Abstract
Background and Objectives
The progression of glaucoma can be reduced or delayed by reducing intraocular pressure (IOP). The properties of femtosecond laser surgery, such as markedly reduced collateral tissue damage, coupled with the ability to achieve isolated subsurface surgical effects in the sclera, make this technology a promising candidate in glaucoma management. In this pilot study we demonstrate the in vivo creation of partial thickness subsurface drainage channels with the femtosecond laser in the sclera of rabbit eyes in order to increase aqueous humor (AH) outflow.
Study Design/Materials and Methods
A femtosecond laser beam tuned to a 1.7 μm wavelength was scanned along a rectangular raster pattern to create the partial thickness subsurface drainage channels in the sclera of one eye of each of the four rabbits included in this pilot study. IOP was measured before and 20 minutes after the laser treatment to evaluate the acute effect of the procedure.
Results
OCT images verified the creation of the partial thickness subsurface scleral channels in the eyes of the in vivo rabbits. Comparison of pre- and postoperative IOP measurements in treated and control eyes revealed a reduction in the intraocular pressure due to the increased rate of AH outflow resulted in by the presence of the partial thickness scleral channels.
Conclusions
The creation of partial thickness subsurface drainage channels was demonstrated in the sclera of in vivo rabbit eyes with a 1.7 μm wavelength femtosecond laser. Reduction in IOP achieved by the partial thickness channels suggests potential utility in the treatment of elevated IOP.
INTRODUCTION
Glaucoma is among the leading causes of blindness [1], with primary open-angle glaucoma (POAG) being the most common form of the disease [2]. Risk factors for glaucoma include elevated IOP, vascular disease, and myopia [2]. While increased IOP is associated with developing glaucoma [3], lowering IOP reduces visual field loss [4] and delays glaucomatous progression [5]. Elevated IOP is related to impaired AH flow dynamics of the eye and initially treated using topical drugs that decrease AH formation or increase AH outflow from the eye. While effective, poor patient compliance with dosing regimens limits the efficacy of medical therapy [6]. Shunt, a valve implanted to increase AH flow, is frequently used as a reliable treatment [7, 8], but has unavoidable scar and bleeding due to surgical insertion. Trabeculectomy, the most widely used incisional glaucoma surgery, can lower IOP more effectively, but is associated with a higher risk of severe complications and substantial long term failure [6, 9, 10]. Laser Trabeculoplasty (LT), though widely utilized using various laser sources, has modest success rates that diminish over time [10–13]. As a result of these therapeutic limitations, there continues to be demand for improved glaucoma treatments. Femtosecond laser technology has been successfully used in high precision corneal surgery, demonstrating reduced collateral tissue damage to adjacent tissue [14]. At the 1.7 μm wavelength, femtosecond laser pulses can be delivered through the translucent conjunctiva and sclera to perform high precision, photodisruptive surgical procedures without damage to superficial or adjacent tissues [16]. Partial thickness subsurface channels through trabecular pathway created with this method in ex vivo human and rabbit eyes have been shown to increase outflow facility, suggesting the potential to reduce IOP [16, 17]. In this pilot study, we demonstrate that partial thickness subsurface channels can be created with a femtosecond laser through the trabecular pathway effectively in vivo using a rabbit model. Treated eyes demonstrated a reduction from pre-operative IOP, suggesting potential clinical utility.
MATERIALS AND METHODS
Laser Source
A Ti:Sapphire laser system was tuned to the 1.7 μm wavelength using an optical parametric amplifier (OPA). The system includes an oscillator, regenerative amplifier and optical parametric amplifier (Coherent; Santa Clara, CA) (see Fig. 1). The laser system was controlled by a computer built in the laser system using LabView (National Instruments, Austin, TX). The Ti: sapphire laser produced 130 fs laser pulses at 800 nm wavelength amplified to 0.3 mJ pulse energy at a 5 KHz repetition rate. The wavelength of the femtosecond laser pulses was then converted to the 1.7 μm wavelength using an optical parametric amplifier (Coherent; Santa Clara, CA). The diameter of the laser beam was expanded through a 4x beam expander and focused with a 0.5 numerical aperture (NA) aspheric lens (Thorlabs; Newton, NJ) (see Fig. 2).
Figure 1.
