Summary
Cultured ES cells can form different classes of neurons, but whether these neurons can acquire specialized subtype features typical of neurons in vivo remains unclear. We show here that mouse ES cells can be directed to form highly specific motor neuron subtypes in the absence of added factors, through a differentiation program that relies on endogenous Wnts, FGFs, and Hh – mimicking the normal program of motor neuron subtype differentiation. Molecular markers that characterize motor neuron subtypes anticipate the functional properties of these neurons in vivo: ES motor neurons grafted isochronically into chick spinal cord settle in appropriate columnar domains and select axonal trajectories with a fidelity that matches that of their in vivo generated counterparts. ES motor neurons can therefore be programmed in a predictive manner to acquire molecular and functional properties that characterize one of the many dozens of specialized motor neuron subtypes that exist in vivo.
Keywords: lateral motor column, embryonic stem cells, Hox, FoxP1, motor neurons, limb, neural patterning, transplantation, motor pool, cell migration, axon guidance, spinal cord, neural tube
Introduction
During development, the emergence of specialized cell types is orchestrated by signaling events that progressively restrict the fates of progenitor cells. Most tissues contain a limited cellular repertoire, but even so, their generation from stem cells in vitro has remained a challenge, largely because of an incomplete understanding of relevant pathways of differentiation. The problem of cell specification is especially daunting in the mammalian central nervous system (CNS), where hundreds of primary neuronal classes are generated, many of which are further diversified into subtypes. The CNS contains, for example, a dozen or so dopaminergic neuronal classes, about two dozen retinal ganglion and amacrine neuronal subtypes, several dozen spinal motor neuron subtypes, and hundreds of receptor-specific olfactory sensory neurons (Buck and Axel, 1991; Dasen and Jessell, 2009; Liss and Roeper, 2008; MacNeil and Masland, 1998; Rockhill et al., 2002). The diversity of CNS neurons contributes to the richness of central circuits and their encoded behaviors, and can correlate with, or confer selective neuronal vulnerability in neurodegenerative diseases.
Of many classes of neurons known to exhibit subtype diversity, programs of spinal motor neuron diversification have been characterized in particular detail (Dasen and Jessell, 2009; Jessell, 2000). The overall program of spinal motor neuron diversification can be deconstructed into a series of developmental steps, in which `generic' motor neurons progressively acquire subtype identities that match features of their muscle targets (Dasen et al., 2003; Dasen et al., 2005; Jessell, 2000; Kania et al., 2000; Sockanathan et al., 2003). Initially, motor neurons acquire columnar identities - median (MMC), hypaxial (HMC), or lateral (LMC) – that dictate their settling positions in the ventral spinal cord as well as the selection of axial, body wall or limb muscles as innervation targets. LMC neurons then acquire divisional identities that dictate the innervation of ventral or dorsal limb muscles, respectively (Kania et al., 2000). Finally, LMC neurons acquire diverse motor pool identities that direct their connections to specific muscles in the limb (Dasen et al., 2005). The existence of dozens of muscle groups in the limbs of most mammals demands an equivalent diversity of motor neuron pool subtypes.
The high degree of LMC diversification makes this a potentially informative population with which to resolve strategies of neuronal subtype specification from embryonic stem (ES) cells. Prior studies have shown that mouse and human ES cells can be converted into spinal motor neurons of generic character, through a program of retinoid and Sonic hedgehog exposure (Lee et al., 2007; Li et al., 2005; Wichterle et al., 2002). But ES cell derived motor neurons (ES motor neurons) generated under these conditions exhibit a rostral cervical, MMC-like, identity (Soundararajan et al., 2006; Wichterle et al., 2002), raising the issue of whether other columnar classes of neurons, and their inherent subtypes, can be generated. And if so, do these specialized motor neuron subtypes express molecular and functional characteristics that reflect those of their in vivo generated counterparts?
The emergence of LMC columnar, divisional and motor pool identities is controlled by the interplay between retinoid and FGF signals and a Hox transcriptional response network (Dasen et al., 2003; Dasen et al., 2005; Liu et al., 2001). At forelimb levels, LMC columnar identity requires the induction of Hox5, Hox6, and Hox8 expression by low level FGF signaling (Dasen et al., 2003). The later emergence of divisional identity within the LMC is directed by paracrine sources of retinoids that promote lateral LMC fate (Sockanathan and Jessell, 1998). In contrast, the diversification of motor pools at a single segmental level has been suggested to depend on the cell-by-cell resolution of an intrinsic Hox repressor network (Dasen et al., 2005). Once established, these motor neuron transcriptional programs govern the settling position, axonal trajectory and trophic factor sensitivity of LMC neuronal subsets.
Using this developmental program as a guide, we have been able to define conditions under which ES cells can be differentiated into motor neurons with LMC columnar, divisional and pool identities, in the absence of any added inductive factors. We also provide evidence that the emergence of LMC divisional and pool identity in individual neurons can occur independently of signals provided by other LMC neurons, and probably by any limb-level specific signals. Most critically, we use isotopic and isochronic grafts of ES cell derived LMC neurons into host spinal cord to show that the transcriptional profile of ES cell derived LMC neuron subsets predicts their settling position within the lateral motor column, their axonal trajectory to the forelimb, and their sensitivity to target derived inductive factors.
Results
Generation of motor neurons with LMC character from ES cells
Mouse ES cells exposed to retinoic acid (RA) and Hedgehog (Hh) receptor agonists generate motor neurons that exhibit rostral cervical identity (Wichterle et al., 2002). We therefore sought to define differentiation conditions that promote the generation of motor neurons of brachial LMC character. In vivo, brachial LMC identity is achieved by exposure of cells to FGF signals and evasion of the rostralizing influence of retinoids (Dasen et al., 2003; Liu et al., 2001) (Figure S1A). Accordingly, we tested whether omission of RA from the culture medium would promote formation of brachial level motor neurons from ES cells.
We cultured ES cells at low density, to minimize the influence of BMP signals that suppress neural fate in ES cells (Watanabe et al., 2005) (Figure S1K). Using this low density, retinoid-free, culture condition we screened a series of basal media and supplements for their ability to promote the generation of GFP+ motor neurons from Hb9-GFP (HBG3) ES cells (Wichterle et al., 2002). We found that Advanced D-MEM/F12/Neurobasal medium supplemented with 10% Knockout serum replacement (referred to as caudalizing/ventralizing [CV] medium) resulted in the generation of spinal motor neurons of brachial LMC character. With this CV differentiation condition, motor neurons constituted 8 ± 1% (mean ± s.e.m.; n=3 independent experiments) of cells in embryoid bodies, an efficiency 4- 5-fold lower than that typically obtained through the use of RA/Hh inductive signals (Supplemental Table 1).
