 | 1.8 | .50 | 2.8 | 1.0 | 5.1 | 2.0 | .30 |
| Death | 1.7 | 0.8 | 0.8 | 0.8 | .43 | … | … | … | … | … |
| Major stroke | 1.7 | 0.8 | 4.2 | 1.8 | .20 | 1.7 | 0.8 | 5.1 | 2.0 | .11 |
| Minor stroke | 1.7 | 0.8 | 5.0 | 2.0 | .13 | 2.6 | 1.0 | 5.9 | 2.2 | .17 |
Abbreviations: MI, myocardial infarction; SE, standard error.
Values are percentages unless indicated otherwise.
Table 5.
Kaplan-Meier estimates for outcome events at 30 days and 1 year based on age strata: asymptomatic patients a
| 30 Days | 1 Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤75 d | >75 d | ≤75 d | >75 d | |||||||
| Outcome Event | Event Rate |
SE | Event Rate |
SE | P Value | Event Rate |
SE | Event Rate |
SE | P Value |
| Death/stroke/MI | 3.3 | 0.6 | 9.1 | 1.6 | .001 | 3.8 | 0.7 | 9.5 | 1.7 | .002 |
| Death/any stroke | 2.4 | 0.5 | 7.5 | 1.5 | .001 | 3.0 | 0.6 | 7.9 | 1.6 | .004 |
| Death/major stroke | 1.2 | 0.4 | 3.2 | 1.0 | .06 | 1.2 | 0.4 | 3.6 | 1.1 | .04 |
| Death | 0.5 | 0.2 | 0.7 | 0.5 | .71 | … | … | … | … | … |
| Major stroke | 1.1 | 0.4 | 2.9 | 1.0 | .09 | 1.1 | 0.4 | 3.3 | 1.0 | .04 |
| Minor stroke | 1.2 | 0.4 | 4.3 | 1.2 | .01 | 1.8 | 0.5 | 4.3 | 1.2 | .05 |
Abbreviations: MI, myocardial infarction; SE, standard error.
Values are percentages unless indicated otherwise.
Potential effects on the outcome of interventionalist experience and specialty training were examined. Years of experience or numbers of stent cases submitted before the lead-in phase did not affect outcomes in the lead-in phase. Operator specialty was recorded in 1,521 cases: 637 cases (42%) were performed by interventional cardiologists, 337 cases (22%) by vascular surgeons, 242 cases (16%) by interventional radiologists, 186 cases (12%) by interventional neuroradiologists, and 119 cases (8%) by neurosurgeons. The remaining cases were performed by neurologists and operators with mixed training backgrounds.
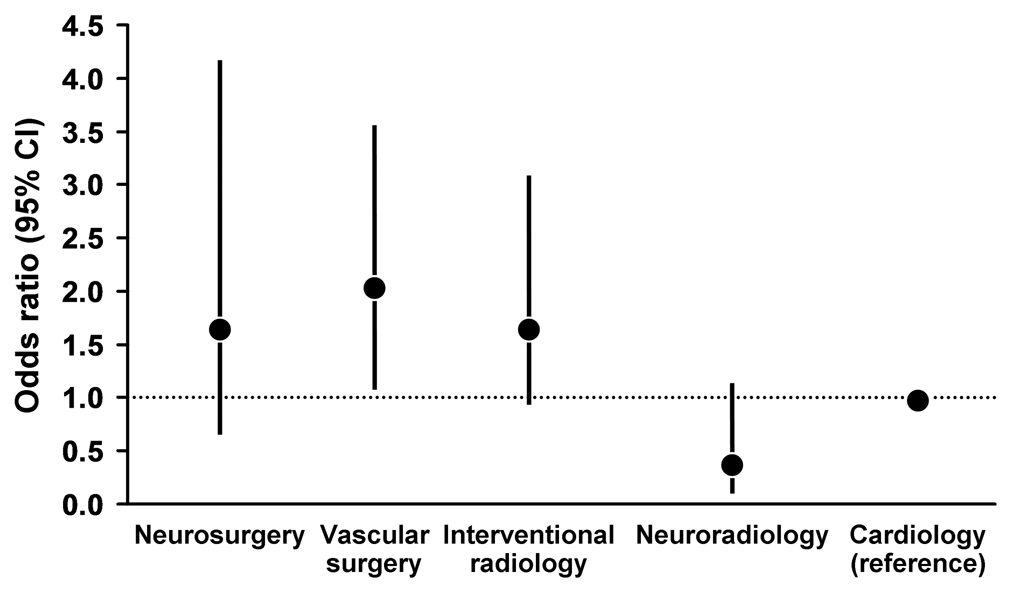
Rates of stroke, MI, and death at 30 days by specialty were as follows: neurosurgery, 6.7% (8/119); neuroradiology, 1.6% (3/186); interventional radiology, 6.6% (16/242); vascular surgery, 7.7% (26/337); and cardiology, 3.9% (25/637) (Table 6). Because it had largest sample size, interventional cardiology was selected as the reference group (OR, 1.0). Univariate analysis showed a higher rate of stroke, MI, or death at 30 days for vascular surgeons and interventional radiologists, relative to interventional cardiologists. In the multivariable models, only age and operator specialty training remained significant (P<.01) (Table 7). As shown in Figure 4, after adjustment for age, vascular surgeons had a higher event rate than interventional cardiologists (OR, 2.05; 95% CI, 1.18–3.56). Event rates did not differ significantly among interventional radiologists (OR, 1.66; 95% CI, 0.89–3.08), neurosurgeons (OR, 1.66; 95% CI, 0.66–4.16), and interventional neuroradiologists (OR, 0.39; 95% CI, 0.13–1.15).