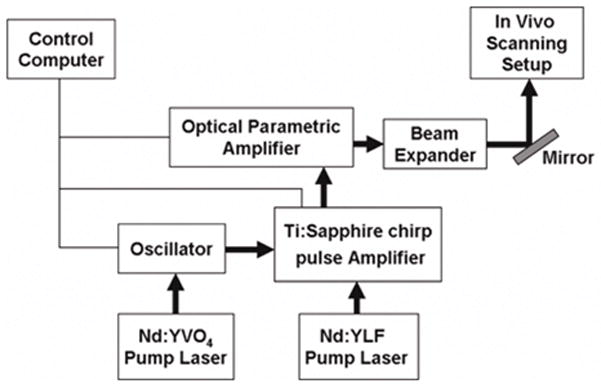
Figure 2.
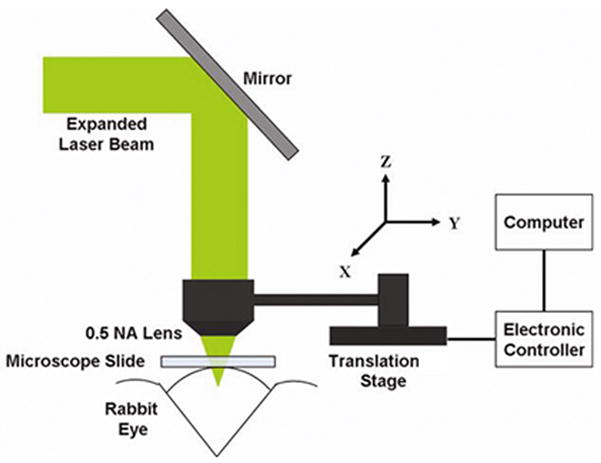
Laser and Beam Delivery System
Scanning Delivery System
A schematic of the scanning mechanism is displayed in Fig. 2. A microscope slide was placed at the limbus of the rabbit eye where the channel was to be created to minimize light scattering on the surface. The laser beam was reflected by the 45° mirror so that the focused beam propagated down to the eye in normal direction through the surface of microscope slide. The laser beam was scanned along a 3 dimensional rectangular raster pattern to create the subsurface drainage channels. A 3 dimensional Cartesian coordinate system is set up to describe scanning parameters so that the Z axis of the coordinate system is parallel with the propagation of the laser light and the X axis is tangential to the limbus (see Fig. 2). The 0.5 NA aspheric lens was connected to the translation stage driven by a stepper motor along each axis as shown in Fig. 2. The stepper motors were connected to the computer through the electronic controller. LabView 7.1 (National Instruments, Austin, TX) was used to control the movements of the lens in each direction along a predetermined pattern. The rabbit holder was mounted on a height adjustable stage so that the eye could be positioned at its optimal position for the laser treatment.
Experimental Procedures
Four New Zealand white rabbits, 4 to 5 kg, were used during this pilot evaluation of the in vivo subsurface procedure. All experiments were performed in compliance with the guidelines of the Institutional Animal Care and Use Committee at the University of California, Irvine and the ARVO Statement for Use of Animals in Ophthalmic and Vision research. The rabbits were anesthetized with intramuscular xylazine 5 mg/kg (Bayer Corp., Shawnee Mission, KS) and ketamine 35 mg/kg (Fort Dodge Laboratories, Fort Dodge, IA). IOP was measured pre- and postoperatively with a tonopen (Reichert Analytical Instrument; Depew, NY) with the average of 10 readings recorded.
