Abstract
Börjeson-Forssman-Lehmann syndrome (BFLS) is a rare X-linked mental retardation syndrome that is caused by germline mutations in PHF6. We describe a 9-year old male with BFLS, who developed T-cell acute lymphoblastic leukemia (T-ALL). The PHF6 gene is located on the X chromosome and encodes a protein with two PHD-type zinc finger domains and four nuclear localization sequences. Previously, overexpression of Phf6 was observed in murine T-cell lymphomas. Our observation indicates that BFLS may represent a cancer predisposition syndrome and that mutations of PHF6 contribute to T-ALL.
Keywords: Börjeson-Forssman-Lehmann syndrome, PHF6, T-cell ALL/lymphoma
INTRODUCTION
Several onco- and tumor suppressor genes that are somatically altered in cancer have also been found to be mutated in the germline of individuals with inherited conditions, supporting the notion that, at least in part, oncogenesis and embryogenesis share common pathways. Examples include germline mutations in the oncogene KRAS in patients with Noonan syndrome [1], germline HRAS mutations in patients with Costello syndrome [2] and germline mutations of the tumor suppressor gene PTEN in individuals with Cowden disease [3]. Moreover, constitutional mutations have been uncovered in several leukemia associated transcription factors such as RUNX1 [4] (familial thrombocytopenia with propensity to AML) and GATA1 (congenital dyserythropoietic anemia with macrothrombocytopenia) [5]. Not surprisingly, inherited conditions that are caused by germline mutations of cancer-related genes are often associated with a spectrum of malignancies occurring at variable frequencies [1–4]. Patients with genetic developmental disorders who develop cancer are of scientific interest because the mutated gene underlying their condition may confer a cancer predisposition. Additionally, disease causative genes of such inherited conditions may be somatically mutated in cancer. In this regard, germline mutations of the ATRX gene are the cause of several X-linked intellectual disability syndromes while somatic mutations have been identified in patients with pre-leukemic alpha-thalassemia myelodysplastic syndrome (ATMDS) [6]. Thus, the clinical description of such rare associations may have broad implications in the understanding of cancer biology and lead to the focused identification of novel cancer genes.
CASE REPORT AND RESULTS
We report on a 9-year old male with Börjeson-Forssman-Lehmann syndrome (BFLS; OMIM 301900) (Figure 1), a rare X-linked disorder associated with intellectual disability, distinctive facial features, truncal obesity and gynecomastia, which is caused by germline mutations of PHF6 (reviewed in [7]). Our patient was diagnosed with T-cell acute lymphoblastic leukemia (T-ALL) at age 7 when he presented with hyperleukocytosis and enlarged cervical lymphnodes. Flow cytometric study of bone marrow cells showed blast cells expressing CD45, CD5, CD4, CD8, CD2, CD7, CD9, CD1a, and cytoplasmic CD3 consistent with T-cell phenotype. Cytogenetic analysis of leukemia cells revealed a normal male karyotype. Treatment (high-risk arm of ANZCCSG ALL Study VII) was complicated by transient liver failure during the induction phase; however, the patient recovered and is currently receiving maintenance chemotherapy. Mutation analysis of DNA extracted from peripheral blood taken during remission showed a mutation of PHF6, c.1024C>T, that results in a premature termination codon (p.R342X) within exon 10. Leukemia cells were not available for analysis.
Figure 1.
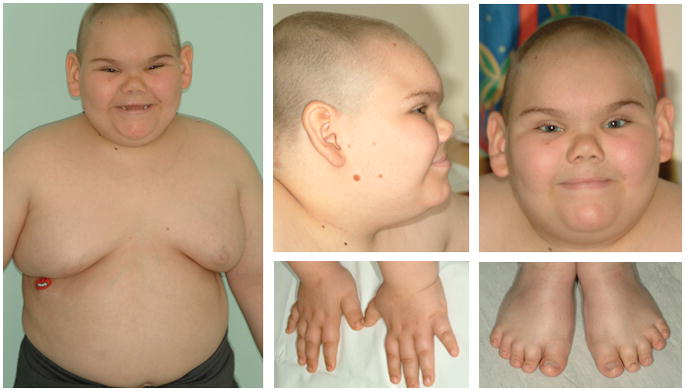
Patient with T-ALL and Börjeson-Forssman-Lehmann syndrome, 9 years. Note deep-set eyes, large, fleshy, posteriorly rotated ears, central obesity, breast enlargement, and tapering fingers.
DISCUSSION
The PHF6 gene is a member of a large family of zinc-finger genes and is ubiquitously expressed. The gene product, a 365-amino-acid protein, contains four nuclear localization sequences but its function remains unknown. Expression of green fluorescent labeled PHF6 showed subcellular localization to the nucleus and nucleolus (reviewed in [7]). The latter serves as a key organelle in the synthesis and assembly of ribosomal subunits and is linked to cell growth and proliferation. PHF6 also contains two imperfect plant homeodomain-type zinc fingers (PHD), resembling transcriptional regulators such as MLL, which is frequently disrupted by leukemia-associated translocations [7]. The data suggest a role for PHF6 as a transcriptional regulator.
