Abstract
Background
Atherosclerotic renovascular disease is associated with an increased risk of cardiovascular disease (CVD) events. This study examines associations between Doppler-derived parameters from the renal artery and renal parenchyma and all-cause mortality and fatal and nonfatal CVD events in a cohort of elderly Americans.
Study Design
Cohort study.
Setting
A subset of participants from the Cardiovascular Health Study (CHS). Through an ancillary study, 870 (70% recruitment) Forsyth County, NC, CHS participants consented to undergo renal duplex sonography to define the prevalence of renovascular disease in the elderly, resulting in 726 (36% men; mean age, 77 years) technically adequate complete studies included in this investigation.
Predictor
Renal duplex sonography–derived Doppler signals from the main renal arteries and renal parenchyma. Spectral analysis from Doppler-shifted frequencies and angle of insonation were used to estimate renal artery peak systolic and end diastolic velocity (both in meters per second). Color Doppler was used to identify the corticomedullary junction. Using a 3-mm Doppler sample, the parenchymal peak systolic and end diastolic frequency shift (both in kilohertz) were obtained. Resistive index was calculated as (1 – [end diastolic frequency shift/peak systolic frequency shift]) using Doppler samples from the hilar arteries of the left or right kidney with the higher main renal artery peak systolic velocity.
Outcomes & Measurements
Proportional hazard regression analysis was used to determine associations between renal duplex sonography–derived Doppler signals and CVD events and all-cause mortality adjusted for accepted cardiovascular risk factors. Index CVD outcomes were defined as coronary events (angina, myocardial infarction, and coronary artery bypass grafting/percutaneous coronary intervention), cerebrovascular events (stroke or transient ischemic attack), and any CVD event (angina, congestive heart failure, myocardial infarction, stroke, transient ischemic attack, and coronary artery bypass grafting [CABG]/percutaneous transluminal coronary intervention [PTCI]).
Results
During follow-up, 221 deaths (31%), 229 CVD events (32%), 122 coronary events (17%), and 92 cerebrovascular events (13%) were observed. Renal duplex sonography–derived Doppler signals from the renal parenchyma were associated independently with all-cause mortality and CVD outcomes. In particular, increased parenchymal end diastolic frequency shift was associated significantly with any CVD event (HR, 0.73; 95% CI, 0.62-0.87; P < 0.001). Marginally significant associations were observed between increases in parenchymal end diastolic frequency shift and decreased risk of death (HR, 0.86; 95% CI, 0.73-1.00; P = 0.06) and decreased risk of cerebrovascular events (HR, 0.78; 95% CI, 0.61-1.01; P = 0.06). Parenchymal end diastolic frequency shift was not significantly predictive of coronary events (HR, 0.84; 95% CI, 0.67-1.06; P = 0.1).
Limitations
CHS participants showed a “healthy cohort” effect that may underestimate the rate of CVD events in the general population.
Conclusion
Renal duplex sonographic Doppler signals from the renal parenchyma showed significant associations with subsequent CVD events after controlling for other significant risk factors. In particular, a standard deviation increase in parenchymal end diastolic frequency shift was associated with 27% risk reduction in any CVD event.
Keywords: Renovascular disease, resistive index, intrarenal Doppler, renal duplex sonography, prospective, population based, cardiovascular events, Cardiovascular Health Study (CHS)
Atherosclerotic renovascular disease is a recognized cause of secondary hypertension and chronic kidney disease (ie, excretory renal insufficiency or ischemic nephropathy).1-3 Renal duplex sonography is an accurate noninvasive means to diagnose hemodynamically significant renovascular disease in patients with severe hypertension and/or renal insufficiency.2,4 The presence of renovascular disease assessed using renal duplex sonography has been associated with increased severity of hypertension and decreased renal function.5,6 Moreover, it appears that the presence of renovascular disease is associated with increased risk of subsequent cardiovascular disease (CVD) events.7,8
In addition to providing information about hemodynamically significant atherosclerotic lesions of the main renal artery, renal duplex sonographic Doppler signals can be obtained from the renal hilum and renal parenchyma. Recent studies involving selected patient populations have shown strong associations between renal duplex sonographic measures of renal artery resistance in the hilum (an alternative measure of disease in the renal parenchyma) and severity of hypertension, progressive renal failure, and mortality.9,10
In prior analyses of a Cardiovascular Health Study (CHS) cohort that had undergone renal duplex sonography, we showed strong associations between renal duplex sonographic measures from the renal parenchyma and severity of hypertension, as well as kidney disease.11 In contrast, the present study constitutes the first examination of renal duplex sonographic measures from the renal parenchyma and risk of subsequent CVD events in the Forsyth County, NC, CHS cohort participating in the renal duplex sonography ancillary study.
The aim of this study is to examine relationships between renal duplex sonography–derived Doppler signals from the main renal arteries, hilar renal arteries, and renal parenchyma and follow-up mortality and CVD events to better describe previously established links between atherosclerotic kidney disease and CVD morbidity and mortality.
METHODS
The CHS
The design of the CHS has been described previously.12 Briefly, it is a longitudinal multicenter observational cohort study of CVD risk factors, morbidity, and mortality in Americans aged ≥65 years. The initial CHS cohort was recruited from a randomly selected sample of Medicare-eligible individuals in 4 US communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Allegheny County, PA). Initial recruitment was performed between April 1989 and May 1990, with a supplemental cohort of African American participants recruited using the same method from June 1992 to June 1993.
