Abstract
Objective
The general goal of this study was to advance our understanding of Type 2 diabetes (T2D)-cognition relationships in older adults by linking and testing comprehensive sets of potential moderators, potential mediators, and multiple cognitive outcomes.
Method
We identified in the literature 13 health-related (but T2D-distal) potential covariates, representing four informal domains (i.e., biological vitality, personal affect, subjective health, lifestyle activities). Cross-sectional data from the Victoria Longitudinal Study (age range = 53-90 years; n = 41 T2D and n = 458 control participants) were used. We first examined whether any of the 13 potential covariates influenced T2D-cognition associations, as measured by a comprehensive neuropsychological battery (15 measures). Next, using standard regression-based moderator and mediator analyses, we systematically tested whether the identified covariates would significantly alter observed T2D-cognition relationships.
Results
Six potential covariates were found to be sensitive to T2D associations with performance on seven cognitive measures. Three factors (systolic blood pressure, gait-balance composite, subjective health) were significant mediators. Each mediated multiple cognitive outcomes, especially measures of neurocognitive speed, executive functioning, and episodic memory.
Conclusions
Our findings offer a relatively comprehensive perspective of T2D-related cognitive deficits, comorbidities, and modulating influences. The implications for future research reach across several fields of study and application. These include (a) neuropsychological research on neural and biological bases of T2D-related cognitive decline, (b) clinical research on intervention and treatment strategies, and (c) larger-scale longitudinal studies examining the potential multilateral and dynamic relationships among T2D status, related comorbidities, and cognitive outcomes.
Keywords: Aging, Cognition, Type 2 Diabetes, Mediator, Moderator
The prevalence of Type 2 diabetes (T2D) is increasing among North American older adults (e.g., National Institute of Diabetes and Digestive and Kidney Diseases, 2008; Votey & Peters, 2009; Wild, Roglic, Green, Sicree, & King, 2004). Although not a neurological disease, T2D is linked to a heightened risk of cognitive deficits (e.g., Awad, Gagnon, & Messier, 2004) and dementia (e.g., Arvanitakis, Wilson, Bienias, Evans, & Bennett, 2004; Xu, Qiu, Wahlin, Winblad, & Fratiglioni, 2004). T2D is conceptualized as a disease cluster with continua of multiple non-specific etiological causes or processes, but with aging and several well-established generic risk factors (e.g., obesity, low physical activity, and genetic predisposition; The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 2003). The neurobiological mechanisms through which diabetes may exacerbate cognitive deficits of normal aging are not yet known (Nilsson & Wahlin, 2009). Working theories identify level and variability in insulin or blood glucose as partly responsible for deleterious vascular effects in key regions of the brain (e.g., hippocampus and cerebral cortex; Gispen & Biessels, 2000; Messier, 2005), all of which may have consequences in core aspects of cognition. Potential mechanisms through which T2D may affect cognitive performance are thought to be (a) indirect, gradual, and cumulative, (b) susceptible to modification by a set of proximal (diabetes-related) biological conditions, or (c) subject to qualification by (more distal) comorbidities or other factors (Nilsson & Wahlin, 2009).
Inconsistencies and qualifications in the T2D-cognition literature are notable (e.g., Nilsson, 2006; Okereke et al., 2008). The patterns are mixed for some cognitive domains, such as episodic memory, fluency, and global cognition (e.g., Arvanitakis, Wilson, & Bennett, 2006; Hassing et al., 2003; Nilsson, Fastbom, & Wahlin, 2002). The patterns for other domains are uncontradicted but require replication (e.g., neurocognitive speed, executive functioning; Fischer, de Frias, Yeung, & Dixon, 2009; Yeung, Fischer, & Dixon, 2009). Further research will benefit from comparative testing across a wide range of cognitive domains and samples, but which also explore key potential covariates and comorbidities that are associated with the inconsistencies (Manschot et al., 2006; Strachan, Frier, & Deary, 1997; Yeung et al., 2009).
Several methodological issues are among the reasons for inconsistencies and qualifications in published results. Two of these issues are of direct concern to the present study. First, the optimal multi-faceted data set for examining cognitive effects of T2D may not yet exist (Strachan et al., 1997). On the one hand, some epidemiological studies excel in T2D-related biological indicators (e.g., blood glucose level, HbA1c), markers of the T2D disease cluster (e.g., insulin signalling and utilization, glucose tolerance), diagnostic criteria, and large well-characterized samples. However, some relevant cognitive neuropsychological domains may be under-represented or imprecisely measured, and the cognitive effects of T2D may be domain-specific or they may emerge selectively in specific temporal patterns. The breadth or efficiency of the neuropsychological test battery is an important consideration (e.g., Yeung et al., 2009). On the other hand, ongoing neuropsychological studies of T2D and aging may offer theoretically and clinically comprehensive coverage of well-measured cognitive domains, but they may be correspondingly weaker in tapping the basic neurobiological and disease severity continua. Arguably, the rougher biological brushstrokes may limit the extent to which theoretically representative and well-measured neuropsychological performance can be linked to basic biological disease patterns (Nilsson & Wahlin, 2009). Nevertheless, progress can be made by balancing and merging different methodological strengths and weaknesses.
A second methodological issue results from the confluence of three important T2D-cognition observations. First, because T2D and cognition are multidimensional clusters of phenomena, the underlying biological mechanisms linking them are indirect and multidirectional. Second, the cognitive effects of T2D may be initially small in magnitude, restricted in range, and in early cases masked by normal aging-related decline. Third, T2D-cognition relationships may be qualified by multiple conditions, ranging from proximal comorbidities (disease-related biological conditions) to distal covariates (contextual, personal, or neurocognitive factors). In sum, the detection of T2D-related cognitive deficits may be affected by unmodified, uncontrolled, or unmeasured biological, health, fitness, affective-psychological, or lifestyle variables. For example, T2D-related cognitive deficits in older adults may be exacerbated when coupled not only with proximal biological complications (e.g., impaired glucose tolerance, dyslipidemia; Nilsson & Wahlin, 2009; Ryan & Geckle, 2000) but also with more distal lifestyle or health characteristics (e.g., clinical depression or poor physical fitness; Stewart & Liolitsa, 1999). Regarding the latter, although relatively little T2D-cognition research has included markers of these more distal factors, their potential importance to the expression and detection of diabetes-cognition relationships in community-dwelling older adults has been noted (Coates & Rae, 2006; Hendrickx, McEwen, & van der Ouderaa, 2005). These distal (i.e., not specifically disease-related) covariates may be potential moderators or mediators of T2D effects on cognition.
Potential Covariates of T2D-Cognition Relationships
In neuropsychological studies of T2D-cognition relationships, the influences of some potential distal covariates are controlled either statistically or through exclusionary criteria (e.g., dementia, cardio/cerebrovascular conditions). Perhaps the most common covariate is blood pressure (e.g. Elias et al., 1997; Hassing, et al., 2004), due to its relationship with vascular health. Specifically, when T2D presents comorbid with systolic hypertension, the risk associated with cognitive decline or dementia is increased (Hassing, et al., 2004; Xu et al., 2004), although its function at non-clinical levels is not clear (Fontbonne, Berr, Ducimetiere, & Alperovitch, 2001; Gregg et. al., 2000; Yeung et al., 2009). Other relatively distal factors may operate to influence both biological and cognitive T2D-related consequences (e.g., Grimley & Areosa, 2003). Although regularly mentioned in the literature, few have been systematically tested (Connell, 1991; Padgett, Mumford, Hynes, & Carter, 1988; Whittemore, Melkus, & Grey, 2005). We identified 13 such factors, sorting them informally into four conceptual domains: biological vitality (fitness), personal affect, subjective and functional health, and lifestyle activities. Lack of empirical precedent has led us to test each variable (marking a single domain) separately.
Biological vitality
Several markers of biological vitality have been identified as qualifying observed cognitive deficits in normal aging (Anstey & Smith, 1999; MacDonald, Dixon, Cohen, & Hazlitt, 2004), Alzheimer's disease (Buchman, Schneider, Wilson, Bienias, & Bennett, 2006), and possibly T2D (McEwen, 2006; Stewart & Liolitsa, 1999). The present markers representing this domain are body mass index (BMI), peak expiratory flow, grip strength, and gait and balance. When presenting with T2D, obesity (often measured as BMI) could affect cognitive performance through a variety of peripheral processes (e.g., sleep disturbance and apnoeic episodes; see McEwen, 2006; Stewart & Liolitsa, 1999). Peak expiratory flow (a pulmonary functioning indicator) and grip strength (a peripheral muscular measure) have been related to multiple domains of cognition among older adults, both concurrently (Wahlin, MacDonald, de Frias, Nilsson, & Dixon, 2006) and across a 12-year longitudinal period (MacDonald et al., 2004). Also related to fitness, markers of gait and balance are thought to reflect neurological compromises related to normal aging decline (particularly executive functioning), non-Alzheimer's dementia, and Alzheimer's disease (e.g., Holzer, Verghese, Xue, & Lipton, 2006; van Iersel, Kessels, Bloem, Verbeek, & Olde Rikkert, 2008; Verghese et al., 2002). Given that T2D may disrupt executive functioning (Fischer et al., 2009), a testable connection to markers of this domain is apparent.
Personal affect
This domain includes depression and well-being (e.g., Anderson, Freedland, Clouse, & Lustman, 2001; Hendrickx et al., 2005; Ryan & Geckle, 2000; Watari et al., 2006). Depression is both more prevalent and serious in older adults with T2D than in controls (Awad et al., 2004). Although the mechanism linking depression and diabetes is not known, their comorbidity may influence cognitive performance through exacerbated risk for (a) apathy or non-compliance of treatment regimens and (b) social indifference or isolation. Regarding well-being, more serious diabetes-related comorbidities have been linked to greater negative affect (e.g., hypertension: Jonas & Lando, 2000; acute hyperglycemia: Sommerfield, Deary, & Frier, 2004), and such combinations may lower cognitive performance. On the other hand, a positive sense of well-being may be linked to enhanced attention to health-promoting behaviors and health information (Moskowitz, Epel, & Acree, 2008), thus potentially supporting cognitive performance (Naor, Steingrüber, Westhoff, Schottenfeld-Naor, & Gries, 1997).