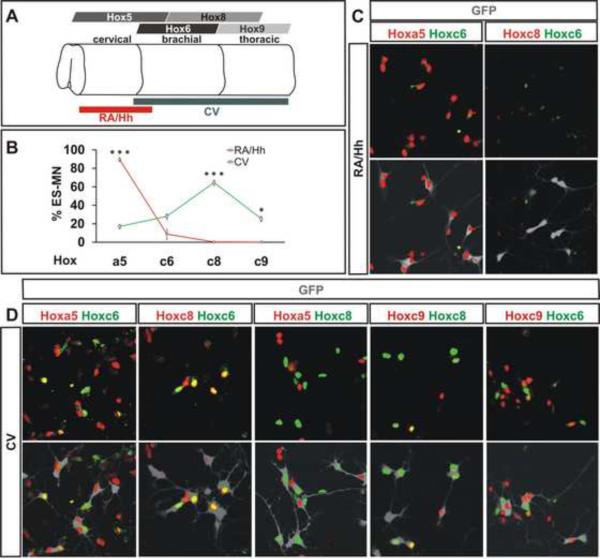
To define the rostrocaudal positional identity of CV differentiated Hb9:GFP+ motor neurons we analyzed their Hox protein expression status. 15 ± 2% of ES motor neurons expressed Hoxa5, 28 ± 8% expressed Hoxc6, 64 ± 2% expressed Hoxc8, and 25 ± 6% expressed Hoxc9 (Figure 1 B, D) (see Supplemental Table 2 for quantification). The vast majority of CV generated ES motor neurons exhibited mutually exclusive expression of Hoxa5 and Hoxc8, and of Hoxc6 with Hoxc9 (Figure 1D), consistent with the reciprocity of expression of these Hox protein pairs observed in vivo (Dasen et al., 2005). Moreover the pattern of Hox gene expression achieved under RA/Hh and CV differentiation conditions was dramatically different: with RA/Hh exposure 89 ± 2% of GFP+ motor neurons expressed Hoxa5 and virtually none expressed Hoxc8 or Hoxc9 (Figure 1 B, C). Thus, ES cells differentiated under CV, but not RA/Hh, conditions give rise to motor neurons of caudal brachial (Hoxc8+) and thoracic (Hoxc9+) positional character.
Figure 1. Characterization of Hox Gene Expression in ES Motor Neurons.
A) Pattern of Hox gene expression in the developing spinal cord. Red and grey lines represent positional identity of ES motor neurons derived under RA/Hh and CV exposure, respectively.
B) Quantification of Hox expression in ES motor. ES motor neurons derived under RA/Hh and CV conditions exhibited differences in Hoxa5 (p<0.001), Hoxc8 (p<0.001) and Hoxc9 (p=0.01) expression. Data from three independent experiments (mean ± standard error of the mean, SEM).
C) RA/Hh generated Hb9-GFP+ ES motor neurons (grey) expressed Hoxa5, low levels of Hoxc6, but not Hoxc8.
D) Most CV differentiated ES motor neurons (grey) expressed Hoxc8, while smaller subsets express Hoxa5, Hoxc6 and Hoxc9. Note mutually exclusive expression of Hoxa5/Hoxc8 and Hoxc6/Hoxc9.
We next addressed whether CV differentiation conditions promote the generation of motor neurons through a program that resembles that operating in vivo. Since Wnt signals underlie the initial specification of spinal cord identity in vivo (Nordstrom et al., 2006), we examined the fate of CV differentiated ES cells exposed to the Wnt antagonist Dickkopf-1 (Dkk1). In the presence of Dkk1, ES cells grown under CV conditions exhibited forebrain/midbrain rather than spinal positional character, revealed by the absence of Hox5 to Hox8 protein expression, the lack of Hb9∷GFP+ neurons, and the prevalence of Otx2+ cells (Figure S1D). Within the context of spinal positional character, FGF signaling plays a role in assigning caudal positional identity (Liu et al., 2001). We therefore examined the Hox profile of CV differentiated ES cells grown in the presence of a pan-FGF receptor antagonist (PD173074; Mohammadi et al., 1998). The resulting ES motor neurons exhibited a rostralized positional character – expressing Hoxa5 rather than Hoxc8 and Hoxd9 (Figure S1E). Thus, low-density retinoid-free conditions are conducive to embryoid body expression of endogenous Wnt and FGF factors that conspire to caudalize induced neural cells.
We next tested whether motor neuron differentiation under CV differentiation conditions reflects an endogenous supply of ventralizing factors (Jessell, 2000). Clusters of Shh+, FoxA2+, Brachyury+ notochord as well as Shh+, FoxA2+, Brachyuryoff floor plate cells were detected in embryoid bodies grown under CV conditions (Figure S1J–L). This endogenous source of hedgehog protein is functional, since exposure of ES cells to a potent Hh receptor antagonist (C61414; Williams et al., 2003) blocked the differentiation of ventral spinal progenitor cells and of motor neurons (Figure S1H, I). Thus ES cells grown under CV conditions are neuralized, caudalized and ventralized solely through the actions of endogenous patterning signals, generating motor neurons of a caudal positional character. We note that our induction protocol differs markedly in outcome from prior FGF and Hh-free protocols that result in the generation of rostralized neural cells (Gaspard et al., 2008; Watanabe et al., 2005),
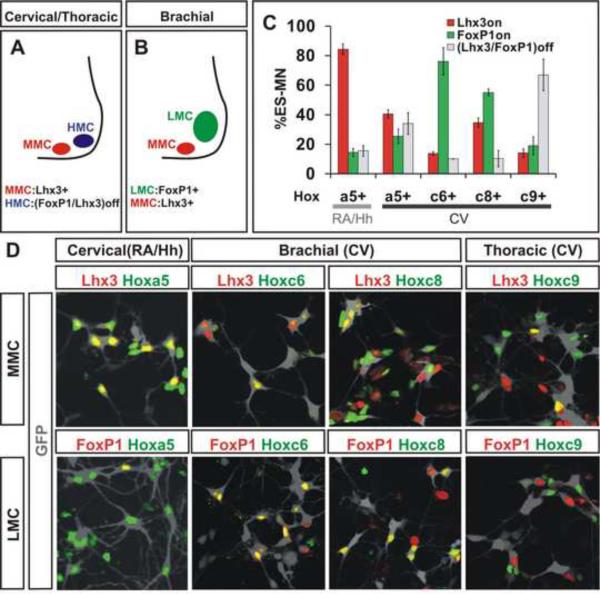
Do CV generated ES motor neurons acquire definitive columnar and pool characters that conform to their Hox profiles? To assess the columnar character of Hb9:GFP+ motor neurons we monitored expression of Lhx3 and FoxP, markers of MMC and LMC, identity (Dasen et al, 2005). We inferred HMC identity by the absence of Lhx3 and FoxP1 (Dasen et al., 2008). We found that 38 ± 2% of CV differentiated motor neurons expressed FoxP1 in the absence of Lhx3, conversely 28 ± 2% expressed Lhx3 in the absence of FoxP1, and 34 ± 1% lacked both FoxP1 and Lhx3 (Figure S2). As in vivo (Dasen et al., 2008), >4-fold more Hoxc6+ motor neurons exhibited LMC (FoxP1+) than MMC (Lhx3+) character, and >4-fold more Hoxc9+ motor neurons exhibited HMC (FoxP1off, Lhx3off) than MMC character (Figure 2C, D). In addition, we found that 19 ± 6% of motor neurons co-expressed Hoxc9+ and FoxP1, a molecular profile predictive of preganglionic motor column (PGC) neurons (Dasen et al., 2008) (Figure 2C, D). The tight correlation between columnar identity and Hox status provides further evidence that CV differentiation conditions favor the formation of motor neurons of coherent caudal brachial or rostral thoracic character.
