Abstract
Our Bioengineering Research Partnership grant, “High Frequency Ultrasound Arrays for Cardiac Imaging”, including the individuals cited at the end of this paper - Douglas N. Stephens (UC Davis), Matthew O’Donnell (UW Seattle), Kai Thomenius (GE Global Research), Aaron M. Dentinger (GE Global Research), Douglas Wildes (GE Global Research), Peter Chen (St. Jude Medical), K. Kirk Shung (University of Southern California), Jonathan M. Cannata (University of Southern California), Butrus (Pierre) T. Khuri-Yakub (Stanford University), Omer Oralkan (Stanford University), Aman Mahajan (UCLA School of Medicine), Kalyanam Shivkumar (UCLA School of Medicine) and David J. Sahn (Oregon Health & Science University) – is in its sixth year of NIH funding, having proposed to develop a family of high frequency miniaturized forward and side-looking ultrasound imaging devices equipped with electrophysiology mapping and localization sensors and eventually to include a family of capactive micromachined ultrasonic transducer (cMUT) devices – a forward-looking cMUT MicroLinear array and a ring array capable of 3-dimensional imaging and a 5Fr lumen large enough to admit an electrode and ablation devices.
I. INTRODUCTION
While fluoroscopic electroanatomical navigation methods are still the most widely used imaging modalities for guiding interventional electrophysiology (EP) procedures, the actual desire to see tissue structure has provided opportunities for transesophageal and intracardiac imaging. Intracardiac echocardiography (ICE) imaging catheters are increasingly being used to guide EP therapeutic procedures because they offer real-time, direct observations and improved procedural guidance over that of fluoroscopy alone [1]. Improved interventional image guidance can certainly lead to improved clinical outcomes. Recent reports have shown procedural improvements for atrial fibrillation using ICE integrated with other available imaging modalities [2], [3]. We have taken this integration approach by building and testing a multifunctional catheter capable of both EP sensing and ICE imaging functions.
II. Arrhythmias and Interventional Procedure in Electrophysiology
Atrial fibrillation (AF), the most common cardiac dysrhythmia, now affects more than 2.2 million adults in the United States alone and was the discharge diagnosis for 465,000 hospitalizations in 2003 [4]. Because cardiac dysrhythmia is more prevalent in ages beyond 60 years, the yearly rate of increase in the patient population with AF is expected to peak by 2030 due to the growing population of aging baby boomers, resulting in an expected 5.6 million U.S. patients by 2050 [5].
Since x-ray fluoroscopy guidance is the standard for EP interventional procedures, the potential for significant radiation exposures is a true concern if there happens to be a case combining a high-exposure fluoroscope with a lengthy procedure especially in a young patient. An example is given [6] of an atrophic indurated plaque forming two years later on the skin of a 17 year old following an EP ablation procedure that used approximately 100 min of fluoroscopy; the corresponding dose to skin was estimated to be 10 Gray. One study [7] conducted as a survey of diagnostic fluoroscopy machines from various hospitals in the Netherlands showed that there were substantial variations in exposure rates, with the highest exposure rate at 15 times that of the least. With the highest-dose fluoroscopic device, a patient could receive 1 Sievert (Sv) in as little as 7 min of exposure. Thus a lengthy, but not uncommon, 50-min procedural exposure with this level of radiation could produce an alarming total equivalent dose of more than 7 Sv to a region of the chest.
There have been only a few studies focused specifically on fluoroscopic radiation exposures to patients during EP procedures; attention to patient exposure rates and measurement accuracy is still in development. One recent study [8] stated that patients undergoing RF ablation procedures for paroxysmal AF with long duration exposures that averaged 57 min produced an effective dose of (only) 0.0011 Sv on average. Improvements in radiation exposimetry in a clinical setting are apparently still in development because another author [9] strongly questions this dubious result. Many investigators now are using “effective dose,” for example, which converts a higher radiation dose delivered to a small portion of the body into an equivalent uniform dose to the entire body that carries the same radiation-induced risk.
Serious efforts to diminish unnecessary radiation exposure have been conducted by groups integrating various mapping tools to display electrical data collected along with anatomical 3-D location. The CARTO system has been employed by investigators [10], [11], [12], [13], all showing significantly decreased radiation exposure. Additionally, the NavX system [10], [14], [15], [16], provided marked reductions in radiation and shortening of procedure time that offer compelling rationale for the utility of these guidance systems.