There are less than 30 unrelated cases of BFLS with confirmed PHF6 mutations reported in the literature. Germline mutations associated with BFLS are predominantly missense and truncation mutations in and around exons 2 and 10 [7]. The c.1024C>T (p.R342X) mutation found in our patient results in a premature termination within exon 10, affecting the 5’-CpG dinucleotide codon for the aminoacid arginine which was previously identified in the original BFLS family and is a recurrent mutation in BFLS [8]. Although, BFLS is not recognized as a cancer predisposition syndrome, another patient with BFLS has been reported to have developed Hodgkin lymphoma [9].
Interestingly, a study by Landais et al. implicated Phf6 overexpression in T-cell lymphoma in mice [10]. Specifically, the highly leukemogenic radiation leukemia retrovirus VL3 (RadLV/VL3) induced T-cell lymphoma in a murine model by retroviral integrations in c-myc, Pim1, Notch1, and Kis2 (Kaplan Integration Site 2) loci. Kis2 rearrangements occurred in 11% of murine lymphomas and resulted in the overexpression of the nearby Phf6 gene implicating a role for Phf6 in T-cell lymphoma development [10]. In independent studies, the Kis2 locus was found to represent a common viral integration site in SL3-3 and Moloney murine leukemia models. Moreover, in another study, comparative genomic hybridization (CGH) identified DNA imbalances in 19% (29) of analyzed cases of human T-cell lymphoma samples with gains related to either the whole or part of the X chromosome that includes bands Xq26-27 containing the PHF6 gene locus [11]. Lastly, in gene expression profiling PHF6 has been identified as a direct target gene of NOTCH1, which is frequently mutated in T-ALL [12]. Murine Phf6 protein expression analysis demonstrated mild to moderate levels of expression of Phf6 in many adult organs including the thymus and spleen [13]. Taken together, these observations suggest for a role of PHF6 in T-cell ALL/lymphoma tumorigenesis. Moreover, because the gene is located on the X chromosome, this may explain the preponderance of TALL/lymphoma in males. Ongoing and future functional and genetic studies such as PHF6 mutation analysis in primary human T-ALL/lymphoma cells are necessary to further characterize the role of PHF6 in human cancer.
ADDENDUM
We have subsequently sequenced DNA from pediatric cases of T-ALL for somatic mutations in PHF6. We screened 9 unselected patients who had been treated at the Division of Pediatric Hematology and Oncology, Department of Pediatrics and Adolescent Medicine, University of Freiburg, Germany. Notably, in blasts from one male pediatric patient with T-ALL we found a novel mutation of PHF6 (c.987T>A) predicting a p.H329Q substitution. After submission of this paper, a report from Van Vlierberghe and colleagues described somatic mutations and deletions of PHF6 in patients with T-ALL (Nature Genetics, advance online publication, March 14, 2010).
Acknowledgments
C.P.K.’s work was supported by the Intramural Research Program of the US National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors do not have any conflict of interest to disclose.
References
- 1.Schubbert S, Zenker M, Rowe SL, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38(3):331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 2.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 3.Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16(1):64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 4.Song WJ, Sullivan MG, Legare RD, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23(2):166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 5.Nichols KE, Crispino JD, Poncz M, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24(3):266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons RJ, Wada T, Fisher CA, et al. Mutations in the chromatin-associated protein ATRX. Hum Mutat. 2008;29(6):796–802. doi: 10.1002/humu.20734. [DOI] [PubMed] [Google Scholar]
- 7.Gecz J, Turner G, Nelson J, et al. The Borjeson-Forssman-Lehman syndrome (BFLS, MIM #301900) Eur J Hum Genet. 2006;14(12):1233–1237. doi: 10.1038/sj.ejhg.5201639. [DOI] [PubMed] [Google Scholar]
- 8.Lower KM, Solders G, Bondeson ML, et al. 1024C> T (R342X) is a recurrent PHF6 mutation also found in the original Borjeson-Forssman-Lehmann syndrome family. Eur J Hum Genet. 2004;12(10):787–789. doi: 10.1038/sj.ejhg.5201228. [DOI] [PubMed] [Google Scholar]
- 9.Carter MT, Picketts DJ, Hunter AG, et al. Further clinical delineation of the Börjeson-Forssman-Lehmann syndrome in patients with PHF6 mutations. Am J Med Genet A. 2009;149A(2):246–250. doi: 10.1002/ajmg.a.32624. [DOI] [PubMed] [Google Scholar]
- 10.Landais S, Quantin R, Rassart E. Radiation leukemia virus common integration at the Kis2 locus: simultaneous overexpression of a novel noncoding RNA and of the proximal Phf6 gene. J Virol. 2005;79(17):11443–11456. doi: 10.1128/JVI.79.17.11443-11456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renedo M, Martinez-Delgado B, Arranz E, et al. Chromosomal changes pattern and gene amplification in T cell non-Hodgkin's lymphomas. Leukemia. 2001;15(10):1627–1632. doi: 10.1038/sj.leu.2402248. [DOI] [PubMed] [Google Scholar]
- 12.Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A. 2006;103(48: supplement):18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss AK, Gamble R, Collin C, et al. Protein and gene expression analysis of Phf6, the gene mutated in the Börjeson-Forssman-Lehmann Syndrome of intellectual disability and obesity. Gene Expr Patterns. 2007;7(8):858–871. doi: 10.1016/j.modgep.2007.06.007. [DOI] [PubMed] [Google Scholar]