Table 6.
Number of procedures, events, and event rate per 100 patients by specialty of the operator
| Number of Operators |
Number of procedures |
Number of events within 30 days |
Event rate/100 | |
|---|---|---|---|---|
| Neurosurgery | 13 | 119 | 8 | 6.7 |
| Neuroradiology | 28 | 186 | 3 | 1.6 |
| Interventional radiology | 26 | 242 | 16 | 6.6 |
| Vascular surgery | 37 | 337 | 26 | 7.7 |
| Cardiology | 73 | 637 | 25 | 3.9 |
Table 7.
Odds ratios (95% CI) of stroke, death or MI within 30 days
| Patient Model Only | Patient (Age) + Operator Characteristics | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariable | Most parsimonious | Multivariable | Most parsimonious | ||
| Patient* | Age >75 | 2.74 (1.66–4.51) | 3.44 (1.91–6.20) | 2.74 (1.66–4.51) | 2.56 (1.54–4.26) | 2.62 (1.59–4.33) |
| Race (white) | 1.02 (0.42–2.45) | 1.09 (0.39–3.00) | ||||
| Sex (Male) | 0.88 (0.55–1.43) | 0.93 (0.54–1.59) | ||||
| Symptomatic status (sympt) | 1.31 (0.77–2.24) | 1.40 (0.80–2.44) | ||||
| Diabetes | 1.42 (0.91–2.21) | 1.49 (0.89–2.49) | ||||
| Hypertension | 1.26 (0.62–2.55) | 1.47 (0.65–3.33) | ||||
| Dyslipidemia | 0.73 (0.41–1.28) | 0.72 (0.38–1.35) | ||||
| Current Smoker | 1.52 (0.74–3.11) | 0.90 (0.45–1.82) | ||||
| Eccentric lesion | 0.96 (0.64–1.46) | 1.17 (0.75–1.83) | ||||
| Ulcerated lesion | 0.83 (0.52–1.32) | 0.63 (0.34–1.17) | ||||
| CABG | 1.06 (0.59–1.90) | 1.11 (0.61–2.02) | ||||
| Stenosis ≥ 80% | 0.86 (0.57–1.31) | 0.78 (0.48–1.28) | ||||
| Lesion length ≥ 16 | 1.10 (0.70–1.73) | 1.18 (0.68–2.02) | ||||
| Operator Model Only | ||||||
| Univariate | Multivariable | Most parsimonious | ||||
| Operator | Total procedures performed in
Lead–in (tertiles) |
1.83 (1.05–3.19) | 1.57 (0.92–2.69) | 1.46 (0.85–2.53) | ||
| 16–35 (26 operators) | 0.88 (0.48–1.62) | 0.84 (0.45–1.58) | 0.81 (0.44–1.52) | |||
| 9–15 (43 operators) | reference | reference | Reference | |||
| 1–8 (114 operators) | ||||||
| Specialty | ||||||
| Neurosurgery | 1.80 (0.72–4.47) | 1.80 (0.84–3.86) | 1.80 (0.72–4.47) | 1.69 (0.76–3.74) | 1.66 (0.66–4.16) | |
| Vascular surgery | 2.09 (1.21–3.61) | 1.95 (1.19–3.20) | 2.09 (1.21–3.61) | 1.96 (1.19–3.24) | 2.05 (1.18–3.56) | |
| Interventional radiology | 1.70 (0.93–3.13) | 1.60 (0.86–2.99) | 1.70 (0.93–3.13) | 1.57 (0.83–2.97) | 1.66 (0.89–3.08) | |
| Neuroradiology | 0.40 (0.14–1.18) | 0.43 (0.13–1.35) | 0.40 (0.14–1.18) | 0.41 (0.13–1.30) | 0.39 (0.13–1.15) | |
| Cardiology | reference | reference | reference | reference | reference | |
Figure 4.