Subjective health
All three forms of subjective health are available in this study. These include (a) veridical beliefs about health status (e.g., overall health ratings), (b) personal or affective impressions of comparative health status (e.g., ratings requiring comparisons to others of same or different ages), and (c) subjective judgments of the functional effect of one's health on everyday life activities (Liang, Bennett, Whitelaw, & Maeda, 1991; Sargent-Cox, Anstey, & Luszcz, 2008). Overall, subjective health beliefs have performed as predictors and covariates of cognition in relatively healthy older adults (e.g., Wahlin et al., 2006) and are typically reported as background or control variables in neuropsychological studies of aging. Regarding diabetes, older adults with T2D have produced lower self-ratings of health than controls (Connell, 1991; Fischer et al., 2009). Moreover, diabetes severity and subsequent health outcomes (e.g., death, Benjamins, Hummer, Eberstein, & Nam, 2004; depression, Sargent-Cox et al., 2008) have been associated with both lower subjective health and poorer everyday instrumental activities (Reynolds & Silverstein, 2003). Arguably, the diagnosis and continuing management demands of T2D could differentially affect overall subjective health among older adults. If so, subjective health may operate to moderate or mediate T2D-cognition relationships.
Lifestyle activities
Engagement in a cluster of everyday lifestyle activities (including social, cognitive, physical) may provide sufficient cognitive reserve to delay normal aging-related decline or even diagnoses of dementia (e.g., Stern, 2007). Regarding T2D, only indirect clinical evidence has appeared. For example, clinicians have noted that the success of T2D treatment regimens is enhanced when patients are socially engaged (Ciechanowski, Katon, & Russo, 2005; Connell, Fisher, & Houston, 1992; Ford, Tilley, & McDonald, 1998) or physically active (Frier, Yang, & Taylor, 2006). Maintaining or enhancing social engagement or support are often components of diabetes interventions and evaluation studies, for they are thought to facilitate treatment effects, thereby leading to overall better disease outcomes (e.g., Ingram, Gallengos, & Elenes, 2005; Naor et al., 1997; Taylor, Keim, Sparrer, Van Delinder, & Parker, 2004) and perhaps prevention or postponement of later disability and death (Kuo, Raji, Peek, & Goodwin, 2004). Cognitive engagement may encourage maintenance of cognitive reserve (Stern, 2007) in typical older adults through such mechanisms as practice and compensation (e.g., Dixon, Garrett, & Bäckman, 2008). For T2D patients, cognitive engagement (e.g., educational intervention) may enhance diabetes management (Padgett et al., 1988). Specific diabetes treatment-related biological mechanisms potentially supporting cognitive performance (e.g., triglyceride levels, Perlmuter et al., 1988; glycemic control, Kanaya, Barrett-Connor, Gildengorin, & Yaffe, 2004) are unclear, but factors that affect T2D biology may also affect cognitive reserve and neuropsychological performance (Grimley & Areosa, 2003). Overall, the loss of lifestyle engagement in the everyday lives of T2D adults may reduce the effectiveness of disease treatments, leading to vulnerabilities in cognitive health.
The Present Study
Although the growing attention to the potential role of distal covariates in modulating T2D-cognition relationships testifies to an important methodological concern about uncontrolled sources of variance in clinical studies, it does not delineate whether the potential covariates operate in ways that are consistent with moderation or mediation models (Baron & Kenny, 1986). Whereas a moderator variable would affect the direction or strength of the T2D-cognition relationship, a mediator variable would directly account for some or all of the observed relationship. Regression-based moderation and mediation analyses (Baron & Kenny, 1986) have made significant contributions to related literatures, including research on neuropsychological populations such as aging and disease groups (e.g., Thornton, Deria, Gelb, Shapiro, & Hill, 2007; Wahlin, 2004). For example, a recent study determined that two of the present candidate covariate domains (i.e., subjective health and biological vitality) selectively qualified age and gender effects in cognitive performance among typically aging adults (Wahlin et al., 2006). To our knowledge, the T2D-cognition relationship has not been systematically studied in terms of moderators or mediators, although reviewers have argued for the importance of doing so.
The Victoria Longitudinal Study (VLS; Dixon & de Frias, 2004) is well-positioned to contribute to the investigation of potential moderators and mediators in T2D-cognition relationships. In two previous VLS studies, we (a) applied all recommended exclusionary criteria and statistically controlled for systolic blood pressure, (b) examined a multidimensional spectrum of cognitive neuropsychological tests, (c) observed robust diabetes-related deficits in specific measures of cognitive speed and speed-intensive executive functioning, and (d) replicated cross-sectional results (Yeung et al., 2009) and longitudinally (3-year interval; Fischer et al., 2009). For the present study, we returned to our original cross-sectional data set (Yeung et al., 2009), as it included our largest group of T2D participants and controls. We expanded the original data set substantially by (a) assembling markers of 13 commonly cited potential covariates (from the four reviewed domains) of T2D-cognition relationships, and (b) including additional participants in the control group (i.e., those who had been previously excluded for subclinical depression). Our goal was to test whether indicators from the domains of biological vitality, personal affect, subjective health, and lifestyle activities served as significant covariates (moderators or mediators) of T2D-related group differences in cognitive performance. In addition to replication of the basic patterns of group differences (cf. Yeung et al., 2009), the present study focused on three unique research questions. First, considering the broad range of candidate covariates from the four clusters noted above, are there significant covariates beyond the standard systolic blood pressure? Second, do any identified significant covariates moderate the effect of diabetes on any cognitive measure? Third, do any of the identified significant covariates mediate the relationship between diabetes and any cognitive measure? Our research plan benefits from a broad neuropsychological battery and a multifaceted set of potential covariates. However, it is exploratory in that specific predictions about which variables may be significant covariates (moderators or mediators) are not available in the literature. Therefore, we use univariate regression-based statistical procedures, standard in this area, to examine the three main research questions.
Method
Participants
The Victoria Longitudinal Study (VLS) is an ongoing multi-cohort study of initially healthy, community-dwelling, volunteer older adults from a medium-sized Canadian city who are tested on cognitive, neuropsychological, health, and physiological measures (for further information see Dixon & de Frias, 2004). We drew participants from VLS Sample 3, Wave 1 (baseline n = 577; age range 53 – 90 years; M age = 68.29 years; SD = 8.60). Initial exclusion removed n = 7 participants (due to missing data or type 1 diabetes status). Further T2D diagnostic information and exclusionary criteria were applied to the remaining pool (n = 570).
T2D classification
A multiple-stage classification procedure involved the sequential application of strict criteria (Yeung et al., 2009). Inclusion into the final T2D group required that participants meet all of the following conditions: (a) concurrent self-report of both formal diabetes diagnosis (by a physician) and a corresponding severity rating, (b) concurrent report of T2D onset over the age of 31, (c) concurrent report of specific method of treatment (i.e., oral medication, insulin, diet and exercise, no control, or any combination), (d) concurrent presence of objective prescription and non-prescription medications for participants who reported this form of treatment, and (e) three-year follow-up validity information, including complete and matching confirmation of the continuing presence of these criteria. Additional biomedical information pertaining to diabetes diagnosis (e.g., blood glucose levels or HbA1c levels) is unavailable for the VLS, but the present classification procedures have been documented and exceed the standards of self-reports (e.g., Arvanitakis, Wilson, Li, Aggarwal & Bennett, 2006; Gregg et al., 2000; Luchsinger, Tang, Stern, Shea, & Mayeux, 2001; Nilsson, 2006). As a result of the concurrent criteria (a-d above), n = 48 were identified as potential T2D participants. After the 3-year follow-up validity check (e above), n = 4 potential T2D participants were removed. This left n = 44 confirmed T2D participants, and n = 522 in the provisional control group.
Exclusionary criteria
The remaining participants in both groups were evaluated on three common exclusionary criteria. First, we confirmed that no participants had been previously diagnosed with Alzheimer's disease or vascular dementia. Second, participants scoring less than 26 on the Mini-Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, 1975) were removed (diabetes n = 0, control n = 16). Third, based on the VLS intake health inventory, we inspected the following clusters of potential comorbid diseases: (a) neurological conditions (i.e., self-reported stroke, Parkinson's disease, epilepsy, and head injury), (b) cardiovascular disease (i.e., myocardial infarction, unstable angina, hyper/hypotension, and atherosclerosis), (c) other related health conditions (i.e., spinal and back conditions, thyroid complications), and (d) psychiatric/adjustment conditions (i.e., alcohol or drug dependence, antipsychotic medication). As in previous studies, we treated the two groups slightly differently (e.g., Yeung et al., 2009). Any T2D participant self-reporting moderate conditions for any of the four clusters of health comorbidities was selected for individualized follow-up cognitive assessment. By a priori standard, we excluded any T2D participant who both indicated a moderate comorbid condition and scored 1 SD (or more) below the diabetes group mean on any test in a standard cognitive reference test battery (i.e., VLS word recall, story memory, vocabulary, simple reaction time). We excluded n = 3 T2D participants on this basis. The health exclusionary procedure for the control group was straightforward (presence of any of the comorbidity criteria), and resulted in n = 46 exclusions. Because of our interest in depressive affect as a potential covariate, we retained n = 36 control group participants who had either reported depression or used anti-depression medication, but who were excluded in the previous study (Yeung et al. 2009). In addition, n = 2 control participants were removed due to missing or extreme values on speed tests.
Final group characteristics
Characteristics of the final T2D group (n = 41; age range = 55 to 81 years) and control group (n = 458; age range = 53 to 90 years) are shown in Table 1. The two groups were similar in age, education, gender proportions, as well as marital and dwelling status. No group differences were found for global cognition, visual acuity (Close Vision Task, Snellen Fractions), or audio acuity (dB).
Table 1. Demographic Characteristics of Participants in the Diabetes and Control Groups.
| Diabetes (n = 41) |
Controls (n = 458) |
|
|---|---|---|
| Age | M = 68.59 (SD = 7.16) | M = 67.50 (SD = 8.45) |
| Range | 55 – 81 | 53 – 90 |
| Gender (% female) | 56.1 | 70.7 |
| Education | M = 15.12 (SD = 3.44) | M = 15.27 (SD = 2.91) |
| MMSE | M = 28.78 (SD = 1.08) | M = 28.86 (SD = 1.06) |
| Marital status (% married) | 56.1 | 60.7 |
| Dwelling status (%) | ||
| Live alone | 31.7 | 35.2 |
| Live with at least one other | 68.3 | 64.7 |
| Perceived audition | M = 2.48 (SD = .88) | M = 1.98 (SD = .91) |
| Perceived vision | M = 2.34 (SD = .66) | M = 2.17 (SD = .76) |
| Tobacco use (%) | ||
| Yes | 4.9 | 4.8 |
| Previously | 53.7 | 53.1 |
| Never | 41.5 | 42.1 |
| Alcohol consumption (%) | ||
| Yes | 61.0 | 88.6 |
| Previously | 24.4 | 4.6 |
| Never | 14.6 | 6.8 |
| Diabetes duration | M = 8.29 (SD = 7.27) | - |
| Diabetes severity (%) | ||
| Mild | 53.7 | - |
| Moderate | 43.9 | - |
| Serious | 2.4 | - |
| Depression (% reporting) all levels | 31.6 | 21.0 |
| Reported medication use (%) | ||
| Anti-diabetic | 68.3 | - |
| Antidepressant | 7.3 | 7.2 |
| Antihypertensive | 51.2 | 18.0 |
Note. Age, age range, diabetes duration, and education are presented in years. Perceived audition and perceived vision are self-rated relative to perfect state based on a scale from 1 – 5 (1 = very good, 5 = very poor)
Cognitive Tests
We began with the same 15 cognitive measures used in our previous diabetes studies (e.g., Fischer et al., 2009). All measures and all factorially-based composite variables are fully described elsewhere (e.g., Dixon & de Frias, 2004; Hultsch, Hertzog, Dixon, & Small, 1998).