Figure 2. Rostro-Caudal and Columnar Identities of ES Motor Neurons.
A) At cervical and thoracic levels, motor neurons are organized into median (MMC) and hypaxial (HMC) motor columns. MMC neurons express Lhx3. HMC neurons lack FoxP1 and Lhx3 expression.
B) At brachial level, HMC is replaced by FoxP1+ lateral motor column (LMC).
C) Quantification of Hox gene expression in the context of FoxP1+ and Lhx3+ ES motor neurons derived under RA/Hh or CV condition. ANOVA analysis of data presented in Supplemental Table 2. Data from three independent experiments (mean ± SEM).
D) FoxP1 and Lhx3 expression in Hoxa5, Hoxc6, Hoxc8, or Hoxc9 expressing Hb9-GFP+ ES motor neurons (grey) derived under RA/Hh or CV conditions.
Position-independent programming of motor pool identity
Soon after their generation, LMC motor neurons acquire specialized divisional and pool identities. Lateral divisional identity is imposed on late-born LMC neurons as they migrate through earlier-born medial LMC neurons that serve as a neuronal source of retinoid inductive signals (Sockanathan and Jessell, 1998). In parallel, the segment by segment diversification of LMC neurons into specific pool subtypes has been attributed to the probabilistic outcome of cross-repressive interactions between members of a Hox transcriptional network (Dasen et al., 2005). The scattered arrangement of LMC neurons within CV differentiated embryoid bodies provided us with an opportunity, not available in vivo, to test the requirement for close proximity between LMC neurons in the emergence of LMC divisional and pool character.
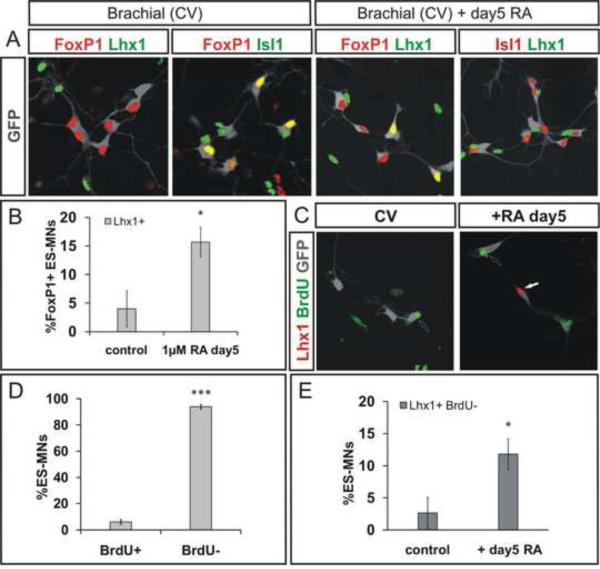
We first examined the emergence of lateral LMC divisional character. Very few CV differentiated motor neurons acquired lateral LMC divisional character, as assessed by expression of Lhx1 in GFP+ motor neurons (Figure 3A). However, exposure of embryoid bodies to RA (1 μM) on day 5 of differentiation elicited a 3-fold increase in the number of Lhx1+, GFP+ motor neurons (p=0.04 vs control RA-free conditions; Figure 3A, B). This retinoid mediated induction of Lhx1 occurred in post-mitotic motor neurons, since BrdU addition at the time of retinoid exposure labeled few if any Lhx1+ motor neurons (Figure 3C–E). We also verified that retinoid treatment did not alter rostrocaudal positional identity of motor neurons, assessed by the profile of Hox expression (Figure S3A, B). Thus under CV differentiation conditions, the progression of LMC neurons to a lateral LMC fate cannot be driven by signals emanating from neighboring LMC neurons, and requires supplemental retinoid exposure.
Figure 3. Retinoid Signaling Specifies Lateral LMC Divisional Identity in Post-mitotic ES Motor Neurons.
A) Expression of Lhx1 and Isl1 in CV generated GFP+ ES motor neurons (grey) on day 7 of differentiation under control conditions and following 1μM RA treatment on day 5. Note mutually exclusive expression of Isl1 and Lhx1.
B) Increase in Lhx1+ FoxP1+ ES motor neurons after retinoid treatment (p=0.044). Results from three independent experiments (mean ± SEM).
C) Labeling of motor neurons born after day 5 in cultures by BrdU treatment on days 5–7.
D) A majority of ES motor neurons (94 ± 2%) do not incorporate BrdU supplemented between day 5 and 7 of differentiation. Results from three independent experiments (mean ± SEM).
E) RA treatment on day 5 significantly increases the fraction of post-mitotic, BrdU− ES motor neurons that express Lhx1 (p=0.028). Results from three independent experiments (mean ± SEM).
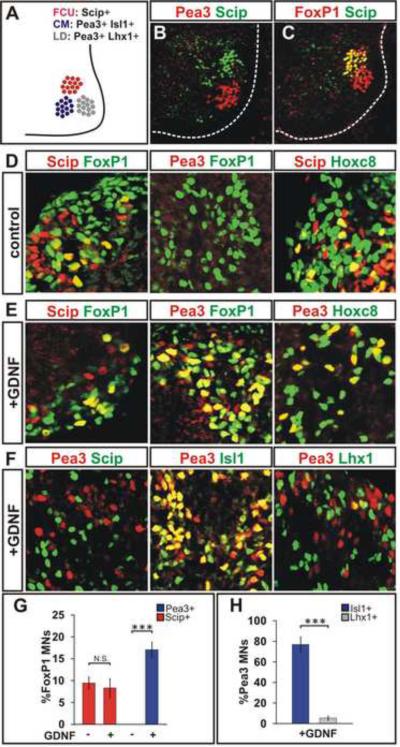
We next examined whether the emergence of motor pool character in an individual LMC neuron can be achieved in the absence of signals provided by neighboring LMC neurons. To address this issue, we focused on three pools generated within caudal brachial LMC: Scip+, Isl1+ motor neurons that innervate the flexor carpi ulnaris (FCU) muscle, Pea3+, Lhx1+ motor neurons that innervate latissimus dorsi (LD) muscle, and Pea3+, Isl1+ motor neurons that innervate the cutaneus maximus (CM) muscle (Figure 4A–C). Pea3 expression is induced in CM and LD motor neurons as a consequence of Hox profiles that confer neuronal competence to a permissive limb-derived signal, glial cell line-derived neurotrophic factor (GDNF) (Dasen et al., 2005; Haase et al., 2002).
Figure 4. ES Motor Neurons Acquire Defined Caudal Brachial Motor Pool Identities.