The 30-day event rates compared by operator training background specialty. Odds ratios for the composite event of death, stroke, or myocardial infarction are shown constructed using multivariate logistic regression with the event rates for the largest group of operators, interventional cardiology, shown as unity. The most parsimonious multivariate model contains age (hazard ratio, 2.62 [95% confidence interval, 1.59–4.33]) in addition to specialty.
Discussion
This article describes the most extensive credentialing process for carotid interventionalists yet reported. The process involved assessment of the clinical and technical acceptability to enter a credentialing registry by a multidisciplinary IMC, rigorous per-protocol assessment of outcomes in a lead-in registry, and approval for CREST by the IMC, largely on the basis of data from the registry.
The CREST lead-in phase is the largest federally funded study of its type, with 414 symptomatic subjects and 1,151 asymptomatic subjects enrolled at 98 centers. The IMC met 137 times to review 10,164 cases submitted by 427 interventionalists. On the basis of those reviews, 224 interventionalists were approved for subsequent participation in the CREST randomized study. All operators followed a well-defined protocol and were monitored by an on-site CREST research coordinator. Patients underwent independent neurologic evaluation before and after the procedure, an external CEC assessed complications, and independent core laboratories evaluated duplex and angiographic findings.
The 30-day results from the lead-in phase are encouraging with regard to the learning curve of the CREST interventionalists. The stroke and death rate was 2.9% when patients older than 75 years were excluded. For NASCET, in patients with 70% to 99% stenosis, the corresponding rate for symptomatic patients was 5.8%, and octogenarians were not eligible (1). For the Asymptomatic Carotid Atherosclerosis Study (ACAS) (2), the corresponding rate in asymptomatic patients was 2.3% (1.5%, excluding angiography complications), and octogenarians were not eligible. The ACAS rate is the lowest reported for CEA in a randomized carotid surgery trial. In addition, the eligibility criteria for ACAS and for NASCET were narrower than those for the lead-in phase of CREST, so some patients were enrolled in the lead-in study with risk-related characteristics that would have made them ineligible for ACAS or NASCET. From the lead-in phase results, we inferred that the IMC-approved CREST interventionalists were well qualified as they entered the randomized phase. This might be in contrast to the interventionalists participating in other large randomized trials of stenting. For example, the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) study (13,14) and the Stent-Protected Angioplasty vs Carotid Endarterectomy (SPACE) study (15) involved training and supervision of operators, but the methods have not been described in detail. No large supervised credentialing study was performed.