Episodic memory
Two VLS episodic memory tasks were combined to create one composite measure (Dixon et al., 2004). Specifically, the standard VLS word recall task used the average number of words recalled immediately from two lists (each 30 words, with six words from five categories). The VLS story recall task used the proportion of gist recall after reading two standard structurally equivalent stories (about 300 words, 60 propositions, in 24 sentences). The scores for each task were converted to z-scores and averaged for a composite episodic memory indicator. In addition, two typical scores from the Rey Auditory Verbal Learning Test (RAVLT; Vakil & Blachstein, 1993) were used. By standardized convention, the scores for the RAVLT Trial B (acquisition) and Trial A6 (retention) were used (e.g., Yeung et al., 2009).
Semantic memory
The vocabulary test measured total number of correct responses on a 54-question multiple-choice recognition vocabulary test from the Educational Testing Service (ETS) kit of factor-referenced tests (Ekstrom, French, Harman, & Dermen, 1976). Fact recall was measured using the average score on two different 40-item tests of general information (e.g., from history, arts, sports) derived from a normed battery (Nelson & Narens, 1980).
Verbal fluency
We measured fluency using three subtests of the VLS Verbal Fluency task: Opposites, Figures of speech, and Similarities (Hultsch et al., 1998). Participants wrote as many correct words as possible within a given time. Raw scores for each subtest were recorded.
Executive functioning
Four tests of executive functioning have been normed, validated and documented (e.g., Bielak, Mansueti, Strauss, & Dixon, 2006; de Frias, Dixon, & Strauss, 2006, 2009). Two tests reflected inhibition: (a) the Hayling Sentence Completion Test measured initiation speed (Section A) and response suppression (Section B) in finding suitable words to complete a series of sentences rapidly (Burgess & Shallice, 1997), and (b) the Stroop Test (Regard, 1981) required participants to ignore the automatic response of reading a printed word (attending to verbal content) and instead name the color of ink in which it is printed (interference). Two tests measured shifting: (a) the Brixton Spatial Anticipation Test, in which participants deduced simple and changing patterns, measured their ability to abstract logical rules (Andrés & Van der Linden, 2000), and (b) the Color Trails Test (Part 2) (CTT-2; D'Elia, Satz, Uchiyama, & White, 1996), which minimizes the influence of language.
Neurocognitive speed
From the five standard VLS speed tests (Dixon et al., 2007; Hultsch et al., 1998), we created two two-indicator composite variables and one single-measure variable. The reaction time composite was comprised of two reaction time tests (using summed average standardized latency scores): (a) Simple Reaction Time (SRT) test measured the average latency of 50 trials and (b) Four-Choice Reaction Time (CRT4) measured the average latency across 20 trials. The semantic speed composite variable was created (using summed average standardized latency scores): (a) Lexical Decision task involved the average of 60 trials wherein participants indicated whether a string of letters formed a plausible word, and (b) Sentence Verification task required participants to judge whether sentences were meaningful or not. The fifth test, the Digit Symbol Substitution Test (DSS; Wechsler, 1991) measured switching and perceptual speed. Participants had 90 seconds to match symbols and numbers in test boxes. The score was the number of correctly transcribed items.
Candidate Covariate Variables
Based on our review, we assembled 13 measures representing four domains of potential moderators or mediators of T2D-cognition relationships in older adults.
Personal affect
We used three measures: (a) the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977), and (b) the Bradburn Affect Balance Scale (ABS) positive and negative affect scales (Bradburn, 1969). For the CES-D participants indicated (on a 4-point Likert scale, 20 items, range = 0-60) how they felt in the last week. Higher scores indicated more depressive symptoms. For ABS well-being, participants indicated whether they had felt 10 emotions over the past month, including five positive (e.g., proud) and five negative (e.g., upset). Each summed score was used (Maitland, Dixon, Hultsch, & Hertzog, 2001).
Subjective health
Three measures combined for a composite variable (Wahlin et al., 2006). For two traditional measures varying in point of reference, participants rated their health on a 5-point Likert scale (1 = very good, 5 = poor) relative to (a) a perfect state of health, and (b) their age-peers. Functional health was assessed with a 7-item instrument reflecting whether health changes in the last three years had affected the ability to do everyday activities (e.g., chores around the house). A 6-point scale (1 = gave up activity, 6 = improved activity) was used. The subjective health composite variable required (a) reverse-coding the functional health measure, (b) converting raw scores to z-scores, and (c) computing participant means.
Biological vitality (fitness)
Six standardized measures were used to assess participants' physical fitness (e.g., MacDonald et al., 2004). Body mass index (BMI; kg/m2) was calculated from baseline measurements of weight and height. Peak expiratory flow (L/minute) was measured using a MiniWright Peak Flow Meter wherein participants exhaled as quickly and forcefully as possible into the meter (score was highest volume exhaled on three attempts). Grip strength (kg force) was measured for each hand using a Smedley hand dynamometer (score was best of two attempts per hand). Both mean systolic and diastolic blood pressure (mmHg) was calculated over eight readings across two testing sessions. Gait and balance were assessed using two timed tasks: (a) walk over a distance of 20 feet (10 feet each way, including turn), and (b) 360-degree turn requiring one complete circle in place (scores were mean latency (ms) for each task). As a result of high task similarity and between-test correlations, we converted performance to standardized z-scores and averaged to create a composite gait-balance index.
Lifestyle
We used the VLS Activities Lifestyle Questionnaire (VLS-ALQ; Hultsch, Hertzog, Small, & Dixon, 1999) to measure typical engagement over the past two years in three domains of everyday activity: (a) physical activities (4 items), (b) social engagement (7 items), and (c) cognitive activities (novel information processing scale, 27 items). The VLS-ALQ uses a 9-point scale reflecting frequency of involvement in each activity; higher scores indicated greater frequency of activity. Scores were summed and averaged across each domain separately.
Data Analyses
To test the three research questions, three sets of analyses were conducted. All statistical analyses were performed using SPSS version 17.0 statistical software. To facilitate replication checks and subsequent covariate analyses (cf. Yeung et al., 2009), we used nontransformed variables (tests and composites) for all analyses.
The goals of the first set of analyses were to (a) identify and select specific candidate covariate variables, and (b) reduce the number of covariate and cognitive variables (and statistical tests) required for the main moderator/mediator analyses. First, we selected (and excluded) covariates by a series of one-way ANOVAs in order to check baseline group (T2D status) differences, indicative of potential covariate status. We used univariate tests for three related reasons: (a) the purpose was to identify candidate covariates for later analyses and not to interpret group differences, (b) T2D status differences on most of these measures have not previously been systematically explored, and (c) information at the level of individual variables would be valuable for archival purposes. Accordingly, we selected for further analyses only those candidate covariate variables associated with significant (set at p < .05) group differences. The default exploratory expectation was for differences in favor of the control group. Second, we selected (and excluded) potential cognitive outcomes by conducting tests of simple group differences on cognitive performance. The purposes were to (a) follow-up (with some new composite variables and additional participants) our earlier results (Yeung et al., 2009), and (b) identify and select candidate cognitive measures for further analyses. The prediction was for differences in favor of the control group on speed and executive function. This selection process identified the most promising covariate and cognitive variables, and excluded the remainder.
The second set of analyses was conducted on only the selected covariate and cognitive variables. For the moderator analyses, we conducted hierarchical regressions (Pedhazur, 1982; Wahlin et al., 2006) to examine whether any of the covariates identified in the preliminary analyses moderated the effect of T2D status on the selected cognitive measures. The hierarchical analyses for each cognitive measure included three steps or blocks, each corresponding to entry of a single variable: (a) Block 1: T2D status; (b) Block 2: selected covariate; and (c) Block 3: T2D status × covariate interaction term. By convention, a significant (set at p < .01) interaction term (Block 3) was taken as indicative of a moderating effect.
For the third set of analyses we used established regression-based mediator analyses (Baron & Kenny, 1986; Kenny, 2008). As is standard, mediators were identified and tested using two steps (e.g., Wahlin et al., 2006). The first step was to partition variability with results reported as percentage attenuation. Two regression analyses varying only in order of entry were conducted. The first analysis had the following order: Block 1: T2D status, and Block 2: potential mediator [ΔR2 (A)]. The second had the opposite order: Block 1: potential mediator, and Block 2: T2D status [ΔR2 (B)]. The value, ΔR2 (A), corresponds to the amount of variability that is shared between T2D status and cognitive performance. The value, ΔR2 (B), corresponds to the amount of variability that is shared between T2D status and cognitive performance after the potential mediator variability has been removed. The percentage of variability accounted for by the potential mediator was calculated to produce the proportion of variability that is shared between the potential mediator variable and the cognitive measure. In the second step we tested the significance (set at p < .01) of the potential mediators, utilizing the Sobel test (Baron & Kenney, 1986; Preacher & Leonardelli, 2003). Three regression analyses were conducted for this test: (a) T2D status predicting cognitive performance, (b) T2D status predicting the potential mediator variable, and (c) T2D status and potential mediator variable entered together to predict cognitive performance. As is stipulated in the Sobel test procedure, if all three analyses were to be significant, the unstandardized regression coefficient (B) and the corresponding standard error (SE) for the regressions were tested to determine if there was a significant difference between the direct effect path (T2D status to cognitive performance) and the indirect effect path (T2D status working through the mediator variable to cognitive performance).
Results
We report results in three sections based on the three research questions.