Guiding interventional EP therapies is clearly challenging. Among the issues are: 1) adequate endocardial electrical mapping; 2) identification of appropriate landmarks and recognition of individual variants in anatomy; 3) specific site guidance of ablation devices; and 4) the determination of therapeutic success while the heart is in motion, and importantly, while radiation exposure is held to a minimum.
III. A Family of Integrated ICE Catheters
Our Bioengineering Research Partnership has targeted several integrated imaging catheter designs specifically for electrophysiology therapy guidance. The first of 3 devices, the “HockeyStick” (HS) [17], is a 9 French (Fr, where 3Fr = 1 mm) combination EP mapping and ICE catheter designed to be easily deployed with standard introducer sheaths, possess dual direction steering capability, and incorporate fully integrated EP mapping electrodes near the imaging tip. A 64-element array was chosen in the first design to operate at a center frequency in the range of 7 to 12 MHz with a fractional bandwidth of 50% or greater. The design of this catheter is discussed in more detail in the companion paper [18]. The HockeyStick catheter is depicted in Fig. 1 as it has been used in the right side of the pig heart (Figs. 2 & 3).
Fig 1.
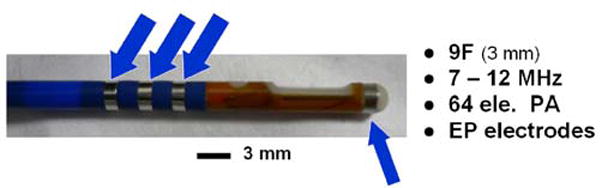
HockeyStick catheter with extra electrodes to enable electrofield 3D localization and image overlay. The phased array (PA) catheter is 9Fr.
Fig 2.
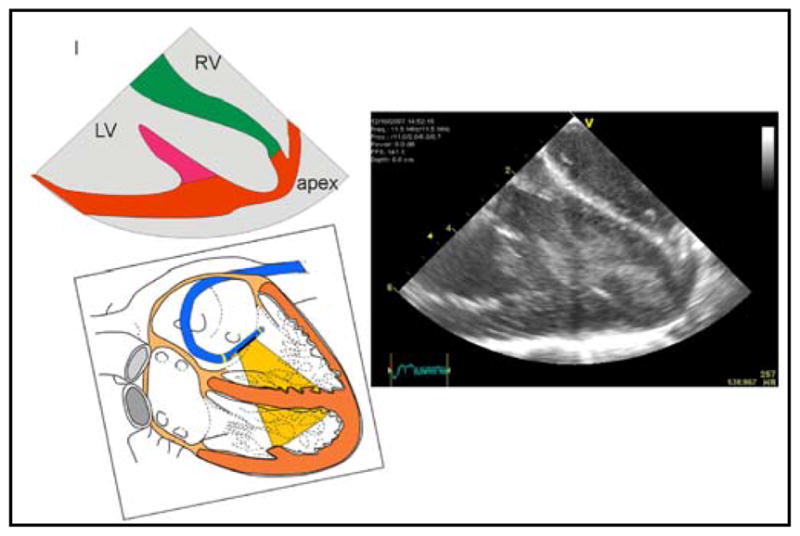
HockeyStick in RV (r-l invert of LV and RV post ions in diagram) capable of imaging the entire LV in a 60 kg pig.
Fig 3.
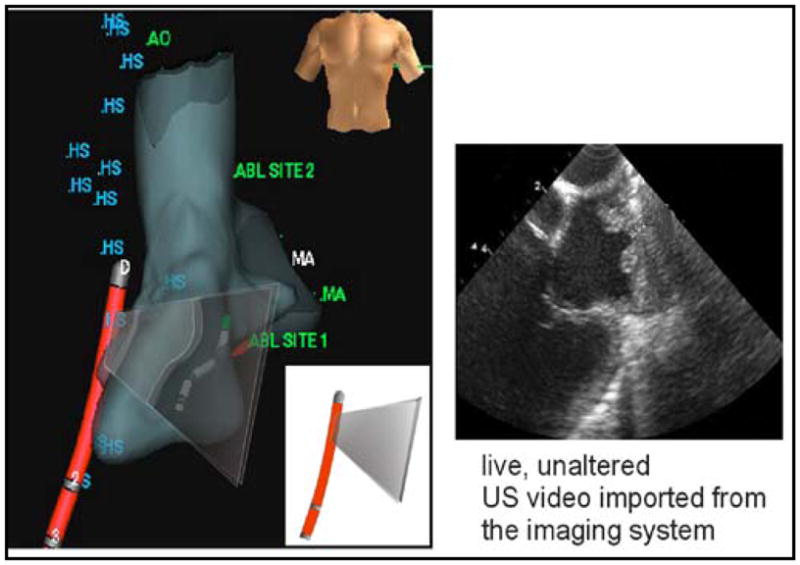
An example of a NavX overlay image plane (left), and image of LA and pulmonary veins with side looking HockeyStick catheter.
