Abstract
In this paper we review the parameters that define the ECT electrical stimulus and discuss their biophysical roles. We also present the summary metrics of charge and energy that are conventionally used to describe the dose of ECT and the rules commonly deployed to individualize the dose for each patient. We then highlight the limitations of these summary metrics and dosing rules in that they do not adequately capture the roles of the distinct stimulus parameters. Specifically, there is strong theoretical and empirical evidence that stimulus parameters (pulse amplitude, shape, and width, and train frequency, directionality, polarity, and duration) exert unique neurobiological effects that are important for understanding ECT outcomes. Consideration of the distinct stimulus parameters, in conjunction with electrode placement, is central to further optimization of ECT dosing paradigms to improve the risk/benefit ratio. Indeed, manipulation of specific parameters, such as reduction of pulse width and increase in number of pulses, has already resulted in dramatic reduction of adverse side effects, while maintaining efficacy. Furthermore, the manipulation of other parameters, such as current amplitude, which are commonly held at fixed, high values, might be productively examined as additional means of targeting and individualizing the stimulus, potentially reducing side effects. We recommend that ECT dose be defined using all stimulus parameters rather than a summary metric. All stimulus parameters should be noted in treatment records and published reports. To enable research on optimization of dosing paradigms, we suggest that ECT devices provide capabilities to adjust and display all stimulus parameters.
Keywords: electroconvulsive therapy, stimulus, parameters, dosage, charge
I. Introduction
In the early years of electroconvulsive therapy (ECT) practice, the commonly accepted belief was that the parameters that describe the electrical stimulus were clinically irrelevant as long as a seizure was induced.1,2 Over the last 7 decades, this belief was refuted by a series of studies demonstrating that ECT-induced seizures can differ markedly in efficacy and side effects, depending upon manipulations of ECT stimulus parameters.3–5 Thus, in ECT, as in the rest of medicine, treatment response depends upon dosage. The observation that ECT stimulus parameters matter may be key to discovering the mechanisms of the unparalleled efficacy of ECT as well as to the ability to optimize dosage to reduce side effects while preserving efficacy.6 However, the best metric(s) to describe the ECT dose and the optimal method of individualizing ECT dosage remain unresolved challenges.
In pharmacotherapy, the dosage of a medication is described by its physical quantity in milligrams (mg), and dosage is individualized using a dosing rule (e.g., 2 mg/kg, or raising the dose until therapeutic effect is perceived or until side effects become prominent) that is based on theoretical and empirical considerations of the specific pharmacokinetic and pharmacodynamic properties of each medication. The most appropriate analogue to “mg” in ECT is not known, nor is the optimal rule to individualize the dosage. Indeed, the ECT stimulus has multiple characteristics, including electrode placement and stimulus parameters that describe the electrical pulse (e.g., shape, width, and amplitude) and the pulse train (e.g., frequency, directionality, polarity, and duration).
Confronted with the wide parameter space and limited knowledge of the role of the individual stimulus parameters, the field has adopted summary metrics to describe the “amount of electricity” delivered by the stimulus. The commonly used summary dose metric was initially the total energy of the stimulus train (in joules, J),7 later superseded by total charge (in millicoulombs, mC),8 which is still the metric of choice.9 Thus, it is common in ECT practice and research to think of charge as the dose (e.g., 96 mC), and to use empirical dosing strategies (e.g., 6 × seizure threshold in mC). Charge-based dosing has been successfully used with good clinical outcomes for decades, and it has been productively deployed in research studies that have identified dose–response relationships. However, a major drawback to this approach is that it obscures the significance of the individual stimulus parameters, thereby limiting our ability to refine the dosage to optimize clinical outcomes. For example, the amount of charge needed to induce a seizure is dramatically lower with a 0.3-ms pulse width than with a 1.5-ms pulse width,5 thus defining the individual seizure threshold in terms of charge does not yield a unique quantity (see also discussion in Sec. II.4).
In this paper we discuss the role of the stimulus parameters in the context of brain stimulation biophysics and current ECT dosing practices and recommend future directions for ECT dosing research. Better understanding of the significance of the ECT electrical parameters could lead to more rationally designed dosing strategies that improve the risk/benefit ratio and reduce interindividual variability of clinical outcomes.
II. Present state of the art of ECT dosing
II.1. The elements of ECT dose
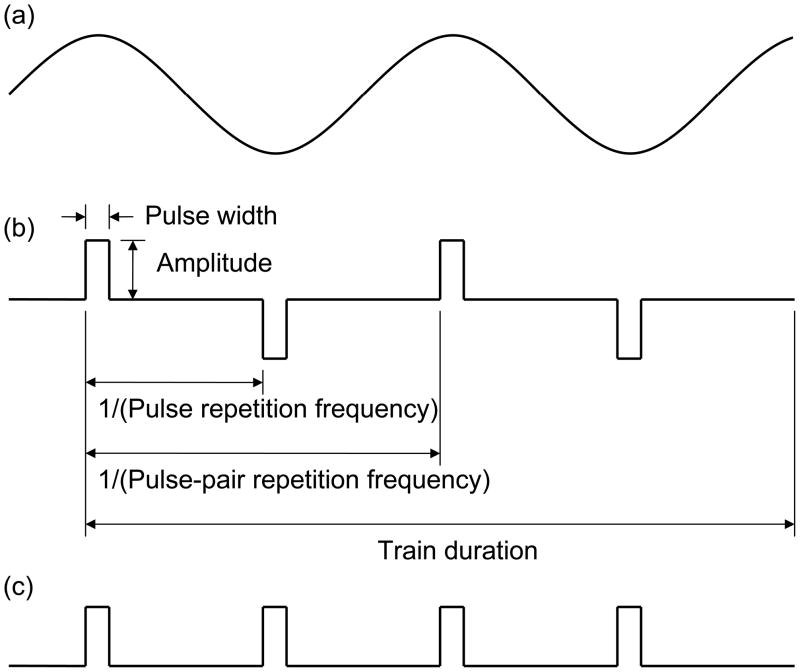
The ECT dose is described by the shape, size, and position of the stimulating electrodes held on the scalp and by the characteristics of the electric current waveform applied through those electrodes. Common ECT waveforms and parameter definitions are shown in Fig. 1. Modern ECT devices, such as the MECTA Spectrum (MECTA Corp., Tualatin, OR) and the Thymatron System IV (Somatics LLC, Lake Bluff, IL), produce trains of rectangular, constant-current pulses with alternating polarity, as shown in Fig. 1(b). As indicated in Fig. 1(b), the waveform is characterized by amplitude (the current strength during the pulse), pulse width (PW, the duration of each pulse), pulse-pair repetition frequency (the number of pairs of a positive and a negative pulse per second), and stimulus train duration. The current amplitude is reported in amperes (A) or in milliamperes (mA). In the now obsolete constant-voltage devices, the pulse amplitude was reported in volts (V). The pulse width is typically reported in milliseconds (ms), and the train duration—in seconds (s). The frequency is conventionally reported in hertz (Hz); however, this unit may refer to either pulse pairs per second (typically for the bidirectional stimuli used in modern ECT devices), or pulses per second (typically for unidirectional stimuli). The pulses per second are twice the pulse pairs per second since each pulse pair is comprised of two pulses. For consistency, in this paper we will indicate whether we are referring to pulse frequency (in pulses per second, pps) or pulse-pair frequency (in pulse-pairs per second). Sometimes it is useful to think of the train length in terms of number of pulses which equals the train duration times the pulse repetition frequency (Equation 1).
Figure 1.
Example ECT stimulus waveforms: (a) sine wave, (b) bidirectional rectangular pulses with indicated parameter definitions, and (c) undirectional rectangular pulses.
| (1) |
II.2. Summary metrics to describe ECT dose
By convention, in ECT practice the stimulus parameters are condensed into a summary metric to describe the ECT dose. Presently, total delivered charge is the most frequently used summary metric, whereas total delivered energy was more commonly used prior to the 1980’s.10 The total charge is the integral of the current magnitude over the duration of the stimulus train. For constant-current, rectangular-pulse trains used by modern ECT devices, the total charge is equal to the product of the pulse current amplitude, width, repetition frequency, and train duration (Equation 2).
| (2) |
The total energy is the integral of the power delivered during the stimulus train, which is equal to the product of the total charge, the current amplitude, and the dynamic head impedance (the impedance during the pulse train) for constant-current, rectangular-pulse trains (Equation 3).
| (3) |
In current ECT practice, the individual dose is typically adjusted by setting the total charge of the stimulus, as discussed in the next section.
II.3. Conventional procedures to individualize ECT dose
Modern ECT devices provide either individual dials or software menu settings for independent adjustment of stimulus parameters (e.g., amplitude, frequency, pulse width, and train duration) or a single dial (e.g., ‘Percent Energy’) that increments the total charge of the stimulus by a percentage of maximal charge by automatically setting the various stimulus parameters following a pre-programmed schedule. Conventionally, the stimulus dose is controlled by adjusting the number of pulses in the train by changing either the train duration or frequency; in some cases the pulse width is modified as well.11 The current amplitude is conventionally held fixed at the device maximum (e.g., 800 mA or 900 mA). In single-dial devices, manufacturers allow the user to select from a set of factory programs or to input a custom program. For example, the device can be programmed to first increment the stimulus duration until the device limit is reached and then to increment the train frequency, while keeping the current and pulse width fixed. An advantage of multi-dial devices is that there is more transparency over the individual parameter settings, whereas single-dial devices are simpler to use and may reduce operator error.
Modern ECT devices have constant-current output, meaning that the strength of the current during each pulse is regulated to be at a constant level regardless of the impedance seen between the electrodes.10 Thus, the main advantage of constant-current devices is that the delivered current is largely insensitive to the electrode–scalp impedance which may vary significantly among different patients, scalp preparation techniques, and amount of pressure applied to the electrodes. Nevertheless, the clinician has to ensure adequate scalp preparation and electrode coupling to avoid high electrode–scalp impedance that can result in delivery of excessive voltages, local heating, or failure to maintain the current at the prescribed level.9,12
In clinical practice, three approaches have been used to determine the ECT stimulus dose to be administered.9 With the first approach, the dose is set based on one or more factors that predict seizure threshold, such as electrode placement, sex, age, anesthetic dosage, and concomitant medication.13 In the second approach, the dose is specified as a multiple of the charge needed to induce a seizure in the patient in the first treatment session (the individual seizure threshold). The seizure threshold is determined via a method-of-limits procedure that involves delivering stimulus trains with increasing charge until a seizure is elicited.14 The third approach amounts to administering fixed high charge to all subjects; it is used mostly with unilateral electrode placement.15,16 The current APA Task Force Report on the Practice of ECT recommends that the dosage be individualized for each subject by adjusting it relative to either age/sex or seizure threshold.9
Below we present arguments regarding the limitations of charge as a dosing metric and propose alternative dosing strategies focusing on the individual stimulus parameters.
II.4. Limitations of defining ECT dose by total stimulus charge
Even though charge is widely used for dosing ECT in clinical practice and research studies, there are compelling arguments that ECT dose should be defined by the individual waveform parameters (e.g., pulse amplitude, shape, width, repetition frequency, train duration) rather than by a summary metric like total charge that absorbs these distinct parameters. The individual waveform parameters that constitute charge have disparate neurophysiological effects, as discussed in the subsequent sections. Lumping these parameters into the single summary metric of charge runs the risk of obscuring their distinct roles.
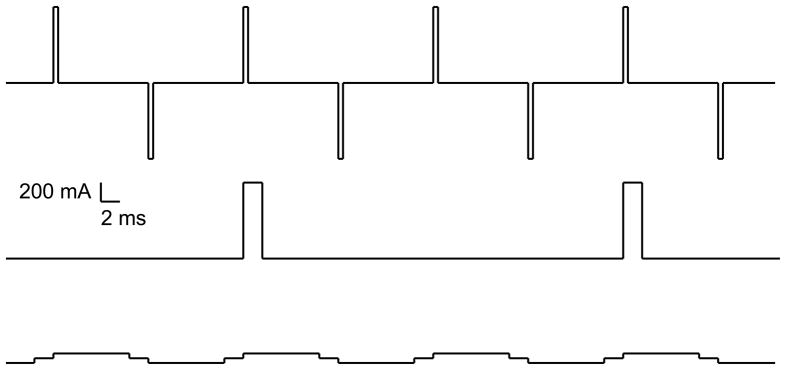
Infinitely many combinations of pulse width, amplitude, frequency, and train duration can result in the same total charge, as illustrated in Fig. 2, but the biological action of these different combinations can be quite distinct. For example, Swartz et al. showed that for fixed total stimulus charge, seizures are induced with a significantly higher success rate with briefer versus longer pulse widths.17,18 In these studies the briefer pulse width trains contained twice as many pulses as the longer pulse width trains in order to maintain fixed charge. Thus, variations in the pulse width and the number of pulses are not equivalent even if they result in the same charge. Furthermore, it has been shown that the individual seizure threshold in units of charge is lower when it is determined by incrementing the train duration than when it is determined by incrementing the train frequency19 and is lower for briefer pulse widths compared to longer pulse widths,5 indicating that these ingredients of charge have distinct contributions to the neurophysiological effect of the stimulus that are not captured by the total charge metric. If charge were the only salient metric, then the individual seizure threshold in units of charge would be the same regardless of the constituent parameter settings. Finally, total charge does not correlate with the safety of the stimulus either. This is clearly demonstrated in transcranial direct current stimulation (tDCS), a subconvulsive brain stimulation paradigm.20,21 During a typical tDCS session, a current of 1 mA is applied for 20 min through scalp electrodes; thus, the total administered electrical dose in terms of charge is 1,200 mC. This amount of charge is more than ten times the typical seizure threshold in ECT; yet, due to the low amplitude of the current, tDCS does not trigger a seizure and, in fact, barely produces any scalp sensation.