Research Question 1a: Identification of Potential T2D–Cognition Covariates
From the pool of 13 candidate covariates we observed significant group differences for six (see Table 2, left column): (a) two from personal affect (depression, negative affect), (b) subjective health composite, and (c) three from biological vitality (BMI, systolic blood pressure, gait-balance composite). As expected, for each the control group displayed better mean outcomes than the T2D group (means are not presented in the table or interpreted substantively). By a priori plan, only these six significant covariates were retained for further analyses.
Table 2. Analysis of Variance for Potential Covariates and Neuropsychological Variables.
| Potential Covariate | df | F | Neuropsychological Measures | df | F |
|---|---|---|---|---|---|
| Depression | 489 | 3.90* | Episodic Memory Composite | 494 | 7.30** |
| Negative Affect | 497 | 5.47* | RAVLT Trial B | 497 | .02 |
| Positive Affect | 497 | 1.11 | RAVLT Trial A6 | 497 | .06 |
| Subjective Health Composite | 497 | 27.0*** | Vocabulary | 497 | .54 |
| BMI | 493 | 27.5*** | Fact Recall | 497 | .22 |
| Peak Expiratory | 474 | 0.88 | VLS Opposites | 495 | 1.36 |
| Grip Strength | 474 | 0.04 | VLS Figures of Speech | 495 | 1.45 |
| Systolic Blood Pressure | 483 | 16.5*** | VLS Similarities | 495 | 2.88 |
| Diastolic Blood Pressure | 483 | 2.80 | Hayling | 479 | 12.3*** |
| Gait-balance Composite | 493 | 8.57** | Stroop | 486 | 4.15* |
| ALQ physical activities | 491 | 3.69 | Brixton | 484 | 3.64 |
| ALQ social engagement | 490 | 1.59 | Color Trails Test 2 | 488 | 10.5** |
| ALQ novel information processing | 491 | .07 | Reaction Time Composite | 489 | 10.6** |
| Semantic Speed Composite | 489 | 19.8*** | |||
| Digit Symbol Substitution | 489 | 6.12* |
Note. All significant mean differences are in accordance with the expectation that the control group would perform (or display) better (or more) of the attribute. Means (and SDs) are not displayed, as they are not interpreted.
p < .05
p < .01.
Research Question 1b: Selection of Target Cognitive Variables
Table 2 (right column) presents the ANOVA results for the simple group comparisons on cognitive performance. As expected, group differences (a) favored the controls over the T2D patients and (b) were consistent with previous results in a similar data set (Yeung et al., 2009). Selected for further moderator-mediator analyses were these seven cognitive measures: (a) episodic memory composite, (b) two executive function (inhibition) tests (Stroop, Hayling), (c) one executive function (shifting) test (CTT-2), and (d) three neurocognitive speed measures (semantic speed composite, reaction time composite, perceptual speed). Again, by a priori stipulation, the means are not presented in the table or interpreted further.
Research Question 2: Testing and Identification of Moderator Effects
Blockwise hierarchical regression analyses were conducted only on the covariates and cognitive measures selected from the previous analyses (see Table 3 for significant results). As is standard in testing for moderator effects, we entered T2D status in Block 1, the covariate score in Block 2, and the interaction term in Block 3 (e.g., Wahlin et al., 2006). Overall, the Block 1 results confirm the unsurprising finding that T2D status is a significant predictor of performance for these tasks (they were selected on this basis). Block 2 results are less uniform but show that some covariates (especially systolic blood pressure, gait-balance, subjective health) are associated with cognitive performance after removing variance associated with T2D status. For present purposes, one key result is that only two of the interaction terms (Block 3) approached significance (p < .05), indicating that most candidate covariate variables did not robustly affect the strength of specific T2D-cognition associations. For archival purposes, we describe briefly the two covariates producing near-significant moderator effects. Notably, both were personal affect variables and both moderated a neurocognitive speed indicator. Depression moderated (p < .05) the T2D status effect on reaction time (ΔF = 6.2, p < .05). Follow-up within-group depression-speed correlations revealed opposite patterns (r = .16 for controls and r = -.20 for T2D group). Negative affect moderated (p < .05) the T2D status effect on semantic speed (ΔF = 6.5, p < .05); the within-group affect-speed correlations revealed opposite patterns (r = -.10 for control group and r = .25 for diabetes group). (Higher performance indicates slower speed.)
Table 3. Hierarchical Multiple Regressions Examining Interaction Effects Associated With Potential Moderators of Type 2 Diabetes-Cognition Relationships.
| Episodic Memory | Stroop | Hayling | Color Trails Test 2 | Semantic Speed | Reaction Time | Digit Symbol Substitution | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2Δ | FΔ | β | R2Δ | FΔ | β | R2Δ | FΔ | β | R2Δ | FΔ | β | R2Δ | FΔ | β | R2Δ | FΔ | β | R2Δ | FΔ | |
| Systolic Blood Pressure | |||||||||||||||||||||
| B1 | -.12 | .01 | 7.1** | .09 | .01 | 4.2* | -.16 | .03 | 12.2** | .15 | .02 | 10.4** | .20 | .04 | 19.6** | .15 | .02 | 10.7** | -.11 | .01 | 6.2* |
| B2 | -.12 | .01 | 6.9** | - | - | - | - | - | - | .12 | .01 | 6.7* | - | - | - | .13 | .02 | 8.0** | -.17 | .03 | 14.0** |
| B3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Body Mass Index | |||||||||||||||||||||
| B1 | -.12 | .01 | 7.1** | .10 | .01 | 4.5* | -.16 | .03 | 12.6** | .14 | .02 | 10.0** | .19 | .04 | 18.8** | .16 | .02 | 12.5** | -.11 | .01 | 6.1* |
| B2 | - | - | - | - | - | - | -.10 | .01 | 4.2* | - | - | - | - | - | - | - | - | - | - | - | - |
| B3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Gait-Balance Composite | |||||||||||||||||||||
| B1 | -.12 | .02 | 7.4** | .09 | .01 | 4.3* | -.16 | .03 | 12.1** | .15 | .02 | 10.6** | .20 | .04 | 20.6** | .15 | .02 | 10.9** | -.11 | .01 | 6.4* |
| B2 | -.24 | .06 | 29.7** | .12 | .01 | 6.8* | -.21 | .04 | 21.4** | .25 | .06 | 33.3** | .29 | .08 | 44.6** | .43 | .19 | 113.** | -.32 | .10 | 56.3** |
| B3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Subjective Health | |||||||||||||||||||||
| B1 | -.12 | .02 | 7.3** | .09 | .01 | 4.2* | -.16 | .03 | 12.3** | .14 | .02 | 10.5** | .20 | .04 | 19.8** | .14 | .02 | 10.6** | -.11 | .01 | 6.1* |
| B2 | -.14 | .02 | 9.1** | - | - | - | - | - | - | .13 | .02 | 8.2** | .16 | .02 | 12.0** | .18 | .03 | 15.0** | -.14 | .02 | 9.6** |
| B3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Depression | |||||||||||||||||||||
| B1 | -.11 | .01 | 5.8* | .10 | .01 | 4.6* | -.17 | .03 | 13.4** | .12 | .02 | 7.3** | .19 | .04 | 18.6** | .15 | .02 | 11.0** | -.11 | .01 | 6.0* |
| B2 | -.12 | .02 | 7.6** | - | - | - | - | - | - | .09 | .01 | 4.0* | .12 | .02 | 7.7** | .12 | .02 | 7.3** | - | - | - |
| B3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | -.22 | .01 | 6.2* | - | - | - |
| Negative Affect | |||||||||||||||||||||
| B1 | -.12 | .02 | 7.3** | .09 | .01 | 4.2* | -.16 | .03 | 12.3** | .14 | .02 | 10.5** | .20 | .04 | 19.8** | .14 | .02 | 10.6** | -.11 | .01 | 6.1* |
| B2 | - | - | - | - | - | - | - | - | - | - | - | - | -.05 | .00 | 1.4 | -.10 | .01 | 5.0* | .12 | .02 | 7.3** |
| B3 | - | - | - | - | - | - | - | - | - | - | - | - | .19 | .01 | 6.5* | - | - | - | - | - | - |
Note. B1 Block 1 = diabetes. B2 Block 2 = potential moderator. B3 Block 3 = diabetes × potential moderator variable.
p < .05.
p < .01 or p < .001.