The second member of the EP-ICE family is the “MicroLinear” (ML) catheter. The most recent design is a 9Fr EP capable catheter with a 24-element, 14 MHz phased array mounted at the tip for high definition, high-frame rate, forward-looking imaging. A preferred design configuration for the ML catheter will include a metal ablation tip surrounding the distal array allowing both radio-frequency ablation (RFA) and imaging simultaneously. Prototypes of the MicroLinear forward-looking catheter are shown in Fig. 4 and Figs. 5 and 6 with our latest design, a cMUT version, shown in Figs. 7 and 8.
Fig 4.
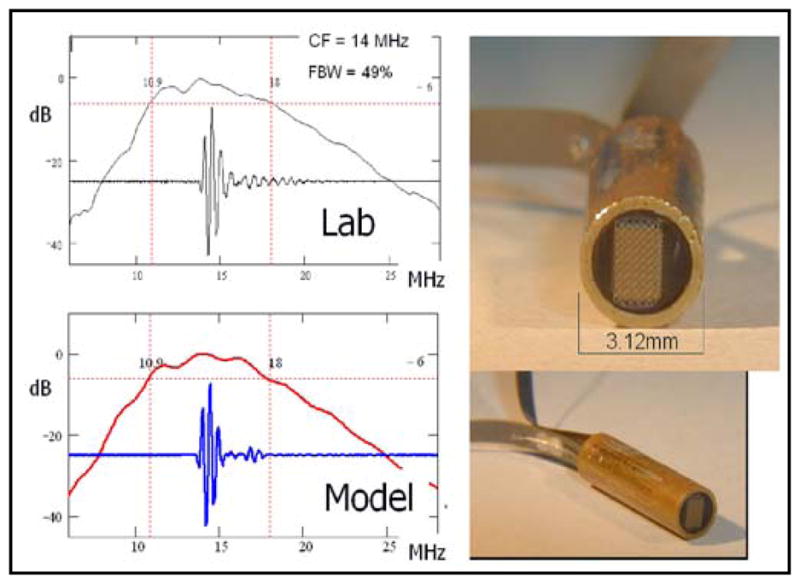
Early prototype tip assemblies of the piezoceramic MicroLinear forward looking ICE catheter showing good match between lab and model.
Fig 5.
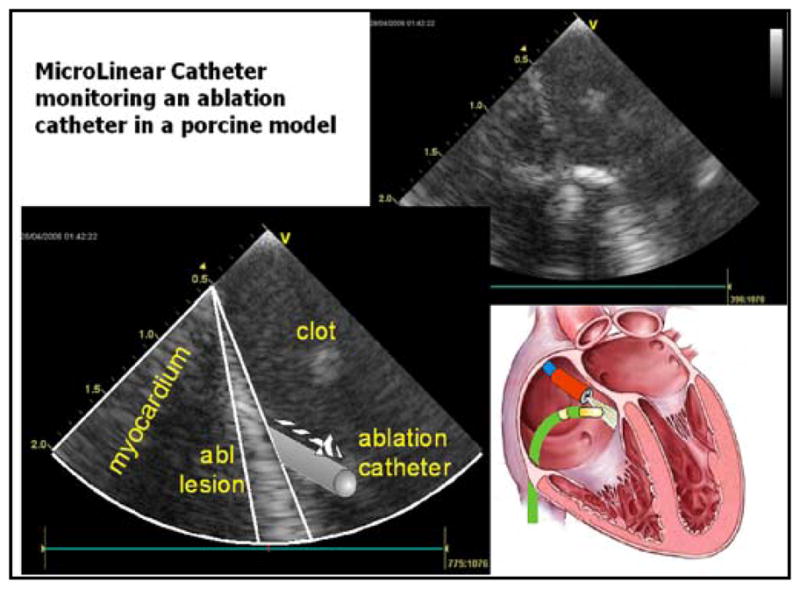
Forward looking catheter imaging ablation of the right atrium near the ostium of the coronary sinus.
Fig 6.