All CHS enrollees underwent a baseline examination consisting of a detailed medical history and clinical examination. Annual follow-up examinations were performed to update medical data, assess the occurrence of cardiovascular events, and repeat portions of the clinical examination at previously defined intervals. Clinical examination included physical examination, blood pressure measurement, phlebotomy, electrocardiography, and pulmonary function testing. Serum creatinine was measured in conjunction with renal duplex examination.
Renal Duplex Sonography
The renal duplex sonographic examination was performed as part of an ancillary study to the CHS to define the prevalence of renovascular disease in a community-dwelling elderly cohort.13 For this ancillary study, which was approved by the Wake Forest University Human Subjects Review Committee (Winston-Salem, NC), 1,245 Forsyth County participants were invited to participate on return for the annual examination between January 1995 and February 1997.
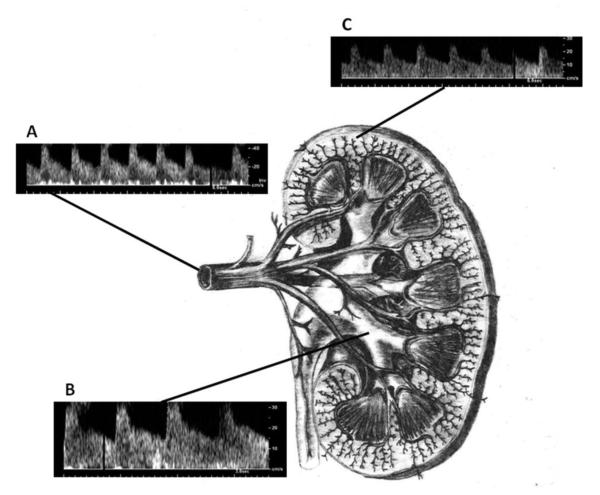
The technique of renal duplex sonography has been described previously.2,13 Briefly, renal duplex sonography was performed by 2 registered vascular technologists using an Ultramark-9 HDI Ultrasound System (Advanced Technologies Laboratories, Bothell, WA) during the participant's return visit for his or her annual examination. After written informed consent and an overnight fast, sagittal B-mode scan images were obtained of the upper abdominal aorta, celiac axis, and superior mesenteric arteries using a 2.25- or 3.0-MHz ultrasound probe with the participant in the supine position. Identification of these 3 vessels was confirmed by the characteristic fasting waveforms from each vessel. Next, using the left renal vein as a reference, the aortic origins of the main renal arteries were identified. While maintaining an angle of insonation <60°, Doppler samples were obtained from each renal artery from aortic origin to renal hilum, for a total of 10 Doppler sample sites per renal artery. Spectral analysis from the Doppler-shifted frequencies and angle of insonation were used to estimate the renal artery peak systolic and end diastolic velocities (both in meters per second). Renal artery peak systolic velocity was confirmed using a flank approach with the participant in the right or left lateral decubitus positions, along with B-mode scan imaging of each kidney to determine the greatest longitudinal kidney length. While in the decubitus position, color Doppler was used to identify the corticomedullary junction. Using a 3-mm Doppler sample, the parenchymal peak systolic and end diastolic frequency shift (both in kilohertz) were obtained. Resistive index was calculated as (1 – [end diastolic frequency shift/peak systolic frequency shift]) using Doppler samples from the hilar arteries of the left or right kidney with the higher main renal artery peak systolic velocity. In analyses, resistive index was examined both as a continuous measure and dichotomized at <0.8 or ≥0.8, a cutoff value established in prior studies as predictive of parenchymal disease.9,10 Figure 1 shows a schematic of the kidney with renal artery, renal hilum, and renal parenchyma. Examples of Doppler signal wave forms in each vascular bed also are shown.
Figure 1.
Schematic representation of renal anatomy and associated Doppler spectra from the (A) main renal artery, (B) hilar vessels, and (C) renal parenchyma.
All B-mode and Doppler spectral data were collected on super VHS tape and transferred to an electronic database. This process was repeated and data were compared for agreement. Three percent discordance in electronic data was adjudicated from review of the original duplex study.
Adverse Events
An updated participant medical history was obtained through the annual CHS clinical examination and biannual telephone calls. Adverse cardiovascular events occurring during the surveillance period were reviewed and confirmed using a defined protocol. Adverse coronary events were defined as any confirmed fatal or nonfatal myocardial infarction (MI), hospitalized angina, or coronary revascularization (coronary artery bypass grafting [CABG] or angioplasty and/or stent placement [percutaneous transluminal coronary intervention (PTCI)]). Adverse cerebrovascular events were defined as any confirmed fatal or nonfatal stroke or transient ischemic attack (TIA). A CVD event was defined as any coronary or cerebrovascular event, as well as fatal or nonfatal hospital admission for congestive heart failure (CHF). Index events were defined as the first adverse coronary event, cerebrovascular event, or any CVD event occurring after the renal duplex sonographic examination through the end of the surveillance period (July 2002) regardless of prevalent CVD at the time of renal duplex sonographic examination. For time-to-event analyses, event times were calculated from the date of renal duplex sonographic examination to the date of occurrence of the index coronary, cerebrovascular, or any CVD event or time to death.
Statistical Analysis
After ancillary study data were keyed and verified, renal duplex sonographic results were matched with participant data provided by the CHS Coordinating Center. Demographic, risk-factor, and prevalent CVD variables for all participants were defined using the most current data available from all CHS examinations before or coincident with the renal duplex sonographic examination.