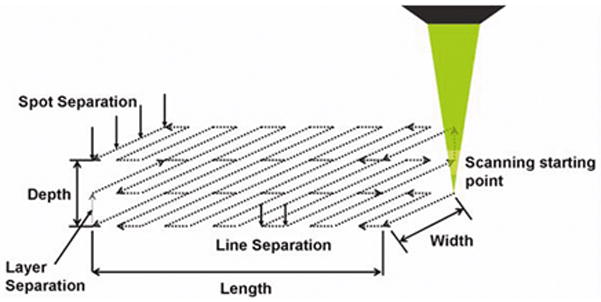
For treatment, each rabbit was placed in a holder with an adjustable head support. The head of the animal was secured to the head support. The eye was positioned at the optimal position for the treatment so that the limbus was oriented normal to the propagation direction of laser beam. A microscope slide held in an adjustable holder was placed on the eye normal to the propagation direction of the laser beam. The applanation of the microscope slide in addition to the localized mounting of the animal’s head to the adjustable head support was sufficient to hold the eye steady during the laser procedures while the animals were under full anesthesia. After aligning the eye a single channel was created in one eye of each animal using the following scanning procedure. Initially, the focal point of the laser beam was moved to the lower surface of the microscope slide by positioning the focusing lens along the Z axis. This marked the zero depth position at the anterior surface of the limbus. The energy of laser beam was set to be 22 μJ. To start the scanning the focus point of the beam was first moved approximately 100 μm below the posterior surface of sclera. Then, it was scanned along the raster pattern shown in Fig. 3 by moving the focusing objective with stepper motors. The pattern was scanned layer by layer from posterior to anterior direction. This scanning procedure ensured a clear opening of the channel to the anterior chamber. Scanning parameters were set to be a 2 μm spot separation, a 4 μm line separation and a 2 μm layer separation. The scanning was stopped approximately 100 μm below the top surface of sclera to make sure that the channel did not reach the anterior surface of the sclera avoiding damage to the conjunctiva and subconjuctival layers. Saline solution (Bausch & Lomb, Rochester, NY) was dropped periodically to the sclera surface not covered by microscope slide to prevent the dehydration during the treatment. The time duration of the treatments was less than 25 minutes for each animal. At the end of the scanning process the microscope slide was removed. Identical IOP measurements to those made preoperatively were repeated 20 minutes post-operatively to monitor the effect of the channel on the intraocular pressure. Then, the shape of the channel was observed in vivo with a home built non-contact spectral domain OCT imaging device working at 890 nm wavelength [18, 19] to evaluate the quality of channel creation. After sacrificing the animals the sclera containing the channel was dissected and observed under a second harmonic imaging microscope (SHIM) tuned to 800 nm wavelength (Axiovert 200, Zeis, Germany).
Figure 3.
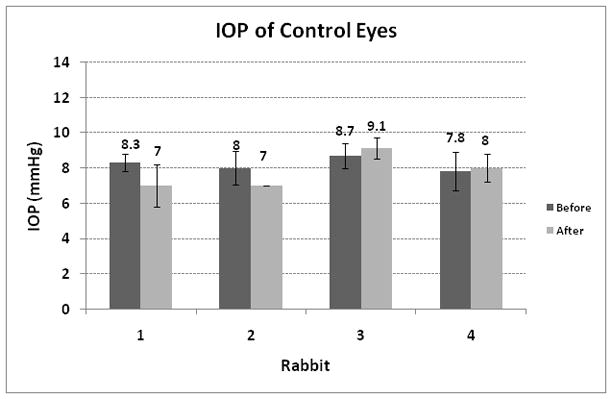
Control experiments were conducted on 4 untreated eyes. In the control studies, the rabbits were treated using the same experimental protocol used in the direct studies described above with the exception of the laser treatment. The IOP in the control studies was measured 20 min after the microscope slide was removed from the eyes.
RESULTS
Representative OCT images of the created channels are shown in Fig. 4(A) and (B). The channel dimensions measured in the OCT images in each animal are shown in Table 1. The SHIM image of the preserved sclera containing the channel is shown in Fig. 4(C). Figures 4(A) and 4(C) verified that the channels were created through the sclera so that a thin layer of tissue remained intact at the surface. The OCT images obtained in vivo also verified the rectangular shape of the channels. The SHIM image shown in Fig. 4(C) indicates an irregular channel shape that may be due to tissue processing after sacrificing the animal. However, the image clearly indicates an intact layer of sclera at the tissue surface. The measured values of pre- and post-operative IOP on each animal are displayed in Fig. 5. The results of the IOP measurements performed in the control studies are shown in Fig. 6. The acute effect of the partial thickness subsurface drainage channel on IOP was determined by calculating the difference of pre- and post-operative IOP levels. As shown in Figures 5 and 6, the post-operative IOP was reduced in all treated animals while the IOP of the control eyes was slightly increased or decreased with a considerably smaller amount than the IOP decrease in the treated animals. Therefore, it can be concluded that IOP reduction in treated animals is due to the increase in AH outflow through the partial thickness channel.
Figure 4.