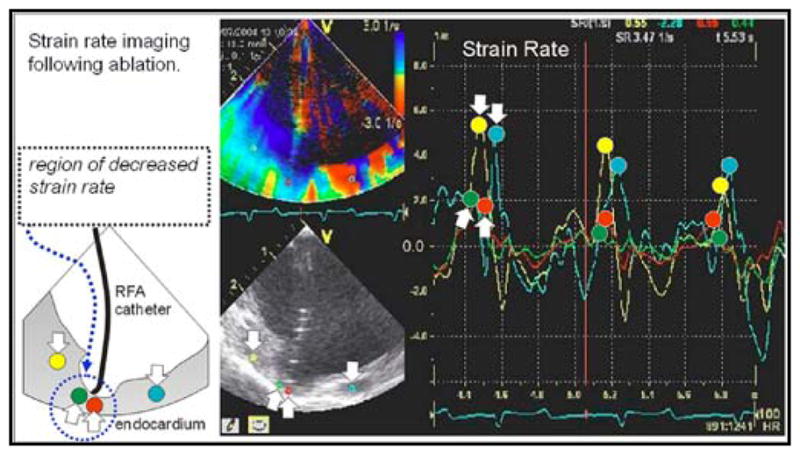
MicroLinear forward looking catheter, imaging ablation (“crater”) and altered strain rate mechanics in ablated region.
Fig 7.
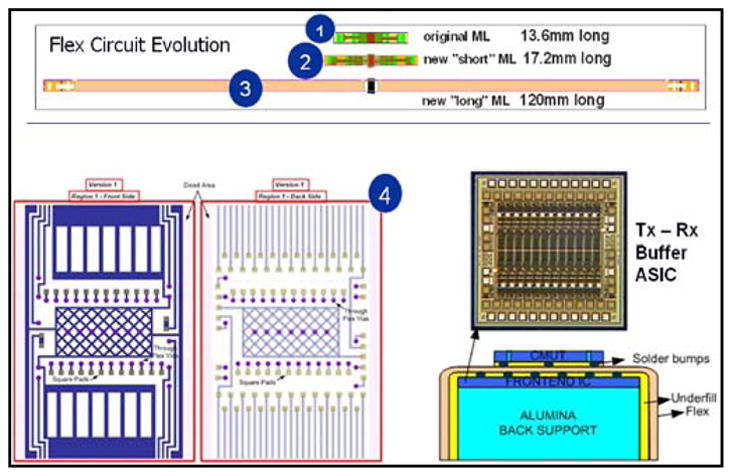
Forward looking cMUT based MicroLinear array fabrication.
Fig 8.
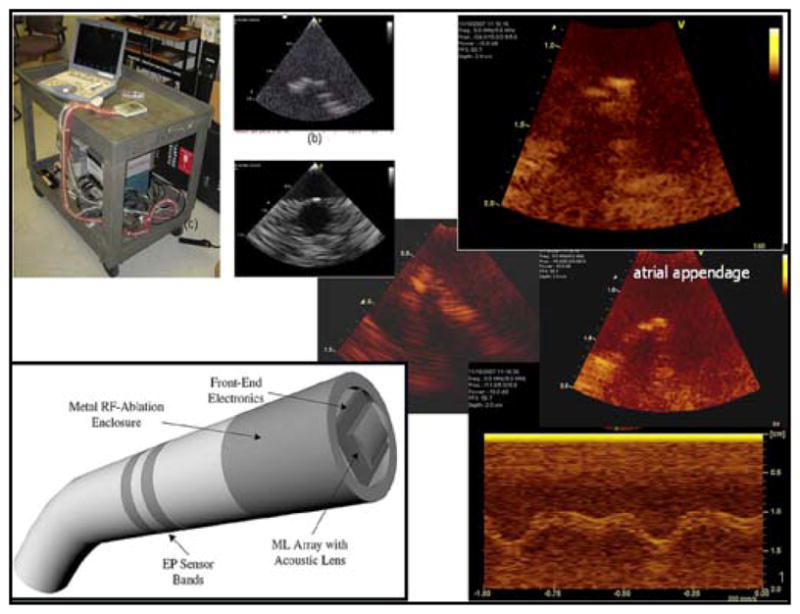
Imaging of the atrium and atrial appendage of a Langendorff heart preparation with the forward looking cMUT based array mounted on a breadboard beneath a water tank and diagram of catheter configuration for cMUT MicroLinear array.
The third device is a 9Fr forward-looking 64 capacitive micromachined ultrasonic transducer (cMUT) element ring array catheter operating at 10 MHz that ultimately will allow the central catheter lumen to be used as a conduit for any of many small wire, fiber, or electroded therapy devices that can be used simultaneously with forward-looking imaging (Fig. 9). The ring array has been used with synthetic aperture imaging techniques in laboratory testing to demonstrate its usefulness (Figs. 10 & 11). Work to incorporate this ring design into a catheter is in progress.