Descriptive statistics were computed for demographic characteristics, renal duplex sonographic parameters (including body surface area–adjusted kidney length14) and CVD risk factors. Univariable comparison of characteristics between participants with hilar artery resistive index ≥0.8 versus <0.8 were performed using t tests for continuous factors and χ2 tests for dichotomous factors. Multivariate models for time from renal duplex sonographic examination to index nonfatal adverse event or death were constructed using proportional hazards regression. Separate models were constructed for time to first occurrence of each of the following: (1) death from any cause; (2) any CVD event (hospitalized angina or CHF, MI, CABG/PTCI, stroke, or TIA); (3) coronary events (hospitalized angina, MI, or CABG/PTCI); and (4) cerebrovascular events (stroke or TIA). Six independent models with renal duplex sonographic parameters were fitted for each outcome. Each model included 1 parenchymal parameter paired separately with 1 renal artery velocity and renal resistive index, and finally a model with both parenchymal frequency shift parameters (end diastolic and peak systolic frequency shift). Akaike's information criterion was used to judge the pairing of parenchymal and renal artery velocity parameters to use in final model selection.15 Proportionality assumptions for renal duplex sonographic parameters were assessed by examining log (– log[survival]) plots.
A backward-elimination model selection procedure was used to select final multivariable models to predict follow-up events. All models included renal duplex sonographic parameters, estimated glomerular filtration rate using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation,16 and systolic and diastolic blood pressures as forced variables. Demographic and atherosclerotic risk factors were included as candidate covariates, as well as coexisting measures of prevalent clinical and subclinical CVD. The following additional variables were considered for inclusion in regression models: age and weight (kilograms) at renal duplex sonographic examination; sex; race; history of smoking; left ventricular wall motion abnormality or decreased ejection fraction from cardiac echocardiography; lipid-lowering medication (any); hypertensive medication use (any); history of hospitalized angina, MI, coronary revascularization procedure (CABG/PTCI), atrial fibrillation, or pacemaker placement; diabetes mellitus, clinical hypertension; CHF; claudication or lower-extremity revascularization; major electrocardiographic abnormality; stroke or TIA; abdominal aortic aneurysm; carotid artery stenosis ≥75% or endarterectomy; carotid artery stenosis ≥25%, common carotid intimal-medial thickness >1.18 mm, or internal carotid intimal-medial thickness >1.81 mm on carotid ultrasound; ankle-brachial index ≤0.90; serum cholesterol level (milligrams per deciliter); high-density lipoprotein cholesterol level (milligrams per deciliter); and low-density lipoprotein cholesterol level (milligrams per deciliter). Models were constructed by eliminating variables one by one, beginning with the least significant, until only variables with P < 0.10 remained. Estimated hazard ratios (HRs) for continuous renal duplex sonographic parameters are presented for a 1-standard deviation (SD) increase in the covariate. All analyses were performed using SAS/STAT, version 9.1 software (SAS Institute Inc, www.sas.com).
RESULTS
Between January 1995 and February 1997, a total of 870 (70%) Forsyth County CHS participants consented to undergo renal duplex sonography to define the prevalence of renovascular disease in the elderly. This resulted in 726 (83%) studies with complete data for the main renal artery, renal hilum, and parenchyma on at least 1 side. Participants with complete renal duplex sonographic data included in analyses were compared with the 144 patients excluded because of incomplete scans or missing data. Excluded participants tended to be older and heavier. No other significant differences in demographics or disease histories were found.
Table 1 lists demographics, clinical and subclinical CVD histories, and risk factors for the study group. Mean age of the group was 77.0 ± 4.8 years. The study group consisted of 264 men (36%), and 52 participants (7%) were African American. At the time of the renal duplex sonographic examination, prevalent coronary artery disease (history of hospitalized angina, MI, or CABG/PTCI) was present in 157 participants (22%). Prevalent cerebrovascular disease was present in 51 participants (7%). A history of clinical CVD (prevalent coronary artery or cerebrovascular disease; history of CHF, atrial fibrillation, abdominal aortic aneurysm repair, claudication, or peripheral vascular disease; or history of internal carotid artery stenosis (<75%) or carotid endarterectomy was present in 261 (36%) participants.
Table 1.
Descriptive statistics overall and by hilar renal artery resistive index (RI) dichotomized at 0.8.
