Short QT syndrome (SQTS) is an inheritable primary electric disease of the heart characterized by abnormally short QT intervals on the ECG and an increased propensity to develop atrial and ventricular tachyarrhythmias.1–3 It is a relatively recent addition to the list of inherited channelopa-thies responsible for sudden cardiac death (SCD) in individuals with structurally normal hearts. Cases of SQTS have been reported with presentation as early as in the first year of life, suggesting that it could be one of the etiologies underlying sudden infant death syndrome.4 SQTS was first described as a new clinical entity by Gussak et al in 2000.1 The familial nature and arrhythmic potential of the disease was further highlighted by Gaita et al.5 They described 6 patients of SQTS in 2 unrelated European families with strong family history of sudden death in association with short QT intervals on the ECG. Since its initial introduction in 2000, significant progress has been made in defining the clinical, genetic,6 and ionic basis of the disease as well as approaches to therapy. The purpose of this review is to summarize the available data concerning SQTS from bench to bedside.
How Short Is Too Short?
The definition of a pathophysiologic long QT interval evolved over a period of several decades and it may take some time to define what constitutes a pathophysiologic short QT interval. Several large scale population studies have shown that the corrected QT interval (QTc, using the Bazett formula) of healthy individuals conforms to a gaussian normal distribution, that is, a bell-shaped curve.7–10 Based on this distribution, the “normal QTc” interval may be defined as values that fall within 2 standard deviations (SD) from the mean. This approach will categorize 95% of the population as having normal QTc interval and the remaining 5% as either short or long QTc interval. These studies suggest that the normal QTc of healthy individuals should fall within a range of 350 to 450 ms for males and 360 to 460 ms for females; hence, QTc <350 for males and 360 for females should be considered as short QT interval. When this population-based approach is used for defining the lower limit of QT interval, it must be emphasized that the Bazett formula used for calculating QTc in these studies tends to underestimate QTc at lower heart rates and overestimates QTc at higher heart rates.11 This can lead to misclassification of an upward of 20% of apparently healthy individuals as having short QT interval, especially if the ECGs are recorded during sinus bradycardia.9 On the other hand, as seen in patients with long QT syndrome, there may be significant overlap of QTc values of normal individuals and those with SQTS; hence some of the SQTS patients may have QTc interval that may fall within normal range, that is, >350 ms in males and >360 ms in females.
An alternative approach is to calculate a predicted QT interval (QTp) as proposed by Rautaharju et al,12 a method that does not use a rate correction formula. They investigated the QT interval in 14 379 healthy individuals and established the formula by which the QT interval can be predicted as QT predicted (QTp)=656/(1+heart rate/100). In this study, the prevalence of QT interval shorter than 88% of QTp (QT/QTp <88%, equivalent to 2 SD below the mean) was 2.5%; and the prevalence of QT intervals shorter than 80% of QTp (QT/QTp <80%) was 0.03%. Thus, a QT interval <88% of QTp (2 SD below mean predicted value) at a particular heart rate might be considered as the lower limit of normal. At a heart rate of 60 beats per minute (bpm), QTp would be 410 ms and 88% of QTp would be 360 ms.
Thus, as a starting point for discussion, a QT (not QTc) value of ≤360 ms at heart rate of 60 bpm might reasonably be considered to be a shorter than normal QT interval. Because rate correction of the QT interval (QT-RR relationship) in SQTS patients is less pronounced, we prefer to use the method of Rautaharju et al as opposed to using the Bazett formula for estimation of QT interval adjusted for heart rate.
What Constitutes a Pathophysiologic Short QT Interval?
An arrhythmogenic potential of shorter than normal QT intervals was first proposed by Algra et al in 1993. These authors reported that in addition to long QT intervals, shorter than normal QT intervals (<400 ms) are associated with increased risk of SCD.13 In his retrospective study, relative risk of SCD in patients with QT interval <400 ms was 2.4 compared with people with normal QT interval.13 Viskin et al14 also reported shorter than normal QT interval (QTc <360 ms in males and <370 ms in females) in patients with idiopathic ventricular fibrillation. Short QT intervals have been reported before and after runs of ventricular tachycardia (VT)/ventricular fibrillation (VF).15,16 Interestingly, short QT interval is a normal ECG finding in species such as kangaroo, and these animals have a very high incidence of SCD.17,18
Contrary to the above description, recent population studies have questioned the arrhythmogenic significance of short QT intervals in healthy individuals. Gallagher et al8 recently reported that in healthy Italian males, QTc interval <330 is extremely rare and the presence of QT interval in the lowest 0.5% does not imply significant risk of sudden death over a period of over 8 years. Similarly, Anttonen et al19 reported that in a middle-aged Finnish population, prevalence of QT interval <340 is only 0.3% and it is not associated with increased risk of death over a follow-up period of 30 years. Recently, in a study involving healthy Japanese population, Moriya et al20 reported a prevalence of 0.01% of QTc interval <350 ms without increased risk of SCD. Although these studies clearly demonstrate that prevalence of short QT intervals in healthy populations is rare, they were underpowered to assign any prognostic significance to isolated presence of short QT intervals in the ECG.21 It is noteworthy that shorter than normal QT intervals may prevail as a part of the normal bell-shaped distribution in healthy individuals. As will be discussed later, abbreviated repolarization may not suffice to create the arrhythmogenic substrate. The ion channelopathies that cause SQTS not only abbreviate repolarization but they significantly increase dispersion of repolarization, thus creating the cellular basis for both the substrate and trigger necessary for the initiation of reentry. This distinction may help us understand why short QT intervals are highly arrhythmogenic in some individuals but not in others.