Fig 9.
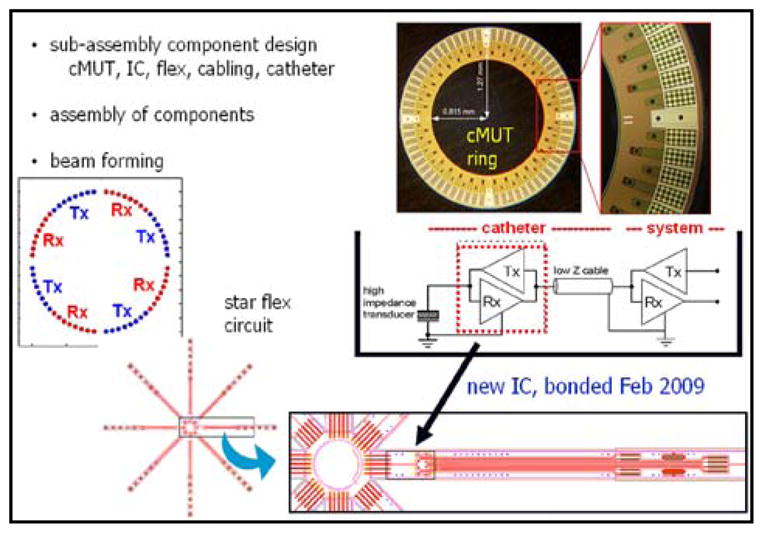
Design elements, fabrication and connections for the cMUT ring array catheter device.
Fig 10.
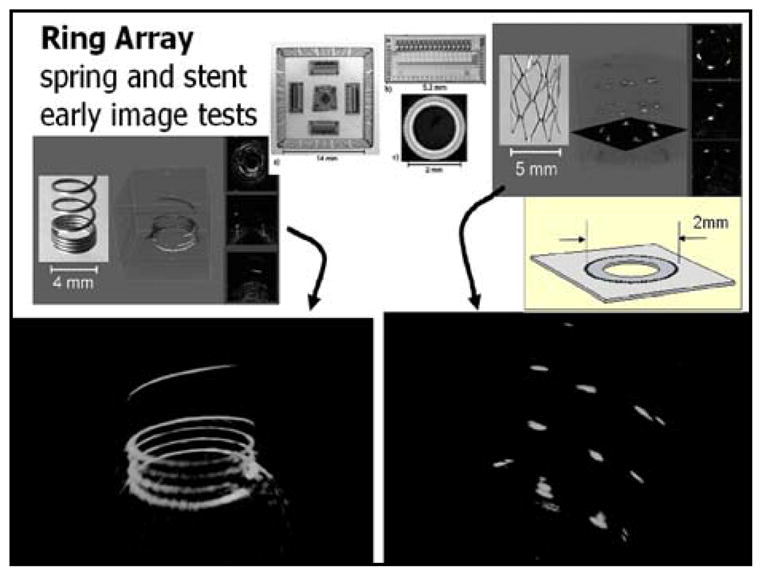
Bench imaging of springs and stents in water using the cMUT ring array device.
Fig 11.
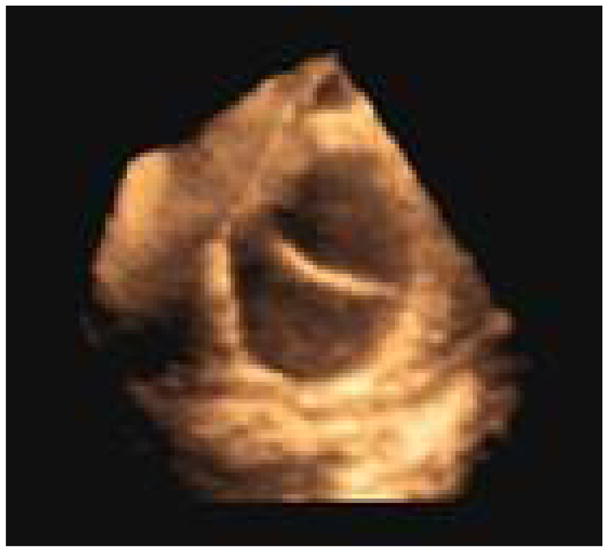
Prototype imaging with a 4D intracardiac imaging catheter, of a curved catheter placed through the left atrial appendage into the LV (seen in short axis view) in an open-chest pig experiment.