| Overall | RI ≥ 0.8 | RI < 0.8 | ||
|---|---|---|---|---|
| Variable | N = 726† | N = 184‡ | N = 542€ | P-value |
| Age in years* (mean ± SD) | 77.0 ± 4.8 | 78.6 ± 5.5 | 76.5 ± 4.4 | <0.001 |
| African-American race** (frequency [%]) | 161 (22.2) | 40 (21.7) | 121 (22.3) | 0.9 |
| Men** (frequency [%]) | 264 (36.4) | 60 (32.6) | 204 (37.6) | 0.2 |
| Systolic blood pressure*, mmHg (mean ± SD) | 136.1 ± 20.3 | 139.8 ± 22.6 | 134.9 ± 19.3 | 0.01 |
| Diastolic blood pressure*, mmHg (mean ± SD) | 72.0 ± 10.3 | 69.0 ± 10.2 | 73.1 ±10.1 | <0.001 |
| Height*, cm (mean ± SD) | 170 ± 24 | 171.1 ± 33.1 | 169.2 ± 19.9 | 0.5 |
| Weight*, kg (mean ± SD) | 71.9 ± 13.3 | 72.3 ± 14.2 | 71.8 ± 12.9 | 0.6 |
| Current or former of cigarette smoker* (frequency [%]) | 348 (47.9) | 91 (49.5) | 257 (47.4) | 0.6 |
| Estimated GFR*, mL/min/1.73m2 from 4-variable MDRD# equation (mean ± SD) | 69.9 ± 15.5 | 68.6 ± 17.0 | 70.3 ± 15.0 | 0.2 |
| Serum creatinine*, mg/dL (mean ± SD) | 1.00 ± 0.27 | 1.03 ± 0.29 | 0.99 ± 0.26 | 0.09 |
| Renal insufficiency*, estimated GFR <60 mL/min/1.73m2 (frequency [%]) | 59 (8.2) | 23 (12.6) | 36 (6.7) | 0.01 |
| History of Coronary Artery Disease (CAD): myocardial infarct, hospitalized angina, or coronary revascularization* (frequency [%]) | 157 (21.6) | 53 (28.8) | 104 (19.2) | 0.01 |
| History of stroke or transient ischemic attack* (frequency [%]) | 51 (7.0) | 22 (12.0) | 29 (5.4) | 0.002 |
| Prevalent CAD or CVD, history of CHF, atrial fibrillation, AAA, claudication or peripheral vascular disease, or a history of internal carotid artery stenosis (>75%) or carotid endarterectomy* (frequency [%]) | 261 (36.0) | 84 (45.7) | 177 (32.7) | 0.002 |
| Hypertension: SBP > 160 mmHg, DBP > 95 mmHg, or use of antihypertensive agent* (frequency [%]) | 368 (50.7) | 114 (62.0) | 254 (46.9) | <0.001 |
| Use of any antihypertensive medication at time of RDS* (frequency [%]) | 401 (55.2) | 122 (66.3) | 279 (51.5) | <0.001 |
| Hypercholesterolemia by National Cholesterol Education Program guidelines* (frequency [%]) | 257 (35.4) | 64 (34.8) | 193 (35.6) | .8 |
| Diabetes by fasting serum glucose > 140 mg/dL, 2-hour post glucose load serum glucose >200 mg/dL, or insulin/oral hypoglycemic agent use* (frequency [%]) | 168 (23.1) | 57 (31.0) | 111 (20.5) | 0.004 |
| Peripheral vascular disease (PVD) by ankle-brachial index ≤ 0.90 (frequency [%]) | 64 (8.9) | 22 (12.1) | 42 (7.8) | 0.08 |
| ECG: any major ECG abnormality (frequency [%]) | 216 (29.8) | 61 (33.2) | 155 (28.6) | 0.2 |
| Echo: LV ejection fraction <50% or major wall motion abnormality (frequency [%]) | 27 (3.8) | 8 (4.4) | 19 (3.5) | 0.6 |
| BSA-adjusted kidney length, cm/m2 (mean ± SD) | 5.77 ± 0.63 | 5.78 ± 0.66 | 5.77 ± 0.62 | 0.9 |
| RA peak systolic velocity (PSV), max of left or right side, m/sec (mean ± SD) | 1.26 ± 0.50 | 1.30 ± 0.47 | 1.24 ± 0.51 | 0.2 |
| RA end diastolic velocity, max of left or right side, m/sec (mean ± SD) | 0.31 ± 0.15 | 0.26 ± 0.10 | 0.33 ± 0.16 | <0.001 |
| Parenchymal peak systolic frequency shift (PSF). max of left or right side, kHz (mean ± SD) | 1550 ± 543 | 1595 ± 541 | 1535 ± 543 | 0.2 |
| Parenchymal end diastolic frequency shift (EDF), max of left or right side, kHz (mean ± SD) | 380 ± 132 | 348 ± 120 | 391 ± 134 | <0.001 |
| Hilar renal artery resistive index (RI), from side with max PSV, % (mean ± SD) | 0.76 ± 0.06 | |||
| RI ≥ 0.8, from side with max PSV (frequency [%]) | 184 (25.3) |
Parameter recorded/updated at nearest visit prior to or concurrent with the renal duplex examination
Parameter recorded at initial baseline examination
N=723 for estimated GFR, serum creatinine, and renal insufficiency; N=724 for kidney length; N=718 for PVD
N=183 for estimated GFR, serum creatinine, and renal insufficiency; N=182 for PVD and echo parameters
N=540 for estimated GFR, serum creatinine, renal insufficiency, and kidney length; N=536 for PVD; N=538 for echo parameters
Table 1 also lists factors compared according to hilar artery resistive index (obtained from the kidney with the greatest renal artery peak systolic velocity) dichotomized at 0.8. Participants with resistive index ≥0.8 were older (78.6 ± 5.5 vs 76.5 ± 4.4 years; P < 0.001); had higher systolic blood pressure (139.8 ± 22.6 vs 134.9 ± 19.3 mm Hg; P = 0.01); lower diastolic blood pressure (69 ± 10.2 vs 73.1 ± 10.1 mm Hg; P < 0.001); higher prevalence of decreased kidney function defined as estimated glomerular filtration rate ≤60 mL/min/1.72 m2 (12.6% vs 6.7%; P = 0.01); history of coronary artery disease (28.8% vs 19.2%; P = 0.01); history of stroke or TIA (12% vs 5.4%; P = 0.002); prevalent CVD (45.7% vs 32.7%; P = 0.002); hypertension (62.0% vs 46.9%; P < 0.001) and antihypertensive medication use (66.3% vs 51.5%; P < 0.001); diabetes (31% vs 20.5%; P = 0.004); lower mean renal artery end diastolic velocity (0.25 ± 0.1 vs 0.33 ± 0.12 m/s; P < 0.001); and lower mean parenchymal end diastolic frequency shift (348 ± 120 vs 391 ± 134 kHz; P < 0.001).