This study confirms previous reports of increased risk of carotid stenting with advancing age. The degree of risk with increasing age is similar to that reported in the SPACE study (15,16). For example, in the lead-in phase of CREST, the 30-day risk of stroke and death for symptomatic patients older than 75 years was 8.0% compared with 11.0% in the SPACE study. Subjects in that age group in the SPACE study treated with CEA fared significantly better. Excess tortuosity and calcification of the carotid artery are age-associated anatomic risks that may contribute to excess morbidity and mortality. Unfortunately, the importance of these vessel characteristics was not appreciated when the SPACE study was initiated, and data were not collected prospectively. Increasing medical risk likely also contributes to the increasing interventional risk. The influence of age also may be amplified in a study in which operators are in earlier phases of their learning curves (17–19).
Neither the experience in performing carotid intervention nor the number of cases performed before the lead-in phase was associated with 30-day outcomes. This does not lead us to conclude that experience is not an important consideration, however. Measurement of experience in years and number of cases was by self-report. In addition, we did not measure total catheter experience, total endovascular treatment experience, or total carotid treatment experience. Finally, as noted earlier, the more experienced interventionalists were required to perform fewer cases in the lead-in phase than the less experienced interventionalists, making the benefits of experience more difficult to detect from the lead-in phase results.
Operator training and specialty have also been suggested as potential considerations in stent trials. In the CREST lead-in, we observed higher event rates for procedures performed by vascular surgeons and marginally higher rates for interventional radiologists, compared with cardiologists. These differences could be related to the specialty or possibly to the complexity of the cases referred to specific specialties. We have attempted to remove this factor by covariate adjustment for potential confounders, including symptomatic status, degree of stenosis, and age. Differences may also be anticipated because of variability within specialties in experience with catheter-based therapies and carotid stenting in particular. Specifically, interventional cardiologists as a group have had early experience with embolic protection devices used in saphenous vein graft stenosis and rapid exchange systems used in carotid stenting.
The differences in procedural risk raise several issues. Importantly, multivariate modeling is always incomplete with regard to controlling for known confounders and cannot control for potential confounders yet to be identified. Nonetheless, the duration and intensity of training in catheter-based diagnosis and treatment were substantial for interventional cardiology and interventional neuroradiology specialists. Those elements of training may not be less substantial for interventional radiology; however, the daily practice of interventional cardiology and interventional neuroradiology involves catheter-based techniques that differ substantially from those involved in interventional radiology. However, day-to-day practice of interventional cardiology and interventional neuroradiology involves catheter-based techniques that differ substantially from interventional radiology. Compared with practitioners in other specialties in the United States, interventional cardiologists were early adopters of the carotid stenting technique, and many of those participating in CREST have rich experience with the procedure. In addition, critical risk-related aspects concerning anatomy and patient selection have been widely disseminated within the interventional cardiology and interventional neuroradiology communities.
The relationship of interventionalist experience to 30-day outcomes is not clear from previous CAS trials and registries. In EVA-3S (13), the primary endpoint – the 30-day incidence of any stroke or death – was higher for patients undergoing stenting compared with those undergoing CEA. Although the authors reported no association between experience and procedural outcomes, their definition of experience was based on the number of procedures done during EVA-3S. Investigators characterized only 10 of the 105 EVA-3S interventionalists as experienced. Because stenting was attempted in 265 patients, the numbers of events would not be adequate to detect an effect of experience if such an effect were present. In the other recent large randomized trial comparing CEA and carotid stenting, the SPACE study, (15) interventionalists were required to show proof of 25 consecutive transluminal angioplasty or stent procedures; a minimum requirement for carotid procedures was not stated. For carotid stent registries, the 30-case relationship of operator experience to outcome has been reported for the Carotid Acculink/Accunet Post Approval Trial to Uncover Rare Events (CAPTURE) registry and the Emboshield and Xact Post Approval Carotid Stent Trial (EXACT), both in higher surgical risk patients with symptomatic and asymptomatic carotid artery disease. In CAPTURE, there was no difference in outcomes based on physician experience as defined in that analysis (20). The most experienced interventionalists treated only 8.1% of the patients. The majority of operators had performed a minimum of 10 carotid stent procedures before entering the study. The EXACT registry showed trends toward increased 30-day events with decreasing levels of experience (8,21). A recent subset analysis showed operator experience to be a significant factor in the SPACE study (22).