Research Question 3: Testing and Identification of Mediator Effects
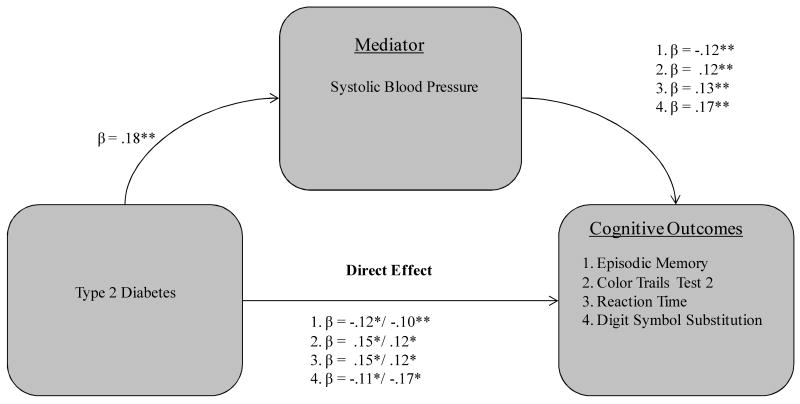
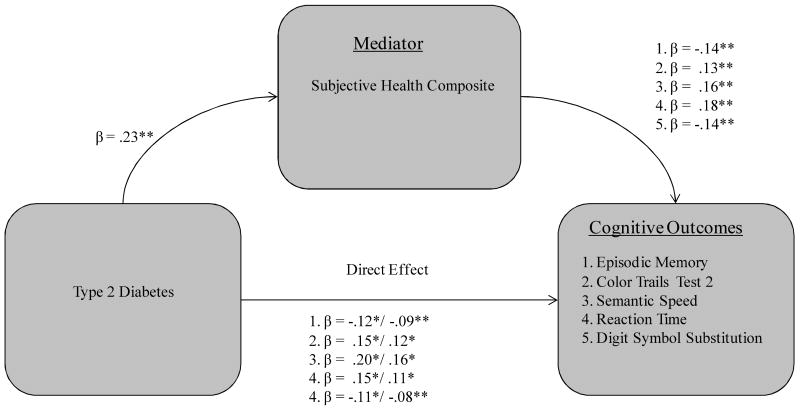
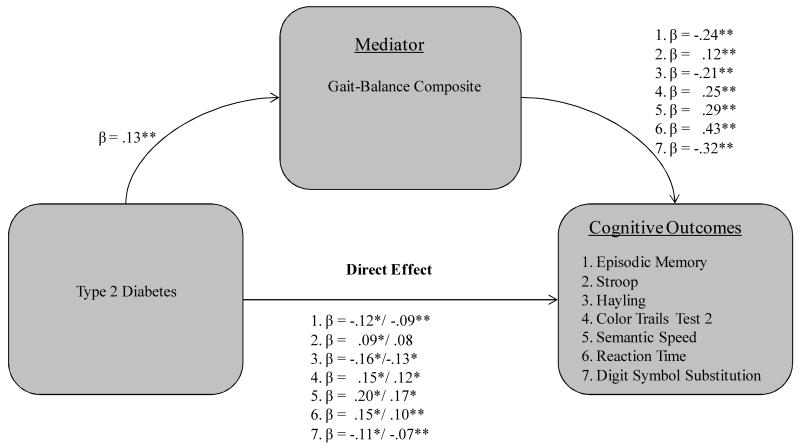
Three of the six covariates functioned as significant mediators in qualifying the T2D-cognition relationship, including two biological vitality markers (systolic blood pressure, gait-balance composite) and subjective health composite. First, systolic blood pressure significantly mediated the effect of T2D on four cognitive measures (see Fig. 1): (a) episodic memory (36% attenuation, Sobel test = -2.02, p < .05); (b) CTT-2 (29% attenuation, Sobel test = 2.17, p < .05); (c) reaction time (32% attenuation, Sobel test = 2.23, p < .05) and (d) DSS (46% attenuation, Sobel test = -2.78, p < .01). Second, the subjective health composite was a mediator for five cognitive measures (see Fig. 2): (a) episodic memory (47% attenuation, Sobel test = -2.61, p < .01); (b) CTT-2 (38% attenuation, Sobel test = 2.51, p < .05); (c) semantic speed (36% attenuation, Sobel test = 2.87, p < .01); (d) reaction time (48% attenuation, Sobel test = 3.12, p < .01); and (e) digit symbol substitution (50% attenuation, Sobel test = -2.67, p < .01). Third, the gait-balance composite was a significant mediator for all seven of the cognitive measures (see Fig. 3): (a) episodic memory (47% attenuation, Sobel test = -2.58, p < .01); (b) Stroop (33% attenuation, Sobel test = 1.95, p < .05); (c) Hayling (32% attenuation, Sobel test = -2.48, p < .05); (d) CTT-2 (38% attenuation, Sobel test = 2.61, p < .01); (e) semantic speed (34% attenuation, Sobel test = 2.68, p < .01); (f) reaction time (59% attenuation, Sobel test = 2.83, p < .01); and (g) DSS (62% attenuation, Sobel test = -2.73, p < .01).
Figure 1.
Systolic blood pressure as a mediator of four T2D-cognition relationships in older adults.
Note. Standardized coefficients after the slash indicate weight after inclusion of specified mediator. *p < .05. **p < .01 and p < .001.
Figure 2.
Subjective health composite as a mediator of five T2D-cognition relationships in older adults.
Note. Standardized coefficients after the slash indicate weight after inclusion of specified mediator. *p < .05. **p < .01 and p < .001.
Figure 3.
Gait-Balance composite as a mediator of seven T2D-cognition relationships in older adults.
Note. Standardized coefficients after the slash indicate weight after inclusion of specified mediator. *p < .05. **p < .01 and p < .001.
Discussion
The general goal of this study was to advance our understanding of T2D-cognition relationships in older adults by linking and testing comprehensive sets of potential moderators, potential mediators, and multiple cognitive outcomes. This study contributed (a) broad coverage of relevant cognitive domains, (b) multiple indicators of some of these domains, (c) measures of numerous potential distal covariates relevant to T2D-cognition relationships, and (d) systematic testing of moderator and mediator effects. Results for three research questions are summarized.
Research Question 1: Selection of Covariate and Cognitive Measures
In the first (covariate) phase, six of the 13 candidate covariates from three of the four conceptual domains (biological vitality, personal affect, subjective health, but not lifestyle) were associated with significant group differences, all in the direction of control participants displaying higher (or more advantageous) levels than T2D participants. The results confirmed the relevance of systolic blood pressure, a commonly applied covariate in the neuropsychological T2D-cognition literature. The remaining five significant covariates (i.e., BMI, gait-balance, depression, negative affect, subjective health) have been informally noted but not previously tested. Future T2D-cognition researchers may find it useful to further investigate the roles of these covariates. The current results did not support the conceptual and clinical observations that the domain of lifestyle activities (as measured in this study, i.e., social engagement, cognitive activity, physical activity) affected adjustment and outcome to T2D status. Conceivably, alternative measures of lifestyle activities or more clinically severe T2D cases could be explored (Stern, 2007). In the second (cognitive) phase, analyses showed that seven measures produced group differences in the expected direction: episodic memory, Hayling, Stroop, CTT-2, semantic speed, reaction time, and DSS. The effects closely replicated our own previous T2D research (Fischer et al., 2009; Yeung et al., 2009). One minor exception is the new episodic memory difference, which has been observed elsewhere (Nilsson, 2006) and was based here on a composite measure not tested previously. In sum, seven cognitive variables (eight excluded) and six covariate variables (seven excluded) were selected empirically for the main analyses.
Research Question 2: Moderator Effects?
In neuropsychological research, significant moderators are expected to affect the strength (not the direction) of the direct effect of the predictor (i.e., T2D status) on the criterion variable (i.e., cognition). Accordingly, we tested whether a potential moderator would either weaken or enhance the T2D-cognition association. Either pattern would be signalled by a significant interaction term in the regression (see Table 3). The first result (see Block 1) confirmed that T2D status was related to performance on these cognitive tests. The second result (see Block 2) is more novel, in that it shows that several covariates are associated with cognitive performance after removing variance attributable to T2D status. Most prominently indicated were systolic blood pressure, gait-balance, subjective health, and depression, all of which were related to performance on at least four of the cognitive tasks (as they can be in normal aging; Wahlin, 2004). On the cognitive side, the predominant effects were on speed-intensive tasks and the episodic memory composite. The Block 2 results support the recommendations that future research in T2D-cognition relationships (a) include a broader base of covariates from several distal domains of influence and (b) investigate alternative T2D-related indicators (e.g., HbA1C levels; Nilsson & Wahlin, 2009), cognitive indicators (e.g., speed and inhibition are under-represented in the literature), and even predictor-criterion arrangements.
The third result is that only two of the moderator-related interaction terms (see Block 3, Table 3) approached significance (p < .05; a priori set at p < .01). Therefore, the candidate covariate variables were related to cognition in this sample but they did not robustly affect the strength of specific T2D-cognition associations. For archival purposes, we comment briefly on these two moderators, both of which were from the personal affect category. Specifically, depression and negative affect tended to moderate the effects of T2D on speeded performance tasks. Regarding depression, follow-up correlations (depression-speed in each group) showed different patterns, with a generally expected (e.g., Bonin-Guillaume, Blin, & Hasbroucq, 2004) exacerbated slowing associated with greater depression scores for the older control participants, but the opposite pattern for the T2D participants. Future researchers may wish to examine in more clinical detail how the acknowledged complexities of depression in older adults with T2D (e.g., Hendrickx et al., 2005; Ludman et al., 2004; Lustman, & Clouse, 2007) may be associated with relatively preserved neurocognitive functioning as compared with typical older adults. Conceivably, diabetes-type depression is relatively autonomous from diabetes-related cognitive deficits (Brands et al., 2007), and perhaps especially for relatively early and managed cases as measured by relatively straightforward speed tests. Regarding negative affect, the T2D group showed a differential tendency for slower performance on semantic speed as levels of negative affect increased. Notably, negative affect may have complicating implications for cognition in older adults with T2D (e.g., Fisher et al., 2007; Kressin, Spiro III, & Skinner, 2000; Van der Does et al., 1996). For example, symptom perception in T2D is influenced not only by actual blood glucose levels, but also by cognitive and emotional responses, particularly negative affectivity (Van der Does et al., 1996). In sum, (a) two moderator results were not significant by the a priori criterion, (b) observed patterns were not robust across all other speed tests, (c) personal affect attributes were displayed at non-clinical levels, but (d) future research should explore in more clinical detail how affective attributes can play moderating T2D-cognition roles.
Research Question 3: Mediator Effects
As can be seen in Figures 1-3, the significant mediators of substantial T2D-cognition relationships were: systolic blood pressure, subjective health, and gait-balance. We explore, in turn, possible mechanisms linking T2D and cognition for each of these three mediators.
Systolic blood pressure
Systolic blood pressure is typically included as a covariate in T2D-cognition studies (e.g., Hassing et al., 2004), although it has not been previously tested as a moderator or mediator. We observed that systolic blood pressure attenuated the T2D-cognition relationship by approximately 30–50% for four cognitive measures (in three different domains): episodic memory composite, neurocognitive speed (reaction time, DSS), and executive function (shifting: CTT-2). This suggests that a connection between T2D and cognitive deficits may operate through processes represented by clinically relevant systolic blood pressure levels (e.g., Kumari, Brunner, & Fuhrer, 2000), and may be associated with T2D-related vascular disturbance. Systolic blood pressure has been related to cognitive decline in some T2D-related conditions (Waldstein, Giggey, Thayer, & Zonderman, 2005; Xu et al., 2004), with a recent report showing that episodic memory, neurocognitive speed and cognitive shifting are affected (van den Berg, Kloppenborg, Kessels, Kappelle, & Biessels, 2009). However, the modulating effects of hypertension on cognitive function in T2D have been observed under several conditions (e.g., Elias et al., 1997; Hassing et al., 2004; Manschot et al., 2007) but not uniformly (den Heijer et al., 2003; Kanaya et al., 2004). One possibility is that T2D and hypertension may be interacting parts of a larger metabolic syndrome, including hyperglycemia and insulin resistance. Notably, each of these is a risk factor for cerebrovascular damage, cortical and subcortical atrophy, and impaired cognition (Akisaki et al., 2006; den Heijer et al., 2003), especially when observed together (Kodl & Seaquist, 2008). Regarding continuous glucose-related conditions, both hyperglycemia and hypoglycemia (a common side effect of T2D) can contribute to neuro-vascular disturbance, which is also associated with subsequent and proportionate cognitive decline (Roriz-Filho et al., 2009). Regarding insulin resistance (and subsequent insulinimia), subsequent higher levels of inflammation can exacerbate macrovascular disease, which may contribute to cognitive decline among some adults with T2D. Overall, some vascular-related changes associated with T2D can lead to a variety of conditions known to be risk factors for cognitive dysfunction (Lu, Lin, & Kuo, 2009). Clearly, sub-clinical systolic blood pressure, hypertension, and associated vascular conditions merit further T2D-cognition research.