Definition, Diagnosis, and Differential Diagnosis
SQTS is best defined as a congenital, inherited, primary electric disorder of the heart characterized by abnormally short QT intervals on the surface ECG (<360 ms) and an increased proclivity to develop atrial and/or ventricular tachyarrhythmias.2,3,22,23 By definition, secondary causes of short QT interval such as hyperkalemia, acidosis, hypercalcemia, hyperthermia, effects of drugs like digitalis, effect of acetylcholine or catecholamine, and abbreviation of QT interval related to activation of KATP current must be ruled out before considering the diagnosis of SQTS (Table 1). A rare but interesting paradoxical ECG phenomenon called deceleration-dependent shortening of QT interval (shortening of QT interval associated with a decrease in heart rate) should also be considered in a differential diagnosis.24 Activation of the KA-Ch current caused by strong parasympathetic stimuli to the heart is thought to be responsible for this phenomenon.
Table 1.
Secondary Causes of SQT Interval
| Hyperkalemia |
| Hypercalcemia |
| Hyperthermia |
| Acidosis |
| Effect of catecholamine |
| Activation of KACh |
| Activation of KATP |
| Effects of drugs such as digitalis |
A comprehensive battery of tests is also recommended by the guidelines from the Joint Steering Committees of UCARE and IVF-US,25 including but not limited to resting ECG, exercise stress testing, echocardiogram, 24-hour Holter monitoring, and cardiac MRI to rule out the presence of organic heart disease. Diagnosis of SQTS should be strongly suspected in young individuals with a short QT interval on 12-lead ECG in conjunction with arrhythmic symptoms, lone atrial fibrillation (AF), primary or resuscitated VF, and a strong family history of arrhythmic events including SCD. The presence of a short QT interval on ECG without associated arrhythmogenic complication warrants further interrogation to rule out SQTS.
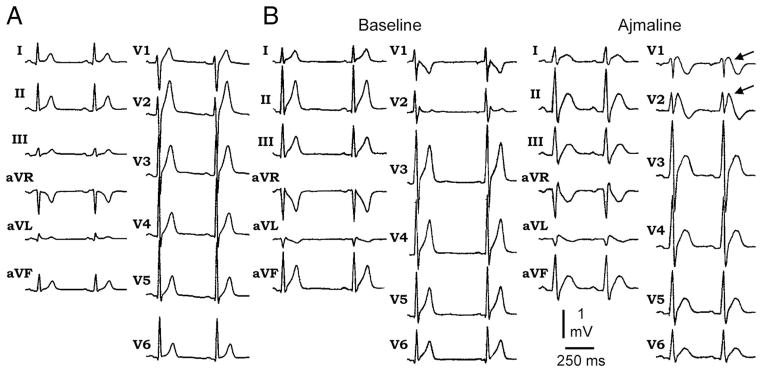
ECG in SQTS is characterized by abnormally short QT intervals, commonly ranging between 220 to 360 ms (Figure 1).1,4,5 When the diagnosis of SQTS is suspected, resting 12-lead ECG should be performed at a heart rate within normal limits. The QT interval should be measured when the heart rate is <100 bpm and preferably less than 80 bpm. The QT-RR relationship is generally less steep (lack of rate dependence) in patients with SQTS (Figure 2).26 As a consequence, QTc corrected by any formula will fail to reflect the true QTc. At rapid rates, QTc will falsely approximate normal values leading to a false-negative diagnosis. This is particularly important for the diagnosis of SQTS in pediatric populations, where resting heart rate is >100 bpm. Holter monitoring or long-term ECG monitoring becomes necessary in such cases to make the correct diagnosis. Another common finding on ECG of SQTS patient is tall, peaked, symmetrical, or asymmetrical T waves in the precordial leads. Another distinctive feature seen on many ECGs is prolonged Tpeak-Tend interval and Tpeak-Tend/QT ratio, suggestive of augmented transmural dispersion of repolarization.27,28 However, asymmetrical T waves with a less steep ascending limb followed by a rapid descending limb have been reported as well.29 In most cases, the ST segment is short or even absent and the T wave originates from the S wave. QT intervals characteristically show lack of adaptation to change in heart rate, as described above. In cases of SQT4 and SQT5, short QT intervals appear together with a Brugada-type ST elevation in right precordial leads V1 and V2 at baseline or after administration of ajmaline.6
Figure 1.
A, Twelve-lead ECG showing characteristic ECG features of SQTS. B, Twelve-lead ECG showing characteristic ECG features of new clinical entity with combined ECG phenotype of Brugada syndrome in addition to SQTS. The ECG shows Brugada-type ST elevation in V1 and V2 after administration of ajmaline in addition to short QT interval. Modified from References 4 and 6 with permission. 4,6
Figure 2.
A, Reduced rate-adaptation of QT interval. The QT-RR relationship is less linear and its slope is less steep in the SQTS patient as compared with control subjects. Quinidine restores the relationship toward control values. QTpV3 denotes the interval from the beginning of QRS complex to peak of T wave, measured in lead V3. Reproduced from Reference 26, with permission.26 B, Holter monitoring showing impaired adjustment of QT interval with change in heart rate.