An important element of the CREST lead-in study was availability of an accurate classification of death, major and minor strokes, and MI in the 30-day periprocedural period. MIs complicating the stenting procedure increased with age and added approximately 1% to the composite endpoint in the cohorts of patients aged 70 to 79 years and those aged 80 years or older. Similarly, death rates (including fatal strokes) increased with age but were stable, with an overall 1% contribution to the composite complication rate for patients older than 60 years. The rate of major strokes increased with age, showing a marked increase in patients older than 75. The rate of minor stroke also increased with age, raising important questions concerning patient selection, technique, and operator experience when considering stenting in elderly patients (17). Future studies ideally should examine the interaction among age, aortic arch anatomy, vessel tortuosity, and vessel calcification.
Conclusions
Large multicenter randomized trials that compare technical procedures ideally include rigorous training and credentialing protocols. Careful selection, training, and lead-in registry analysis of operators pre-randomization are expected to ensure that the results of a randomized trial are applicable to the quality standards expected in the medical community. In the CREST lead-in study, which included both conventional-risk and higher-risk symptomatic and asymptomatic patients, outcomes achieved by the carotid interventionalists were comparable to those reported in previous randomized carotid surgery trials. The large numbers of mid- to high-level operators in this study should ensure that the randomized study findings are generalizable to the broader community. The potential impact of patient and operator characteristics on the outcomes in CREST and in other randomized carotid intervention trials will merit careful analysis.
Acknowledgments
Sources of Funding
This study was supported by the National Institutes of Health, National Institute of Neurological Disorders of Stroke grant number RO1-NS38384. Industry support was provided by Abbott Vascular Solutions (formerly Guidant Endovascular Solutions).
Abbreviations
- ACAS
Asymptomatic Carotid Atherosclerosis Study
- CAPTURE
Carotid Acculink/Accunet Post Approval Trial to Uncover Rare Events
- CAS
carotid artery stenting
- CEA
carotid endarterectomy
- CEC
Clinical Events Committee
- CI
confidence interval
- CREST
Carotid Revascularization Endarterectomy vs Stenting Trial
- DSMB
Data Safety Monitoring Board
- ECG
electrocardiographic, electrocardiogram
- EVA-3S
Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis
- EXACT
Emboshield and Xact Post Approval Carotid Stent Trial
- IMC
Interventional Management Committee
- MI
myocardial infarction
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- NIH
National Institutes of Health
- OR
odds ratio
- SPACE
Stent-Protected Angioplasty vs Carotid Endarterectomy (study)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration: URL: www.ClinicalTrials.gov. Unique identifier: NCT00004732
Conflict of interest: none.
Addendum
CREST Interventional Management Committee Membership
Gary S. Roubin, MD, PhD, Committee Co-Chair, Interventional Cardiology
Robert D. Ferguson, MD, Committee Co-Chair, Interventional Neuroradiology
Jonathan Goldstein, MD, Interventional Cardiology
William A. Gray, MD, Interventional Cardiology
Robert W. Hobson II, MD (1999–2008), Vascular Surgery
L. Nelson Hopkins, MD, Neurosurgery
William Morrish, MD, Interventional Neuroradiology
Barry T. Katzen, MD, Interventional Radiology
Kenneth Rosenfield, MD, Interventional Cardiology
Thomas G. Brott, MD, Ex-Officio, Neurology
Elie Y. Chakhtoura, MD, Ex-Officio, Interventional Cardiology
Disclosures
The authors believe they have no relevant disclosures
References
- 1.North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22(6):711–720. doi: 10.1161/01.str.22.6.711. [DOI] [PubMed] [Google Scholar]