A) Molecularly defined LMC motor pools in caudal brachial spinal cord: Scip+ pool innervates flexor carpi ulnaris (FCU), Pea3+/Isl1+ pool innervates cutaneous maximus (CM), and Pea3+/Lhx1+ motor pool innervates latissimus dorsi (LD) muscles.
B) Scip and Pea3 expression in E13.5 mouse spinal cord.
C) A subset of FoxP1+ LMC neurons expresses Scip at caudal brachial E13.5 mouse spinal cord.
D) Cells in day 6 embryoid bodies express FoxP1, Scip, Pea3 and Hoxc8. A subset of FoxP1+ LMC neurons expresses Scip but not Pea3. Scip+ motor neurons co-express Hoxc8.
E) FoxP1+ ES motor neurons acquire Pea3 expression in response to GDNF treatment on day 5–6. Pea3+ motor neurons express Hoxc8.
F) Mutually exclusive expression of Pea3 and Scip in ES motor neurons. A majority of Pea3+ ES motor neurons express Isl1 but not Lhx1.
G) Quantification of Pea3 and Scip expressing FoxP1+ ES motor neurons in the presence and absence of GDNF. The fraction of Pea3+ but not Scip+ LMC neurons was increased upon GDNF treatment (p<0.001). Results from three independent experiments (mean ± SEM).
H) Quantification of Lhx1 and Isl1 expression in Pea3+ motor neurons. Significantly greater fraction of Pea3+ ES motor neurons expressed Isl1 compared to Lhx1 (p<0.001). Results from three independent experiments (mean ± SEM).
To reflect this permissive target-derived influence, we supplemented basal CV differentiation medium with GDNF (10 ng/ml; from day 5 onwards), and analyzed the transcriptional status of the resulting GFP+ ES motor neurons. We found that 9 ± 1% of FoxP1+ ES LMC neurons co-expressed Scip with Hoxc8 and Isl1 (FCU-like; Figure 4D, E, G). 17 ± 2% of FoxP1+ ES motor neurons co-expressed Pea3 with Hoxc8 in the absence of Scip (CM-like; Figure 4 D–G). Of these Pea3+ LMC neurons, 87 ± 6% co-expressed Isl1, and 8 ± 5% expressed Lhx1 (Figures 4F, H) indicating that most Pea3+ ES motor neurons acquired CM rather than LD character. Moreover, as in vivo, FoxP1+ neuronal co-expression of Pea3 but not Scip was dependent on GDNF (9 ± 1% of FoxP1+ ES motor neurons express Scip in the absence and 8 ± 2% in the presence of GDNF, p=0.70) (Figure 4D, E, G). These results show that LMC neurons generated under CV conditions can acquire motor pool identities consistent with those generated in vivo.
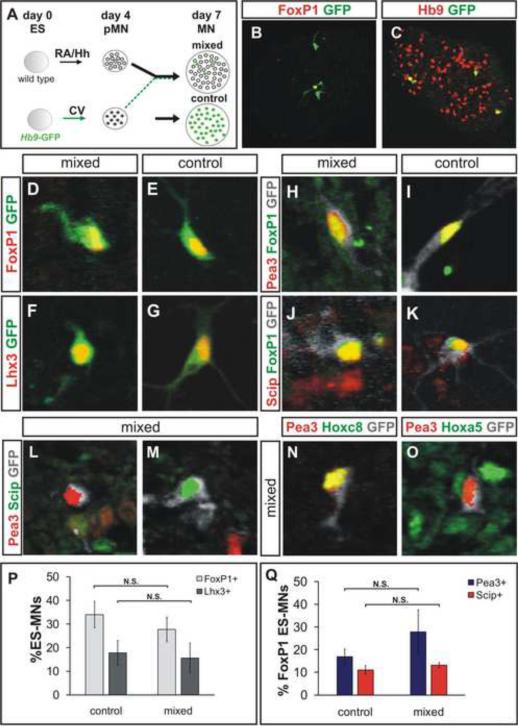
To test the ability of individual ES cell derived LMC neurons to acquire authentic pool character in the absence of signals from their LMC neighbors we devised a method of generating a few isolated LMC neurons within a vast majority of neighboring cervical non-LMC cells. To achieve this condition, we dissociated Hb9-GFP motor neuron progenitors generated under CV conditions on day 4 of differentiation, and mixed them with a 5-fold excess of wild type motor neuron progenitors generated under RA/Hh conditions, and lacking GFP expression (Figure 5A–C). Since under CV conditions, ~8% of cells differentiate into motor neurons, and under RA/Hh conditions ~40% become motor neurons, the resulting ratio of CV: RA/Hh derived motor neurons in reaggregates is predicted to be ~1:25. In practice, we found that 2.6 ± 0.7 % of all Hb9+ ES motor neurons generated under this reaggregate condition expressed GFP (a 1:38 ratio) (Figure 5C, data not shown). And since motor neurons are surrounded by a majority of other cell types, the chances that any two LMC neurons occupy the same microdomain of the embryoid body are exceedingly low.
Figure 5. Microenvironment-Independent Acquisition of Motor Pool Identities.
A) CV differentiated Hb9-GFP ES motor neuron progenitors were dissociated on day 4 and mixed with a five-fold excess of dissociated RA/Hh differentiated wild-type (wt) ES motor neuron progenitors, reaggregated, and cultured until day 7.
B) One of three GFP+ ES motor neurons expresses FoxP1.
C) ES motor neurons derived from wt ES cells (Hb9+/GFPoff) under RA/Hh condition surround scattered brachial GFP+ ES motor neurons.
D–G) ES motor neurons in control and mixed conditions express FoxP1 (D, E) and Lhx3 (F, G).
H–K) Day 5 treatment of mixed aggregates with GDNF induced Pea3 (H, I) and Scip (J, K) expression in FoxP1+ LMC ES motor neurons (grey).
L, M) Expression of Pea3 and Scip in ES motor neurons (grey) does not overlap in mixed aggregates.
N, O) Hoxc8 expressing Pea3+ ES motor neurons (N) are surrounded by Hoxa5+ cervical cells (O).
P) Similar fractions of FoxP1+ and Lhx3+ ES motor neurons in control and mixed cultures (p=0.29 and p=0.81, respectively). Results from three independent experiments (mean ± SEM).
Q) The fractions of FoxP1+ ES motor neurons expressing Pea3 and Scip were not significantly different in control or mixed cultures (p= 0.40 and p=0.44, respectively). Results from three independent experiments (mean ± SEM).
We therefore examined the pool subtype identities of individual CV GFP+ Hoxc8+ ES motor neurons three days after reaggregation. We found that Scip and Pea3 were expressed in a mutually exclusive manner by GFP+, FoxP1+ LMC neurons, and the proportional representation of these two pool subtypes was similar to that found in CV differentiated conditions (Figure 5H–K, L, M, N, Q). These findings indicate that in contrast to the rules of LMC divisional specification, authentic molecular motor pool identities can emerge in the absence of any influence from other LMC neurons.