Figure 2.
Three ECT stimulus trains that each have a total charge of 3.2 mC but have widely different parameters. From top down: (1) bidirectional pulse train, amplitde = 800 mA, PW = 0.5 ms, frequency = 100 pps (50 pulse-pairs per second), 8 pulses; (2) unidirectional pulse train, amplitde = 800 mA, PW = 2 ms, frequency = 25 pps, 2 pulses; (3) bidirectional pulse train, amplitde = 50 mA, PW = 8 ms, frequency = 100 pps (50 pulse-pairs per second), 8 pulses.
Thus, as the field came to appreciate that all seizures are not equal, we also have evidence that all charge is not equal. Thinking of the ECT dose solely in terms of total charge may limit understanding of the biophysical mechanisms of ECT, impede attempts to further optimize dosing paradigms, and confound reporting and interpretation of treatment results and cognitive side effects. For example, seizure threshold titrations that increment frequency and/or pulse width deliver relatively inefficient stimuli to patients with high seizure thresholds, which result in measured seizure thresholds that are higher than they would be if the stimulus were titrated by increasing the train duration. Consequently, the difference between low- and high- seizure threshold patients is amplified by a reduction of the stimulus effectiveness at high doses. This is an artifact of the titration schedule itself, rather than a true indication of biological differences between patients. In addition, seizure threshold titration schedules vary across clinical programs, research groups, studies, and devices. These factors may have contributed to the very wide range of seizure thresholds reported in the literature (up to a factor of 50-fold in units of charge11,22). Furthermore, the common practice of setting the treatment stimulus charge at 6 times seizure threshold in unilateral ECT may be relatively less effective if it is implemented by increasing the frequency and/or pulse width than if it is implemented by lengthening the train duration, although the same total charge is delivered in both cases. The clinical relevance of this is still unknown, but it could be one reason for different outcomes using ostensibly equivalent doses based on charge.
Focusing exclusively on charge as the ECT dosing metric has also obscured the significance of some key stimulus parameters. For example, the role of current amplitude has been largely unexplored in modern ECT studies.9 The majority of ECT studies use fixed amplitude of 800 mA or 900 mA, but the theoretical or empirical justification for this practice is unclear. Further, total charge is insensitive to other stimulus parameters such as directionality (bidirectional versus unidirectional) and polarity (anode and cathode assignment for unidirectional stimulation) that may significantly affect the neurophysiological response to ECT.22,23
It should be noted that no other brain stimulation paradigm uses summary metrics such as total charge or energy to quantify the stimulus. In repetitive transcranial magnetic stimulation (rTMS), the pulse amplitude and the train frequency and duration are explicitly specified and adjusted.24 Similarly, the doses of tDCS and of deep brain stimulation (DBS)25 are defined by the individual stimulus parameters, not by charge or energy.
III. Understanding the ECT stimulus parameters
Various authors have acknowledged that there is insufficient understanding of the role that ECT stimulus parameters play in clinical outcome and have called for more research in optimizing these parameters.10,26–28 In this section we attempt to link the biophysics of ECT to empirical evidence of the role of individual stimulus parameters, which may guide future work on a rational approach to optimizing ECT dosage.
III.1. Overview of biophysical mechanisms of ECT
ECT induces an electric field in the brain which results in modulation of neural activity and seizure induction. Here we focus on the biophysical mechanisms linking the electrical stimulus parameters to neural activation and seizure induction, which have received relatively little coverage in the ECT literature to date. Understanding of these mechanisms can inform empirical studies to optimize the ECT parameters on a physiological basis. This analysis can be readily extended to magnetic seizure therapy (MST) which uses magnetically induced currents, instead of currents delivered through cranial electrodes, to elicit therapeutic seizures.29,30 For a discussion of the putative therapeutic mechanisms of seizures, the reader is referred to other publications.16,22
III.1.a. Induced Electric Field
The ECT device applies an electrical potential across the ECT stimulating electrodes held on the scalp. This induces an electric field in the brain that varies in strength depending on the location within the brain. When a train of electrical potentials (pulses) is applied, the induced electric field varies over time synchronously with the electrical potentials.
At the relatively low frequencies used in ECT, the electric field can be modeled using the quasistatic approximation.31–33 Essentially, the quasistatic approximation assumes that at the macroscopic level the head tissues are purely resistive, ignoring tissue capacitance. Even though the electrode–scalp impedance is nonlinear and has capacitive components,7,10 these impedance characteristics do not substantially affect the shape of the current pulses injected in the head since modern ECT devices have constant-current output. Thus, the electric field variation over time is directly proportional to the electrode current waveform for constant-current ECT devices. That is, if we measure the electric field waveform at any point inside the brain, it will look like the waveform of the ECT stimulus current. Importantly, the magnitude of the electric field is directly proportional to the stimulus current amplitude. The distribution of the electric field strength in the head (i.e., the strength of the electric field at one location in the brain versus another) depends on the electrode geometry, size, and position; the spread of the electrode gel; and the geometry and conductivity of the various tissues in the head (head anatomy).
The key insights from this analysis are that (1) the ECT electrode configuration and the patient’s head anatomy determine the electric field distribution in the brain, (2) the strength of the electric field can be directly controlled by adjusting the amplitude of the ECT stimulus current, and (3) the temporal variation of the electric field seen by the individual neurons approximately matches the ECT stimulus current waveform. Thus, the ECT operator can manipulate the electrode configuration and the stimulus parameters in order to control the electric field characteristics.
III.1.b. Neural Response to Induced Electric Field
The electric field induced by ECT affects brain activity by depolarizing and hyperpolarizing neuronal membranes.34 Stimulation with extracellular electric fields initiates action potentials in the axon, and not in the soma or dendrites.35–39 The sites where action potentials are most likely to occur are the initial segment of the axon close to the soma, the axonal synaptic terminals, or sharp bends in axons such as those of pyramidal neurons.36,40–45 An action potential is followed by depolarizing and hyperpolarizing afterpotentials of the neural membrane46,47 as well as by modulations of activity in the associated neural networks,48 which can alter the effect of subsequent pulses.
III.1.c. Mechanisms of Seizure Induction
Work with in vitro brain slices has revealed some aspects of the mechanisms involved in seizure elicitation by electrical stimulation.49 A cascade of reactions involving metabotropic glutamate receptors and GABA50 eventually result in accumulation of extracellular potassium that can sustain persistent neural activity over relatively long periods of time.51 The activity of individual neurons becomes synchronized via a combination of mechanisms including synaptic interactions and non-synaptic field effects. Repetitive synchronous discharges are likely to promote epileptic activity by potentiating excitatory synapses,52 and by decreasing the efficacy of inhibitory synapses.53,54 Thus, the elicitation and maintenance of ictal activity involves complex acute and cumulative processes at the level of the individual neuron and neural ensembles.
It should be noted that a seizure is not sufficient for therapeutic benefit. Generalized tonic-clonic seizures can be therapeutically ineffective, as in the case of low-dose right unilateral (RUL) ECT4,55,56 and ultrabrief-pulse bilateral (BL) ECT.5 The electrical stimulus affects clinical outcome beyond its role as an ictal trigger, as exemplified by the efficacy of markedly suprathreshold RUL ECT.4,5 Thus, besides inducing a seizure, the stimulus train may effect other neuromodulatory processes that could be therapeutically salient26 (see also discussion in Sec. II.2.h).
III.2. Role of individual ECT stimulus parameters
In the subsequent sections we review evidence of the role of individual stimulus parameters in seizure induction and in the clinical outcome of ECT, and attempt to link it to underlying biophysical mechanisms.
III.2.a. ECT electrode configuration
The geometry of the ECT electrodes and their position on the head are the major determinants of the spatial distribution of the induced electric field, as discussed in Sec. III.1.a. Therefore, it is not surprising that the electrode configuration plays a central role in the therapeutic action and side effects of ECT. The electrode configuration determines the relative degree of stimulation of the various brain regions and can, therefore, be used to target specific structures that may confer therapeutic benefit, while avoiding other regions that may contribute adverse side effects. Electrodes spaced close together produce more focal electric field but they also effect more current shunting in the scalp and a weaker electric field in the brain (which could be compensated for by administering higher amplitudes as long as the charge density in the scalp stays within safe limits). When the electrodes are close together, as in some unilateral configurations, the electric field maximum in the cortex lies between the two electrodes, whereas when the electrodes are far apart, as in conventional BL configurations, there are local electric field maxima under each electrode.57 Further, smaller electrodes are more focal than larger electrodes, although shunting in the scalp limits the focality achievable in the brain.22,57
Here we provide a brief overview of electrode configurations used in ECT which are more thoroughly discussed in the article by Kellner et al. in this edition. The two most commonly used ECT electrode configurations are the conventional bifrontotemporal BL placement in which the electrodes are positioned at the midpoint between the canthus and the tragus, and the d’Elia RUL placement in which one electrode is located frontotemporally and the other is located parietally, lateral to the vertex.9 Brief-pulse bifrontotemporal ECT is highly effective with 70–80% response rate; however, it may result in significant adverse cognitive side effects.4,5,56 Adequately dosed RUL ECT can be as effective as BL ECT but with fewer cognitive side effects,4,5,15,58 putatively because it produces more focal stimulation with relatively less involvement of temporal regions.3,59,60
In addition to the conventional BL and RUL placements, a number of alternative electrode configurations have been explored as a means of enhancing therapeutic efficacy and reducing side effects. These include RUL with more closely spaced electrodes,61,62 frontotemporal–mastoid,63 and left unilateral placement.16 There is significant interest in anterior electrode positions that target frontal circuits involved in the etiology of depression and in treatment response64–66 while diminishing temporal lobe stimulation which is linked to cognitive side effects.67 Proposed configurations include bifrontal,68–78 left anterior–right temporal (LART),79–81 fronto–vertex,82 right fronto–parietal,83–86 right fronto (small electrode)–partietal (large electrode) (for focal electrically administered seizure therapy, FEAST),22,23 as well as frontal coil positions in magnetic seizure therapy (MST).30,87,88
The biophysics of ECT argues that refinements of electrode configuration are key to targeting the intervention, but the optimal electrode configuration remains a matter of considerable debate. Advancements in understanding of the neural circuits involved in depression66,89 coupled with anatomically-accurate computer models of the induced electric fields90–92 could be harnessed to elucidate the mechanisms of ECT with conventional electrode placements as well as to aid the development of novel, rationally-designed electrode configurations.
III.2.b. Pulse amplitude
The magnitude of the induced electric field is directly proportional to the amplitude of the ECT current pulses, as noted in Sec. III.1.a. Depolarization and hyperpolarization of neural membranes are, in turn, proportional to the local electric field magnitude.34 Neurons that are depolarized to their firing threshold generate action potentials. Therefore, of all waveform parameters, the pulse amplitude provides the most immediate control over the volume of neural tissue that is directly activated by the ECT stimulus.
The central role of the pulse amplitude in controlling the volume of stimulated neurons is widely recognized in DBS and in rTMS; the pulse amplitude is, therefore, a key dosing parameter in these brain stimulation therapies. In clinical practice, the DBS pulse amplitude is empirically adjusted to be below the threshold of adverse side effects which is linked to the extent of electric field spread beyond the targeted brain structures.93 Recently developed detailed computer simulations can predict accurately the volume of neural activation by DBS94,95 which could be used as a more systematic approach to choosing the pulse amplitude than trial and error. In rTMS, the pulse amplitude is routinely set relative to the patient’s motor threshold which is derived by single-pulse amplitude titration.24 In some cases, the rTMS pulse amplitude is also adjusted based on the coil-to-cortex distance.96,97
Within a brain region, incrementing the pulse amplitude increases the number of recruited neurons until the entire neuronal population is activated, as demonstrated by measurements of motor input-output curves with transcranial electrical stimulation (TES) and TMS.98 On a larger scale, the dependence of the volume of neural activation on the pulse amplitude has been directly demonstrated by measuring the latency of corticospinal volleys evoked by TES and TMS for various pulse amplitudes in anesthetized humans99 and nonhuman primates.100 Increasing the amplitude of the TES pulses shifts the site of action potential initiation from the level of the cortex to deeper locations, reaching as deep as the brain-stem.