It is important to emphasize that a short QTc should not be the primary or sole criterion for establishing a diagnosis of SQTS. Differential diagnosis must include a comprehensive assessment of all ECG features as well as comprehensive clinical and family history.
Genetic Basis of SQTS
SQTS is a genetically heterogeneous disease. Mutations in 5 different genes (Table 2) have been associated with SQTS thus far, and these have been labeled SQT1 to SQT5, based on the chronology of their discovery. At the present time, SQT1 and SQT3–5 have been reported in a familial setting, and SQT2 is reported only in a single patient in a sporadic setting. The mode of transmission is autosomal dominant.
Table 2.
Genetic Basis of SQTS
| Subtype | Inheritance | Locus | Ion Channel | Gene | Electrophysiologic Characteristics of Mutant Current/Channel | Net Effect of Mutation |
|---|---|---|---|---|---|---|
| SQT1 | AD | 7q35 | IKr | KCNH2, HERG | Shift of voltage dependence of inactivation of IKr by +90 mV out of the range of the action potential | Gain of function of IKr |
| SQT2 | AD | 11p15 | IKs | KCNQ1, KvLQT1 | Shift of voltage dependence of activation of IKs by −20 mV and acceleration of activation kinetics | Gain of function of IKs |
| SQT3 | AD | 17q23.1–24.2 | IK1 | KCNJ2, Kir2.1 | Increase in outward IK1 at potentials between −75 mV and −45 mV | Gain of function of IK1 |
| SQT4 | AD | 12p13.3 | ICa | CACNA1C, Cav1.2 | Decrease in amplitude of the inward calcium current | Loss of function of ICa |
| SQT5 | 10p12.33 | ICa | CACNB2b, Cavβ2b |
Mutations in KCNH2-SQT1
The KCNH2 gene, often referred to as human-ether-go-go-related gene (HERG), encodes the α subunit of the rapidly activating delayed rectifier potassium channel, IKr. A mutation in KCNH2 was the first reported gene mutation in SQTS. Using the candidate gene approach, Brugada et al30 reported 2 different missense mutations in KCNH2 in 2 unrelated families. Both mutations resulted in substitution of lysine for asparagine at position 588 of KCNH2. This residue is located at the S5-P loop region of HERG at the outer mouth of the channel. In the voltage clamp studies, N588K mutation led to loss of normal rectification of the IKr at physiological range of voltages and shifted the voltage-dependent inactivation by +90 mV, resulting in a large gain of function in IKr during the plateau phase of the action potential leading to marked abbreviation of action potential.30,31 Interestingly, the N588K mutation reduced the affinity of the IKr channel for Class III antiarrhythmic agents such as d-sotalol, which has direct implication on the treatment of SQT1, as will be discussed below. The reduced affinity is due to the fact that the inactivated state of the channel normally stabilizes the inter- action of the channel with most IKr blockers31 and the mutant channel fails to enter the inactivated state.30–32
Recently, Itoh et al33 reported a novel mutation R1135H in the KCNH2 gene a 34-year-old man with short QT interval. When expressed in a heterologous expression system, mutant channels display significantly slow deactivation leading to a gain of function of IKr.
Mutation in KCNQ1-SQT2
The KCNQ1 gene encodes a subunit of the cardiac potassium channel KvLQT1, which, in association with the β-subunit mink encoded by KCNE1, forms the slow component of the cardiac delayed rectifying potassium current, IKs. A mutation in KCNQ1 was identified by Bellocq et al34 in a 70-year-old man with resuscitated ventricular fibrillation and short QT interval—a single sporadic case. A candidate gene approach was used to identify a mutation in KCNQ1 predicting substitution of valine at position 307 by Leucine (V307L). Functional studies revealed that the mutation caused a shift of −20 mV in the half-activation potential and acceleration of activation kinetics resulting in gain in function of IKs. It is noteworthy that a similar gain of function mutation in KCNQ1 has been reported previously in the setting of familial atrial fibrillation.35
Mutations in KCNJ2-SQT3
The KCNJ2 gene encodes the inwardly rectifying Kir2.1 (IK1) channel. A mutation in KCNJ2 associated with SQTS was identified by Priori et al29 in an asymptomatic 5-year-old child and 35-year-old father displaying extremely short QT intervals (315 and 320 ms, respectively). In contrast to SQT1 and SQT2, which characteristically display symmetrical T waves in the ECG, SQT3 patients exhibited asymmetrical T waves with a slow ascending limb and rapid terminal phase. Genetic testing revealed a mutation in KCNJ2 that predicted substitution of aspartic acid by asparagines at position 172 (D172N). Whole-cell patch-clamp studies of heterologously expressed D172N channels demonstrated a gain of function in outward IK1.
Mutation in CACNA1c and CACNB2b-SQT4 and SQT5
We recently described a new clinical entity characterized by a combined ECG phenotype of the Brugada syndrome and shorter than normal QT intervals.6 While SQT1–3 are associated with gain of function mutations in outward potassium currents, this entity is caused by loss of function mutations in genes encoding cardiac L-type calcium channel. The cardiac L-type calcium channel is a protein complex formed by α1, β, and α2δ subunits. The pore-forming Cav1.2 α1-subunit is encoded by CACNA1C and the β-subunit is encoded by CACNB2b. Genetic screening of 3 families with this clinical phenotype revealed missense mutations in CACNA1C (A39V and G490R) and CACNB2b (S481L).6 Biophysical analysis showed that these mutations all cause a marked loss of function of ICa, responsible for abbreviation of the action potential as well as ST-segment elevation.6 Confocal microscopy unmasked a trafficking defect in the case of A39V CACNA1C mutation.