IV. Results
More than 10 pigs weighing in the range of 34 to 55 kg have been studied. ICE imaging was performed using a VingMed Vivid 7 ultrasound system in standard imaging modes, including color and pulsed Doppler, tissue Doppler, TVI, SRI, and tissue synchronization (TSI) imaging. High frame rates were commonly used at 150 F/sec. Digital scan line data were transferred to an offline EchoPac-PC (GE Healthcare, Milwaukee, WI) for further analysis.
The pig studies yielded useful ultrasound imaging guidance indicators while simultaneous tissue ablation was performed using a separate ablation catheter with 50 Watts of RF power delivery capability.
V. Discussion
A. Imaging Utility of Multifunctional ICE Catheters
Early animal studies targeted general B-Mode imaging of intracardiac features and ablation catheters with attention to evaluation of resynchronization pacing using the multifunctional nature of the EP-ICE combination catheters equipped with integrated EP sensors. The EP-ICE catheters were usually advanced to the heart to perform studies of the RA and RV without fluoroscopic guidance. In one animal, the EP-ICE catheter entered the patent foramen ovale in the intra-atrial septal wall and entered the LA without difficulty. Clear delineation of bubble production after prolonged RF ablation was observed.
Both the side-looking HockeyStick catheter and the forward-looking MicroLinear catheter designs have been successful in obtaining very useful images of therapeutic RF catheter ablations.
The HockeyStick has been used very successfully to track tissue synchrony using the high frame rate SRI modality available on the imaging system platform. This ability can be valuable in the assessment of cardiac arrhythmias. High frame rate SRI imaging (Fig. 6) allows a mechanical survey of the effects of the electrical activation and improves the ability to detect early contractions in the monitored regions of the myocardium that move first using this tissue-tracking technique.
B. Imaging with 3-D Electroanatomical Guidance
Individual intracardiac ECG channel evaluations of arrhythmias have evolved toward simultaneous, multichannel mapping, producing much more detail in the temporal characterization of specific arrhythmias. With the sheer bulk and complexity of the temporo-spatial information, it has become increasingly more difficult to maintain a clear perspective on the large number of channels of ECG data and as well interpret their significance with respect to their specific anatomic locations. Within the last decade, the development of computer-based mapping that better records and presents both the spatial and temporal characteristics of cardiac activity has become more popular as a natural solution to this issue, and in particular as it addresses the need for procedural guidance of therapeutic ablation treatments for problems related to arrhythmias.
Electro-anatomical mapping using either electric or magnetic fields in particular has become a significant guidance tool. One technique uses patient isolated electrical field gradients established by a set of patch electrodes attached to the patient’s body in at least 5 key positions. The electrical field gradients can be sensed by either a single electrode on a single intracardiac catheter or on as many as 64 electrodes from many different catheters.
A series of pre-clinical studies have been performed by our partnership that have combined ICE imaging capability with catheter localization and tracking in 3-D space in real time. Following an initial volumetric mapping with the NavX catheter, the HockeyStick catheter itself could be tracked continuously within the volume, and with the multiple electrode feature, the orientation of the ICE catheter could also be placed accurately in the intracardiac chamber (Fig. 3). Since the HockeyStick has a separate EP connector to allow for ECG signal monitoring, the electrodes connected to this EP connector allow for a very easy means of connection to the NavX system connectors. It is only this simple interconnect that is necessary for the NavX system to track the electrode positions in 3-D space. This capability can potentially yield a very powerful strategy to enhance the clinical utility of ICE by enabling therapeutic procedures, guided by intracardiac echocardiography, with much less dependence on hazardous fluoroscopic image guidance. In one of our studies, the navigation and manipulation time for achieving ultrasound imaging of an ablation procedure was substantially reduced by more than 75% compared with fluoroscopic visualization only.
VI. Conclusions
Future intracardiac therapies will likely include devices which have multiple capabilities to improve clinical outcomes with superior guidance features and less dependence on fluoroscopy with its potential for hazardous radiation exposure.
A 3-D road map projection of the heart anatomy through the use of electroanatomical mapping can be successfully combined with ICE catheters in a very seamless fashion, which suggests a great future for the success of this technology integration. The future combination of electroanatomical mapping and ICE may offer a significant means for improving the identification accuracy of therapeutic targets, lessen the lengthy procedural times, and decrease the dependence on potentially hazardous fluoroscopic guidance.