ES cell derived LMC neurons display functional character after intraspinal transplantation
We next turned to the issue of whether CV generated LMC neurons exhibit functional attributes of their in vivo generated counterparts. Adhering to the principle of in vivo veritas, we examined the behavior of CV generated LMC neurons after isochronic grafting into embryonic chick spinal cord, focusing on the intraspinal settling position and peripheral axonal trajectories of grafted neurons (Wichterle et al., 2002; 2009). In addition, we devised a method to compare directly the behavior of CV and RA/Hh ES motor neurons within a single host embryo.
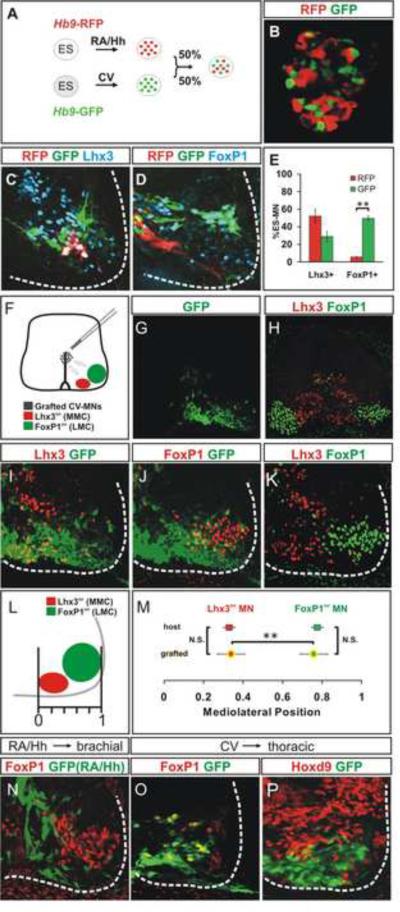
We first generated a new ES cell line (HBR) that carries a motor neuron specific Hb9-RFP transgene, and used this line to generate rostral cervical RFP+ ES motor neurons under RA/Hh differentiation conditions, and the classic HBG3 GFP line to generate caudal brachial GFP+ motor neurons under CV differentiation conditions. RFP+ and GFP+ motor neurons were dissociated and mixed in equal numbers (Figures 6A, B, S6C). Aggregates were harvested on day 6 and transplanted into the brachial or thoracic spinal cord of Hamburger-Hamilton (HH) stages 15–17 chick embryos, at the time of endogenous motor neuron generation. Under both differentiation conditions, the vast majority (94 ± 2%) of ES motor neurons were post-mitotic at the time of transplantation, as revealed by the lack BrdU incorporation, when added from day 5 of differentiation (Figure 3D). Chick embryos were harvested three days after grafting and motor neuron settling preference and axon pathfinding analyzed. We estimate that 30–50% of transplanted ES motor neurons survive at 3 days after transplantation (Supplemental Experimental Procedures).
Figure 6. Column-Specific Settling of Transplanted ES Motor Neurons.
A) Hb9-RFP and Hb9-GFP ES cell lines differentiated under RA/Hh and CV conditions, respectively. RFP+ and GFP+ motor neuron aggregates were transplanted into HH stage 15–17 brachial spinal cord and analyzed 3 days later.
B) Intermixing of RFP+ and GFP+ ES motor neurons in aggregates.
C, D) ES derived neurons in host ventral spinal cord (white outline) express Lhx3 (C) and FoxP1 (D).
E) FoxP1 and Lhx3 expression in RFP+ and GFP+ motor neurons 3 days after transplantation. More transplanted GFP+ neurons express FoxP1 (p=0.004). Results from three transplanted embryos (mean ± SEM).
F) CV differentiated ES motor neurons transplanted into HH stage 15–17 brachial spinal cord.
G) Ventrally localized GFP+ motor neurons three days after transplantation.
H–K) Spinal cord section (from G) triple labeled for Lhx3, FoxP1 and GFP. White dashed line delineates spinal cord margin.
L) Measuring the settling position of transplanted cells.
M) Settling preferences of transplanted and endogenous motor neurons expressing Lhx3 or FoxP1. The settling positions of host and grafted FoxP1+ or Lhx3+ motor neurons are not (N.S.) different (p=0.85, resp. p=0.92). Settling position of transplanted Lhx3+ ES motor neurons is different (p<0.01) from that of FoxP1+ ES motor neurons. Results from four transplanted embryos (mean ± SEM).
N) Lack of FoxP1 expression in RA/Hh differentiated ES motor neurons grafted into brachial spinal cord.
O, P) FoxP1 expression by CV ES motor neurons transplanted into Hoxd9+ thoracic spinal cord (P).
We first examined whether transplanted motor neurons retain their subtype-specific molecular character in the chick spinal cord. Three days after transplantation, 50 ± 3% of GFP+ ES motor neurons expressed FoxP1 and 29 ± 6% expressed Lhx3 (Figures 6C–E). In addition, 52 ± 8% of RFP+ ES motor neurons expressed Lhx3 and 5.6 ± 0.4% FoxP1 (Figure 6C–E). These values are consistent with the proportional allocation of columnar subtypes in culture (Figure S2), providing evidence for maintenance of columnar character after transplantation. We did detect a decrease in the representation of Lhx3+ neurons (52 ± 8% in vivo compared to 82.0 ± 0.3% in vitro, p=0.022) (Figures 6E, S2). As a consequence, 42 ± 5% of grafted RFP+ ES motor neurons exhibited a transcriptional profile (Lhx3off/FoxP1off) indicative of HMC columnar character (Figure 6C–E). The loss of Lhx3 expression resembles the normal developmental program of Lhx3 extinction during the maturation of spinal motor neurons (Agalliu et al., 2009; Sharma et al., 1998). Thus, ES motor neurons largely maintain their columnar identities after transplantation, permitting analysis of their settling behavior and axonal trajectory.
In the ventral spinal cord in vivo, MMC neurons are positioned medially whereas LMC neurons settle laterally (Figure 6F). To examine whether grafted ES motor neurons settle in distinct domains according to their columnar identities we monitored the segregation of CV differentiated Lhx3+ and FoxP1+ ES motor neurons 72 hours after transplantation into host chick embryos (equivalent to HH stage 28), by which time endogenous MMC and LMC columns are well separated (Figures 6F–K). The position of individual transplanted and endogenous motor neurons was assigned a mediolateral (m-l) positional value between 0.0 (medial) and 1.0 (lateral) (Figure 6L). CV generated Lhx3+ ES motor neurons settled medially (m-l value 0.28 ± 0.07) close to endogenous MMC neurons (m-l value 0.33 ± 0.06) (Figures 6I, K, M, S5F–H), whereas FoxP1+ ES motor neurons settled laterally (m-l value 0.68) close to endogenous LMC neurons (m-l value 0.77 ± 0.08) (Figures 6J, K, M, S5I–K). Thus, CV generated ES motor neurons are found in distinct ventral domains, appropriate for their columnar subtype identities.