Table 2 lists the index adverse cardiovascular events (the first occurrence during post–renal duplex sonographic follow-up of any CVD, coronary artery, or cerebrovascular event, as examined in analyses), as well as specification of fatal event causes. With an average post–renal duplex sonographic surveillance follow-up of 81.2 months (range, 0.5-100.4 months), there were 229 index CVD events, including 17 that were fatal. There were 122 coronary artery disease–specific index events, including 19 fatal, and 92 cerebrovascular-specific index events, including 3 fatal. Of the 221 total fatal events, 33 (15%) were attributed to cardiovascular causes, and the remaining were noncardiovascular.
Table 2.
First occurrence during post-RDS follow-up of cardiovascular disease (CVD) event, coronary artery disease (CAD), or cerebrovascular disease (CBV).
| Any adverse cardiovascular events | Non-fatal | Fatal | |
| Hospitalized angina | 19 | 0 | |
| Hospitalized congestive heart failure | 63 | 2 | |
| Myocardial infarction | 34 | 4 | |
| Coronary artery angioplasty or stent | 13 | 0 | |
| Coronary artery bypass surgery | 6 | 0 | |
| Transient ischemic event | 15 | 0 | |
| Stroke | 62 | 2 | |
| Coronary artery related death (other) | 0 | 9 | |
| Total | 212 | 17 | |
| Cardiac events | Non-fatal | Fatal | |
| Hospitalized angina | 38 | 0 | |
| Myocardial infarction | 41 | 4 | |
| Coronary artery angioplasty or stent | 16 | 0 | |
| Coronary artery bypass surgery | 8 | 0 | |
| Coronary artery related death (other) | 0 | 15 | |
| Total | 103 | 19 | |
| Cerebrovascular events | Non-fatal | Fatal | |
| Transient ischemic event | 15 | 0 | |
| Stroke | 74 | 2 | |
| Cerebrovascular death (other) | 0 | 1 | |
| Total | 89 | 3 | |
| Fatal events | Fatal | ||
| Myocardial infarction | 4 | ||
| Coronary artery related death | 15 | ||
| Stroke | 2 | ||
| Cardiovascular accident | 7 | ||
| Cardiovascular disease | 2 | ||
| Congestive heart failure | 3 | ||
| Non-cardiovascular disease death | 188 | ||
| Total | 221 |
Before implementing general model selection procedures to select important covariates, models containing only renal duplex sonographic parameters measured in the main renal arteries, hilar arteries, and parenchyma were examined to select the optimal measure from each. Results of model comparison are listed in Table S1 (available in the supplementary material available with this article at www.ajkd.org). Comparison of models including parenchymal end diastolic frequency shift versus peak systolic frequency shift and continuous versus dichotomized resistive index showed similar predictive ability. However, Akaike information criterion values were consistently smaller for models including parenchymal end diastolic frequency shift and dichotomized resistive index with renal artery peak systolic velocity, which led to inclusion of those factors as renal duplex sonographic parameters in the final model selection. In the selected models containing only renal duplex sonographic parameters, parenchymal end diastolic frequency shift, renal artery peak systolic velocity, and hilar vessel resistive index were associated significantly with all-cause mortality and CVD events. Parenchymal end diastolic frequency shift was the only renal duplex sonographic parameter significantly associated with all 4 outcomes. Plots of the log (−log[survival]) versus log of survival time showed no substantial departures from proportionality.
Table 3 lists results of backward variable elimination. HRs with 95% confidence intervals and P values are shown for all variables forced into the models. Other covariates selected for inclusion are listed for each outcome. The following associations between an SD increase in end diastolic frequency shift and each outcome were observed: a 14% decrease in risk of all-cause mortality (HR, 0.86; P = 0.06); 27% decrease in risk of CVD event (HR, 0.73; P < 0.001); 16% decrease in risk of coronary artery disease event (HR, 0.84; P = 0.1); and 22% decrease in risk of cerebrovascular event (HR, 0.78; P = 0.06). After inclusion of parenchymal end diastolic frequency shift and selection of other factors through backward elimination, the only additional renal duplex sonographic parameter found to be predictive of follow-up events was renal artery peak systolic velocity, which was associated significantly with a 16% increased risk of CVD events (HR, 1.16; P = 0.02).
Table 3.
Results of backwards elimination model selection procedures for time-to-event outcomes.