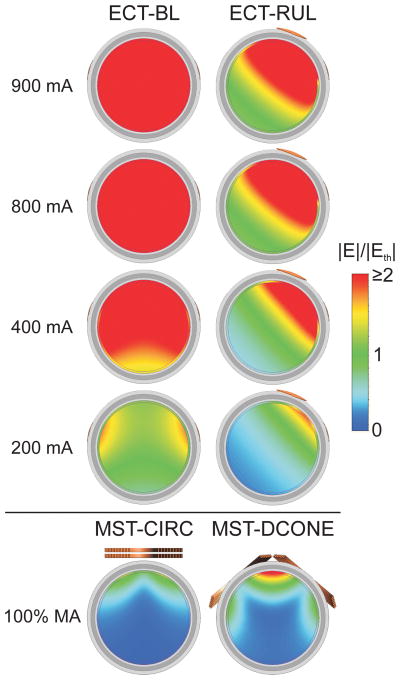
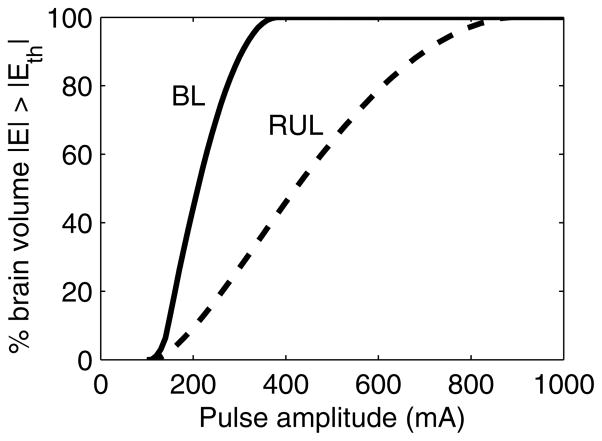
Fig. 3 illustrates with a computer simulation the effect of current amplitude on the volume of neurons directly activated by individual ECT pulses. At the conventional 800/900 mA current levels, the whole brain is exposed to suprathreshold electric fields in both BL and RUL ECT.60 Lowering the current amplitude reduces the activated brain volume. This relationship is more linear for the RUL configuration which is fundamentally more focal. Finally, a simulation of MST provided for comparison in Fig. 3(a) demonstrates that electric fields that are substantially more focal and less intense than those in ECT can be used for seizure induction. This simulation suggests that reduction of the current amplitude along with the use of focal electrode configurations could achieve more targeted stimulation in ECT, though the clinical impact of such manipulations will need to be determined.
Figure 3.
(a) Computer simulation with a spherical head model illustrating the electric field strength (E) relative to neural activation threshold (Eth) for conventional bilateral (BL) and right unilateral (RUL) ECT with a range of current amplitudes (PW = 0.5 ms). Coronal view is shown with tissue layers consisting of (from outer shell inward) scalp, skull, cerebrospinal fluid, gray matter, and white matter. Simulations of MST with circular (CIRC) and double-cone (DCONE) coils at 100% maximum amplitude (MA) of the Magstim Theta device (Magstim Co., Whitland, UK) are shown for comparison. Simulation methods are described by Deng et al.60 (b) Percentage suprathreshold stimulated brain volume as a function of ECT current amplitude derived from the spherical head model simulation.
Given its central role in governing the spatial extent of neural activation, it is surprising that pulse amplitude has received relatively little attention in contemporary ECT studies.9 As previously noted, conventional ECT paradigms use a fixed current amplitude of 800 mA or 900 mA. The lack of attention to the concept of pulse amplitude modification is reflected in the design of ECT devices which provide only a fixed current amplitude (e.g., 900 mA for Thymatron System IV) or a narrow range of adjustability (500–800 mA for MECTA Spectrum). The choice of these current levels has not been rigorously justified and may have originated as a consequence of the relatively short train durations (≤ 2 s) used in early models of these devices, since briefer train durations require larger current amplitude to induce seizures (see Sec. III.2.h). Indeed, while present practice is to leave the current setting fixed at 800 mA or 900 mA for all patients, below we discuss evidence that current amplitudes lower than these levels can trigger adequate seizures and can have therapeutic effect with potentially reduced side effects.
Based on computer simulations, the motor excitation threshold for TES with a unilateral electrode configuration in humans has been estimated to be 175–269 mA corresponding to pulse widths of 1–0.05 ms, respectively.101 These data are consistent with measurements in anesthetized humans where motor thresholds of 170–420 mA for 0.05-ms pulse width were reported.102 Further, cadaver measurements and computational modeling show that for 800-mA, 0.5-ms current pulses, the electric field in the brain exceeds the threshold for robust neural activation by a factor of five or more.60,90,103 Finally, computational modeling studies have also shown that the individual variability of anatomical parameters such as scalp and skull thickness and conductivity, and skull-to-cortex distance can substantially alter the electric field magnitude in the brain with conventional electrode placements and fixed current.60 To compensate for these individual differences, the current amplitude could be individualized to yield comparable cerebral electric field magnitude.
Few studies have measured the seizure threshold in terms of pulse amplitude in humans, but available data support the presence of significant individual variability and the adequacy of currents lower than 800/900 mA. Friedman and colleagues reported thresholds of less than 100 mA with half-sine pulses (vertex–temporal, unidirectional, PW = 8.3 ms, frequency = 60 pps continuous or in bursts repeating at ≤ 10 Hz, duration = 0.1–4 s).104,105 Liberson reported current thresholds of 233–540 mA (vertex–temporal, unidirectional, PW = 0.5–0.7 ms, frequency = 120 pps, 120 pulses) and 400–650 mA (bitemporal, unidirectional, PW = 0.7 ms, frequency = 120 pps, 156 pulses), with older patients having higher thresholds.106 Liberson claimed that this ECT technique had excellent therapeutic benefit with minimal side effects. Weaver et al. reviewed the ECT literature and found a current amplitude range of 30–2,000 mA used for seizure induction.107 In their own study, this group determined pulse amplitude seizure thresholds of 110–250 V (corresponding to 363–825 mA at the reported nominal impedance of 303 Ω) (RUL, bidirectional, PW = 1 ms, frequency = 91 pps, 150–300 pulses),108 and later summarized the current amplitude data from various protocols yielding a mean of 587 mA and a range from 300 mA to over 900 mA.109 Gordon observed typical current thresholds in the 250–600 mA range (unidirectional, PW = 1 ms, frequency = 50 or 100 pps), with some patients seizing with currents as low as 70 mA.110,111 Zyss et al. quoted a seizure threshold range of 300 mA for younger patients to 500 mA for elders.112 Furthermore, some studies have used fixed pulse amplitudes that are significantly lower than those used in modern ECT. Hilkevitch reported successful seizure induction with current amplitudes of 250 mA (unidirectional, PW = 2 ms, frequency = 200 pps).113 Finally, MST can induce generalized tonic-clonic seizures with electric field pulse amplitudes and pulse widths much lower than those induced by conventional ECT.60,88,114
The wide range of current amplitudes in the above reports is likely due in part to differences in the other pulse train parameters (such as pulse width, frequency, and number of pulses) and other aspects of the ECT procedure (such as anesthesia). Nevertheless, it is clear that (1) current amplitudes below the conventionally used 800/900 mA can elicit adequate seizures, and (2) there likely is variability in the individual current amplitude threshold that could be due to differences in head anatomy, age, neural excitability, and other physiological factors. Indeed, the variability of ECT seizure threshold and clinical outcome10,11 may be due, in part, to anatomical variability which is not properly accounted for with conventional train duration and frequency titration, but could be accounted for by amplitude adjustment.
Notably, early attempts to introduce low-amplitude, ultrabrief-pulse ECT paradigms may have failed to gain ground in part due to the lack of significant cognitive disruption.62 Since anesthesia was not used in early ECT, the patients could remain conscious and remember the painful sensation associated with the initial moments of stimulation with low-amplitude, ultrabrief pulses before a grand mal seizure is induced, which would cause apprehension of continuing the treatment.106,115,116 In contrast, the conventional sine-wave technique would produce instant unconsciousness and complete amnesia of the treatment session.115,116 Since anesthesia is used in modern ECT, the diminished ability of low-amplitude, ultrabrief pulses to induce unconsciousness and amnesia appears to be beneficial rather than detrimental as it could be indicative of reduced adverse cognitive sequelae.
III.2.c. Pulse shape
Modern ECT devices use trains of monophasic rectangular pulses with alternating current direction [Fig. 1(b)]. Rectangular pulses are universally used for neural stimulation;117 however, the alternating polarity of the pulses is unique to ECT. The origins of the shape of the ECT pulse train are historical. Originally, ECT was delivered with sine waves at the power-line frequency (50 Hz in Europe, 60 Hz in the USA).118 The sine wave has both positive and negative phases [Fig. 1(a)]. After it was recognized that the long duration and the slow rise and fall times of the line-frequency sine wave are physiologically inefficient and cause adverse side effects, the pulses were made shorter by substituting brief rectangular pulses where the peaks of the sine wave would have been.7 A biophysical rationale for this characteristic waveform of ECT has not been presented, and there is renewed interest in the use of unidirectional pulse trains [Fig. 1(c)] for improved specificity of stimulation22,23,26 (see also discussion in Sections III.2.e–d).
Regardless of the directionality of the stimulus train, it has been established that brief rectangular pulses with almost instantaneous rise time are more efficient for neuronal excitation than 50/60 Hz sine waves (PW = 8.3/10 ms).8,119 In this case, the advantage of the rectangular pulse likely stems not only from its brevity, but also from its almost instantaneous rise and fall times compared to the slow rise and fall times (~ 4 ms) of the sine wave, since slow rise times cause accommodation resulting in an increased action potential threshold.10,117 By dramatically lowering the cognitive side effects of ECT without sacrificing efficacy, the shift from sine-wave to brief-pulse stimuli represented a major advance in the risk/benefit ratio of ECT.
Over the years, in addition to sine-wave and rectangular pulses, a variety of other pulse shapes have been used in ECT devices;105,110,120,121 these shapes, however, typically resulted from limitations of contemporary technology rather than from sound biophysical considerations. Offner theoretically proved that a rising exponential current waveform uses the least amount of energy to depolarize a neuron, but noted that rectangular pulses use only 22% more energy and recommended use of the latter since they are easier to generate electronically.122 Indeed, it has been pointed out the shape of the pulse is of less importance than the pulse width which should be close to the chronaxie.119 Thus, rectangular pulses appear a sound choice for the ECT stimulus; research efforts should be directed at optimizing the parameters of the rectangular pulses (width and amplitude) and of the pulse train (frequency, directionality, etc.).
III.2.d. Pulse width
Modern ECT uses rectangular, constant-current pulses that are briefer that the 8.33–10 ms sine pulses of the original ECT technique. Pulse widths in the 0.5–2.0 ms range, classified as “brief,” have been standard since the 1980’s. However, interest in ultrabrief-pulse (PW < 0.5 ms) paradigms is growing due to evidence of their comparable efficacy but reduced side effects relative to the conventional brief-pulse technique.
Some early studies provided evidence of preserved efficacy and lower side effects with brief and ultrabrief stimuli compared to sine-wave ECT.62,68,106,119,120 Other studies, however, concluded that ultrabrief pulses were not superior to wider pulse widths.121,123 Some of these studies were poorly controlled and were confounded by simultaneous variations in multiple parameters124 and may have used suboptimal stimulus dosing.22 It was not until the 1980’s that conclusive evidence emerged that brief-rectangular-pulse ECT could match the efficacy of sine-wave ECT while dramatically reducing the adverse cognitive side effects.3,125,126 Since then there has been growing interest in reducing pulse width into the ultrabrief range to further enhance the risk/benefit ratio.