Subjective health
This composite variable was a significant mediator of T2D-cognition relationships, as demonstrated by significant attenuation (between 35 – 50%) of the direct effect. Fully five cognitive outcome measures were affected (see Fig. 2): (a) episodic memory, (b) three speed markers (semantic speed, reaction time, and DSS), and (c) executive function (shifting, CTT-2). By design, the subjective health represented a recommended broad range of the construct reflecting a personal summary of an individual's knowledge and beliefs about a variety of health-related aspects (e.g., Jylhä, Volpato, & Guralnik, 2006; Sargent-Cox et al., 2008). Among older adults strong measures of subjective health have had notable associations with objective health conditions, physician's ratings of health, psychological resources and attitudes, and social engagement or networks (Moor, Zimprich, Schmitt, & Kliegel, 2006; Sargent-Cox et al., 2008; Wahlin et al., 2006), as well as basic biological markers (e.g., high density lipoprotein cholesterol, white cell count; Goldman, Glei, & Chang, 2004; Tomten & Høstmark, 2007). Once diagnosed, T2D is a disease that obligates awareness along all of the dimensions of subjective health (Jylhä et al., 2006), including direct and indirect health information (e.g., chronicity, severity, prognosis) and everyday functional implications (e.g., health burden, management requirements, effects on everyday life). Even when managed in the context of available health care (e.g., the present VLS Canadian participants), the presence of T2D would be associated with (a) biological changes (e.g., increased insulin resistance), (b) constant T2D management requirements for self-care, emotion, and psychosocial stress (Lekander, Elofsson, Neve, Hansson, & Unden, 2004; Meyer et al., 2005), and (c) likely lower levels of subjective health (e.g., Bjorner et al., 1996; Svedberg, Gatz, & Pedersen, 2009; Undén et al., 2008).
In turn, subjective health has been related to cognitive performance among healthy older adults (e.g., Wahlin et al., 2006), with speculation that even stronger relationships might be found for more diverse populations with specific health conditions (Hultsch, Hammer, & Small, 1993). Regarding diabetes patients, subjective health measures recently predicted several important T2D-related complications, including cardiovascular and cerebrovascular events (e.g., myocardial infarctions, stroke; Hayes et al., 2008), which are known risk factors for cognitive deficits (e.g., Anstey, Mack, & von Sanden, 2006; Mansueti, de Frias, Bub, & Dixon, 2008; Wahlin, 2004). In addition to mechanisms associated with actual health conditions, subjective health may mediate T2D-cognition relationships through subjective-affective comorbidities. Specifically, T2D may exacerbate levels of psychosocial stress, depression, and (lower) health self-efficacy—all of which may negatively affect motivation for performance on cognitive tests (Mulsant, Ganguli, & Seaberg, 1997). With T2D, processes of interoception may detect inner biological stimuli of discomfort or nutritional deficits that could be associated with lower subjective health ratings and, by extension, cognitive performance (Jylhä et al., 2006). In sum, we found that subjective health (broadly measured) is an important consideration in understanding the extent and manner of T2D-cognition relationships. Although it is common in the neurospychology literature for single subjective health measures to be included in background participant information, future research may wish to enrich the assessment of subjective health and include new indices as covariates in assessing the effects of T2D on outcomes reflecting medical and cognitive health.
Gait-Balance
The gait-balance composite was a mediator of T2D-cognition relationships for all seven cognitive measures, spanning the domains of speed, executive functions, and episodic memory (see Fig. 3). Moreover, the attenuation effects were substantial, ranging between 32 and 62%. As foreshadowed in the review, the results are consistent with the possibility that T2D may operate on cognition partially through its intermediate effects on (a) a set of specific neural mechanisms (e.g., vestibular system) that are associated with decrements in both gait-balance and specific aspects of cognition or (b) mechanisms which diffusely affect the brain such that multiple overlapping areas associated with gait-balance and cognition are both affected. Although gait-balance has been conceptually linked to T2D and cognition (Hassing et al., 2004; Holzer et al., 2006), no previous studies have tested or reported that it serves a mediator role in the association. Therefore, the present discussion focuses on aging-related research linking T2D to gait-balance and then on research linking gait-balance to cognition.
With typical aging, walking can become a complex task requiring dynamic integration from sensory (e.g., exterioceptive senses such as touch and balance, as well as proprioceptive senses), neural, and cognitive modalities (Hausdorff, Yogev, Springer, Simon, & Giladi, 2005). In addition, awareness of the severe health risk of falls likely informs gait-balance behaviour in older adults. Moreover, with increases in individual vulnerabilities (e.g., gait-balance-related impairment, risk for reduced strength or mobility, vascular risk factors) or task complexities (e.g., cognitive load, competing resource demands), gait and balance performance may decrease further (Holzer et al., 2006; Lövdén, Schaefer, Pohlmeyer, & Lindenberg, 2008). T2D (and its common physical comorbidities) has been associated with elements of gait-balance performance, such as slower walking speed, lack of balance episodes, and increased falls (Gregg, & Brown, 2003). These T2D-related gait-balance deficits may be the result of central damages to the vestibular, somatic, and autonomous system as a result of: (a) vascular (e.g., microcirculation) changes associated with poor glycemic control (Petrofsky, Lee, & Bweir, 2005), (b) lesion location and presence of projections to hippocampus or cerebral cortex (Hanes & McCollum, 2006), or (c) interactive effects. The mechanisms of T2D-related postural and gait alterations are not firmly fixed in the literature, but some researchers have pointed to peripheral somatosensory neuropathy and subsequent sensitization of the vestibular system (Darlington, Erasmus, Nicholson, King, & Smith, 2000; Horak & Hlavacka, 2001; Petrofsky et al., 2005). In addition, the cognitive effects that might be expected from gait-balance decrements are multifactorial in that they are related to several potential cortical mechanisms and cognitive processes (Holzer et al., 2006). Specifically, neurocognitive speed, executive control, and episodic memory have been identified as key components of walking speed in older adults and are likely markers or correlates of gait-balance decrements (e.g., Holzer et al., 2006; van Iersel et al., 2008; Verghese et al., 2002). As illustrated in our results (see Fig. 3), these are indeed the effects we observe as outcomes of the gait-balance mediation of T2D-cognition relationships.
By design our gait and balance tasks were basic ones, not requiring any concurrent or competing physical or cognitive activity. For typical (and cognitively vulnerable) older adults, increments of cognitive and physical load during gait-balance tasks are associated with decrements in performance (e.g., Lövdén et al., 2008). The present tasks were designed to permit an external focus of attention, such that participants could fully monitor their movement operations. Given the present unique results with basic gait-balance tasks, future research with advanced techniques and more diverse samples may permit (a) identification of specific mechanisms being affected by T2D that, in turn, affect cognitive performance, and (b) evaluation of T2D-related foot conditions (e.g., diabetic foot) that could affect other samples.
Summary and Conclusion
The results of this study show that (a) multiple relatively distal covariates may influence T2D-cognition relationships, and (b) three of these covariates functioned as mediators of substantial T2D-cognition relationships. Specifically, measures of vascular health (e.g., systolic blood pressure), functional and subjective health, and gait-balance integrity play an important role in modulating the effects of T2D on cognition in older adults. To be sure, T2D-cognition relationships may also be affected by disease-proximal biological complications, such as glycemia, insulin resistance, and micro-infarcts (e.g., Manschot et al., 2007). Future research may be directed at further explicating the mechanisms within (and interactions among) candidate covariates from both relatively proximal and distal domains. Distal covariates may be especially relevant to understanding T2D-cognition relationships in earlier phases or in better-managed cases of T2D. A testable implication is that more disease-proximal biological covariates may become especially relevant in later phases, older age groups, or less-managed cases.
Several strengths and limitations of the present study should be noted. First, our design was cross-sectional and thus we are unable to establish temporal relationships. The VLS and other studies will eventually have multi-wave longitudinal data for investigations of moderators or mediators of changes in T2D-cognition relationships. Second, our volunteer sample included fairly well-educated Canadian older adults, most of whom benefit from a national health care system. Although our results are likely representative of a large and growing group of aging adults (e.g., western society boomers), they are not necessarily generalizable to all populations of older adults, including very old adults. Third, our T2D diagnosis procedures follow a strict and multi-faceted protocol (e.g., objective medication data), but the VLS does not currently have more precise biological measures (e.g., HbA1c) which would be especially useful in confirming that no controls are in undiagnosed or pre-clinical T2D conditions (Nilsson, 2006). The practical implications are relatively minor, as our control group size is substantial and a few such cases would only make our tests slightly more conservative. Fourth, although it would be preferable to have a larger number in our T2D group, it is within the range of other comparable neuropsychological studies and is sufficient for the analyses we conducted. In addition, the percentage of T2D participants in this sample is similar to the prevalence rate in Canada. Fifth, among the strengths of this study is the extensive VLS battery itself, which provided the bases for systematic tests of (a) a theoretically pertinent set of cognitive neuropsychological measures and (b) a set of candidate covariate markers well-matched to the literature. Further investigations using either the same or alternative operational definitions of candidate covariates are encouraged, especially as they may supplement or interact with select proximal (T2D-related) biological covariates as well as related neurobiological conditions (infarcts, white matter lesions; Manschot et al. 2006, 2007).
In sum, the present report assembled clinical observations, empirical results, and suggested research directions regarding T2D-cognition relationships in aging. Our findings offer a relatively comprehensive perspective of T2D-related cognitive deficits, comorbidities, and modulating influences. The implications for future research reach across several fields of study and application. These include neuropsychological research on neural bases of T2D-related cognitive decline, clinical research on intervention and treatment strategies, and larger-scale longitudinal epidemiological studies, all of which will help clarify the multilateral (and possibly dynamic) relationships and mechanisms of T2D, related comorbidities, and cognitive outcomes.