| Mortality (216 events) | CVD Events (224 events) | CAD Events (121 events) | CBV Events (N=89) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (death by any cause) | (Angina, CHF, MI, CABG, PTCI, Stroke, or TIA) |
(Angina, MI, CABG, or PTCI) | (Stroke or TIA) | |||||||||
| Variable Forced in Models | Hazard Ratio |
95% Confidence Interval |
P-value | Hazard Ratio |
95% Confidence Interval |
P-value | Hazard Ratio |
95% Confidence Interval |
P-value | Hazard Ratio |
95% Confidence Interval |
P-value |
| Parenchymal end diastolic frequency shift (EDF)* |
0.86 | (0.73, 1.00) | 0.058 | 0.73 | (0.62, 0.87) | <0.001 | 0.84 | (0.67, 1.06) | 0.14 | 0.78 | (0.61, 1.01) | 0.064 |
| Renal artery peak systolic velocity (PSV)* | 1.09 | (0.98, 1.21) | 0.12 | 1.16 | (1.02, 1.33) | 0.023 | 1.04 | (0.88, 1.23) | 0.6 | 1.11 | (0.92, 1.34) | 0.3 |
| D-segment Resistive index (RI) ≥ 0.8 | 0.86 | (0.62, 1.19) | 0.3 | 1.10 | (0.81, 1.50) | 0.5 | 1.14 | (0.76, 1.72) | 0.5 | 0.94 | (0.58, 1.53) | 0.8 |
| Estimated GFR at renal duplex (RDS)* | 0.80 | (0.65, 0.99) | 0.043 | 0.89 | (0.72, 1.08) | 0.2 | 0.73 | (0.57, 0.95) | 0.017 | 0.76 | (0.55, 1.04) | 0.085 |
| SBP at RDS* | 0.91 | (0.77, 1.07) | 0.3 | 1.00 | (0.84, 1.18) | 0.9 | 1.19 | (0.95, 1.48) | 0.14 | 0.96 | (0.75, 1.24) | 0.8 |
| DBP at RDS* | 1.00 | (0.85, 1.18) | 0.9 | 1.02 | (0.87, 1.21) | 0.8 | 0.90 | (0.72, 1.13) | 0.4 | 1.13 | (0.87, 1.46) | 0.4 |
| Other covariates included in final model: | Age at RDS; former or current smoker; history of diabetes mellitus; HDL cholesterol; on lipid-lowering medication(s); History of CHF; history of Stroke or TIA; history of abdominal aortic aneurysm; ankle-arm index ≤ 0.90; Internal carotid IMT ≥ 1.81 mm |
History of diabetes mellitus; on anti- hypertensive medication(s); on lipid- lowering medication(s); history of atrial fibrillation or pace maker; history of claudication or lower extremity procedure; history of hospitalized angina; history of Stroke or TIA; history of abdominal aortic aneurysm; history of carotid artery stenosis (>25% stenosis); LV ejection fraction or wall motion abnormality |
Total cholesterol; on anti- hypertensive medication(s); weight at renal duplex (RDS); history of atrial fibrillation or pace maker; history of claudication or lower extremity procedure; history of hospitalized angina; history of Stroke or TIA; history of carotid artery stenosis (>25% stenosis) |
Age at RDS; history of diabetes mellitus |
||||||||
Parameter estimates based upon one standard deviation change
DISCUSSION
This report examines relationships between Doppler-shifted frequencies obtained using renal duplex sonography of the renal parenchyma and subsequent morbid and fatal outcomes in an elderly cohort. At a mean follow-up of 81 months, an SD increase in parenchymal end diastolic frequency shift was associated significantly with a 27% decrease in risk of all CVD events and marginally significant risk reductions of 14% for all-cause mortality and 22% for cerebrovascular events. A nonsignificant 16% decrease in risk of coronary artery events also was observed. Although main renal artery peak systolic velocity and hilar vessel resistive index showed significant associations with all-cause mortality and any CVD event in models that included only renal duplex sonographic parameters (Table S1), only peak systolic velocity remained a significant predictor of CVD events after full model selection was performed (Table 3). Associations between parenchymal end diastolic frequency shift and outcomes were independent of other accepted risk factors for cardiovascular-related events, such as renal function, hypertension, diabetes, and history of coronary artery or cerebrovascular events.
We have previously reported strong associations between the presence of renovascular disease (defined as ≥60% stenosis based on renal artery peak systolic velocity ≥1.8 m/s or renal artery occlusion using renal duplex sonography) and clinical and subclinical manifestations of CVD.7,17 In the present report, continuous measures of renal artery peak systolic velocity were associated with all-cause mortality and CVD events in models containing only renal duplex sonographic parameters, but these relationships did not persist in final models that included important CVD risk factors selected using backward variable elimination.
Renal duplex sonographic Doppler signals from the renal parenchyma have correlated with cardiovascular-related outcomes. In a select group of 566 hypertensive individuals without clinical evidence of atherosclerotic vascular or kidney disease, resistive index ≥0.7 correlated with higher systolic blood pressure, carotid artery intima-media thickness, left ventricular mass index, and echocardiographic evidence of diastolic dysfunction.18 In a previous study of the CHS cohort,11 we observed strong cross-sectional relationships between decreased parenchymal end-diastolic frequency shift and, to a lesser extent, increased resistive index, with increased severity of hypertension.
Others have examined resistive index and found that it correlated with increased serum creatinine level and decreased creatinine clearance in a group of selected patients with chronic kidney disease, particularly those with diabetes mellitus.19 Resistive index also may predict worsening renal dysfunction because Radermacher et al9 showed that resistive index ≥0.8 was predictive of worsened renal function and progression to dialysis therapy in a group of patients with newly diagnosed renal disease, as well as all-cause mortality. Investigation of a cohort of patients with serial renal duplex sonographic examinations who also underwent renal revascularization at our center also found resistive index ≥0.8 to be predictive of all-cause mortality.10 Moreover, resistive index appears to correlate, at least in part, with transplant renal allograft function and may serve as a marker of rejection.20 Others have examined the ability of resistive index to predict outcomes after renal artery revascularization for patients with renovascular disease.21-23 These studies identified resistive index >0.8 or 0.7 (diastolic-systolic ratio <0.3) to be associated with worsened clinical responses to renal artery revascularization.