It has been firmly established that briefer pulses require less total energy and charge to induce seizures than do longer pulses.5,109,119,120,127–129 It has also been shown that briefer pulses result in weaker seizure expression and fewer EEG abnormalities following treatment.107,119,130 Modern studies providing evidence that ultrabrief-pulse ECT can be as effective as brief-pulse ECT, while having reduced side effects, have appeared only recently. Sackeim et al. compared ultrabrief (PW = 0.3 ms) and brief (PW = 1.5 ms) stimuli in RUL ECT at 6 × seizure threshold and in BL ECT at 2.5 × seizure threshold (bidirectional, 800 mA, 80–100 pps).5 Ultrabrief BL ECT was found to be ineffective, whereas the other three conditions were equally effective (within the limitations of the study size of 22–23 patients per group). The lack of benefit with ultrabrief BL ECT was an unexpected outcome that complements the data on inefficacy of RUL ECT at doses close to seizure threshold4 to provide a stark demonstration that an “adequate” seizure is not sufficient for efficacy and that therapeutic benefit is powerfully affected by the electrical stimulus parameters. The two ultrabrief ECT groups had less cognitive side effects compared to the brief groups, which is remarkable since the former received 1.5–3 times more pulses during treatment than the latter. Studies by Loo and colleagues replicated the findings of equivalent efficacy and lesser cognitive side effects of ultrabrief (0.3 ms) relative to brief (1 ms) RUL ECT, although they noted slower response rate with the ultrabrief stimulus.129,131 This group has also noted diminished adverse cardiac effects with ultrabrief pulses (0.3–0.7 ms) compared to brief pulses (1 ms).78 Some reports of the use of ultrabrief RUL ECT in hospital settings have supported its efficacy and reduced adverse sequelae,132 although McCormick et al. have shown slower response and diminished efficacy with this paradigm compared to brief BL ECT.133 It should be noted that in the McCormick et al. study the tabulated ultrabrief treatment dose was only 4 × seizure threshold, even though it was reported as 6 × seizure threshold, which could have resulted in reduced efficacy. Sienaert et al. compared ultrabrief (0.3 ms) unilateral and bifrontal ECT, and found both to be efficacious and lacking cognitive side effects.77,134 Finally, Pisvejc et al. compared brief (1 ms) and ultrabrief (0.2–0.4 ms) unilateral ECT in a sample of mostly schizophrenic patients, and reported marked efficacy and lack of deleterious effects on cognition and memory, with no differences between the two groups.135 However, the latter study is confounded by variation in multiple parameters besides the pulse width between the two groups.
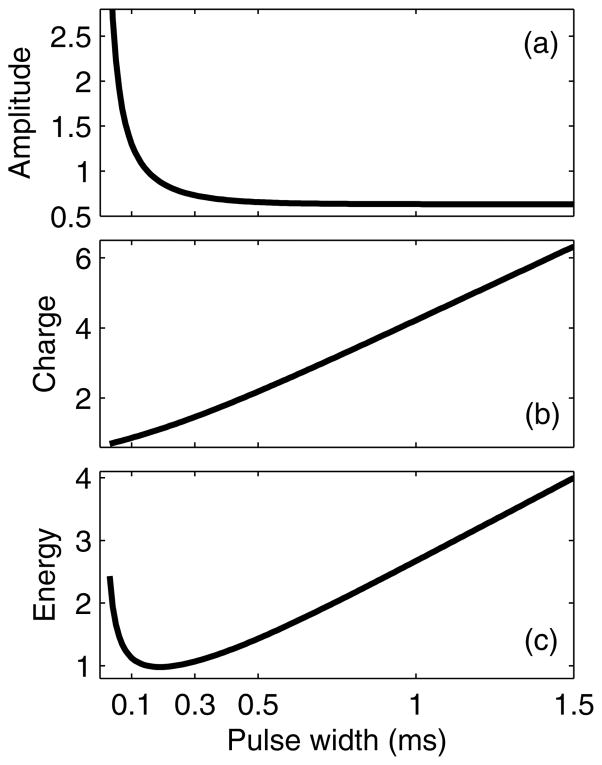
Reduced charge and energy required for seizure elicitation with briefer pulses has a relatively simple explanation when the biophysics of neural stimulation with extracellular electric fields is considered. The electrical response of neuronal membranes is associated with a characteristic time constant (or chronaxie); therefore, the membrane depolarization resulting from the externally applied electric field depends not only on the electric field pulse amplitude, but also on the pulse shape and width.136 For pulse widths below the chronaxie, the depolarization is directly proportional to pulse width, whereas for larger pulse widths the depolarization reaches a plateau that is independent of pulse width [Fig. 4(a)]. The chronaxie for transcranial stimulation is around 0.1–0.2 ms matching the values of axonal chronaxie35,137–139 since extracellular electric fields preferentially activate axons rather than soma.35,36,45 Consequently, the relation between the pulse width and the threshold current amplitude required to induce neuronal firing is described by the well-known strength–duration curve shown in Fig. 5(a).10 Similar curves can be constructed for the pulse charge and pulse energy for threshold stimulation, as shown in Fig. 5(b) and Fig. 5(c), respectively.140 The longer the pulse is relative to the chronaxie, the more charge and energy are used to depolarize the neuron to the action potential threshold. Therefore, it is not surprising that ECT paradigms using briefer pulses are more efficient in terms of total charge and energy delivered.
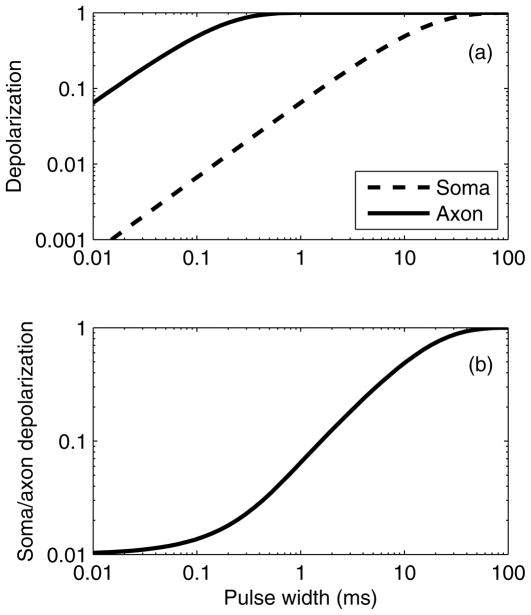
Figure 4.
(a) Soma and axon depolarization induced by a single rectangular electric field pulse. Curves are normalized to depolarization by direct current (infinite PW). The soma and axon are assumed to have chronaxies of 10 ms and 0.1 ms, respectively. (b) Ratio of soma depolarization to axon depolarization.
Figure 5.
Strength–duration (a), charge–duration (b), and energy–duration (c) curves for ECT, assuming neural chronaxie of 0.1 ms. The curves are normalized to unity for PW equal to the chronaxie.
Whereas the reduction of total charge and energy with briefer pulses is largely explained by the charge–duration and energy–duration curves, the reduction of cognitive side effects with briefer ECT pulses is not readily accounted for. The charge density and charge per pulse have been linked to tissue damage in neural stimulation,141,142 and the total energy is proportional to tissue heating;122 there is, however, no evidence of tissue damage in ECT.12,143–146 A more plausible explanation is that decreasing the pulse width reduces the volume of directly stimulated tissue, since the briefer pulses produce less membrane depolarization. From the plot in Fig. 4(a), it can be shown that a 0.3-ms ECT pulse induces 14% less axonal depolarization than a 1.5-ms pulse, implying that some neurons that are activated by the 1.5-ms pulses will not be activated by the 0.3-ms pulses. Further, briefer pulses preferentially affect the axons, whereas longer pulses may also have a significant effect on the soma and dendrites.27 The membrane of the soma and dendrites has a time constant that is two orders of magnitude larger than that of the axon;35 therefore, the axonal and somatic/dendritic membrane potentials can have substantially different dynamic response when exposed to various pulse widths, as shown in Fig. 4(a). Fig. 4(b) indicates that the ratio of soma and dendrite depolarization to axon depolarization is more than 4 times larger for PW = 1.5 ms compared to PW = 0.3 ms. These estimates are derived from a very simplistic model, but they do illustrate the possibility of differential impact of pulse width on the distinct neuronal processes (soma and dendrites versus axons) which could have wide-ranging implications on how individual neurons and neural networks respond to ECT with various pulse widths.
Finally, the above discussion suggests that there is no compelling reason to push pulse width below 0.2 ms, because for PW < 0.2 ms the differential effects between the axons and soma/dendrites disappear [Fig. 4(b)], tissue capacitive effects start to reduce the effective electric field magnitude,33 and exponentially higher voltages are required to induce adequate neural depolarization [Fig. 5(a)]. Thus, empirical evidence and biophysical considerations suggest that the pulse width should be kept around 0.2 ms.
III.2.e. Directionality
Even though most modern ECT devices deliver bidirectional stimuli [Fig. 1(b)], unidirectional stimuli in which the pulses are of only one polarity [e.g., see Fig. 1(c)] have also been widely used in various ECT devices and studies.58,104,106,110,120,121,147 There is renewed interest in the use of unidirectional pulse trains as a means of enhancing the stimulus efficiency and focality;22,23,26 however, there still is a notable paucity of research to elaborate the significance of current directionality.
Early ECT studies with unidirectional stimuli reported substantially lower seizure thresholds, comparable clinical efficacy, and significantly lower memory deficits compared with bidirectional ECT.68,104,105,148,149 In these studies, however, current directionality was confounded with other factors such as pulse amplitude, shape, and width, and train frequency and duration. Other authors have asserted that there is no substantial difference between unidirectional and bidirectional stimulation threshold for after-discharge induction150 and seizure elicitation.119
More recently, rTMS studies have compared the neuromodulatory effectiveness of unidirectional and bidirectional stimulation. rTMS is typically given with devices that induce bidirectional current flow in the brain; however, a series of findings indicates that unidirectional rTMS produces stronger cortical inhibition and facilitation at low and high frequencies, respectively, relative to bidirectional pulse trains.151–157 These observations support a reexamination of unidirectional stimulation in ECT.
Based on work showing polarity-specific response characteristics in TES,40,41 Sackeim hypothesized that unidirectional currents coupled with different size electrodes (FEAST) would increase the focality and efficiency of the ECT stimulus.22 To evaluate this idea, Spellman et al. compared unidirectional and bidirectional currents in nonhuman primates, showing that unidirectional stimulation has 12.8% and 8.1% lower seizure threshold with FEAST and BL electrode configurations, respectively.23
A factor contributing to the threshold reduction with unidirectional stimuli could be their non-zero average current which is not present in bidirectional stimuli where each pulse is counteracted by an identical pulse in the opposite direction. The average current of unidirectional rectangular stimuli equals the product of the pulse amplitude, width, and repetition frequency. For example, for amplitude = 800 mA, PW = 0.5 ms, and frequency = 100 pps, the average current is 40 mA, which is 40 times the current typically used in tDCS. This average current component could produce polarization effects in the soma and dendrites which have large (10–30 ms) time constants35 and, therefore, act as integrators. However, if this effect is present it must be small. Liberson experimented with introducing a direct current bias in the unidirectional stimulus to make the average current zero and concluded that this bias did not affect the seizure threshold, although he did not present quantitative data to support this claim.119 Of course, there is the possibility that the non-zero average stimulus current may affect other aspects of the neural response besides the seizure threshold.
Finally, no safety concerns specific to unidirectional ECT pulse trains have been reported. Thus, in view of the evidence of some potential advantages of this paradigm, unidirectional ECT stimuli should be explored further.
III.2.f. Polarity
Polarity is irrelevant to conventional bidirectional ECT where the pulses alternate polarity; however, it may have significance in unidirectional ECT paradigms. The use of unidirectional stimulation enables one to spatially separate the anode (positive electrode) from the cathode (negative electrode), whereas in conventional bidirectional ECT the two electrodes alternate between serving as the anode and the cathode during the stimulus train.
Studies with TES have found lower thresholds for motor response when the motor cortex is stimulated with the anode than when it is stimulated with the cathode.158,159 Similarly, direct stimulation of motor and somatosensory cortices has revealed lower thresholds with anodal than with cathodal stimulation.160 Furthermore, anodal stimulation appears to excite white matter fibers resulting in a highly consistent response, whereas cathodal stimulation activates the initial axonal segment close to the soma producing a more variable response.40,41 The threshold and latency of motor evoked potentials induced by TMS depends on the orientation and polarity of the current flow in the motor strip suggesting that different neural populations or different axonal sites are recruited.161,162 Finally, work with tDCS which stimulates below threshold for action potentials indicates that the anode potentiates whereas the cathode inhibits endogenous activity and responses to suprathreshold stimulation.20,21
On the other hand, there is evidence that the sensitivity of neurons to the polarity of pulsed stimulation is limited. The application of extracellular electric fields results in both regions of depolarization and regions of hyperpolarization within the same neuron.45,163 Even when the electric field pulse produces initial hyperpolarization in certain parts of the neuron, the neuron can still fire after the end of the pulse due to a depolarizing overshoot of the membrane potential during recovery from hyperpolarization (anodic break)117,164 and/or due to transfer of charge from depolarized compartments of the neurons such as the soma.45 It has also been noted that the polarity of unidirectional stimuli is relatively unimportant for after-discharge induction.150 Thus, electric field pulses of either polarity may trigger action potentials with comparable thresholds.