The ECG in this clinical entity, designated SQT4 and SQT5, typically shows ST-segment elevation in precordial leads V1 and V2 either at baseline or after ajmaline administration. Tall peaked T waves are visible in some of the ECGs, and QTc intervals tend to be longer (330 to 360 ms) when compared with the other forms of SQTS. A less steep QT-RR relationship was also observed in these patients as with SQT1 patients.6
Clinical Presentation
The clinical presentation of SQTS patients is quite varied. Initial presentation and clinical course differs among families and members of the same family. In the largest available case series of 29 SQTS patients reported by Giustetto et al,4 approximately 25% of patients had a mutation in KCNH2 (SQT1). Mutations in KCNQ1 and KCNJ2 were not detected and CACNA1c and CACNB2b were not screened. The first clinical manifestation of the disease has been reported as early as first year of life and as late as at 80 years of age. Approximately 62% of the patients were symptomatic. Cardiac arrest was the most frequently (34%) reported symptom, and in 28% of patients it was the first clinical presentation. Cardiac arrest occurred in the first year of life in 2 patients, suggesting that SQTS may be a cause of sudden infant death syndrome. The available data suggest that patients are at risk throughout a lifetime. Palpitations were the second most frequently reported symptom (31%), followed by syncope (24%). AF was the first presenting symptom in 17% of patients. Many patients had frequent ventricular extrasystoles. Approximately 38% patients were asymptomatic and were diagnosed due to strong family history. Strong family history of arrhythmic symptoms including SCD is a common finding. The circumstances of onset of symptoms are highly variable, and episodes of SCD have been reported during or following loud noise, at rest, during exercise, and during daily activities.
The only reported patient of SQT2 is a 70-year-old man who was successfully resuscitated after an episode of ventricular fibrillation.34 There are 2 reported cases of SQT3: a 5-year-old girl who was asymptomatic and a 35-year-old father, who had frequent episodes of sudden awakening at night with seizure-like activity followed by shortness of breath and palpitations.29 SQT4 has thus far been reported in 2 patients of unrelated families.6 One patient was a 41-year-old man with a family history of SCD who presented with AF in conjunction with QTc of 346 ms and the second patient was a 44-year-old man with fascioscapulohumeral muscular dystrophy and a family history of syncope and SCD. SQT5 has been described in 7 patients belonging to a family of European descent.6 The proband, a 25-year-old man, presented with a QTc of 330 ms and had an episode of aborted SCD. His 23-year-old brother had frequent syncope as well. The rest of the family was asymptomatic.
Most patients with SQTS have QTc ≤340 ms with range of 210 to 320 ms. However, in patients with SQT4 and SQT5, QTc intervals are generally a bit longer (330 to 360 ms). No correlation has been identified as yet between the extent of QT interval abbreviation and risk of arrhythmic events.4
Electrophysiological Study
Invasive electrophysiological studies have reported extremely short atrial and ventricular effective refractory periods (ERP) in patients with SQTS.4–6,29,34 The ventricular ERP at the right ventricular apex at cycle length of 500 to 600 ms varies between 140 to 180 ms and at pacing cycle length of 400 to 430 ms varies between 130 to 180 ms. Atrial ERP measured in the high lateral right atrium at a cycle length of 600 ms varies between 120 and 180 ms. The programmed electric stimulation with 2 to 3 premature stimuli induces AF in atria and VF in ventricles in 60% of SQTS patients. In the case series presented by Giustetto et al,4 VF was inducible in only 3 of 6 patients with clinically documented VF, suggesting that sensitivity of for inducibility of VF is not high.
Cellular Basis of Arrhythmogenesis in SQTS
An increase in net outward current due to either a reduction in inward depolarizing currents such as INa or ICa or augmentation of outward repolarizing currents such as Ito, IK1, IK-ATP, IACh, IKr, or IKs or a combination will favor early repolarization leading to abbreviation of action potential and QT interval. Experimental studies suggest that the abbreviation of action potential in SQTS is heterogeneous with preferential abbreviation of either epicardium or endocardium, giving rise to an increase in transmural dispersion of repolarization (TDR). Dispersion of repolarization and refractoriness serve as substrate for reentry in that it promotes unidirectional block. Marked abbreviation of wave length (product of refractory period and conduction velocity) is an additional factor promoting the maintenance of reentry. Mutations giving rise to a gain-of-function of outward K currents has been identified in SQT1–329,30,34 and a loss of function in inward ICa-L have been identified in SQT4–5.6 Moreover, the Tpeak-Tend interval and Tpeak-Tend/QT ratio, an ECG index of spatial dispersion of repolarization, and perhaps TDR, are significantly augmented in cases of SQTS.27,28 Interestingly, this ratio is more amplified in patients who are symptomatic.36,37
Evidence supporting the role of augmented TDR in arrhythmogenesis in SQTS derives from the work experimental studies using the canine left ventricular wedge preparation. In the first experimental model of SQTS, the KATP activator pinacidil was used to abbreviate repolarization time.38,39 With availability of a specific IKr agonist, PD-118057, we created a cellular model of SQT1 that mimics the cellular condition of gene mutation.40 As shown in Figure 3A, augmentation of IKr by PD-118057 significantly abbreviates the QT interval with a preferential abbreviation of the epicardial versus M-cell action potential, leading to an increase in TDR. The pseudo-ECG recorded in our experiment was able to recapitulate ECG features of SQTS in clinical practice (Figure 3A). Using programmed electric stimulation delivered from epicardium, polymorphic ventricular tachycardia (pVT) was induced in 50% of wedge preparations (Figure 3B). Infusion of quinidine reversed the effect of PD-118057 on QT interval but the augmented TDR persisted. However, pVT was no longer inducible as a consequence of the increase in refractory period causing an increase in the wave length.