In contrast to these studies, we found end-diastolic frequency shift from Doppler samples obtained directly in the renal parenchyma to be a better predictor of adverse CVD events and mortality than resistive index measurements from the hilar arteries. Frequency shifts in Doppler signals from the renal parenchyma reflect flow in small arteries, arterioles, and the renal microcirculation. It has been theorized that decreases in parenchymal frequency shifts result from arteriosclerosis, interstitial changes related to renal scarring, or decreases in intrarenal microvascular area. Platt et al24 correlated decreased Doppler shifts with tubulointerstitial disease, including acute tubular necrosis and vasculitis. Others have correlated histologic parenchymal changes associated with hypertensive nephrosclerosis and active lupus nephritis. Further support for the relationship between histologic changes in the renal parenchyma and Doppler-derived measures comes from studies involving patients with progressive systemic sclerosis, tubulointerstitial nephropathy, hypertensive nephrosclerosis, and chronic kidney disease.25-27
Although this study emphasizes microvascular flow in the kidney, associations between microvascular changes in other vascular beds and adverse outcomes have been examined. Changes in retinal microvasculature, such as focal arteriolar narrowing and arterial-venous nicking documented using retinal photography, have been associated with subclinical and clinical manifestations of CVD and subsequent CVD events.28,29 We have recently reported, for this same CHS cohort, significant cross-sectional associations between retinopathy and increases in serum creatinine level.30 Moreover, participants with retinopathy were 3 times more likely to have a >20% decrease in estimated glomerular filtration rate during follow-up. Impaired myocardial tissue perfusion also has been correlated with inferior cardiac results after coronary revascularization. Impaired tissue-level perfusion after intervention assessed using TIMI (thrombolysis in myocardial infarction) flow grade is independently correlated with postprocedural increases and impaired resolution in serum cardiac-specific troponin levels.31 This so-called “no-reflow” phenomenon has been correlated with microvascular damage and may have effects on myocardial remodeling after infarct. Even more relevant to the findings of this study may be those of impaired myocardium flow using magnetic resonance imaging or contrast echocardiography, which correlate with subsequent functional recovery of myocardium after infarction.32,33 Hence, there appears to be increasing evidence to suggest that microvascular changes in differing vascular beds correlate, at least in part, with the increased risk of subsequent CVD events typically associated with large-vessel atherosclerotic vascular disease.
To our knowledge, no other study has examined relationships between Doppler measurements from the renal parenchyma and cardiovascular outcomes. Advocates of resistive index correctly note that obtaining the ratio of diastolic to systolic frequency shifts in the same Doppler spectrum eliminates error in velocity estimation related to the angle of insonation. Unfortunately, changes in resistive index may be influenced by a number of intrinsic and extrinsic renal factors and have not proved to be as reliable a measure of renal parenchymal changes or predictive of renal function outcomes after surgical repair of renovascular disease in patients at our center.10 It is possible that the best measure of parenchymal microvascular circulatory changes is the individual parameters measured in the parenchyma (ie, end diastolic and peak systolic frequency shift) rather than the ratio of hilar vessel end diastolic and peak systolic frequency shifts.
Although this study uses a carefully constructed community-dwelling elderly cohort, several study weaknesses deserve comment. The recruitment strategy used by the CHS did not produce a true randomly selected population-based sample. Index participants were selected for initial contact based on a random sampling of Medicare eligibility lists, but age-eligible members of the same household also were recruited. As a result, a significant number (30%) of participants were enrolled from shared households. This recruitment strategy was designed to enhance longitudinal follow-up; however, it unavoidably introduced selection bias because significant differences existed between the randomly selected individuals who chose to enroll in the CHS and those who declined. In general, participants enrolling were younger, more highly educated, more likely to be married, and more likely to have previously quit smoking.34 This ”healthy cohort” effect, combined with a similar trend in renal duplex sonographic ancillary study participants and nonparticipants,13 may have resulted in a lower prevalence of clinical CVD and subsequent CVD events in studied individuals.
Results of this study describe significant independent associations between renal duplex–derived Doppler signals and subsequent fatal events and fatal and nonfatal CVD events. In particular, renal duplex sonographic Doppler-shifted frequencies from the renal parenchyma showed significant independent associations with CVD events. These results are consistent with previous reports describing associations between microvascular changes and clinical and subclinical CVD and suggest that renal duplex sonographic–derived Doppler signals may predict subsequent CVD events independent of their associations with blood pressure and renal function.
Supplementary Material
ACKNOWLEDGEMENTS
Support: This research is supported in part by contract numbers N01-HC-85079–N01-HC-85086, N01-HC-35129, N01-HC-15103, N01-HC-55222, N01-HC-75150, N01-HC-45133, and grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke; and grant 1R01DK47414 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
SUPPLEMENTARY MATERIAL
Note: Supplementary material associated with this article (doi:10.1053/j.ajkd.2009.10.044) can be found, in the online version, at www.ajkd.org.