- 2.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421–1428. [PubMed] [Google Scholar]
- 3.Hobson RW, 2nd, Howard VJ, Roubin GS, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40(6):1106–1111. doi: 10.1016/j.jvs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Moore WS, Vescera CL, Robertson JT, et al. Selection process for surgeons in the Asymptomatic Carotid Atherosclerosis Study. Stroke. 1991;22(11):1353–1357. doi: 10.1161/01.str.22.11.1353. [DOI] [PubMed] [Google Scholar]
- 5.Moore WS, Young B, Baker WH, et al. Surgical results: a justification of the surgeon selection process for the ACAS trial. J Vasc Surg. 1996;23(2):323–328. doi: 10.1016/s0741-5214(96)70277-x. [DOI] [PubMed] [Google Scholar]
- 6.Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44(2):258–268. doi: 10.1016/j.jvs.2006.03.044. Erratum in: J Vasc Surg. 2007;45(1):226. [DOI] [PubMed] [Google Scholar]
- 7.Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69(3):341–348. doi: 10.1002/ccd.21050. [DOI] [PubMed] [Google Scholar]
- 8.Howard VJ, Voeks JH, Lutsep HL, et al. Does sex matter? Thirty-day stroke and death rates after carotid artery stenting in women versus men: results from the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) lead-in phase. Stroke. 2009;40(4):1140–1147. doi: 10.1161/STROKEAHA.108.541847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliasziw M, Smith RF, Singh N, et al. Further comments on the measurement of carotid stenosis from angiograms. Stroke. 1994;25(12):2445–2449. doi: 10.1161/01.str.25.12.2445. [DOI] [PubMed] [Google Scholar]
- 10.Rautharju PM, Calhoun HP, Chaitman BR. NOVACODE serial ECG classification system for clinical trials and epidemiologic studies. J Electrocardiol. 1992;24 Suppl:179–187. doi: 10.1016/s0022-0736(10)80041-x. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Hobson RW., 2nd CREST (Carotid Revascularization Endarterectomy versus Stent Trial): background, design, and current status. Semin Vasc Surg. 2000;13(2):139–143. [PubMed] [Google Scholar]
- 13.Mas JL, Chatellier G, Beyssen B, et al. Carotid angioplasty and stenting with and without cerebral protection: clinical alert from the Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial. Stroke. 2004;35(1):e18–e20. doi: 10.1161/01.STR.0000106913.33940.DD. [DOI] [PubMed] [Google Scholar]
- 14.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 15.Ringleb PA, Allenberg J, Bruckmann H, et al. 30 Day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368(9543):1239–1247. doi: 10.1016/S0140-6736(06)69122-8. Erratum in: Lancet. 2006;368(9543):1238. [DOI] [PubMed] [Google Scholar]
- 16.Stingele R, Berger J, Alfke K, et al. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol. 2008;7(3):216–222. doi: 10.1016/S1474-4422(08)70024-3. [DOI] [PubMed] [Google Scholar]
- 17.Chiam PT, Roubin GS, Iyer SS, et al. Carotid artery stenting in elderly patients: importance of case selection. Catheter Cardiovasc Interv. 2008;72(3):318–324. doi: 10.1002/ccd.21620. [DOI] [PubMed] [Google Scholar]
- 18.Henry M, Henry I, Polydorou A, et al. Carotid angioplasty and stenting in octogenarians: is it safe? Catheter Cardiovasc Interv. 2008;72(3):309–317. doi: 10.1002/ccd.21574. [DOI] [PubMed] [Google Scholar]
- 19.Velez CA, White CJ, Reilly JP, et al. Carotid artery stent placement is safe in the very elderly (> or =80 years) Catheter Cardiovasc Interv. 2008;72(3):303–308. doi: 10.1002/ccd.21635. [DOI] [PubMed] [Google Scholar]
- 20.Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc Interv. 2007;70(7):1025–1033. doi: 10.1002/ccd.21359. [DOI] [PubMed] [Google Scholar]
- 21.Wholey MH, Barbato JE, Al-Khoury GE. Treatment of asymptomatic carotid disease with stenting: pro. Semin Vasc Surg. 2008;21(2):95–99. doi: 10.1053/j.semvascsurg.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Fiehler J, Jansen O, Berger J, et al. Differences in complication rates among the centres in the SPACE study. Neuroradiology. 2008;50(12):1049–1053. doi: 10.1007/s00234-008-0459-6. [DOI] [PubMed] [Google Scholar]