Figure 3.
PD-118057 (IKr agonist) model of SQTS in canine left ventricular wedge. A, PD-118057 induced abbreviation of QT interval and increase in TDR. Preferential abbreviation of epicardial action potential results in an increase in TDR. Each panel shows transmembrane action potentials simultaneously recorded from an epicardial (Epi) and a deep subendocardial M cell in an arterially perfused LV wedge preparation, together with a pseudo-ECG. Basic cycle length, 2000 ms. B, Programmed electric stimulation applied to epicardium induced polymorphic VT in the presence of PD-110857 but not after addition of quinidine. Basic cycle length, 2000 ms. Modified with permission.40
In the clinic, only 1 patient had been continuously monitored during onset of pVT.41 Observation from this patient, along with data obtained by interrogating implantable cardioverter-defibrillators (ICDs) implanted in SQT patients, indicates that closely coupled premature ventricular extrasystoles precede the onset of pVT (Figure 4).41,42 The cellular basis for these closely coupled extrasystoles is not known but most likely related to phase 2 reentry or late phase 3 early afterdepolarizations.43
Figure 4.
Self-terminating episode of polymorphic VT in a patient with SQTS: Lead V3. The episode is precipitated by an extrasystole with a very short coupling interval.
In a recent study exploring the cellular basis of the U wave in SQTS patients, Schimpf et al44 elegantly demonstrated that electric repolarization in SQTS patients terminates significantly earlier as compared with the mechanical contraction. Similar striking dissociation of electric and mechanical systole has been reported previously in kangaroos.45,46 In kangaroos, the occurrence of an extrasystole during the period of electromechanical dissociation has been shown to reexcite the myocardium despite the fact that the contraction induced by the previous beat is still not complete, thus creating a state of incomplete tetanus.45,46 Interestingly, Watanabe et al47 recently reported that patients with SQTS have a higher prevalence of early repolarization pattern in the ECG and that the presence of early repolarization is strongly associated with arrhythmic events. These observations suggest that an early repolarization pattern may be useful in identifying SQTS patients at risk and that the molecular mechanism in the SQTS cohort may overlap with that responsible for J-wave syndromes (Brugada syndrome, early repolarization syndrome, and some forms of idiopathic ventricular fibrillation).48
The role of autonomic nervous system in arrhythmogenesis in SQTS is still unclear as VF is observed during sleep as well as after intense autonomic stimulation.4 As the repolarization heterogeneity as well as the abnormal QT-RR relationship is more pronounced at slower heart rates, it likely that episodes of tachyarrhythmia will more likely to occur at slower than faster heart rates.
Treatment in SQTS
The ICD
SQTS patients are at a high risk of SCD because of malignant ventricular arrhythmia. ICD implantation is strongly recommended for secondary prevention of SCD, unless absolutely contraindicated or refused by the patient.23 There is a scarcity of data delineating the natural history of SQTS, and features identifying patients at higher risk of SCD are not clear. Consequently, there is no clear role for ICD in primary prevention strategies. Based on a malignant family history, physicians and patients may choose to implant an ICD for primary prevention, but clearly this approach is not evidence-based at this point in time. SQTS patients offered an ICD for primary prevention of SCD should be informed that the ICD may abort a future episode of SCD or may not fire at all during his or her lifetime but may be associated with a high risk of complication over the span of a lifetime. It should be emphasized that the sensitivity of electrophysiological study for VF inducibility is on the order of about 50%, and noninducibility at electrophysiological study does not rule out future risk of SCD.4,42 The decision to insert ICD should be based on clinical grounds (short QT interval on ECG in conjunction with arrhythmic symptoms and strong family history of SCD) rather than genetic or electrophysiological data at this point, until further guidelines for risk stratification are available.
Oversensing of the T wave is a frequent clinical problem in patients with SQTS who receive an ICD.49 The tall, peaked, and closely coupled T waves are often mistakenly sensed as R waves, leading to inappropriate ICD shocks. Reprogramming the decay delay, sensitivity, or both generally prevents inappropriate discharges.
Pharmacological Therapy
Although the ICD is the mainstay of therapy for SQTS, pharmacological therapy may be useful as an adjunct to the ICD or may be used for primary prevention in cases in which the patient refuses an ICD or in young children in whom the implantation of an ICD may be problematic.