Acknowledgments
This work was supported in part by the National Heart, Lung & Blood Institute under Grant 2 R01 HL067647-06A1.
Contributor Information
David J. Sahn, Email: sahnd@ohsu.edu, Professor of Pediatrics, Diagnostic Radiology, OB/GYN & Biomedical Engineering at Oregon Health & Science University, Portland, OR 97239 USA (phone: 503-494-2191; fax: 503-494-2190.
Douglas N. Stephens, Email: dnstephens@ucdavis.edu, Director of Transducer Research, Department of Bioengineering, University of California, Davis, CA 95616.
Jonathan M. Cannata, Email: cannata@usc.edu, Research Assistant Professor of Biomedical Engineering at the University of Southern California, Los Angeles, CA 90089.
K. Kirk Shung, Email: kkshung@usc.edu, Professor of Biomedical Engineering at the University of Southern California, Los Angeles, CA 90089.
Ömer Oralkan, Email: ooralkan@stanford.edu, Senior Research Engineer in the E.L. Ginzton Laboratory at Stanford University, Stanford, CA 94305.
Amin Nikoozadeh, Graduate Student in Biomedical Engineering at Stanford University, Stanford, CA 94305.
B. T. (Pierre) Khuri-Yakub, Email: Khuri-yakub@stanford.edu, Research Professor of Electrical Engineering and Director of the E.L. Ginzton Laboratory at Stanford University, Stanford, CA 94305.
Hien Nguyen, Email: hnguyen@sjm.com, Catheter Engineer at the Atrial Fibrillation Division, St. Jude’s Medical, Irvine, CA 92614.
Peter Chen, Email: pchen@sjm.com, President of the Center for Innovation and Strategic Collaboration at St. Jude’s Medical, Irvine, CA 92614.
Aaron M. Dentinger, Email: dentinge@crd.ge.com, Electrical Engineer at General Electric Global Research, Niskayuna, NY 12309.
Douglas Wildes, Email: wildes@crd.ge.com, Physicist at General Electric Global Research, Niskayuna, NY 12309.
Kai E. Thomenius, Email: thomeniu@crd.ge.com, Chief Technologist, Imaging Technologies, at General Electric Global Research, Niskayuna, NY 12309.
Aman Mahajan, Email: amahajan@mednet.ucla.edu, Associate Professor and Chief of Cardiac Anesthesiology at the David Geffen School of Medicine, University of California, Los Angeles, CA 90095.
Kalyanam Shivkumar, Director, UCLA Cardiac Arrhythmia Center and Electrophysiology Program, David Geffen School of Medicine, University of California, Los Angeles, CA 90095.
Matthew O’Donnell, Email: odonnel@engr.washington.edu, Frank & Julie Jungers Dean of Engineering and Professor of Bioengineering, College of Engineering, University of Washington, Seattle, WA 98195.
References
- 1.Jongbloed M, Schalij M, Zeppenfeld K, Oemrawsingh P, van der Wall E, Bax J. Clinical applications of intracardiac echocardiography in interventional procedures. Heart. 2005;91:981–990. doi: 10.1136/hrt.2004.050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wazni O, Tsao H, Chen S, Chuang H, Saliba W, Natale A, Klein A. Cardiovascular imaging in the management of atrial fibrillation. J Am Coll Cardiol. 2006;48(10):2077–2084. doi: 10.1016/j.jacc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 3.Burke M, Roberts M, Knight B. Integration of cardiac imaging and electrophysiology during catheter ablation procedures for atrial fibrillation. J Electrocardiol. 2006 Oct;39(4):S188–S192. doi: 10.1016/j.jelectrocard.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 4.American Heart Association. Heart disease and stroke statistics, 2005 update. Available www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf.