We considered whether differential survival of ES motor neurons within columnar `niches' might underlie the differential distribution of Lhx3+ and FoxP1+ ES motor neurons. Against this idea, we observed that FoxP1+ ES motor neurons survived when transplanted into the thoracic spinal cord (Figure 6P), a region that lacks an endogenous LMC niche (Figure 6O). Moreover, these neurons settled laterally (Figure 6O, P). Thus the survival and molecular character of LMC neurons is not contingent on the presence of endogenous LMC, or a specialized limb-level niche. Similarly, heterotopic transplantation of RA/Hh induced cervical ES motor neurons into the brachial spinal cord did not increase the incidence of FoxP1+ neurons (Figure 6D, N). The differential segregation and settling pattern of ES motor neurons with distinct columnar identities is likely therefore to reflect the recognition of intraspinal cues that normally establish the columnar architecture of the spinal motor system.
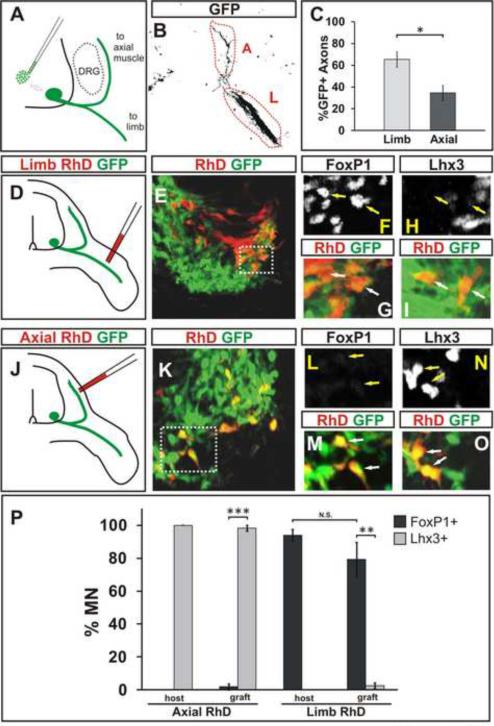
We next examined whether ES cell derived LMC neurons pursue a characteristic axonal trajectory to the limb. Aggregates containing an equal mixture of RFP+ RA/Hh-induced cervical and GFP+ CV-induced brachio-thoracic motor neurons were transplanted into brachial level chick spinal cord. Three days later, embryos were harvested and the contribution of RFP+ and GFP+ ES motor neuron axons to axial- and limb-directed nerve branches was examined (Figure 7A, B, S6D). If the axons of ES motor neuron columnar subtypes pursued a trajectory that conforms to that of their endogenously generated counterparts, the subtype composition of grafted motor neurons (RFP+: 52 ± 8% MMC, 39 ± 13% HMC; GFP+: 50 ± 3% LMC, 20 ± 5% HMC, 29 ± 6% MMC) predicts that greater fraction of RFP+ axons (~70%) than GFP+ axons (~30%) will pursue the axial motor trajectory (Dasen et al., 2008) (Figure S6A, B). Our analysis of GFP axonal projections revealed that 65 ± 7% of GFP+ motor axons were found in the limb-directed nerve branch and only 35 ± 7% within the axially-directed branch (Figures 7B, C). Conversely, 59 ± 5% of RFP+ motor axons projected axially and 41 ± 5% projected into the limb (Figure S6D, E). The axonal trajectories of ES motor neurons of cervical and brachiothoracic character are therefore distinct, and moreover they conform to the expected trajectories of their in vivo generated motor neuron counterparts.
Figure 7. Axons of LMC and MMC ES Motor Neurons Project to Limb and Axial Muscles.
A) Strategy to examine axon pathfinding preference of GFP+ ES motor neurons derived under CV condition three days after grafting into brachial spinal cord (HH stage 15–17).
B, C) A greater fraction of transplanted GFP+ (black) axons is detected in the limb (L) nerve compared to axial (A) nerve branch three days after grafting (p=0.011). Results from six transplanted embryos (mean ± SEM). See also Figure S6D, E.
D) CV differentiated transplanted motor neurons were backfilled with tetramethylrhodamine-dextran (RhD) from the limb nerve branch.
E) Section of a ventral spinal cord containing GFP+ transplanted and RhD+ limb innervating motor neurons (boxed area corresponds to panels F and G).
F–I) Transplanted motor neurons retrogradely labeled from the limb (RhD+/GFP+) express FoxP1 (arrows, F, G) but not Lhx3 (arrows, H, I).
J, K) Transplanted ES motor neurons backfilled with RhD from the axial nerve branch (boxed area in K corresponds to panels L and M).
L–O) ES motor neurons retrogradely labeled from the axial musculature (RhD+/GFP+) expressed Lhx3 (arrows, N, O) but not FoxP1 (arrows, L, M).
P) More axially projecting grafted ES motor neurons expressed Lhx3 than FoxP1 (p<0.001). More limb projecting ES motor neurons expressed FoxP1 than Lhx3 (p=0.002). The fractions of endogenous and grafted FoxP1+ motor neurons labeled from the limb, or Lhx3+ motor neurons labeled from the axial muscles are not different (p=0.26, resp. p=0.37). Results from three embryos each for limb and axial backfills (mean ± SEM).
To provide a more rigorous assessment of the axonal trajectory of grafted CV differentiated ES motor neurons, we compared directly, transcriptional status (Lhx3 and FoxP1 expression) and retrograde tracer accumulation, after injection of tetramethylrhodamine-dextran (RhD) into axial or limb motor nerve branches (Figure 7D, J). We found that 97 ± 2% of transplanted ES motor neurons retrogradely labeled from axial nerve expressed Lhx3 (Figures 7N, O, P) and that 80 ± 10% of ES motor neurons retrogradely labeled from the limb expressed FoxP1 (Figures 7E, F, G, P). Fewer than 5% of ES motor neurons retrogradely labeled from the axial nerve expressed FoxP1 and fewer than 5% of ES motor neurons retrogradely labeled from the limb nerve expressed Lhx3 (Figures 7 H, I, K, L, M, P). The ~20% of ES motor neurons that possessed HMC molecular character (Lhx3off, FoxP1off) were also retrogradely-labeled after limb tracer injection (Figure 7P), consistent with the trajectory of ectopic HMC neurons induced to differentiate at limb levels of mouse spinal cord (Dasen et al., 2008). Thus, LMC and MMC neurons derived from ES cells extended axons along divergent peripheral trajectories. These findings indicate that ES motor neurons acquire functional subtype characters that reflect those of corresponding motor neurons generated in vivo.
Discussion
Studies on the directed differentiation of ES cells to nerve cells have typically relied on transgene expression and/or exposure to exogenous signaling factors (e.g. Andersson et al., 2006; Bain et al., 1995; Wichterle et al., 2002). In this study we have considered whether ES cells might be coaxed into the formation of endogenous signaling centers that direct the generation of specialized cell subtypes with properties that match those of counterpart cells generated in vivo. To address this issue we devised a differentiation protocol for generation of functional motor neuron columnar, divisional and pool subtypes without the need for added factors. With this trick, we have been able to distinguish the relative contributions of late-stage inductive signals and position-independent programs to the specification of motor neuron subtypes, providing insights into the mechanisms of motor neuron differentiation. Moreover, we have used intraspinal transplantation of ES motor neurons to demonstrate a tight link between the molecular character of motor neuron subtypes and two of their critical functional and developmental attributes – neuronal settling position and axonal trajectory.