REFERENCES
- 1.Dean RH, Tribble RW, Hansen KJ, O'Neil E, Craven TE, Redding JF., II Evolution of renal insufficiency in ischemic nephropathy. Ann Surg. 1991;213(5):446–455. doi: 10.1097/00000658-199105000-00010. discussion 455-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen KJ, Tribble RW, Reavis SW, et al. Renal duplex sonography: evaluation of clinical utility. J Vasc Surg. 1990;12(3):227–236. doi: 10.1067/mva.1990.22791. [DOI] [PubMed] [Google Scholar]
- 3.Hansen KJ, Cherr GS, Craven TE, et al. Management of ischemic nephropathy: dialysis-free survival after surgical repair. J Vasc Surg. 2000;32(3):472–481. doi: 10.1067/mva.2000.108637. discussion 481-482. [DOI] [PubMed] [Google Scholar]
- 4.Motew SJ, Cherr GS, Craven TE, et al. Renal duplex sonography: main renal artery versus hilar analysis. J Vasc Surg. 2000;32(3):462–471. doi: 10.1067/mva.2000.108643. [DOI] [PubMed] [Google Scholar]
- 5.Zierler RE, Bergelin RO, Isaacson JA, Strandness DE., Jr Natural history of atherosclerotic renal artery stenosis: a prospective study with duplex ultrasonography. J Vasc Surg. 1994;19(2):250–257. doi: 10.1016/s0741-5214(94)70100-8. [DOI] [PubMed] [Google Scholar]
- 6.Tullis MJ, Zierler RE, Caps MT, Bergelin RO, Cant-well-Gab K, Strandness DE., Jr Clinical evidence of contralateral renal parenchymal injury in patients with unilateral atherosclerotic renal artery stenosis. Ann Vasc Surg. 1998;12(2):122–127. doi: 10.1007/s100169900127. [DOI] [PubMed] [Google Scholar]
- 7.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med. 2005;165(2):207–213. doi: 10.1001/archinte.165.2.207. [DOI] [PubMed] [Google Scholar]
- 8.Edwards MS, Hansen KJ, Craven TE, et al. Relationships between renovascular disease, blood pressure, and renal function in the elderly: a population-based study. Am J Kidney Dis. 2003;41(5):990–996. doi: 10.1016/s0272-6386(03)00196-3. [DOI] [PubMed] [Google Scholar]
- 9.Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39(2 pt 2):699–703. doi: 10.1161/hy0202.103782. [DOI] [PubMed] [Google Scholar]
- 10.Crutchley TA, Pearce JD, Craven TE, Stafford JM, Edwards MS, Hansen KJ. Clinical utility of the resistive index in atherosclerotic renovascular disease. J Vasc Surg. 2009;49(1):148–155. doi: 10.1016/j.jvs.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Pearce JD, Craven TE, English WP, Mondi MM, Reavis SW, Hansen KJ. Renal duplex parameters, blood pressure, and renal function in elderly people. Am J Kidney Dis. 2005;45(5):842–850. doi: 10.1053/j.ajkd.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36(3):443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 14.Mosteller RD. Simplified calculation of body surface area [letter] N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 15.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–723. [Google Scholar]
- 16.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 17.Edwards MS, Hansen KJ, Craven TE, et al. Associations between renovascular disease and prevalent cardiovascular disease in the elderly: a population-based study. Vasc Endovascular Surg. 2004;38(1):25–35. doi: 10.1177/153857440403800103. [DOI] [PubMed] [Google Scholar]
- 18.Tedesco MA, Natale F, Mocerino R, Tassinario G, Calabro R. Renal resistive index and cardiovascular organ damage in a large population of hypertensive patients. J Hum Hypertens. 2007;21(4):291–296. doi: 10.1038/sj.jhh.1002145. [DOI] [PubMed] [Google Scholar]
- 19.Sari A, Dinc H, Zibandeh A, Telatar M, Gumele HR. Value of resistive index in patients with clinical diabetic nephropathy. Invest Radiol. 1999;34(11):718–721. doi: 10.1097/00004424-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Radermacher J, Mengel M, Ellis S, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349(2):115–124. doi: 10.1056/NEJMoa022602. [DOI] [PubMed] [Google Scholar]
- 21.Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344(6):410–417. doi: 10.1056/NEJM200102083440603. [DOI] [PubMed] [Google Scholar]
- 22.Frauchiger B, Zierler R, Bergelin RO, Isaacson JA, Strandness DE., Jr Prognostic significance of intrarenal resistance indices in patients with renal artery interventions: a preliminary duplex sonographic study. Cardiovasc Surg. 1996;4(3):324–330. doi: 10.1016/0967-2109(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 23.Cohn EJ, Jr, Benjamin ME, Sandager GP, Lilly MP, Killewich LA, Flinn WR. Can intrarenal duplex waveform analysis predict successful renal artery revascularization? J Vasc Surg. 1998;28(3):471–480. doi: 10.1016/s0741-5214(98)70133-8. discussion 480-481. [DOI] [PubMed] [Google Scholar]
- 24.Platt JF, Rubin JM, Ellis JH. Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology. 1991;179(2):419–423. doi: 10.1148/radiology.179.2.2014284. [DOI] [PubMed] [Google Scholar]
- 25.Tublin ME, Bude RO, Platt JF. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180(4):885–892. doi: 10.2214/ajr.180.4.1800885. [DOI] [PubMed] [Google Scholar]
- 26.Boddi M, Cecioni I, Poggesi L, et al. Renal resistive index early detects chronic tubulointerstitial nephropathy in normo- and hypertensive patients. Am J Nephrol. 2006;26(1):16–21. doi: 10.1159/000090786. [DOI] [PubMed] [Google Scholar]
- 27.Aikimbaev KS, Canataroglu A, Ozbek S, Usal A. Renal vascular resistance in progressive systemic sclerosis: evaluation with duplex Doppler ultrasound. Angiology. 2001;52(10):697–701. doi: 10.1177/000331970105201006. [DOI] [PubMed] [Google Scholar]
- 28.Wong TY, Klein R, Nieto FJ, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 29.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287(9):1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 30.Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis. 2005;46(2):214–224. doi: 10.1053/j.ajkd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Wong GC, Morrow DA, Murphy S, et al. Elevations in troponin T and I are associated with abnormal tissue level perfusion: a TACTICS-TIMI 18 substudy. Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis in Myocardial Infarction. Circulation. 2002;106(2):202–207. doi: 10.1161/01.cir.0000021921.14653.28. [DOI] [PubMed] [Google Scholar]
- 32.Selvanayagam JB, Porto I, Channon K, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111(8):1027–1032. doi: 10.1161/01.CIR.0000156328.28485.AD. [DOI] [PubMed] [Google Scholar]
- 33.Bolognese L, Ducci K, Angioli P, et al. Elevations in troponin I after percutaneous coronary interventions are associated with abnormal tissue-level perfusion in high-risk patients with non-ST-segment-elevation acute coronary syndromes. Circulation. 2004;110(12):1592–1597. doi: 10.1161/01.CIR.0000142856.56565.56. [DOI] [PubMed] [Google Scholar]
- 34.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.