- 5.Go AS, Hylek EM, Phillips KA, Chang Y, Henault L, Selby J, Singer D. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The anticoagulation and risk factors in AF (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 6.Hirshfeld J, Balter S, Lindsay B, Brinker J, Tommaso C, Kern M, Tracy C, Klein L, Wagner L. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures. J Am Coll Cardiol. 2004;44(11):2259–2282. doi: 10.1016/j.jacc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Wittkampf F, Wever E, Vos K, Geleijns J, Schalij M, van der Tol J, Robles de Medina E. Reduction of radiation exposure in the cardiac electrophysiology laboratory. J Pacing Clin Electrophysiol. 2000;23(11 pt I) doi: 10.1046/j.1460-9592.2000.01638.x. [DOI] [PubMed] [Google Scholar]
- 8.Macle L, Weerasooriya R, Jais P, Scavee C, Raybaud F, Choi K, Hocini M, Clementy J, Haissaguerre M. Radiation exposure during radiofrequency catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol. 2003;26(pt. 2):288–291. doi: 10.1046/j.1460-9592.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 9.Lickfett L, Mahesh M, Vasamreddy C, Bradley D, Jayam V, Eldadah Z, Dickfeld T, Kearney D, Dalal D, Luderitz B, Berger R, Calkins H. Radiation exposure during catheter ablation of atrial fibrillation. Circulation. 2004;110:3003–3010. doi: 10.1161/01.CIR.0000146952.49223.11. [DOI] [PubMed] [Google Scholar]
- 10.Earley M, Showkathali R, Alzetani M, Kistler P, Gupta D, Abrams D, Horrocks J, Harris S, Sporton S, Schilling R. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: A prospective randomized trial. Eur Heart J. 2006;27:1223–1229. doi: 10.1093/eurheartj/ehi834. [DOI] [PubMed] [Google Scholar]
- 11.Kottkamp H, Hugl B, Krauss B, Wetzel U, Fleck A, Schuler G, Hindricks G. Electromagnetic versus fluoroscopic mapping of the inferior isthmus for ablation of typical atrial flutter, a prospective randomized study. Circulation. 2000;102:2082–2086. doi: 10.1161/01.cir.102.17.2082. [DOI] [PubMed] [Google Scholar]
- 12.Tse H, Lee K, Fan K, Lau C. Nonfluoroscopic magnetic electroanatomic mapping to facilitate local pulmonary veins ablation for paroxysmal atrial fibrillation. J Pacing Clin Electrophysiol. 2002;25(1) doi: 10.1046/j.1460-9592.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 13.Sporton S, Earley M, Nathan A, Schilling R. Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: A prospective randomized study. J Cardiovasc Electrophysiol. 2004 Mar;15:310–315. doi: 10.1111/j.1540-8167.2004.03356.x. [DOI] [PubMed] [Google Scholar]; Faulhaber GR. Design of service systems with priority reservation. Conf. Rec. 1995 IEEE Int. Conf. Communications; pp. 3–8. [Google Scholar]
- 14.Ventura R, Rostock T, Klemm H, Lutomsky B, Demir C, Weiss C, Meinertz T, Willems S. Catheter ablation of common-type atrial flutter guided by three-dimensional right atrial geometry reconstruction and catheter tracking using cutaneous patches: A randomized prospective study. J Cardiovasc Electrophysiol. 2004 Oct;15(10):1157–1161. doi: 10.1046/j.1540-8167.2004.04064.x. [DOI] [PubMed] [Google Scholar]
- 15.Rotter M, Takahashi Y, Sanders P, Haissaguerre M, Jais P, Hsu L, Sacher F, Pasquie J, Clementy J, Hocini M. Reduction of fluoroscopy exposure and procedure duration during ablation of atrial fibrillation using a novel anatomical navigation system. Eur Heart J. 2005;26:1415–1421. doi: 10.1093/eurheartj/ehi172. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Granell R, Morell-Cabedo S, Ferrero de Loma A, Garcia-Civera R. Atrioventricular node ablation and permanent ventricular pacemaker implantation without fluoroscopy: Use of an electroanatomic navigation system. J Cardiovasc Electrophysiol. 2005 July;16:793–795. doi: 10.1046/j.1540-8167.2005.40774.x. [DOI] [PubMed] [Google Scholar]
- 17.Stephens D, Shung K, Cannata J, Zhao J, Chia R, Nguyen H, Thomenius K, Dentinger A, Wildes D, Chen X, O’Donnell M, Lowe R, Pemberton J, Burch G, Sahn D. Clinical application and technical challenges for intracardiac ultrasound imaging. Proc. IEEE Ultrason. Symp; 2004. pp. 772–777. [Google Scholar]
- 18.Stephens D, Cannata J, Liu R, Zhao J, Shung K, Nguyen H, Chia R, Dentinger A, Wildes D, Thomenius K, Mahajan A, Shivkumar K, Kim K, O’Donnell M, Sahn D. The acoustic lens design and in vivo use of a multifunctional catheter combining intracardiac ultrasound imaging and electrophysiology sensing. IEEE Trans Ultrason, Ferroelect, Freq Contr. 2008 Mar;55(3):602–618. doi: 10.1109/TUFFC.2008.685. [DOI] [PMC free article] [PubMed] [Google Scholar]