Motor neuron differentiation under retinoid-free conditions
The generation of motor neurons from ES cells requires directed neural differentiation. Early exposure of ES cells to retinoids directs cells along a neurogenic lineage (Bain et al., 1995). The application of retinoids in neural differentiation protocols has therefore become a prevalent practice, despite the fact that retinoids have not been implicated in early stages of neural programming within the intact embryo (Niederreither et al., 1999). But retinoid exposure also consigns progenitor cells in the neural tube to caudal hindbrain and rostral cervical identity (Liu et al., 2001).
To uncover conditions for LMC neuron differentiation from ES cells, we developed a differentiation protocol that more closely mimics the patterning events that operate during vertebrate neural differentiation. Local interactions between early embryonic cells are thought to engage a BMP signaling pathway that suppresses the potential for neural differentiation (Finley et al., 1999; Ying et al., 2003), such that dissociation and maintenance of cells at low density avoids paracrine BMP exposure and permits neural differentiation (Watanabe et al., 2005). Our findings indicate that ES cells grown initially under low density retinoid-free conditions give rise to neural cells of caudal brachial character.
In contrast to previously reported RA-free differentiation conditions which rostralize neural cells (Eiraku et al., 2008; Gaspard et al., 2008; Watanabe et al., 2005; Wataya et al., 2008), neural cells generated under CV conditions exhibit caudal and ventral neural progenitor character, and give rise to motor neuron columnar and pool subtypes normally located at brachial and thoracic levels of the spinal cord. These caudal fates depend on embryoid body-derived Wnt, FGF and Hh signals that mimic the signaling milieu of the intact embryo. Several Wnts (Wnt3, Wnt5b, Wnt8a), FGFs (FGF4, FGF5, and FGF15) and Shh are expressed by cells in embryoid bodies over the period that neural cells first appear (Lako et al., 2001; Stavridis et al., 2007). Moreover, blockade of Wnt, FGF or Hh signaling in embryoid bodies prevents the acquisition of caudal neural character. Thus, the generation of LMC neurons from ES cells is driven solely by the actions of endogenously supplied inductive factors. It remains to be seen whether rational application of Wnt and Fgf signals to differentiating ES cells might increase motor neuron yields without sacrificing the columnar and motor pool subtype diversity achieved under CV differentiation conditions.
Neighbor-dependent and -independent programs of LMC subtype specification
Motor neurons within the LMC acquire divisional and pool identities that can be defined by expression of LIM, POU and ETS transcription factors (Arber et al., 2000; Dasen et al., 2005; Jessell, 2000; Livet et al., 2002; Sockanathan and Jessell, 1998). The acquisition of divisional character within an initially generic set of LMC neurons is driven by a retinoid-mediated paracrine signal from early-born LMC neurons (Sockanathan and Jessell, 1998). Furthermore, assignment of the rostrocaudal position of motor pools within the span of the brachial LMC reflects the ambient balance of retinoid and FGF signaling (Dasen et al., 2003; Liu et al., 2001).
How LMC neurons acquire distinct pool identities remains less clear. Analysis of the segregation of Hox protein expression patterns and of switches in motor pool fate after manipulation of spinal Hox gene activity has led to a model in which the segment by segment diversification of LMC neurons into motor pools is driven by cell-intrinsic cross-repressive interactions between the cohort of Hox proteins that is initially expressed by an LMC equivalence group (Dasen et al., 2005). To date however there has been no direct experimental test of this `intrinsic' view, and thus the possibility that signals supplied by neighboring LMC neurons drive intrasegmental pool diversity has not been falsified. One telling prediction of the intrinsic model is that individual LMC neurons should be able to progress to discrete pool identities when isolated from LMC neurons or other limb-level cells that could serve as potential sources of pool inducing signals. Systematic manipulation of the local position of individual LMC neurons in vivo is a formidable and, so far, unmet challenge.
The identification of conditions that permit the generation of LMC neurons from ES cells provided us with an opportunity to examine motor pool specification under conditions in which individual LMC neurons are forced to differentiate in the virtual absence of neighboring LMC neurons and other limb-level neural cells. Our analysis reveals that ES cell derived brachial LMC neurons grown at limitingly low density in a sea of rostral cervical cells can still progress to molecularly authentic CM and FCU fates. These findings are difficult to reconcile with the view that local signals provided by LMC neurons (or other limb level cells) are needed to assign motor pool identity. By extension, they lend experimental support for the idea that the Hox profile inherited by post-mitotic LMC neurons is the primary determinant of the subsequent intrasegmental diversification of motor pools.
ES motor neuron subtype identity validated by cellular behavior in vivo
The segregation of motor neuron cell bodies into distinct intraspinal columnar domains and the projection of motor axons along different peripheral trajectories are two prominent features of motor neuron subtypes linked to distinct transcriptional profiles (Kania and Jessell, 2003; Kania et al., 2000; Sharma et al., 1998; Sockanathan and Jessell, 1998). Our studies have addressed whether the molecular character of motor neuron subtypes predicts distinct cellular behaviors in the developing embryo. Isochronic grafting of ES motor neurons into embryonic spinal cord reveals that neurons with the transcriptional character of MMC and LMC columnar subtypes segregate into different domains of the ventral spinal cord, and settle close to endogenously generated motor neurons of the same columnar character. Thus, ES motor neurons can sense their position within the host spinal cord and migrate appropriately in response to column-specific settling signals. Close inspection of settling position, however, reveals that ES motor neurons of MMC and LMC character failed to mix with their endogenously generated columnar counterparts, possibly because of mismatches in the surface properties of mouse and chick LMC neurons. In contrast to the striking homing behavior of ES motor neurons when introduced in vivo, columnar segregation is not evident in embryoid bodies (Figure S3C), presumably because relevant migratory substrates and guidance cues are inadequately organized under these in vitro conditions.
Grafted ES motor neurons of LMC and MMC character establish peripheral axonal trajectories appropriate for their columnar subtype - selecting axial and limb nerve branches with a fidelity that approaches that of endogenous motor neurons. A substantial majority of motor neurons of caudal brachial character projected axons into the limb mesenchyme, consistent with the predominance of FoxP1+ LMC neurons. More persuasively, retrograde labeling studies revealed that individual Lhx3+ and FoxP1+ ES motor neurons established peripheral axonal trajectories appropriate for their transcriptional identity with high fidelity. The specificity of the link between molecular identity and axonal trajectory implies that the transcriptional status of ES motor neurons directs expression of guidance receptors that underlie the selection of distinct peripheral trajectories (Marquardt et al., 2005).