Data on the effect of polarity of unidirectional stimuli in ECT are scarce. Working with low-amplitude unidirectional currents, Friedman and Wilcox observed lowest seizure thresholds with negative electrode on left temple and positive electrode on vertex104 and adopted this placement and polarity in other studies.105 More recently, it has been argued that ECT stimuli can be made more targeted by the use of unidirectional stimuli with the anode providing preferential activation, as noted in the previous section.22 Studying the FEAST electrode configuration in nonhuman primates, Spellman et al. did find polarity dependence of the EEG response but not of the seizure threshold:23 positive frontal electrode yielded higher ictal power and more lateralization, whereas negative frontal electrode yielded more post-ictal suppression.
Thus, at present there are limited data on the effects of stimulus polarity; this parameter should be explored further within studies of unidirectional ECT.
III.2.g. Frequency
Even though stimulus frequency is commonly adjusted to individualize the ECT dose, its role in seizure induction and clinical outcome is not well understood. The interpulse interval, equal to the inverse of the pulse repetition frequency, affects the neural response to the stimulus. Each stimulus pulse sets off a cascade of dynamic processes that transiently alter the state of the activated neurons and neural networks. Therefore, the subsequent stimulus pulse impinges upon a modulated state of the system that depends on the time elapsed since the previous pulse, i.e., it depends on the stimulus frequency.
rTMS studies have shown that frequencies around 1 pps have a lasting inhibitory effect, whereas frequencies ≥ 3 pps can have an excitatory effect and could trigger a seizure if delivered with a sufficiently high pulse amplitude and train duration.165,166 Effective frequencies for photic seizure induction are in the 3–20 pps range.167 For the induction of after-discharges, which are considered precursors to a seizure, frequencies below 10 pps are ineffective, frequencies in the range of 25–60 pps are optimal, and frequencies above 100 pps are still effective but less suitable for the production of long-lasting after-discharges.150 In line with this view, high-frequency (> 50 pps) stimulation has been shown to suppress ongoing ictal activity,168 suggesting that high frequencies may not be efficient at triggering seizures. Furthermore, very high frequencies (> 500 pps) may be inefficient due to stimulation of the neurons during their absolute refractory period which lasts for 1–2 ms—this phenomenon has been called “stimulus crowding”.10
Consistent with these considerations, data from ECT studies suggest that low frequencies are more efficient for seizure induction than high frequencies. Weaver et al. studied frequencies in the range of 20–91 pps (RUL placement, bidirectional, amplitude = 200 V, PW = 1 ms, 150 pulses), and chose 32 pps as optimal since for these settings initiation of convulsive activity coincided approximately with the end of the stimulus train, whereas with lower and higher frequencies it occurred before or after the end of the stimulus, respectively.108 The authors also noted that based on clinical observations, 20 pps (10 pulse pairs per second) may be close to the lower limit of usefulness. These data can also be interpreted to indicate that frequencies in the range of 20–32 pps are more efficient than 32 pps, since the convulsion is initiated during the pulse train, i.e., with fewer pulses than required at 32 pps. A study by Swartz and Larson suggests that stimulus frequency of 60 pps was more efficient than 120 pps at inducing seizures (BL placement, bidirectional, amplitude = 800 mA, PW = 1.5 ms, 120 pulses).17 However, a similar study found no difference in seizure induction efficiency between 60 pps and 120 pps frequency (LART placement, bidirectional, amplitude = 900 mA, PW = 0.5 ms and 1 ms, charge = 2.27 mC per patient’s year of age).18 Devanand et al. showed that the seizure threshold is lower when titrated by stimulus duration (0.5–4 s) than when titrated by frequency (40–280 pps) with BL ECT (bidirectional, amplitude = 800 mA, PW = 1 ms),19 supporting the view that lower frequencies are more efficient. Direct evaluation of seizure threshold at 50 pps and 200 pps with BL169 and RUL170 placements (bidirectional, amplitude = 800 mA, PW = 1 ms) confirms that the lower frequency requires a smaller number of pulses (and, hence, total charge) to trigger a seizure. Further, the two frequencies are found to result is equal seizure duration, cardiovascular response, and efficacy, with a trend of lower cognitive side effects with 50 pps.170 Thus, the evidence suggests that low frequencies compared to high frequencies are more efficient for seizure induction, although an optimum frequency has not been determined. The data on frequency dependence of efficacy and side effects are limited.
Finally, above we discussed ECT stimulus trains delivered at a single, constant frequency. There are also neural stimulation paradigms that use temporal pulse patterns having two or more frequency components. For example, theta-burst stimulation consists of brief high-frequency bursts (e.g., 50 pps) repeated at a lower frequency (e.g., 5 pps), and sometimes interrupted at an even lower frequency (e.g., 0.1 pps). Theta-burst stimulation has been used in long-term potentiation (LTP) and long-term depression (LTD) protocols, and in rTMS paradigms to effect rapid, strong neuromodulatory effects that can be excitatory or inhibitory depending on the specific stimulus parameters.171,172 Intermittent ECT stimuli similar to theta-burst have been deployed in a number of older ECT studies,58,68,104,105,120,123,148 were in common use for decades in Europe as a feature of the Siemens Konvulsator device (50-Hz bursts of 4 unidirectional quarter-sine pulses, repeated at 6 Hz),58,147 and are an option is some modern devices such as the Thymatron System IV.173 Some researchers have recommended the use of intermittent stimuli as superior to continuous pulse trains;147 however, a systematic comparison of these two paradigms is lacking and should be addressed in future studies.
III.2.h. Number of pulses
Every ECT stimulus pulse results in the depolarization of a large numbers of neurons. This causes the release of neurotransmitters and other biochemical changes at neuronal level, resulting in modulation of the neural network. When a sufficient number of pulses are delivered, the cumulative effect of these processes yields a seizure (see Sec. III.1.c). In subconvulsive neuromodulation paradigms such as rTMS the duration of neural excitability changes following the stimulus is directly related to the number of pulses in the stimulus train.165,171,174,175
Thus, the number of pulses in the stimulus is a key parameter for seizure induction. Modern ECT devices typically control the dose by adjusting the number of pulses by varying the stimulus train duration and/or frequency, since the number of pulses equals the product of the train duration and frequency (see Equation 1). Conversely, in subconvulsive brain stimulation paradigms such as rTMS the number of pulses is limited to prevent seizures.165,166 Compared to stimulation with fewer pulses, trains consisting of more pulses require smaller pulse amplitude and/or pulse width to elicit a seizure.5,106,107,112,165,176–178 Stimuli with matched total charge are more efficient when containing more pulses than when having longer pulse width or larger amplitude.17,18,179 In RUL ECT, the efficacy of treatment grows with increasing dose (number of pulses) above threshold.4,180
It is remarkable that a very wide range of parameters can be used to induce seizures, ranging from single pulses with large amplitude and pulse width107,181 to hundreds of pulses at relatively low amplitude and/or pulse width.5,105,106 An accumulating body of evidence suggests that ECT paradigms using lower amplitude and/or pulse width, and larger number of pulses can be as effective but have fewer side effects than paradigms using larger amplitude and/or pulse width, and smaller number of pulses.5,28,62,119,182 For example, a RUL treatment dose with PW = 0.3 ms and 429 pulses (on average) had the same high efficacy but substantially fewer side effects than a dose with PW = 1.5 ms and 265 pulses.5 Furthermore, MST induces electric fields with lower amplitude and pulse width, and requires a larger number of pulses to induce a seizure than ECT.88 Studies of ultrabrief (PW = 0.3 ms) RUL ECT at maximum dose (1,920 pulses with a MECTA Spectrum 5000Q device) were effective and well tolerated.182 ECT paradigms with such a large number of pulses are in stark contrast to early sine-wave ECT which typically had stimulus duration less than 0.4 s, corresponding to less than 48 pulses, and which was associated with significantly higher cognitive side effects.3,106,118 The trend toward stimulus trains with larger number of pulses is reflected in the evolution of modern ECT devices such as the MECTA and the Thymatron whose present models offer train durations up to 8 s, constituting an increase of more than two-fold over the capabilities of earlier models.
The large number of pulses used in modern ECT paradigms appears to have importance for the therapeutic effect beyond the stimulus role as a seizure trigger. RUL ECT is typically dosed to deliver six or more times the number of pulses required to elicit a seizure (6 × seizure threshold) since this produces robust therapeutic effect, whereas stimuli close to the seizure threshold are ineffective.4,180 Thus, to confer therapeutic benefit, the stimulus should continue after a seizure is already induced. The implication of this finding is that the electrical stimulus itself, and/or its interaction with an ongoing seizure, is a critical element for treatment outcome. This observation supports a view of ECT as an electric neuromodulation therapy with similarities to subconvulsive neuromodulation interventions such as rTMS rather than as merely an electric means of inducing seizures. rTMS effects lasting changes in neural activity by delivering stimuli with a large number of pulses, typically around 3,000 per session.24 Modern seizure therapies are approaching these values, with maximum ECT and MST doses reaching 2,000 and 1,000 pulses, respectively.129,182,183 Possible clinical advantages of ECT paradigms using large number of pulses, potentially coupled with reduced current amplitude, should be explored further.
IV. Summary and recommendations for ECT stimulus dosage
IV.1. Dosing
Based on the arguments above, we recommend that all ECT stimulus parameters should be considered explicitly in dosage since summary metrics like charge do not uniquely determine the stimulus and its physiological effects. Biophysical considerations and mounting empirical evidence suggest that the individual stimulus parameters have distinct neurostimulation roles and effects on the clinical outcome that are not captured by the summary metrics. ECT practitioners should be familiar with all components of the stimulus; further clinical trials are encouraged to couple this knowledge with clinical practice to ensure that patients receive an individualized treatment that maximizes efficacy and safety, and minimizes cognitive side effects which are the main drawback of ECT.
Spatial targeting of the electrical stimulation is thought to be central to eliciting the desired therapeutic effect while minimizing adverse side effects. The key variables that control the focality of ECT are the electrode configuration and the current amplitude. The most common electrode positions are bifrontotemporal and RUL. When adequately dosed, RUL ECT can match the efficacy of bifrontotemporal ECT while having lower cognitive side effects. Other electrode placements, such as bifrontal and FEAST, are of significant interest as they may provide additional means of reducing side effects. Contemporary understanding of the brain circuits involved in depression, coupled with anatomically-realistic computer simulations of the electric field distribution, should be used to generate testable hypotheses for optimal electrode configurations, as has been done with DBS for the treatment of depression.184
The pulse amplitude is another key variable for targeting as it controls the volume of neural tissue directly activated by the induced electric field. Reducing ECT pulse amplitude, in conjunction with an appropriate electrode configuration, can enable focusing of the neural stimulation to particular brain regions salient to therapeutic effect, while minimizing the electric field strength in areas that could result in undesirable side effects. Furthermore, individual amplitude adjustment appears to be the most appropriate approach to compensate for anatomical variability across patients, as it could provide comparable electric field magnitude in the brains of all patients. For these reasons, the pulse amplitude is a central dosing parameter in other brain stimulation therapies such as rTMS and DBS. Some older ECT studies have used amplitude titration for individual dosing, yielding current amplitudes significantly below presently used values (800/900 mA). However, there has not been significant effort to explore this parameter in recent studies, which could be explained in part by the non-existent or limited amplitude control features of common ECT devices. Amplitude titration could also reduce adverse side effects associated with seizure threshold titration procedures, since it employs a gradual increase of the volume of activated neural tissue until a seizure is elicited, thus avoiding excessive stimulation of large portions of the brain that would occur at conventional current amplitudes. The potential clinical value of lowering pulse amplitude and of amplitude titration to individualize dosage should be examined.
While there are compelling reasons to individualize the current amplitude, there is at present no strong rationale for individualizing other pulse train parameters such as the stimulus train frequency and the pulse width. These parameters should be optimized in careful preclinical and clinical studies for best risk/benefit ratio, and subsequently these fixed optimal values could be used in ECT administration. In contrast, present dosing paradigms commonly scale train frequency, and sometime pulse width, to individualize the stimulus. The available data and theoretical considerations suggest that the optimal pulse width is in the ultrabrief range (~ 0.2–0.3 ms) and that the optimal frequency is on the low end of the currently used frequency range (20–40 pps, or 10–20 pulse pairs per second). Stimulation paradigms with intermittent (burst) pulse patterns have also been successfully used, and their potential advantages should be evaluated further. Unidirectional stimuli should also be studied for potential advantages over conventional bidirectional trains. Finally, the number of pulses is a key parameter for building up the neuromodulatory effects of electrical stimulation, and should be explicitly considered in ECT studies. ECT paradigms with large number of pulses (> 1,000) are becoming common, associated with reduction in the pulse width. At present, the ECT dose is individualized by adjusting the number of stimulus pulses based typically on the patient’s seizure threshold or age. The most efficient way to increment the number of pulses appears to be lengthening the stimulus train duration, rather than increasing the frequency. If individualization of the pulse amplitude is adopted, as suggested above, it may be advantageous to keep the number of pulses at a relatively high value.
