Abstract
Trigeminal afferents convey nociceptive information from the corneal surface of the eye to trigeminal subnucleus caudalis (Vc). Trigeminal afferents, like other nociceptors, are thought to use glutamate and neuropeptides as neurotransmitters. The current studies examined whether corneal afferents contain both neuropeptides and vesicular glutamate transporters. Corneal afferents to Vc were identified using Cholera Toxin B (CTb). Corneal afferents project in two clusters to the rostral and caudal borders of Vc; regions that contain functionally distinct nociceptive neurons. Thus, corneal afferents projecting to these two regions were examined separately. Dual immunocytochemical studies combined CTb with either calcitonin gene-related peptide (CGRP), substance P (SP), vesicular glutamate transporter 1 (VGluT1) or VGluT2. Corneal afferents were more likely to contain CGRP than SP, and corneal afferents projecting to the rostral region were more likely to contain CGRP than afferents projecting caudally. Overall, corneal afferents were equally likely to contain VGluT1 or VGluT2. Together, 61% of corneal afferents contained either VGluT1 or VGluT2, suggesting that some afferents lack a vesicular glutamate transporter. Caudal corneal afferents were more likely to contain VGluT2 than VGluT1; while rostral corneal afferents were more likely to contain VGluT1 than VGluT2. Triple labeling studies combining CTb, CGRP and VGluT2 showed very few corneal afferents contain both CGRP and VGluT2; caudally (1%) and rostrally (2%). These results suggest that most corneal afferents contain a peptide or a VGluT, but rarely both. Our results are consistent with a growing literature suggesting that glutamatergic and peptidergic sensory afferents may be distinct populations.
Keywords: Substance P, calcitonin gene-related peptide, immunocytochemistry, confocal microscopy
INTRODUCTION
The cornea is one of the mostly densely innervated tissues in the body and is supplied by unmyelinated and lightly myelinated axons that appear to encode only noxious sensations (Marfurt and Del Toro, 1987; Maclver and Tanelian, 1993; Belmonte et al., 2004). Corneal nociceptors are responsive to mechanical, chemical, and thermal stimuli (Belmonte et al., 2004) as well as CO2, suggesting that they may be activated by protons or changes in pH. In spite of the potentially diverse responses of corneal afferents to different stimuli, the neurochemical nature of corneal afferents is still poorly understood. Since they represent a small subset of trigeminal afferents, only a few studies have examined corneal-projecting neurons within the trigeminal ganglion to determine their neurochemical signature. Corneal-projecting trigeminal ganglion cells in both rat and mouse contain the neuropeptides CGRP and substance P which are often considered to be reliable markers of a subset of nociceptors (LaVail et al., 1993; Murata and Masuko, 2006; Nakamura et al., 2007). In addition, most trigeminal ganglia cells (Li et al., 2003b) and many synaptophysin-immunoreactive (-ir) axon terminals in the superficial laminae of the trigeminal dorsal horn (Li et al., 2003a) contain vesicular glutamate transporters, suggesting that they may release glutamate. However, none of these studies specifically examined the neurochemical phenotype of central terminals of corneal nociceptive afferents.
The first study in rat that examined the distribution of central projections of corneal afferents to the trigeminal nuclear complex found that corneal afferents terminate in two distinct clusters (Marfurt and Del Toro, 1987): one at the rostral border of the trigeminal subnucleus caudalis (Vc) at the transition between Vc and the interpolaris subnucleus Vi (Vi/Vc) and the other at the caudal border between Vc and the cervical spinal cord (Vc/C1) (Meng and Bereiter, 1996; Martinez and Belmonte, 1996). These regions of Vc also appear to contain functionally distinct nociceptive neurons (Bereiter et al., 2000). Physiological studies suggest that there may be differential modulation of neurons in these two regions by ligands of a number of types of receptors, including neurokinin, opioid and glutamate receptors (Bereiter and Bereiter, 1996; Bereiter et al., 1998; Bereiter et al., 2000). Neurons in these two regions play different roles in effector systems as well, with neurons in rostral Vi/Vc region potentially playing a greater role in the detection of signals related to dry eye and projecting to pathways that mediate tear production (Hirata et al., 2004). By examining Vi/Vc and Vc/C1 separately, we can determine if the neurochemical nature of corneal afferents to these two regions are distinct.