We have not resolved whether ES motor neurons with distinct pool characters direct axonal projections to specific target muscle groups in the limb. The divergence in anatomical and functional organization of mouse forelimb and chick wing musculature (Ferns and Hollyday, 1995; Jones, 1979; Ryan et al., 1998) confounds analysis of innervation patterns in the mouse-to-chick transplantation assays (Ferns and Hollyday, 1995; Jones, 1979; Ryan et al., 1998). Nevertheless, our studies argue that the programming of motor neuron columnar subtypes from naïve ES cells generates neuronal subsets that express cell-specific transcriptional markers and, in parallel, exhibit specialized cellular behaviors in vivo.
The ability to generate defined motor neuron subtypes without transgene expression or exogenous factor exposure may prove beneficial for studies of neuronal diversification in other regions of the mammalian CNS, as well as for disease modeling. Motor neuron subtypes exhibit differential susceptibility to neurodegeneration in two prominent motor neuron diseases, Amyotrophic Lateral Sclerosis (ALS) and Spinal Muscular Atrophy (SMA) (Kanning et al., 2010). The ability to drive the differentiation ES cells into disease-sensitive and -resistant motor neuron subtypes could help to uncover new strategies for therapy in motor neuron disorders.
Experimental Procedures
Differentiation of ES Cells
Differentiation of ES cells under RA/Hh conditions was performed as previously described (Wichterle et al., 2002). For CV differentiation, ES cells (~20,000 cells/ml) were plated in ADFNK medium (see Supplemental Experimental Procedures). Medium was changed on days 1, 2 and 5 of differentiation and embryoid bodies were split 1:4 on day 2 of differentiation.
Transplantation of ES motor neurons into Chick Neural Tube
Transplantation of ES motor neurons into chick developing neural tube was performed as described (Wichterle et al., 2002). Embryoid bodies were harvested on day 5 or day 6 of differentiation and transplanted into lesioned chick spinal cord. Three days later were transplanted embryos fixed with 4% paraformaldehyde (PFA), cryosectioned, and processed for immunohistochemistry.
Retrograde Labeling of ES motor neurons
Retrograde labeling was performed as described (Dasen et al., 2005). Embryos were dissected three days after transplantation and axial or limb GFP+ nerve branches were retrogradely labeled with 3000 MW lysine-fixable tetramethylrhodamine-dextran (RhD, Molecular Probes) under a fluorescence dissection microscope. Embryos were incubated in an oxygenated bath for 3–5 hours at 37°C, fixed with 4% PFA (1 hour at 4°C), and processed for immunohistochemistry.
Statistical Analysis
Two-tail Student's t-test was used for statistical analysis. Relevant p-values (*p=0.01–0.05, **p=0.001–0.01, ***p<0.001) are listed in the Supplemental Table 2. We used one-way ANOVA to test for a difference between groups and a two-tailed t-test for all pairwise comparisons using a Bonferroni correction for multiple testing.
Additional Experimental Procedures
For more detail see Supplemental Experimental Procedures
Highlights.
- ES cell derived motor neurons can be directed to generate highly specialized subtype identities in the absence of added signaling factors.
- The differentiation of spinal progenitors into specific LMC pool subtypes can be achieved independently of signals provided by neighboring LMC neurons.
- In vivo transplantation reveals that ES cell derived LMC neurons settle in appropriate domains in the spinal cord, and extend axons to limb rather than axial muscle targets.
Supplementary Material
Acknowledgments
We thank Roger Tsien for mCherry plasmid, Stephane Nedelec for Hb9-RFP plasmid, Susan Brenner-Morton for antibodies, Ira Schieren for FACS analysis, members of Wichterle and Doetsch labs for critical discussion. This work was supported by Project A.L.S. grant (TMJ and HW), NINDS NS058502, NS055923 grants (HW), and NS033245 (TMJ), NIH T32 HD055165 Ruth L. Kirschstein National Research Service Award (MP), JD is HHMI Early Career Scientist, EOM is the David and Sylvia Lieb Fellow of the Damon Runyon Cancer Research Foundation (DRG-1937-07), and TMJ is a HHMI investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agalliu D, Takada S, Agalliu I, McMahon AP, Jessell TM. Motor neurons with axial muscle projections specified by Wnt4/5 signaling. Neuron. 2009;61:708–720. doi: 10.1016/j.neuron.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. doi: 10.1016/j.cell.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Jessell TM. Current Topics in Developmental Biology. Academic Press; 2009. Chapter Six Hox networks and the origins of motor neuron diversity; pp. 169–200. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Ferns MJ, Hollyday M. Chick wing innervation. III. Formation of axon collaterals in developing peripheral nerves. J Comp Neurol. 1995;357:272–280. doi: 10.1002/cne.903570207. [DOI] [PubMed] [Google Scholar]
- Finley MF, Devata S, Huettner JE. BMP-4 inhibits neural differentiation of murine embryonic stem cells. J Neurobiol. 1999;40:271–287. [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Haase G, Dessaud E, Garces A, de Bovis B, Birling M, Filippi P, Schmalbruch H, Arber S, deLapeyriere O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jones CL. The morphogenesis of the thigh of the mouse with special reference to tetrapod muscle homologies. J Morphol. 1979;162:275–309. doi: 10.1002/jmor.1051620207. [DOI] [PubMed] [Google Scholar]
- Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102:161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Lako M, Lindsay S, Lincoln J, Cairns PM, Armstrong L, Hole N. Characterisation of Wnt gene expression during the differentiation of murine embryonic stem cells in vitro: role of Wnt3 in enhancing haematopoietic differentiation. Mech Dev. 2001;103:49–59. doi: 10.1016/s0925-4773(01)00331-8. [DOI] [PubMed] [Google Scholar]
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liss B, Roeper J. Individual dopamine midbrain neurons: Functional diversity and flexibility in health and disease. Brain Research Reviews. 2008;58:314–321. doi: 10.1016/j.brainresrev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and Retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Nordstrom U, Maier E, Jessell TM, Edlund T. An early role for WNT signaling in specifying neural patterns of Cdx and Hox gene expression and motor neuron subtype identity. PLoS Biol. 2006;4:e252. doi: 10.1371/journal.pbio.0040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JM, Cushman J, Jordan B, Samuels A, Frazer H, Baier C. Topographic position of forelimb motoneuron pools is conserved in vertebrate evolution. Brain Behav Evol. 1998;51:90–99. doi: 10.1159/000006531. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40:97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J Neurosci. 2006;26:3256–3268. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proceedings of the National Academy of Sciences. 2008;105:11796–11801. doi: 10.1073/pnas.0803078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Peljto M, Nedelec S. Stem Cells in Regenerative Medicine. 2009. Xenotransplantation of embryonic stem cell-derived motor neurons into the developing chick spinal cord; pp. 171–183. [DOI] [PubMed] [Google Scholar]
- Williams JA, Guicherit OM, Zaharian BI, Xu Y, Chai L, Wichterle H, Kon C, Gatchalian C, Porter JA, Rubin LL, et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci U S A. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.