The role of the stimulus parameters can be further clarified by considering the basic biophysics of neural stimulation, coupled with empirical studies. In this respect, the ECT field can benefit from research on other brain stimulation paradigms such as rTMS, tDCS, epidural cortical stimulation, and DBS, which share biophysical principles with ECT, and whose mechanisms have attracted a substantial amount of research interest in the past decade. Better understanding of the mechanisms of ECT could lead to more rationally designed dosing strategies that potentially result in high efficacy, low side effects, and reduced interindividual variability of clinical outcome.
IV.2. Reporting
Since summary quantities such as charge and energy do not uniquely determine the stimulus, the independent stimulus parameters (pulse amplitude, shape, and width, and train frequency, directionality, polarity, and duration/number of pulses) should be reported in publications and in clinical records both for titration procedures and for treatment administration, in addition to other treatment variables such as electrode position, anesthesia, etc.185
IV.3. Device capabilities
To enable research on optimization of dosing paradigms, ECT device manufacturers should consider implementing the following features, at least in research versions of their machines and with proper FDA approvals:
Adequate range of current amplitude adjustment (e.g., 100–900 mA).
Pulse width selection down to 0.2 ms or less.
Pulse-train frequency selection down to 20 pps (10 pulse-pairs per second) or less.
Limit on the maximum number of pulses (e.g., ≤ 2,000), rather than limit on the train duration, thus allowing low-frequency pulse trains with adequate length.
Unidirectional pulse train option, with polarity of electrode clearly marked on the ECT paddles.
Option for programming intermittent trains (e.g., theta burst) with adjustable parameters, and/or external pulse trigger feature to enable computer control of the pulse train.
Transparency/display of the stimulus parameter settings, in addition to or in lieu of indication of summary metrics such as charge and energy.
Furthermore, manufacturers should always indicate in the device manual the instrument output specifications and tolerances, including pulse amplitude, shape, width, and rise and fall times.
IV.4. Conclusion
There is strong theoretical and empirical evidence that individual stimulus parameters exert unique neurobiological effects that are important for understanding and controlling the clinical outcome of ECT, and that are not adequately captured in summary metrics such as charge and energy. Therefore, we recommend that ECT dose be defined using all stimulus parameters rather than a summary metric. Dosing paradigms, record-keeping and reporting of studies, and device design should take into account the distinct roles of the stimulus parameters.
Acknowledgments
Sources of support acknowledgement: NYSTAR Faculty Development Award (SHL), NARSAD Independent Investigator Award (JP), NIH MH60884, NIH NCRR 5TL1RR024158-03
Footnotes
Financial Disclosure: A. V. Peterchev, Z.-D. Deng, and S. H. Lisanby are inventors on Columbia University patent applications on TMS and MST technology. A. V. Peterchev and S. H. Lisanby have received equipment support from Magstim and MagVenture, TMS and MST device manufacturers. S. H. Lisanby has received research grants from ANS/St. Jude Medical, Neuronetics, Cyberonics, NIH, AFAR, NARSAD, Stanley Medical Research Foundation, DARPA, and NYSTAR.
References
- 1.Kalinowsky LB, Hippius H. Pharmacological, convulsive, and other somatic treatments in psychiatry. New York: Grune & Stratton; 1969. [Google Scholar]
- 2.Kalinowsky LB. ECT instrumentation. Biol Psychiatry. 1988;24:361–362. [PubMed] [Google Scholar]
- 3.Weiner RD, Rogers HJ, Davidson JR, et al. Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci. 1986;462:315–325. doi: 10.1111/j.1749-6632.1986.tb51266.x. [DOI] [PubMed] [Google Scholar]
- 4.Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–434. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 5.Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulat. 2008;1:71–83. doi: 10.1016/j.brs.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell RD. Electrical factors in electroconvulsive therapy. Acta Psychiatr Scand. 1968;44:436–448. doi: 10.1111/j.1600-0447.1968.tb07648.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiner RD. ECT and seizure threshold: effects of stimulus wave form and electrode placement. Biol Psychiatry. 1980;15:225–241. [PubMed] [Google Scholar]
- 9.APA. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Priviledging: A Task Force Report of the American Psychiatric Association. Washington, DC: American Psychiatric Association; 2001. Treatment procedures; pp. 125–196. [Google Scholar]
- 10.Sackeim HA, Long J, Luber B, et al. Physical properties and quantification of the ECT stimulus: I. Basic principles. Convuls Ther. 1994;10:93–123. [PubMed] [Google Scholar]
- 11.Boylan LS, Haskett RF, Mulsant BH, et al. Determinants of seizure threshold in ECT: benzodiazepine use, anesthetic dosage, and other factors. J ECT. 2000;16:3–18. doi: 10.1097/00124509-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Swartz CM. Safety and ECT stimulus electrodes: I. Heat liberation at the electrode-skin interface. Convuls Ther. 1989;5:171–175. [PubMed] [Google Scholar]
- 13.Petrides G, Fink M. The “half-age” stimulation strategy for ECT dosing. Convuls Ther. 1996;12:138–146. [PubMed] [Google Scholar]
- 14.Sackeim HA, Decina P, Portnoy S, et al. Studies of dosage, seizure threshold, and seizure duration in ECT. Biol Psychiatry. 1987;22:249–268. doi: 10.1016/0006-3223(87)90144-2. [DOI] [PubMed] [Google Scholar]
- 15.Abrams R, Swartz CM, Vedak C. Antidepressant effects of high-dose right unilateral electroconvulsive therapy. Arch Gen Psychiatry. 1991;48:746–748. doi: 10.1001/archpsyc.1991.01810320070010. [DOI] [PubMed] [Google Scholar]
- 16.Abrams R. Electroconvulsive therapy. 4. New York: Oxford Unversity Press; 2002. [Google Scholar]
- 17.Swartz CM, Larson G. ECT stimulus duration and its efficacy. Ann Clin Psychiatry. 1989;1:147–152. [Google Scholar]
- 18.Swartz CM, Manly DT. Efficiency of the stimulus characteristics of ECT. Am J Psychiatry. 2000;157:1504–1506. doi: 10.1176/appi.ajp.157.9.1504. [DOI] [PubMed] [Google Scholar]
- 19.Devanand DP, Lisanby SH, Nobler MS, et al. The relative efficiency of altering pulse frequency or train duration when determining seizure threshold. J ECT. 1998;14:227–235. [PubMed] [Google Scholar]
- 20.Nitsche MA, Seeber A, Frommann K, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang N, Nitsche MA, Paulus W, et al. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- 22.Sackeim HA. Convulsant and anticonvulsant properties of electroconvulsive therapy: toward a focal form of brain stimulation. Clin Neurosci Res. 2004;4:39–57. [Google Scholar]
- 23.Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: A novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seziure induction. Neuropsychopharm. 2009;34:2002–2010. doi: 10.1038/npp.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 26.Lerer B, Isserles M. From Meduna to ultrabrief: New directions for the oldest brain stimulation therapy. Brain Stimulat. 2008;1:84–85. doi: 10.1016/j.brs.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 27.McIntyre C. Brief thoughts on ECT parameter settings. Brain Stimulat. 2008;1:88–88. doi: 10.1016/j.brs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Hyrman V. Optimizing ECT technique. J ECT. 2009;25:147. doi: 10.1097/YCT.0b013e3181855097. [DOI] [PubMed] [Google Scholar]
- 29.Lisanby SH, Schlaepfer TE, Fisch HU, et al. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58:303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- 30.Rowny SB, Benzl K, Lisanby SH. Translational development strategy for magnetic seizure therapy. Exp Neurol. 2009;219:27–35. doi: 10.1016/j.expneurol.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plonsey R, Heppner D. Considerations of quasi-stationarity in electrophysiological systems. Bull Math Biol. 1967;29:657–664. doi: 10.1007/BF02476917. [DOI] [PubMed] [Google Scholar]
- 32.Rattay F. Modeling the Excitation of Fibers under Surface Electrodes. IEEE Trans Biomed Eng. 1988;35:199–202. doi: 10.1109/10.1362. [DOI] [PubMed] [Google Scholar]
- 33.Bossetti CA, Birdno MJ, Grill WM. Analysis of the quasi-static approximation for calculating potentials generated by neural stimulation. J Neural Eng. 2008;5:44–53. doi: 10.1088/1741-2560/5/1/005. [DOI] [PubMed] [Google Scholar]
- 34.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 35.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter I. Evidence from chronaxie measurements. Exp Brain Res. 1998;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- 36.Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res. 1998;118:489–500. doi: 10.1007/s002210050305. [DOI] [PubMed] [Google Scholar]
- 37.Kole MHP, Stuart GJ. Is action potential threshold lowest in the axon? Nat Neurosci. 2008;11:1253–1255. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- 38.McIntyre CC, Mori S, Sherman DL, et al. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115:589–595. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Iles JF. Simple models of stimulation of neurones in the brain by electric fields. Prog Biophys Mol Biol. 2005;87:17–31. doi: 10.1016/j.pbiomolbio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Amassian VE, Cracco RQ. Human cerebral cortical responses to contralateral transcranial stimulation. Neurosurgery. 1987;20:148–155. [PubMed] [Google Scholar]
- 41.Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol. 1990;77:390–401. doi: 10.1016/0168-5597(90)90061-h. [DOI] [PubMed] [Google Scholar]
- 42.Nagarajan SS, Durand DM, Warman EN. Effects of induced electric fields on finite neuronal structures: a simulation study. IEEE Trans Biomed Eng. 1993;40:1175–1188. doi: 10.1109/10.245636. [DOI] [PubMed] [Google Scholar]
- 43.Maccabee PJ, Amassian VE, Eberle LP, et al. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol. 1993;460:201–219. doi: 10.1113/jphysiol.1993.sp019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- 45.McIntyre CC, Grill WM. Excitation of central nervous system neurons by nonuniform electric fields. Biophys J. 1999;76:878–888. doi: 10.1016/S0006-3495(99)77251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon D. The electrical and radiological aspects of ECT. In: Palmer RL, editor. Electroconvulsive Therapy: An Appraisal. New York: Oxford University Press; 1981. pp. 79–96. [Google Scholar]
- 47.McIntyre CC, Grill WM. Extracellular stimulation of central neurons: Influence of stimulus waveform and frequency on neuronal output. J Neurophysiol. 2002;88:1592–1604. doi: 10.1152/jn.2002.88.4.1592. [DOI] [PubMed] [Google Scholar]
- 48.Esser SK, Hill SL, Tononi G. Modeling the effects of transcranial magnetic stimulation on cortical circuits. J Neurophysiol. 2005;94:622–639. doi: 10.1152/jn.01230.2004. [DOI] [PubMed] [Google Scholar]
- 49.Jefferys JG. Models and mechanisms of experimental epilepsies. Epilepsia. 2003;44 (Suppl 12):44–50. doi: 10.1111/j.0013-9580.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- 50.Bracci E, Vreugdenhil M, Hack SP, et al. On the synchronizing mechanisms of tetanically induced hippocampal oscillations. J Neurosci. 1999;19:8104–8113. doi: 10.1523/JNEUROSCI.19-18-08104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaila K, Lamsa K, Smirnov S, et al. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bliss TVP, Collingridge GL. A synaptic model of memory - long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 53.Bracci E, Vreugdenhil M, Hack SP, et al. Dynamic modulation of excitation and inhibition during stimulation at gamma and beta frequencies in the CA1 hippocampal region. J Neurophysiol. 2001;85:2412–2422. doi: 10.1152/jn.2001.85.6.2412. [DOI] [PubMed] [Google Scholar]
- 54.Thompson SM, Gahwiler BH. Activity-dependent disinhibition. 1. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989;61:501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- 55.Sackeim HA, Decina P, Kanzler M, et al. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am J Psychiatry. 1987;144:1449–1455. doi: 10.1176/ajp.144.11.1449. [DOI] [PubMed] [Google Scholar]