Information regarding pharmacological therapy for SQTS is fairly limited, and the majority of available data pertains to SQT1. Gaita et al50 tested 4 different antiarrhythmic drugs including flecainide, sotalol, ibutilide, and hydroquinidine in 6 patients with SQT1. Only hydroquinidine prolonged the QT interval to normal levels, increased ventricular ERP, and rendered VF noninducible. Class IC and III antiarrhythmic drugs failed to do so. Quinidine also restored the QT-RR relationship toward the normal range.26 In a 1-year follow-up, patients treated with hydroquinidine remained asymptomatic and no further episodes of ventricular arrhythmia were detected.
The efficacy of quinidine and the failure of Class IC and III antiarrhythmic drugs in SQT1 is primarily related to a secondary effect of the N588K mutation. The N588K mutation reduces the availability of the inactivated state of the mutant channel, hence reducing the affinity of IKr channels to many drugs with Class III antiarrhythmic activity.30 Electro-physiological studies in heterologous expression systems have shown that N588K mutation reduces the affinity of the channel for quinidine only by 3.5- to 5.8-fold, whereas affinity for E-4031 and d-sotalol it is reduced by 11.5- and 20-fold, respectively.32 30,31,51 The affinity of N588K mutant IKr channel for disopyramide was reduced by only 1.5-fold32 and for amiodarone was reduced by only 4.1-fold.52 Consistent with these bench side observations, Schimpf et al53 have recently reported clinical efficacy of disopyramide in 2 female patients with SQT1. In this pilot study, administration of disopyramide proved to be equally effective as quinidine in prolonging the QT interval and restoring the ventricular ERP toward normal.53 Similarly, amiodarone also prolonged QT interval in 2 patients of SQTS with unknown genotype.41,54
Although its efficacy is proven in patients with SQT1, quinidine, by virtue of being a blocker of multiple K currents including Ito, IK1, IKr, and IKs, should be effective in other forms of SQTS as well, especially in patients with SQT4 and SQT5, in whom the Ito blocking effect will provide a therapeutic edge over other antiarrhythmic drugs by reducing the substrate and trigger for Brugada syndrome. In fact, prolongation of the QT interval with quinidine has been reported in 1 patient with SQT4.6 Unlike SQT1, Class III antiarrhythmic drugs are expected to be clinically useful in SQT2 and SQT3; however, drug testing in these patients is still not available as yet.
AF is another common clinical problem in SQTS. Some SQTS patients exhibit only AF.55 Propafenone has been shown to be effective in preventing frequent paroxysms of AF with no recurrence of arrhythmia for more than 2 years without any effect on QT interval.22 Quinidine is effective as well.
Conclusion
SQTS is a relatively rare recent addition to the growing list of channelopathies associated with SCD in individuals with structurally normal heart, including sudden infant death syndrome. Timely diagnosis and optimal treatment can significantly improve the overall prognosis of the patient and family members. In contrast to its mirror-image disorder long QT syndrome; there is a scarcity of data about SQTS in terms of its clinical presentation, diagnosis, genotype-phenotype correlation, risk-stratification, and treatment. However, impressive progress has been made in defining the molecular genetics and cellular basis of arrhythmogenesis in SQTS since its first description in 2000. An ICD is recommended as first-line treatment for secondary prevention of SCD and may be useful for primary prevention as well. There is a paucity of information concerning the long-term effectiveness of pharmacological therapy; hence, it might best be reserved as an adjunct to ICD treatment. Quinidine has thus far proved to be the most effective pharmacotherapeutic agent.
Acknowledgments
Sources of Funding
This work was supported by grant HL47678 from NHLBI (Dr Antzelevitch) and New York State and Florida Grand Lodges Free and Accepted Masons.
Footnotes
Disclosures
None.
References
- 1.Gussak I, Brugada P, Brugada J, Wright RS, Kopecky SL, Chaitman BR, Bjerregaard P. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 2.Gussak I, Antzelevitch C, Goodman D, Bjerregaard P. Short QT interval: ECG phenomenon and clinical syndrome. In: Gussak I, Antzelevitch C, editors. Cardiac Repolarization. Bridging Basic and Clinical Sciences. Totowa, NJ: Humana Press; 2003. pp. 497–506. [Google Scholar]
- 3.Gussak I, Bjerregaard P. Short QT syndrome: 5 years of progress. J Electrocardiol. 2005;38:375–377. doi: 10.1016/j.jelectrocard.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Giustetto C, Di Monte F, Wolpert C, Borggrefe M, Schimpf R, Sbragia P, Leone G, Maury P, Anttonen O, Haissaguerre M, Gaita F. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006;27:2440–2447. doi: 10.1093/eurheartj/ehl185. [DOI] [PubMed] [Google Scholar]