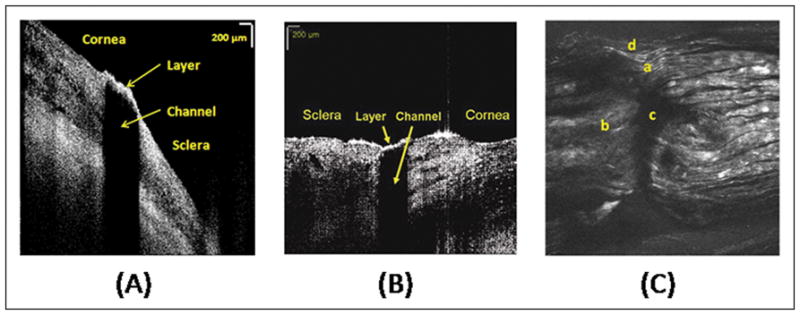
Table 1.
| Rabbit | Channel Dimensions (width × length) |
|---|---|
| 1 | 450μm × 150μm |
| 2 | 600μm × 200μm |
| 3 | 600μm × 220μm |
| 4 | 800μm × 240μm |
Figure 5.

Figure 6.
DISCUSSION
We have demonstrated in vivo femtosecond laser creation and imaging of partial thickness drainage channels through the superficial sclera and conjunctiva. Experiments also indicated reduction of the IOP due to the presence of the partial thickness drainage channels. IOP can be decreased by reduced AH production or increased AH outflow. Pre-operative IOP values in control eyes are slightly lower than those in treated eyes. This is in agreement with the results of a previously published study [20] that showed that IOP in the rabbit eye may be lower than 10 mmHg due to diurnal IOP change. Since the interval between the pre and post-operative IOP measurement is less than 1 hour the natural AH production and outflow rate can be assumed to be unchanged. Therefore it can be concluded that immediate post-procedure IOP reductions in treated eyes may due to the increased AH outflow through the created channels. The minimized collateral damage observed with femtosecond laser photodisruption in other studies [15–17] indicates that damage to the subconjuctival tissue may be avoided with the femtoseocnd laser which may decrease the healing response of the sclera. Therefore, the femtosecond laser procedure has the potential to provide long term solution for IOP reduction which will be investigated in future studies in animal models better suited for healing studies such as the rhesus monkey.
The comparison of Table 1 and Fig. 5 in the current study suggests a possible correlation between channel cross section and IOP reduction, but a larger sample size is needed to establish statistical significance which was beyond the scope of this pilot study. If such a correlation is confirmed in a larger number of eyes, titration of the IOP reduction may be possible via control of this parameter. Additional studies in a model that more closely resembles the glaucomatous human eye are also required to evaluate the long term predictability, stability and repeatability of such minimally invasive femtosecond laser channels.
Various laser treatments have been proposed to increase AH outflow without the complexity of the manual sclerostomy procedure. All of these procedures are aiming the creation of partial thickness drainage channels by ablating the posterior sclera at the irido-corneal angle using excimer laser [21], CO2 laser [22] and holmium laser [23]. However none of the above mentioned lasers can be delivered through the sclera, therefore the creation of an additional port hole is necessary on the eye globe for beam delivery. Additionally, the more confined tissue effect of the femtosecond photodisuption combined with the transcleral beam delivery has the potential for the development of a minimally invasive highly precise and well controlled glaucoma procedure that can be performed in outpatient offices since no surface wound is created on the eye, providing an advantage over currently existing manual and laser procedures.