- 56.Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328:839–846. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 57.Datta A, Elwassif M, Battaglia F, et al. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng. 2008;5:163–174. doi: 10.1088/1741-2560/5/2/007. [DOI] [PubMed] [Google Scholar]
- 58.Lamy S, Bergsholm P, d’Elia G. The antidepressant efficacy of high-dose nondominant long-distance parietotemporal and bitemporal electroconvulsive-therapy. Convuls Ther. 1994;10:43–52. [PubMed] [Google Scholar]
- 59.Daniel WF, Weiner RD, Crovitz HF. Autobiographical amnesia with ECT: an analysis of the roles of stimulus wave form, electrode placement, stimulus energy, and seizure length. Biol Psychiatry. 1983;18:121–126. [PubMed] [Google Scholar]
- 60.Deng Z-D, Lisanby SH, Peterchev AV. Effect of anatomical variability on neural stimulation strength and focality in electroconvulsive therapy (ECT) and magnetic seizure therapy (MST) Conf Proc IEEE Eng Med Biol Soc. 2009:682–688. doi: 10.1109/IEMBS.2009.5334091. [DOI] [PubMed] [Google Scholar]
- 61.Lancaster NP, Steinert RR, Frost I. Unilateral electro-convulsive therapy. J Ment Sci. 1958;104:221–227. doi: 10.1192/bjp.104.434.221. [DOI] [PubMed] [Google Scholar]
- 62.Valentine M, Keddie KM, Dunne D. A comparison of techniques in electro-convulsive therapy. Br J Psychiatry. 1968;114:989–996. doi: 10.1192/bjp.114.513.989. [DOI] [PubMed] [Google Scholar]
- 63.McAndrew J, Berkey B, Matthews C. The effects of dominant and nondominant unilateral ECT as compared to bilateral ECT. Am J Psychiatry. 1967;124:483–490. doi: 10.1176/ajp.124.4.483. [DOI] [PubMed] [Google Scholar]
- 64.Nobler MS, Sackeim HA, Prohovnik I, et al. Regional cerebral blood flow in mood disorders, III. Treatment and clinical response. Arch Gen Psychiatry. 1994;51:884–897. doi: 10.1001/archpsyc.1994.03950110044007. [DOI] [PubMed] [Google Scholar]
- 65.Sackeim HA, Luber B, Katzman GP, et al. The effects of electroconvulsive therapy on quantitative electroencephalograms. Relationship to clinical outcome. Arch Gen Psychiatry. 1996;53:814–824. doi: 10.1001/archpsyc.1996.01830090060009. [DOI] [PubMed] [Google Scholar]
- 66.Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 67.Inglis J. Electrode placement and the effect of E.C.T. on mood and memory in depression. Can Psychiatr Assoc J. 1969;14:463–471. doi: 10.1177/070674376901400507. [DOI] [PubMed] [Google Scholar]
- 68.Alexander L. Treatment of mental disorder. Philadelphia: W. B. Saunders and Co; 1953. [Google Scholar]
- 69.Abrams R, Taylor MA. Anterior bifrontal ECT: a clinical trial. Br J Psychiatry. 1973;122:587–590. doi: 10.1192/bjp.122.5.587. [DOI] [PubMed] [Google Scholar]
- 70.Lawson JS, Inglis J, Delva NJ, et al. Electrode placement in ECT: cognitive effects. Psychol Med. 1990;20:335–344. doi: 10.1017/s0033291700017645. [DOI] [PubMed] [Google Scholar]
- 71.Letemendia FJJ, Delva NJ, Rodenburg M, et al. Therapeutic advantage of bifrontal electrode placement in ECT. Psychol Med. 1993;23:349–360. doi: 10.1017/s0033291700028452. [DOI] [PubMed] [Google Scholar]
- 72.Bailine SH, Rifkin A, Kayne E, et al. Comparison of bifrontal and bitemporal ECT for major depression. Am J Psychiatry. 2000;157:121–123. doi: 10.1176/ajp.157.1.121. [DOI] [PubMed] [Google Scholar]
- 73.Delva NJ, Brunet D, Hawken ER, et al. Electrical dose and seizure threshold: relations to clinical outcome and cognitive effects in bifrontal, bitemporal, and right unilateral ECT. J ECT. 2000;16:361–369. doi: 10.1097/00124509-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Heikman P, Kalska H, Katila H, et al. Right unilateral and bifrontal electroconvulsive therapy in the treatment of depression: a preliminary study. J ECT. 2002;18:26–30. doi: 10.1097/00124509-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Bakewell CJ, Russo J, Tanner C, et al. Comparison of clinical efficacy and side effects for bitemporal and bifrontal electrode placement in electroconvulsive therapy. J ECT. 2004;20:145–153. doi: 10.1097/00124509-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 76.Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10:701–707. doi: 10.1111/j.1399-5618.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 77.Sienaert P, Vansteelandt K, Demyttenaere K, et al. Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: clinical efficacy. J Affect Disord. 2009;116:106–112. doi: 10.1016/j.jad.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Loo C, Stewart P, MacPherson S, et al. Lower incidence of asystole and brachycardia with ultrabrief pulsewidth stimulation and bifrontal electrode placement. J ECT. 2009;25:149–150. [Google Scholar]
- 79.Swartz CM. Asymmetric bilateral right frontotemporal left frontal stimulus electrode placement for electroconvulsive-therapy. Neuropsychobiology. 1994;29:174–178. doi: 10.1159/000119083. [DOI] [PubMed] [Google Scholar]
- 80.Swartz CM, Evans CM. Beyond bitemporal and right unilateral electrode placements. Psychiatr Ann. 1996;26:705–708. [Google Scholar]
- 81.Swartz CM. Left anterior right temporal (LART) electrode placement. J ECT. 2001;17:76–76. [Google Scholar]
- 82.Pridmore S, Rybak M. Supraorbital and d’Elia electrode positions in unilateral ECT? J ECT. 1999;15:170–171. [PubMed] [Google Scholar]
- 83.Muller DJ. Unilateral ECT (One year’s experience at a city hospital) Dis Nerv Syst. 1971;32:422–424. [PubMed] [Google Scholar]
- 84.d’Elia G, Widepalm K. Comparison of frontoparietal and temporoparietal unilateral electroconvulsive therapy. Acta Psychiatr Scand. 1974;50:225–232. doi: 10.1111/j.1600-0447.1974.tb08211.x. [DOI] [PubMed] [Google Scholar]
- 85.Erman MK, Welch CA, Mandel MR. A comparison of two unilateral ECT electrode placements: efficacy and electrical energy considerations. Am J Psychiatry. 1979;136:1317–1319. doi: 10.1176/ajp.136.10.1317. [DOI] [PubMed] [Google Scholar]
- 86.Alexopoulos GS, Young RC, Shamoian CA. Unilateral electroconvulsive therapy: an open clinical comparison of two electrode placements. Biol Psychiatry. 1984;19:783–787. [PubMed] [Google Scholar]
- 87.Sackeim H. Magnetic stimulation therapy and ECT. Convuls Ther. 1994;10:255–258. [PubMed] [Google Scholar]
- 88.Lisanby SH, Luber B, Schlaepfer TE, et al. Safety and feasibility of magnetic seizure therapy (MST) in major depression: Randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharm. 2003;28:1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- 89.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nadeem M, Thorlin T, Gandhi OP, et al. Computation of electric and magnetic stimulation in human head using the 3-D impedence method. IEEE Trans Biomed Eng. 2003;50:900–907. doi: 10.1109/TBME.2003.813548. [DOI] [PubMed] [Google Scholar]
- 91.Suh HS, Kim SH, Lee WH, et al. Realistic simulation of transcranial direct current stimulation via 3-D high-resolution finite element analysis: effect of tissue anisotropy. Conf Proc IEEE Eng Med Biol Soc. 2009:638–641. doi: 10.1109/IEMBS.2009.5333686. [DOI] [PubMed] [Google Scholar]
- 92.Datta A, Bansal V, Diaz J, et al. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulat. 2009;2:201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuncel AM, Cooper SE, Grill WM. A method to estimate the spatial extent of activation in thalamic deep brain stimulation. Clin Neurophysiol. 2008;119:2148–2158. doi: 10.1016/j.clinph.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Butson CR, McIntyre CC. Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng. 2006;3:1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Butson CR, Cooper SE, Henderson JM, et al. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nahas Z, Li X, Kozel FA, et al. Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: A pilot study. Depress Anxiety. 2004;19:249–256. doi: 10.1002/da.20015. [DOI] [PubMed] [Google Scholar]
- 97.Stokes MG, Chambers CD, Gould IC, et al. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J Neurophysiol. 2005;94:4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- 98.Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods. 2001;112:193–202. doi: 10.1016/s0165-0270(01)00468-x. [DOI] [PubMed] [Google Scholar]
- 99.Burke D, Hicks R, Gandevia SC, et al. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edgley SA, Eyre JA, Lemon RN, et al. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120:839–853. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- 101.Suihko V. Modelling the response of scalp sensory receptors to transcranial electrical stimulation. Med Biol Eng Comput. 2002;40:395–401. doi: 10.1007/BF02345071. [DOI] [PubMed] [Google Scholar]
- 102.Calancie B, Harris W, Broton JG, et al. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457–470. doi: 10.3171/jns.1998.88.3.0457. [DOI] [PubMed] [Google Scholar]
- 103.Rush S, Driscoll DA. Current distribution in the brain from surface electrodes. Anesth Analg. 1968;47:717–723. [PubMed] [Google Scholar]
- 104.Friedman E, Wilcox PH. Electrostimulated convulsive doses in intact humans by means of unidirectional currents. J Nerv Ment Dis. 1942;96:56–63. [Google Scholar]
- 105.Friedman E. Unidirectional electrostimulated convulsive therapy. Am J Psychiatry. 1942;99:218–223. [Google Scholar]
- 106.Liberson WT. Brief stimulus therapy - physiological and clinical observations. Am J Psychiatry. 1948;105:28–39. doi: 10.1176/ajp.105.1.28. [DOI] [PubMed] [Google Scholar]
- 107.Weaver L, Ravaris C, Rush S, et al. Stimulus parameters in electroconvulsive shock. J Psychiatr Res. 1974;10:271–281. doi: 10.1016/0022-3956(74)90010-7. [DOI] [PubMed] [Google Scholar]
- 108.Weaver LA, Jr, Ives J, Williams R. Studies in brief-pulse electroconvulsive therapy: the voltage threshold, interpulse interval, and pulse polarity parameters. Biol Psychiatry. 1982;17:1131–1143. [PubMed] [Google Scholar]
- 109.Weaver LA, Jr, Williams RW. Stimulus parameters and electroconvulsive therapy. Ann N Y Acad Sci. 1986;462:174–185. doi: 10.1111/j.1749-6632.1986.tb51252.x. [DOI] [PubMed] [Google Scholar]
- 110.Gordon D. Electro-convulsive therapy with minimum hazard. Br J Psychiatry. 1982;141:12–18. doi: 10.1192/bjp.141.1.12. [DOI] [PubMed] [Google Scholar]
- 111.Gordon D. Electroconvulsive therapy. J Biomed Eng. 1989;11:170–171. doi: 10.1016/0141-5425(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 112.Zyss T, Zieba A, Krawczyk A. Electricity of electroconvulsive therapy. J Tech Phys. 2002;43:543–561. [Google Scholar]
- 113.Hilkevitch A. Frequency and length of stimulus in electric shock therapy. AMA Arch Neurol Psychiatry. 1951;65:245–245. [Google Scholar]
- 114.Kayser S, Bewernick B, Axmacher N, et al. Magnetic seizure therapy of treatment-resistant depression in a patient with bipolar disorder. J ECT. 2009;25:137–140. doi: 10.1097/YCT.0b013e31817dc45a. [DOI] [PubMed] [Google Scholar]
- 115.Arieff AJ. Threshold studies in electrical convulsions with use of a square wave stimulator. Arch Neurol Psychiatry. 1949;62:680–682. [PubMed] [Google Scholar]
- 116.Kalinowsky LB, Hoch PH. Shock treatments, psychosurgery, and other somatic treatments in psychiatry. New York: Grune & Stratton; 1952. 2d rev. and enl. ed. [Google Scholar]
- 117.Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 118.Cerletti U. Old and new information about electroshock. Am J Psychiatry. 1950;107:87– 94. doi: 10.1176/ajp.107.2.87. [DOI] [PubMed] [Google Scholar]
- 119.Liberson WT. Current evaluation of electric convulsive therapy; correlation of the parameters of electric current with physiologic and psychologic changes. Res Publ Assoc Res Nerv Ment Dis. 1953;31:199–231. [PubMed] [Google Scholar]
- 120.Cronholm B, Ottosson JO. Ultrabrief stimulus technique in electroconvulsive therapy. I. Influence on retrograde amnesia of treatments with the Elther ES electroschock apparatus, Siemens Konvulsator III and of lidocaine-modified treatment. J Nerv Ment Dis. 1963;137:117–123. [PubMed] [Google Scholar]
- 121.Robin A, De Tissera S. A double-blind controlled comparison of the therapeutic effects of low and high energy electroconvulsive therapies. Br J Psychiatry. 1982;141:357–366. doi: 10.1192/bjp.141.4.357. [DOI] [PubMed] [Google Scholar]