- 5.Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108:965–970. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 6.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Ober-heiden R, Bhatia A, Hsu LF, Haissaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funada A, Hayashi K, Ino H, Fujino N, Uchiyama K, Sakata K, Masuta E, Sakamoto Y, Tsubokawa T, Yamagishi M. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin Cardiol. 2008;31:270–274. doi: 10.1002/clc.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher MM, Magliano G, Yap YG, Padula M, Morgia V, Postorino C, Di LF, Leo R, Borzi M, Romeo F. Distribution and prognostic significance of QT intervals in the lowest half centile in 12,012 apparently healthy persons. Am J Cardiol. 2006;98:933–935. doi: 10.1016/j.amjcard.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Kobza R, Roos M, Niggli B, Abacherli R, Lupi GA, Frey F, Schmid JJ, Erne P. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm. 2009;6:652–657. doi: 10.1016/j.hrthm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Viskin S. The QT interval: too long, too short or just right. Heart Rhythm. 2009;6:711–715. doi: 10.1016/j.hrthm.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 11.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart J. 1920;7:353–370. [Google Scholar]
- 12.Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- 13.Algra A, Tijssen JGP, Roelandt JRTC, Pool J, Lubsen J. QT interval variables from 24-Hour electrocardiography and the 2-year risk of sudden death. Br Heart J. 1993;70:43–48. doi: 10.1136/hrt.70.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viskin S, Zeltsner D, Ish-Shalom M, Katz A, Glikson M, Justo D, Tekes-Manova D, Belhassen B. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm. 2004;1:587–591. doi: 10.1016/j.hrthm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Fei L, Camm AJ. Shortening of the QT interval immediately preceding the onset of idiopathic spontaneous ventricular tachycardia. Am Heart J. 1995;130:915–917. doi: 10.1016/0002-8703(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 16.Kontny F, Dale J. Self-terminating idiopathic ventricular fibrillation presenting as syncope: a 40-year follow-up report. J Intern Med. 1990;227:211–213. doi: 10.1111/j.1365-2796.1990.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 17.Campbell TJ. Characteristics of cardiac action potentials in marsupials. J Comp Physiol [B] 1989;158:759–762. doi: 10.1007/BF00693014. [DOI] [PubMed] [Google Scholar]
- 18.Rezakhani A, Webster JD, Atwell RB. The electrocardiogram of the eastern grey kangaroo (Macropus giganteus) Aust Vet J. 1986;63:310–312. doi: 10.1111/j.1751-0813.1986.tb08078.x. [DOI] [PubMed] [Google Scholar]
- 19.Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M, Huikuri HV. Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation. 2007;116:714–720. doi: 10.1161/CIRCULATIONAHA.106.676551. [DOI] [PubMed] [Google Scholar]
- 20.Moriya M, Seto S, Yano K, Akahoshi M. Two cases of short QT interval. Pacing Clin Electrophysiol. 2007;30:1522–1526. doi: 10.1111/j.1540-8159.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan MN, Page RL. Short QT: When does it matter? Circulation. 2007;116:686–688. doi: 10.1161/CIRCULATIONAHA.107.720896. [DOI] [PubMed] [Google Scholar]
- 22.Bjerregaard P, Gussak I. Short QT syndrome. Ann Noninvasive Electrocardiol. 2005;10:436–440. doi: 10.1111/j.1542-474X.2005.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjerregaard P, Gussak I. Short QT syndrome: mechanisms, diagnosis and treatment. Nat Clin Pract Cardiovasc Med. 2005;2:84–87. doi: 10.1038/ncpcardio0097. [DOI] [PubMed] [Google Scholar]
- 24.Gussak I, Antzelevitch C, Bjerregaard P, Towbin JA, Chaitman BR. The Brugada syndrome: clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol. 1999;33:5–15. doi: 10.1016/s0735-1097(98)00528-2. [DOI] [PubMed] [Google Scholar]
- 25.Joint Steering Committees of UCARE and IVF-US. Survivors of out-of-hospital cardiac arrest with apparently normal heart. Need for definition and standardized clinical evaluation. Consensus Statement of the Joint Steering Committees of the Unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry of the United States. Circulation. 1997;95:265–272. doi: 10.1161/01.cir.95.1.265. [DOI] [PubMed] [Google Scholar]
- 26.Wolpert C, Schimpf R, Giustetto C, Antzelevitch C, Cordeiro JM, Dumaine R, Brugada R, Hong K, Bauersfeld U, Gaita F, Borggrefe M. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol. 2005;16:54–58. doi: 10.1046/j.1540-8167.2005.04470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anttonen O, Vaananen H, Junttila J, Huikuri HV, Viitasalo M. Electrocardiographic transmural dispersion of repolarization in patients with inherited short QT syndrome. Ann Noninvasive Electrocardiol. 2008;13:295–300. doi: 10.1111/j.1542-474X.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. Tp-e/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 30.Brugada R, Hong K, Dumaine R, Cordeiro JM, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 31.Cordeiro JM, Brugada R, Wu YS, Hong K, Dumaine R. Modulation of IKr inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc Res. 2005;67:498–509. doi: 10.1016/j.cardiores.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 32.McPate MJ, Duncan RS, Witchel HJ, Hancock JC. Disopyramide is an effective inhibitor of mutant HERG K+ channels involved in variant 1 short QT syndrome. J Mol Cell Cardiol. 2006;41:563–566. doi: 10.1016/j.yjmcc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Itoh H, Sakaguchi T, Ashihara T, Ding WG, Nagaoka I, Oka Y, Nakazawa Y, Yao T, Jo H, Ito M, Nakamura K, Ohe T, Matsuura H, Horie M. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol. 2009;137:83–85. doi: 10.1016/j.ijcard.