Acknowledgments
This research is supported by the National Institutes of Health (NIH Grant: R01 EY014456-02). The authors also thank Professor Zhongping Chen for providing the OCT device for the experiments.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Leske MC. Open-angle glaucoma-An epidemiologic overview. Ophthalmic Epidemiology. 2007;14:166–172. doi: 10.1080/09286580701501931. [DOI] [PubMed] [Google Scholar]
- 3.Nemesure B, Honkanen R, Hennis A, et al. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007;114:1810–1815. doi: 10.1016/j.ophtha.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Maier PC, Funk J, Schwarzer G, et al. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomized controlled trials. British Medical Journal. 2005;331:134–139. doi: 10.1136/bmj.38506.594977.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiji A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: result from the Early Manifest Glaucoma Trial. Arch Ophthalmology. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JC, Johnson CC, Kammer JA, et al. The Ahmed Shunt versus the Baerveldt Shunt for Refractory Glaucoma II. Ophthalmology. 2006;113:913–917. doi: 10.1016/j.ophtha.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Minckler DS, Francis BA, Hodapp EA. Aqueous Shunts in Glaucoma. American Academy of Ophthalmology. 2008;115:1089–1098. doi: 10.1016/j.ophtha.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield DS, Sunner IJ, Miller MP, et al. Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996;114:943–949. doi: 10.1001/archopht.1996.01100140151007. [DOI] [PubMed] [Google Scholar]
- 10.Spaeth GL, Baez KA. Argon laser trabeculoplasty controls one third of cases of progressive, uncontrolled, open angle glaucoma for 5 years. Arch Ophthalmol. 1992;110:491–494. doi: 10.1001/archopht.1992.01080160069032. [DOI] [PubMed] [Google Scholar]
- 11.Weber PA, Burton GD, Epitropoulos AT. Laser trabeculoplasty retreatment. Ophthalmic Surgery. 1989;20(10):702–706. [PubMed] [Google Scholar]
- 12.Roberts CJ, Rivera BK, Grzybowski DM, et al. Effect of low fluence diode laser irradiation on the hydraulic conductivity of perfused trabecular meshwork endothelial cell monolayers. Current Eye Research. 2007;32:625–638. doi: 10.1080/02713680701486394. [DOI] [PubMed] [Google Scholar]
- 13.Julia Song, Lee PP, Epstein DL, et al. High failure rated associated with 180° selective laser trabeculoplasty. J of Glaucoma. 2005;14:400–408. doi: 10.1097/01.ijg.0000176939.43681.c2. [DOI] [PubMed] [Google Scholar]
- 14.Vogel A, Noack J, Nahen K, Theisen D, Busch S, et al. Energy balance of optical breakdown in water at nanosecond to femtosecond time scales. Appl Physics B. 1999;68:1–10. [Google Scholar]
- 15.Sacks ZS, Kurtz RM, Juhasz T, et al. High precision subsurface photodisruption in human sclera. J of Biomedical Optics. 2002;7:442–450. doi: 10.1117/1.1482381. [DOI] [PubMed] [Google Scholar]
- 16.Chai D, Chaudhary G, Kurtz R, Juhasz T. Aqueous humor outflow effects of partial thickness channel in ex vivo eyes. In proceedings of SPIE Photonics West Biomedical Optics (BIOS) 2007;6426:64350O. [Google Scholar]
- 17.Chai D, Chaudhary G, Mikula E, Sun H, Juhasz T. 3D finite element model of aqueous outflow to predict the effect of femtosecond laser created partial thickness channels. Lasers in Surgery and Medicine. 2008;40:188–195. doi: 10.1002/lsm.20608. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhary G. PhD thesis. Department of Electrical Engineering & Computer Science. University of California-Irvine; CA 92697: 2008. Visualization assisted high-precision ophthalmic micro-surgery with femtosecond laser. [Google Scholar]
- 19.Chaudhary G, Rao B, Dongyul Chai, et al. Post-operative OCT evaluation of in vivo partial thickness scleral and ophthalmology (ARVO) Fort Lauderdale; Florida, USA: 2008. [Google Scholar]
- 20.McLaren JW, Brubaker RF, FitzSimon JS. Continuous measurement of Intraocular pressure in rabbit by telemetry. Investigative Ophthalmology & Visual Science. 1996;37:966–975. [PubMed] [Google Scholar]
- 21.Brooks AM, Samuel M, Carroll N, et al. Excimer laser filtration surgery. American Journal of Ophthalmology. 1995;119:40–47. doi: 10.1016/s0002-9394(14)73811-5. [DOI] [PubMed] [Google Scholar]
- 22.Beckman H, Fuller TA, Boyman R, et al. Carbon dioxide laser surgery of the eye and adnexa. Ophthalmology. 1980;87:990–1000. doi: 10.1016/s0161-6420(80)35139-7. [DOI] [PubMed] [Google Scholar]
- 23.Iwach AG, Hoskins HD, Drake MV, et al. Subconjunctival THC:YAG (“holmium”) laser thermal sclerostomy ab externo. A one-year report. Ophthalmology. 1993;100:356–366. [PubMed] [Google Scholar]