Acknowledgments
This research was supported by a grant from the U.S. National Institutes of Health (National Institute on Aging) to Roger A. Dixon (R37 AG008235). Dr. Dixon is also supported by the Canadian Research Chairs program. We appreciate the many important contributions of VLS participants and staff (notably Jill Friesen, Terry Perkins, and Sophie Yeung) to the present research. Further information about the VLS may be accessed via: http://www.ualberta.ca/∼clslab/index.html
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Akisaki T, Sakurai T, Takata T, Umegaki H, Araki A, Mizuno S, et al. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabletes intervention trial (J-EDIT) Diabetes/ Metabolism Research and Reviews. 2006;22:376–384. doi: 10.1002/dmrr.632. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Andrés P, Van der Linden M. Age-related differences in supervisory attentional system functions. Journal of Gerontology: Psychological Sciences. 2000;55B:P373–P380. doi: 10.1093/geronb/55.6.p373. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Mack HA, von Sanden C. The relationship between cognition and mortality in patients with stroke, coronary heart disease, or cancer. European Psychologist. 2006;11:182–195. [Google Scholar]
- Anstey KJ, Smith GA. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychology and Aging. 1999;14:605–618. doi: 10.1037//0882-7974.14.4.605. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Bennett DA. Diabetes mellitus, dementia, and cognitive function in older persons. Journal of Nutrition, Health, and Aging. 2006;10:287–291. [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Archives of Neurology. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Li Y, Aggarwal NT, Bennett DA. Diabetes and function in different cognitive systems in older individuals without dementia. Diabetes Care. 2006;29:560–565. doi: 10.2337/diacare.29.03.06.dc05-1901. [DOI] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. Journal of Clinical and Experimental Neuropsychology. 2004;26:1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Benjamins MR, Hummer RA, Eberstein IW, Nam CB. Self-reported health and adult mortality risk: An analysis of cause-specific mortality. Social Science & Medicine. 2004;59:1297–1306. doi: 10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Mansueti L, Strauss E, Dixon RA. Performance on the Hayling and Brixton tests in older adults: Norms and correlates. Archives of Clinical Neuropsychology. 2006;21:141–149. doi: 10.1016/j.acn.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bjorner JB, Söndergaard-Kristensen T, Orth-Gomer K, Tibblin G, Sullivan M, Westerholm P. Self-rated health: A useful concept in research, prevention and clinical medicine. Stockholm: Council for Planning and Coordination of Research; 1996. [Google Scholar]
- Bonin-Guillaume S, Blin O, Hasbroucq T. An additive factor analysis of the effect of depression on the reaction time of old patients. Acta Psychologica. 2004;117:1–11. doi: 10.1016/j.actpsy.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Bradburn NM. The structure of psychological well-being. Oxford, England: Aldine; 1969. [Google Scholar]
- Brands AMA, Biessels GJ, Kappelle LJ, de Haan EHF, de Valk HW, Algra A, et al. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: A comparative study. Dementia and Geriatric Cognitive Disorders. 2007;23:343–350. doi: 10.1159/000100980. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton tests. Thurston, Suffolk, England: Thames Valley Test Company; 1997. [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE. The association of depression and perceptions of interpersonal relationships in patients with diabetes. Journal of Psychosomatic Research. 2005;58:139–144. doi: 10.1016/j.jpsychores.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Coates V, Rae G. Commentary on Whittemore R, Melkus GD & Grey M (2005) Journal of Clinical Nursing. 2006;15:234–236. doi: 10.1111/j.1365-2702.2006.01167.x. [DOI] [PubMed] [Google Scholar]
- Connell CM. Psychosocial contexts of diabetes and older adulthood: Reciprocal effects. The Diabetes Educator. 1991;17:364–371. doi: 10.1177/014572179101700507. [DOI] [PubMed] [Google Scholar]
- Connell CM, Fisher EB, Houston CA. Relationships among social support, diabetes outcomes, and morale for older men and women. Journal of Aging and Health. 1992;4:77–100. [Google Scholar]
- Darlington CL, Erasmus J, Nicholson M, King J, Smith PF. Comparison of visual-vestibular interaction in insulin-dependent and non-insulin dependent diabetes mellitus. NeuroReport. 2000;11:487–490. doi: 10.1097/00001756-200002280-00012. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Structure of four executive functioning tests in healthy older adults. Neuropsychology. 2006;20:206–214. doi: 10.1037/0894-4105.20.2.206. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: From cognitively elite to cognitively impaired. Neuropsychology. 2009;23:778–791. doi: 10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia LF, Satz P, Uchiyama CL, White T. Color Trails Test: Professional manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM. The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsychology, and Cognition. 2004;11:346–376. [Google Scholar]
- Dixon RA, Garrett DD, Bäckman L. Principles of compensation in cognitive neuroscience and neurorehabilitation. In: Stuss DT, Winocur G, Robertson IH, editors. Cognitive neurorehabilitation. 2nd. Cambridge: Cambridge University Press; 2008. pp. 22–38. [Google Scholar]
- Dixon RA, Garrett DD, Lentz TL, MacDonald SWS, Strauss E, Hultsch DF. Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Wahlin Å, Maitland SB, Hultsch DF, Hertzog C, Bäckman L. Episodic memory change in late adulthood: Generalizability across samples and performance indices. Memory & Cognition. 2004;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JEW, Harman HH, Dermen D. Manual for the kit of factor-referenced cognitive tests. Princeton, N.J.: Educational Testing Service; 1976. [Google Scholar]
- Elias PK, Elias MF, D'Agostino RB, Cupples LA, Wilson PW, Silbershatz H, et al. NIDDM and blood pressure as risk factors for poor cognitive performance: The Framingham Study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proceedings of the National Academy of Science of the United States of America. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AL, de Frias CM, Yeung SE, Dixon RA. Short-term longitudinal trends in cognitive performance in older adults with Type 2 diabetes. Journal of Clinical and Experimental Neuropsychology. 31:809–822. doi: 10.1080/13803390802537636. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, et al. Clinical depression versus distress among patients with type 2 diabetes: Not just a question of semantics. Diabetes Care. 2007;30:542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: Results of the Epidemiology of Vascular Aging Study. Diabetes Care. 2001;24:366–370. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- Ford ME, Tilley BC, McDonald PE. Social support among African-American adults with diabetes, part 2. Journal of the National Medical Association. 1998;90:425–432. [PMC free article] [PubMed] [Google Scholar]
- Frier B, Yang P, Taylor AW. Diabetes, aging and physical activity. European Review of Aging Physical Activity. 2006;3:63–73. [Google Scholar]
- Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends in Neurosciences. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Goldman N, Glei DA, Chang MC. The role of clinical risk factors in understanding self-rated health. Annals of Epidemiology. 2004;14:49–57. doi: 10.1016/s1047-2797(03)00077-2. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Brown A. Cognitive and physical disabilities and aging-related complications of diabetes. Clinical Diabetes. 2003;21:113–118. [Google Scholar]
- Gregg EW, Yaffe K, Cauley JA, Rolka DB, Blackwell TL, Narayan KMV, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Archive of Internal Medicine. 2000;160:174–180. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- Grimley EJ, Areosa SA. Effect of the treatment of type II diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database of Systematic Reviews. 2003;1 doi: 10.1002/14651858.CD003804. Art. No.: CD003804. [DOI] [PubMed] [Google Scholar]
- Hanes DA, McCollum G. Cognitive-vestibular interactions: A review of patient difficulties and possible mechanisms. Journal of Vestibular Research. 2006;16:75–91. [PubMed] [Google Scholar]
- Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn G, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: Evidence from a longitudinal study. Age and Ageing. 2004;33:355–361. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- Hassing LB, Johansson B, Pedersen NL, Nilsson SE, Berg S, McClearn G. Type 2 diabetes mellitus and cognitive performance in a population-based sample of the oldest old. Aging, Neuropsychology, and Cognition. 2003;10:99–107. [Google Scholar]
- Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: Gait in the elderly as a complex cognitive task. Experimental Brain Research. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31:795–797. doi: 10.2337/dc07-1391. [DOI] [PubMed] [Google Scholar]
- Hendrickx H, McEwen BS, van der Ouderaa F. Metabolism, mood and cognition in aging: Lifestyle and dietary intervention. Neurobiology of Aging. 2005;26S:S1–S5. doi: 10.1016/j.neurobiolaging.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Holzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: Results from the Einstein aging study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Horak FB, Hlavacka F. Somatosensory loss increases vestibulospinal sensitivity. Journal of Neurophysiology. 2001;86:575–585. doi: 10.1152/jn.2001.86.2.575. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: Relationships to self-reported health and activity life style. Journal of Gerontology: Psychological Sciences. 1993;48:P1–P11. doi: 10.1093/geronj/48.1.p1. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory change in the aged. New York: Cambridge University Press; 1998. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Ingram M, Gallegos G, Elenes J. Diabetes is a community issue: The critical elements of a successful outreach and education model on the U.S.- Mexico border. Preventing Chronic Disease: Public Health Research, Practice, and Policy. 2005 January;2(1) Article 15. Retrieved July 28, 2009, from http://www.cdc.gov/pcd/issues/2005/jan/toc.htm. [PMC free article] [PubMed]
- Jonas BS, Lando JF. Negative affect as a prospective risk factor for hypertension. Psychosomatic Medicine. 2000;62:188–196. doi: 10.1097/00006842-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Jylhä M, Volpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population sample. Journal of Clinical Epidemiology. 2006;59:465–471. doi: 10.1016/j.jclinepi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: A 4-year prospective study on the Rancho Bernardo study cohort. Archives of Internal Medicine. 2004;164:1327–1333. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- Kenny DA. Mediation. 2008 September 18; http://davidakenny.net/cm/mediate.htm.
- Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocrine Reviews. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressin NR, Spiro A, III, Skinner KM. Negative affectivity and health-related quality of life. Medical Care. 2000;38:858–867. doi: 10.1097/00005650-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Kumari M, Brunner E, Fuhrer R. Mechanisms by which the metabolic syndrome and diabetes impair memory. Journal of Gerontology: Biological Sciences. 2000;55:228–232. doi: 10.1093/gerona/55.5.b228. [DOI] [PubMed] [Google Scholar]
- Kuo YF, Raji MA, Peek MK, Goodwin JS. Health-related social disengagement in elderly diabetic patients: Association with subsequent disability and survival. Diabetes Care. 2004;27:1630–1637. doi: 10.2337/diacare.27.7.1630. [DOI] [PubMed] [Google Scholar]
- Lekander M, Elofsson S, Neve IM, Hansson LO, Unden AL. Self-rated health is related to levels of circulating cytokines. Psychosomatic Medicine. 2004;66:559–563. doi: 10.1097/01.psy.0000130491.95823.94. [DOI] [PubMed] [Google Scholar]
- Liang J, Bennett J, Whitelaw N, Maeda D. The structure of self-reported physical health among the aged in the United States and Japan. Medical Care. 1991;29:1161–1180. doi: 10.1097/00005650-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Schaefer S, Pohlmeyer AE, Lindenberger U. Walking variability and working-memory load in aging. Journal of Gerontology: Psychological Sciences. 2008;63B:P121–P128. doi: 10.1093/geronb/63.3.p121. [DOI] [PubMed] [Google Scholar]
- Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS ONE. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. Retrieved June 12, 2009 from www.plosone.org. [DOI] [PMC free article] [PubMed]
- Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. American Journal of Epidemiology. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, et al. Depression and diabetes symptom burden. General Hospital Psychiatry. 2004;26:430–436. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE. Depression in diabetes: The chicken or the egg? Psychosomatic Medicine. 2007;69:297–299. doi: 10.1097/PSY.0b013e318060cc2d. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: Findings from the Victoria Longitudinal Study. Gerontology. 2004;50:64–81. doi: 10.1159/000075557. [DOI] [PubMed] [Google Scholar]
- Maitland SB, Dixon RA, Hultsch DF, Hertzog C. Well-being as a moving target: Measurement equivalence of the Bradburn Affect Balance Scale. Journal of Gerontology: Psychological Sciences. 2001;56B:P69–P77. doi: 10.1093/geronb/56.2.p69. [DOI] [PubMed] [Google Scholar]
- Manschot SM, Brands AMA, van der Grond J, Kessels RPC, Algra A, Kappelle LJ, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with Type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- Manschot SM, Biessels GJ, de Valk H, Algra A, Rutten GEHM, van der Grond J, et al. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansueti L, de Frias CM, Bub D, Dixon RA. Exploring cognitive effects of self-reported mild stroke in older adults: Selective but robust effects on story memory. Aging, Neuropsychology, and Cognition. 2008;15:545–573. doi: 10.1080/13825580701858216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism Clinical and Experimental. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiology of Aging. 2005;26S:S26–S30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, et al. The clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial: Clinical comparison of subgroups with and without the metabolic syndrome. Schizophrenia Research. 2005;80:9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Moor C, Zimprich D, Schmitt M, Kliegel M. Personality, aging self-perceptions, and subjective health: A mediation model. International Journal of Aging and Human Development. 2006;63:241–257. doi: 10.2190/AKRY-UM4K-PB1V-PBHF. [DOI] [PubMed] [Google Scholar]
- Moskowitz JT, Epel ES, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychology. 2008;27:S73–S82. doi: 10.1037/0278-6133.27.1.S73. [DOI] [PubMed] [Google Scholar]
- Mulsant BH, Ganguli M, Seaberg EC. The relationship between self-rated health and depressive symptoms in an epidemiological sample of community-dwelling older adults. Journal of the American Geriatrics Society. 1997;45:954–958. doi: 10.1111/j.1532-5415.1997.tb02966.x. [DOI] [PubMed] [Google Scholar]
- Naor M, Steingrüber HJ, Westhoff K, Schottenfeld-Naor Y, Gries AF. Cognitive function in elderly non-insulin-dependent diabetic patients before and after inpatient treatment for metabolic control. Journal of Diabetes Complications. 1997;11:40–46. doi: 10.1016/1056-8727(95)00106-9. [DOI] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics, 2007 fact sheet. Bethesda, MD: Department of Health and Human Services; 2008. [Google Scholar]
- Nelson TO, Narens L. Norms of 300 general-information questions: Accuracy of recall, latency of recall, and feeling-of-knowing ratings. Journal of Verbal Learning and Verbal Behavior. 1980;19:338–368. [Google Scholar]
- Nilsson E. Unpublished doctoral dissertation. Karolinska Institutet; Stockholm, Sweden: 2006. Diabetes and cognitive functioning: The role of age and comorbidity. [Google Scholar]
- Nilsson E, Fastbom J, Wahlin Å. Cognitive functioning in a population-based sample of very old non-demented and non-depressed persons: The impact of diabetes. Archives of Gerontology and Geriatrics. 2002;35:95–105. doi: 10.1016/s0167-4943(01)00208-4. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Wahlin Å. Diabetes and elevated glycosylated haemoglobin: Episodic memory and utilisation of cognitive support. European Journal of Cognitive Psychology. 2009;21:388–405. [Google Scholar]
- Okereke OI, Kang JH, Cook NR, Gaziano JM, Manson JE, Buring JE, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. Journal American Geriatrics Society. 2008;56:1028–1036. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and psychosocial interventions on management of diabetes mellitus. Journal of Clinical Epidemiology. 1988;41:1007–1030. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- Pedhazur EJ. Multiple regression in behavioral research. 2nd. New York: Holt, Reinehart, & Winston; 1982. [Google Scholar]
- Perlmuter LC, Nathan DM, Goldfinger SH, Russo PA, Yates J, Larkin M. Triglyceride levels affect cognitive function in noninsulin-dependent diabetics. The Journal of Diabetic Complications. 1988;2:210–213. doi: 10.1016/s0891-6632(88)80011-4. [DOI] [PubMed] [Google Scholar]
- Petrofsky J, Lee S, Bweir S. Gait characteristics in people with type 2 diabetes mellitus. European Journal of Applied Physiology. 2005;93:640–647. doi: 10.1007/s00421-004-1246-7. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Leonardelli GJ. Calculation of the Sobel Test. 2003 http://www.people.ku.edu/∼preacher/sobel/sobel.htm.
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Regard M. Unpublished doctoral dissertation. University of Victoria; Victoria BC, Canada: 1981. Cognitive rigidity and flexibility: A neuropsychological study. [Google Scholar]
- Reynolds SL, Silverstein M. Observing the onset of disability in older adults. Social Science & Medicine. 2003;57:1875–1889. doi: 10.1016/s0277-9536(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Roriz-Filho JS, Roriz STS, Foss MP, Foss-Freitas MC, Ferriolli E, Lima NKC, et al. Correlation between Alzheimer's disease, pre-diabetes and diabetes mellitus evaluated by quantitative magnetic resonance imaging. Alzheimer's and Dementia. 2009;5:49. [Google Scholar]
- Ryan CM, Geckle M. Why is learning and memory dysfunction in Type 2 diabetes limited to older adults? Diabetes Metabolism Research and Reviews. 2000;16:308–315. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr141>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Sargent-Cox KA, Anstey KJ, Luszcz MA. Determinants of self-rated health items with different points of reference: Implications for health measurement of older adults. Journal of Aging and Health. 2008;20:739–761. doi: 10.1177/0898264308321035. [DOI] [PubMed] [Google Scholar]
- Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care. 2004;27:2335–2340. doi: 10.2337/diacare.27.10.2335. [DOI] [PubMed] [Google Scholar]
- Stern Y, editor. Cognitive reserve: Theory and applications. New York: Taylor & Francis Group; 2007. [Google Scholar]
- Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabetic Medicine. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- Strachan MWJ, Frier BM, Deary IJ. Cognitive assessment in diabetes: The need for consensus. Diabetic Medicine. 1997;14:421–222. doi: 10.1002/(SICI)1096-9136(199706)14:6<421::AID-DIA382>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Gatz M, Pedersen NL. Genetic and environmental mediation of the associations between self-rated health and cognitive abilities. Experimental Aging Research. 2009;35:178–201. doi: 10.1080/03610730902720372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, Keim KS, Sparrer A, Van Delinder J, Parker S. Social and cultural barriers to diabetes prevention in Oklahoma American Indian women. Preventing Chronic Disease: Public Health Research, Practice, and Policy. 2004 April;1(2) Article 06. Retrieved July 28, 2009, from http://www.cdc.gov/pcd/issues/2004/apr/toc.htm. [PMC free article] [PubMed]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- Thornton WL, Deria S, Gelb S, Shapiro RJ, Hill A. Neuropsychological mediators of the links among age, chronic illness, and everyday problem solving. Psychology and Aging. 2007;22:470–481. doi: 10.1037/0882-7974.22.3.470. [DOI] [PubMed] [Google Scholar]
- Tomten SE, Høstmark AT. Self-rated health showed a consistent association with serum HDL-cholesterol in the cross-sectional Oslo health study. International Journal of Medical Sciences. 2007;4:278–287. doi: 10.7150/ijms.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undén AL, Elofsson S, Andréasson A, Hillered E, Eriksson I, Brismar K. Gender differences in self-rated health, quality of life, quality of care, and metabolic control in patients with diabetes. Gender Medicine. 2008;5:162–180. doi: 10.1016/j.genm.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H. Rey Auditory-Verbal Learning Test: Structure analysis. Journal of Clinical Psychology. 1993;49:883–890. doi: 10.1002/1097-4679(199311)49:6<883::aid-jclp2270490616>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- van den Berg E, Kloppenborg RP, Kessels RPC, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease. 2009;1792:470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Van der Does FE, De Neeling JN, Snoek FJ, Kostense PJ, Grootenhuis PA, Bouter LM, et al. Symptoms and well-being in relation to glycemic control in type II diabetes. Diabetes Care. 1996;19:204–210. doi: 10.2337/diacare.19.3.204. [DOI] [PubMed] [Google Scholar]
- van Iersel MB, Kessels RPC, Bloem BR, Verbeek ALM, Olde Rikkert MGM. Special section: Executive functions are associated with gait and balance in community-living elderly people. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2008;63:1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- Votey SR, Peters AL. Diabetes mellitus, type 2- A review. 2009 Retrieved May 19, 2009, from http://emedicine.medscape.com/article/766143-overview.
- Wahlin Å. Health, disease, and cognitive functioning in old age. In: Dixon RA, Bäckman L, Nilsson LG, editors. New frontiers in cognitive aging. New York: Oxford University Press; 2004. pp. 279–302. [Google Scholar]
- Wahlin Å, MacDonald SWS, de Frias CM, Nilsson LG, Dixon RA. How do health and biological age influence chronological age and sex differences in cognitive aging: Moderating, mediating, or both? Psychology and Aging. 2006;21:318–332. doi: 10.1037/0882-7974.21.2.318. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- Watari K, Letamendi A, Elderkin-Thompson V, Haroon E, Miller J, Darwin C, et al. Cognitive function in adults with type 2 diabetes and major depression. Archives of Clinical Neuropsychology. 2006;21:787–796. doi: 10.1016/j.acn.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-revised. New York: Psychological Corporation; 1991. [Google Scholar]
- Whittemore R, Melkus GD, Grey M. Metabolic control, self-management and psychosocial adjustment in women with type 2 diabetes. Journal of Clinical Nursing. 2005;14:195–203. doi: 10.1111/j.1365-2702.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Xu WL, Qiu CX, Wahlin Å, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- Yeung SE, Fischer AL, Dixon RA. Exploring effects of Type 2 diabetes on cognitive functioning in older adults. Neuropsychology. 2009;23:1–9. doi: 10.1037/a0013849. [DOI] [PMC free article] [PubMed] [Google Scholar]