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 34.Bellocq C, Van Ginneken AC, Bezzina CR, Alders M, Escande D, Mannens MM, Baro I, Wilde AA. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394–2397. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 35.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 36.Anttonen O, Junttila MJ, Maury P, Schimpf R, Wolpert C, Borggrefe M, Giustetto C, Gaita F, Sacher F, Haissaguerre M, Sbragia P, Brugada R, Huikuri HV. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm. 2009;6:267–271. doi: 10.1016/j.hrthm.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 37.Anttonen O, Junttila J, Giustetto C, Gaita F, Linna E, Karsikas M, Seppanen T, Perkiomaki JS, Makikallio TH, Brugada R, Huikuri HV. T-wave morphology in short QT syndrome. Ann Noninvasive Electrocardiol. 2009;14:262–267. doi: 10.1111/j.1542-474X.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short QT syndrome. Circulation. 2004;110:3661–3666. doi: 10.1161/01.CIR.0000143078.48699.0C. [DOI] [PubMed] [Google Scholar]
- 39.Milberg P, Tegelkamp R, Osada N, Schimpf R, Wolpert C, Breithardt G, Borggrefe M, Eckardt L. Reduction of dispersion of repolarization and prolongation of postrepolarization refractoriness explain the antiar-rhythmic effects of quinidine in a model of short QT syndrome. J Cardiovasc Electrophysiol. 2007;18:658–664. doi: 10.1111/j.1540-8167.2007.00813.x. [DOI] [PubMed] [Google Scholar]
- 40.Patel C, Antzelevitch C. Cellular basis for arrhythmogenesis in an experimental model of the SQT1 form of the short QT syndrome. Heart Rhythm. 2008;5:585–590. doi: 10.1016/j.hrthm.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu LX, Zhou W, Zhang X, Cao Q, Yu K, Zhu C. Short QT syndrome: a case report and review of literature. Resuscitation. 2006;71:115–121. doi: 10.1016/j.resuscitation.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Schimpf R, Bauersfeld U, Gaita F, Wolpert C. Short QT syndrome: successful prevention of sudden cardiac death in an adolescent by implantable cardioverter-defibrillator treatment for primary prophylaxis. Heart Rhythm. 2005;2:416–417. doi: 10.1016/j.hrthm.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 43.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schimpf R, Antzelevitch C, Haghi D, Giustetto C, Pizzuti A, Gaita F, Veltmann C, Wolpert C, Borggrefe M. Electromechanical coupling in patients with the short QT syndrome: further insights into the mechano-electrical hypothesis of the U wave. Heart Rhythm. 2008;5:241–245. doi: 10.1016/j.hrthm.2007.100.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Rourke MF, Avolio AP, Nichols WW. The kangaroo as a model for the study of hypertrophic cardiomyopathy in man. Cardiovasc Res. 1986;20:398–402. doi: 10.1093/cvr/20.6.398. [DOI] [PubMed] [Google Scholar]
- 46.Sugishita Y, Iida K, O’Rourke MF, Kelly R, Avolio A, Butcher D, Reddacliff G. Echocardiographic and electrocardiographic study of the normal kangaroo heart. Aust N Z J Med. 1990;20:160–165. doi: 10.1111/j.1445-5994.1990.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe H, Makiyama T, Koyama T, Kannankeril PJ, Seto S, Okamura K, Oda H, Itoh H, Okada M, Tanabe N, Yagihara N, Kamakura S, Horie M, Aizawa Y, Shimizu W. High prevalence of early repolarization in short QT syndrome. Heart Rhythm. 2010;7:647–652. doi: 10.1016/j.hrthm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schimpf R, Wolpert C, Bianchi F, Giustetto C, Gaita F, Bauersfeld U, Borggrefe M. Congenital short QT syndrome and implantable cardio-verter defibrillator treatment: inherent risk for inappropriate shock delivery. J Cardiovasc Electrophysiol. 2003;14:1273–1277. doi: 10.1046/j.1540-8167.2003.03278.x. [DOI] [PubMed] [Google Scholar]
- 50.Gaita F, Giustetto C, Bianchi F, Schimpf R, Haissaguerre M, Calo L, Brugada R, Antzelevitch C, Borggrefe M, Wolpert C. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol. 2004;43:1494–1499. doi: 10.1016/j.jacc.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 51.Cordeiro JM, Brugada R, Hong K, Antzelevitch C, Dumaine R. Short QT syndrome mutation in HERG abolishes inactivation [abstract] Biophys J. 2004;86:134a. [Google Scholar]
- 52.McPate MJ, Duncan RS, Hancox JC, Witchel HJ. Pharmacology of the short QT syndrome N588K-hERG K+ channel mutation: differential impact on selected class I and class III antiarrhythmic drugs. Br J Pharmacol. 2008;155:957–966. doi: 10.1038/bjp.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schimpf R, Antzelevitch C, Hsu LF, Schickel S, Pollevick GD, Cordeiro JM, Haissaguerre M, Veltmann C, Borggrefe M, Wolpert C. The QT-intervalin patients with a Brugada syndrome: is a shortening of the QT-time an existing and relevant ECG-pattern [abstract]? Heart Rhythm. 2007;4:S188. [Google Scholar]
- 54.Mizobuchi M, Enjoji Y, Yamamoto R, Ona T, Funatsu A, Kambayashi D, Kobayashi T, Nakamura S. Nifekalant and disopyramide in a patient with short QT syndrome: evaluation of pharmacological effects and electro-physiological properties. Pacing Clin Electrophysiol. 2008;31:1229–1232. doi: 10.1111/j.1540-8159.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 55.Hong K, Bjerregaard P, Gussak I, Brugada R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16:394–396. doi: 10.1046/j.1540-8167.2005.40621.x. [DOI] [PubMed] [Google Scholar]