- 122.Offner F. Stimulation with minimum power. J Neurophysiol. 1946;9:387–390. doi: 10.1152/jn.1946.9.5.387. [DOI] [PubMed] [Google Scholar]
- 123.Cronholm B, Ottosson JO. Ultrabrief stimulus technique in electroconvulsive therapy. II. Comparative studies of therapeutic effects and memory disturbances in treatment of endogenous depression with the Elther ES electroshock apparatus and Siemens Konvulsator III. J Nerv Ment Dis. 1963;137:268–276. doi: 10.1097/00005053-196309000-00008. [DOI] [PubMed] [Google Scholar]
- 124.Weiner RD. The first ECT devices. Convuls Ther. 1988;4:50–61. [PubMed] [Google Scholar]
- 125.Squire LR, Zouzounis JA. ECT and memory: brief pulse versus sine wave. Am J Psychiatry. 1986;143:596–601. doi: 10.1176/ajp.143.5.596. [DOI] [PubMed] [Google Scholar]
- 126.Kho KH, van Vreeswijk MF, Simpson S, et al. A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT. 2003;19:139–147. doi: 10.1097/00124509-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 127.Hyrman V, Palmer LH, Cernik J, et al. ECT: the search for the perfect stimulus. Biol Psychiatry. 1985;20:634–645. doi: 10.1016/0006-3223(85)90098-8. [DOI] [PubMed] [Google Scholar]
- 128.Lisanby SH, Luber B, Osman M, et al. The effect of pulse width on seizure threshold during electroconvulsive stimulation (ECS) Convuls Ther. 1997;13:56–56. [Google Scholar]
- 129.Loo CK, Sainsbury K, Sheehan P, et al. A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. Int J Neuropsychopharmacol. 2008;11:883–890. doi: 10.1017/S1461145708009292. [DOI] [PubMed] [Google Scholar]
- 130.Weiner RD, Rogers HJ, Davidson JR, et al. Effects of electroconvulsive therapy upon brain electrical activity. Ann N Y Acad Sci. 1986;462:270–281. doi: 10.1111/j.1749-6632.1986.tb51261.x. [DOI] [PubMed] [Google Scholar]
- 131.Loo C, Sheehan P, Pigot M, et al. A report on mood and cognitive outcomes with right unilateral ultrabrief pulsewidth (0.3 ms) ECT and retrospective comparison with standard pulsewidth right unilateral ECT. J Affect Disord. 2007;103:277–281. doi: 10.1016/j.jad.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 132.Kim SW, Grant JE, Rittberg BR, et al. Decreased memory loss associated with right unilateral ultra-brief pulse wave ECT. Minn Med. 2007;90:34–35. [PubMed] [Google Scholar]
- 133.McCormick LM, Brumm MC, Benede AK, et al. Relative ineffectiveness of ultrabrief right unilateral versus bilateral electroconvulsive therapy in depression. J ECT. 2009;25:238–242. doi: 10.1097/YCT.0b013e31819fdff7. [DOI] [PubMed] [Google Scholar]
- 134.Sienaert P, Vansteelandt K, Demyttenaere K, et al. Absence of cognitive side-effects after ultrabrief electroconvulsive therapy. J ECT. 2008;24:105–105. [Google Scholar]
- 135.Pisvejc J, Hyrman V, Sikora J, et al. A comparison of brief and ultrabrief pulse stimuli in unilateral ECT. J ECT. 1998;14:68–75. [PubMed] [Google Scholar]
- 136.Geddes LA. Optimal stimulus duration for extracranial cortical stimulation. Neurosurgery. 1987;20:94–99. doi: 10.1097/00006123-198701000-00023. [DOI] [PubMed] [Google Scholar]
- 137.Ranck J. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 138.Barker AT, Garnham CW, Freeston IL. Magnetic nerve stimulation: the effect of waveform on efficiency, determination of neural membrane time constants and the measurement of stimulator output. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:227–237. [PubMed] [Google Scholar]
- 139.Peterchev AV, Jalinous R, Lisanby SH. A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width (cTMS) IEEE Trans Biomed Eng. 2008;55:257–266. doi: 10.1109/TBME.2007.900540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geddes LA. Accuracy limitations of chronaxie values. IEEE Trans Biomed Eng. 2004;51:176–181. doi: 10.1109/TBME.2003.820340. [DOI] [PubMed] [Google Scholar]
- 141.Agnew WF, McCreery DB. Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery. 1987;20:143–147. doi: 10.1097/00006123-198701000-00030. [DOI] [PubMed] [Google Scholar]
- 142.McCreery DB, Agnew WF, Yuen TGH, et al. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;37:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- 143.Weiner RD. Does Electroconvulsive-Therapy Cause Brain-Damage. Behav Brain Sci. 1984;7:1–22. [Google Scholar]
- 144.Coffey CE, Weiner RD, Djang WT, et al. Brain anatomic effects of electroconvulsive-therapy - a prospective magnetic-resonance-imaging study. Arch Gen Psychiatry. 1991;48:1013–1021. doi: 10.1001/archpsyc.1991.01810350053008. [DOI] [PubMed] [Google Scholar]
- 145.Dwork AJ, Arango V, Underwood M, et al. Absence of histological lesions in primate models of ECT and magnetic seizure therapy. Am J Psychiatry. 2004;161:576–578. doi: 10.1176/appi.ajp.161.3.576. [DOI] [PubMed] [Google Scholar]
- 146.Dwork AJ, Christensen JR, Larsen KB, et al. Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience. 2009;164:1557–1564. doi: 10.1016/j.neuroscience.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.d’Elia G, Ottosson JO, Stromgren LS. Present practice of electroconvulsive therapy in Scandinavia. Arch Gen Psychiatry. 1983;40:577–581. doi: 10.1001/archpsyc.1983.01790050103013. [DOI] [PubMed] [Google Scholar]
- 148.Impastato DJ, Berg S. Convulsive therapy with amplitude modulated unidirectional currents (Reiter) Am J Psychiatry. 1956;112:932–934. doi: 10.1176/ajp.112.11.932. [DOI] [PubMed] [Google Scholar]
- 149.Epstein J, Wender L. Alternating current vs unidirectional current for electroconvulsive therapy-comparative studies. Confin Neurol. 1956;16:137–146. doi: 10.1159/000105284. [DOI] [PubMed] [Google Scholar]
- 150.Ajmone Marsan C. Focal electrical stimulation. In: Purpura DP, Penry JK, Tower DB, et al., editors. Experimental Models of Epilepsy-A Manual for the Laboratory Worker. New York: Raven Press; 1972. pp. 147–172. [Google Scholar]
- 151.Sommer M, Lang N, Tergau F, et al. Neuronal tissue polarization induced by repetitive transcranial magnetic stimulation? Neuroreport. 2002;13:809–811. doi: 10.1097/00001756-200205070-00015. [DOI] [PubMed] [Google Scholar]
- 152.Antal A, Kincses TZ, Nitsche MA, et al. Pulse configuration-dependent effects of repetitive transcranial magnetic stimulation on visual perception. Neuroreport. 2002;13:2229–2233. doi: 10.1097/00001756-200212030-00013. [DOI] [PubMed] [Google Scholar]
- 153.Tings T, Lang N, Tergau F, et al. Orientation-specific fast rTMS maximizes corticospinal inhibition and facilitation. Exp Brain Res. 2005;164:323–333. doi: 10.1007/s00221-005-2253-6. [DOI] [PubMed] [Google Scholar]
- 154.Arai N, Okabe S, Furubayashi T, et al. Comparison between short train, monophasic and biphasic repetitive transcranial magnetic stimulation (rTMS) of the human motor cortex. Clin Neurophysiol. 2005;116:605–613. doi: 10.1016/j.clinph.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 155.Arai N, Okabe S, Furubayashi T, et al. Differences in after-effect between monophasic and biphasic high-frequency rTMS of the human motor cortex. Clin Neurophysiol. 2007;118:2227–2233. doi: 10.1016/j.clinph.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 156.Taylor JL, Loo CK. Stimulus waveform influences the efficacy of repetitive transcranial magnetic stimulation. J Affect Disord. 2007;97:271–276. doi: 10.1016/j.jad.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 157.Hosono Y, Urushihara R, Harada M, et al. Comparison of monophasic versus biphasic stimulation in rTMS over premotor cortex: SEP and SPECT studies. Clin Neurophysiol. 2008;119:2538–2545. doi: 10.1016/j.clinph.2008.07.279. [DOI] [PubMed] [Google Scholar]
- 158.Marsden CD, Merton PA, Morton HB. Direct stimulation of corticospinal pathways through the intact scalp in human subjects. In: Desmedt J, editor. Motor Control Mechanisms in Health and Disease. New York, NY: Raven Press; 1982. pp. 387–392. [Google Scholar]
- 159.Rothwell JC, Thompson PD, Day BL, et al. Motor cortex stimulation in intact man. General characteristics of EMG responses in different muscles. Brain. 1987;110:1173– 1190. doi: 10.1093/brain/110.5.1173. [DOI] [PubMed] [Google Scholar]
- 160.Libet B, Alberts WW, Wright EW, et al. Production of threshold levels of conscious sensation by electrical stimulation of human somatosensory cortex. J Neurophysiol. 1964;27:546–578. doi: 10.1152/jn.1964.27.4.546. [DOI] [PubMed] [Google Scholar]
- 161.Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19:322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 162.Di Lazzaro V, Oliviero A, Pilato F, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 163.Rattay F. Analysis of the electrical excitation of CNS neurons. IEEE Trans Biomed Eng. 1998;45:766–772. doi: 10.1109/10.678611. [DOI] [PubMed] [Google Scholar]
- 164.McIntyre CC, Grill WM, Sherman DL, et al. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 165.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 166.Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bickford RG, Semjacobsen CW, White PT, et al. Some observations on the mechanism of photic and photo-metrazol activation. Electroencephalogr Clin Neurophysiol. 1952;4:275–282. doi: 10.1016/0013-4694(52)90052-7. [DOI] [PubMed] [Google Scholar]
- 168.Durand DM, Bikson M. Suppression and control of epileptiform activity by electrical stimulation: A review. Proc IEEE. 2001;89:1065–1082. [Google Scholar]
- 169.Girish K, Gangadhar BN, Janakiramaiah N, et al. Seizure threshold in ECT: effect of stimulus pulse frequency. J ECT. 2003;19:133–135. doi: 10.1097/00124509-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 170.Kotresh S, Girish K, Janakiramaiah N, et al. Effect of ECT stimulus parameters on seizure physiology and outcome. J ECT. 2004;20:10–12. doi: 10.1097/00124509-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 171.Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 172.Grossheinrich N, Rau A, Pogarell O, et al. Theta burst stimulation of the prefrontal cortex: safety and impact on cognition, mood, and resting electroencephalogram. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 173.Abrams R, Swartz CM. Thymatron(R) System IV Instruction Manual. 13. Somatics LLC; 2006. [Google Scholar]
- 174.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 175.Nyffeler T, Wurtz P, Luscher HR, et al. Extending lifetime of plastic changes in the human brain. Eur J Neurosci. 2006;24:2961–2966. doi: 10.1111/j.1460-9568.2006.05154.x. [DOI] [PubMed] [Google Scholar]
- 176.Walter WG. Therapy by electrically induced convulsions. Brit J Phys Med. 1940;3:146– 149. [Google Scholar]
- 177.Hovorka EJ, Schumsky DA, Work MS. Electroconvulsive thresholds as related to stimulus parameters of unidirectional ECS. J Comp Physiol Psychol. 1960;53:412–414. doi: 10.1037/h0041786. [DOI] [PubMed] [Google Scholar]
- 178.Andrade C, Kurinji S, Sudha S, et al. Effects of pulse amplitude, pulse frequency, and stimulus duration on seizure threshold: A laboratory investigation. J ECT. 2002;18:144–148. doi: 10.1097/00124509-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 179.Sudha S, Andrade C, Mukundan CR, et al. Spectral EEG effects of electroconvulsive shock stimulus parameters: the development of a rationale for the optimization of the ECT stimulus. J ECT. 2003;19:197–210. doi: 10.1097/00124509-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 180.McCall WV, Reboussin DM, Weiner RD, et al. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy - acute antidepressant and cognitive effects. Arch Gen Psychiatry. 2000;57:438–444. doi: 10.1001/archpsyc.57.5.438. [DOI] [PubMed] [Google Scholar]
- 181.Woodbury LA, Swinyard CA. Stimulus parameters for electroshock seizures in rats. Am J Physiol. 1952;170:661–667. doi: 10.1152/ajplegacy.1952.170.3.661. [DOI] [PubMed] [Google Scholar]
- 182.Rosa MA, Serafim AP, Acha MF, et al. Comparison of ultra-brief pulse ECT with fixed high charge or six times threshold for depression. J ECT. 2009;25:148–149. [Google Scholar]
- 183.Peterchev AV, Kirov G, Ebmeier K, et al. Frontiers in TMS technology development: Controllable pulse shape TMS (cTMS) and magnetic seizure therapy (MST) at 100 Hz. Biol Psychiatry. 2007;61:107s–107s. [Google Scholar]
- 184.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weiner RD, Weaver LA, Jr, Sackeim HA. Reporting of technical parameters in ECT publications: recommendations for authors. Convuls Ther. 1988;4:88–91. [PubMed] [Google Scholar]