MATERIALS AND METHODS
Experimental animals and surgery
All protocols were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University. Male Sprague-Dawley rats (n = 16; 250–400 g; Charles River Laboratories) were used. We have developed a method for labeling corneal afferents using an anterograde tracer modified from methods described by other laboratories (Marfurt and Del Toro, 1987; De Felipe et al., 1999). Each rat was initially anesthetized in a Plexiglas chamber with 5% isoflurane in oxygen vaporized by an Isotex Tec3 (Datex-Ohmeda; Madison, WI). Rats were positioned on their side with their heads level on a gauze pillow to maintain the corneal surface as perpendicular to the table as possible. Anesthesia was maintained at 2–3% isoflurane in oxygen delivered through a nose cone for the duration of the procedure. In order to reduce spontaneous eye movements, several drops of ophthalmic Proparacaine Hydrochloride (5% solution) were applied to the animals’ left eye and allowed to penetrate the cornea for several minutes. The eye was gently dried with a surgical swab, then a small metal retaining ring (7 gauge stainless steel tubing, 0.12 inches long, custom, HTX-07R, Small Parts Inc., Miramar, FL) was secured to the surface of the eye with petroleum jelly. The outer epithelial layer of the cornea was chemically abraded with a 1 minute application of 1-heptanol (99%; Alfa Aesar, Ward Hill, MA) inside the retaining ring and then rinsed profusely with physiological saline. This limited removal of the outer epithelial layer allows for tracer uptake without extensive corneal injury (Belmonte et al., 2004). The corneal surface was again swabbed dry and the tracer Cholera Toxin B (CTb; 1% solution in 0.1 M phosphate buffer; List Biological Laboratories, Inc., Campbell, CA) was applied (4–12 μl) to the abraded cornea inside the retaining ring for 30 minutes to allow uptake of the tracer into corneal afferents. In a separate group of animals, isolectin B4 (IB4; 1–4% solution in deionized water; Sigma, St. Louis, MO) was applied in a similar fashion. Following the procedure, excess tracer was rinsed from the surface of the cornea and rats received a subcutaneous injection of ketoprofen (2.5 mg/kg in 0.9% saline) to reduce discomfort. Rats immediately recovered from anesthesia and showed no signs of discomfort.
Perfusion and tissue preparation
Seven days after the corneal application of tract tracer, rats were overdosed with sodium pentobarbital (150 mg/kg) and perfused transcardially through the ascending aorta. Each rat was perfused with the following sequence of solutions: (1) 10 ml of heparinized saline (1000 units/ml), (2) 50 ml of 3.8% acrolein in 2% paraformaldehyde, and (3) 200 ml of 2% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The caudal trigeminal brainstem was removed and placed in 2% paraformaldehyde for 30 min, and then into 0.1 M PB. Blocks of tissue were sectioned at 40 μm on a vibrating microtome (Leica, Malvern, PA) and collected into 0.1 M PB. Prior to immunocytochemical processing, free floating tissue sections were placed in 1% NaBH4 (Sigma) for 30 min to bind remaining free aldehydes and increase the antigenicity of acrolein-perfused tissues and then in 0.5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) in 0.1 M Tris-buffered saline (TS) for 30 minutes to reduce nonspecific binding.
Immunocytochemistry
Dual- and triple-labeling immunocytochemical studies were performed as previously described (Aicher et al., 2003; Winkler et al., 2006). Tissue sections were incubated in primary antibody cocktails made in 0.1% BSA (Sigma) and 0.25% Triton-X (Sigma) in 0.1 M Tris-buffered saline (TS) for 2 nights at 4°C with continuous agitation. Two different antibodies were used to label the beta subunit of Cholera Toxin (CTb) in order to avoid cross-reactivity with the host species of the other antibodies used in these studies: rabbit anti-CTb (Novus Biologicals, Littleton, CA) and goat anti-CTb (List Biological Laboratories, Inc.) antibodies. For the CTb and VGluT dual-labeling studies, the rabbit anti-CTb (1:10,000; Novus Biologicals) primary antibody was preincubated with naïve rat tissue sections for 2 hours at room temperature to decrease non-specific binding. Following the preincubation period, experimental tissue sections were incubated in a primary antibody cocktail of the rabbit anti-CTb (1:10,000; Novus Biologicals) and one of the following antibodies: guinea pig anti-VGluT1 (1:5,000; Chemicon, Temecula, CA) or guinea pig anti-VGluT2 (1:2,500; Chemicon). For the CTb and peptide dual-labeling studies, tissue sections were incubated in a primary antibody cocktail of goat anti-CTb (1:25,000; List Biological Laboratories, Inc.) and either rabbit anti-CGRP (1:8,000; Immunostar, Hudson, WI) or rat anti-SP (1:1,000; Accurate Chemical & Scientific Corporation, Westbury, NY). For triple-labeling studies, the primary antibody cocktail consisted of goat anti-CTb (1:25,000; List Biological Laboratories, Inc.), rabbit anti-CGRP (1:8,000; Immunostar) and guinea pig anti-VGluT2 (1:2,500; Chemicon). Following profuse rinsing, tissue sections were incubated in cocktails of fluorescent secondary antibodies (1:800) made in 0.1% BSA in 0.1 M TS for 2 hours at room temperature with continuous agitation. Secondary antibodies were selected to allow for combinations of fluorophores that would be spectrally distinct. For the CTb and VGluT dual-labeling studies, the CTb primary antibody was visualized using a donkey anti-rabbit antibody conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and the VGluT primary antibodies were visualized using a donkey anti-guinea pig antibody conjugated with Cy5 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). For the CTb and peptide dual-labeling studies, the CTb primary antibody was visualized using a donkey anti-goat antibody conjugated to Alexa Fluor 488 (Invitrogen), the CGRP primary antibody was visualized using a donkey anti-rabbit antibody conjugated to Alexa Fluor 594 (Invitrogen) and the SP primary antibody was visualized using a donkey anti-rat antibody conjugated to Cy5 (Jackson). For triple-labeling studies, the CTb, CGRP and VGluT2 primary antibodies were visualized with Alexa Fluor 488-conjugated donkey anti-goat (Invitrogen), Alexa Fluor 546-conjugated donkey anti-rabbit (Invitrogen) and Cy5-conjugated donkey anti-guinea pig (Jackson ImmunoResearch Laboratories, Inc.), respectively. Tissue sections were then mounted onto gelatin-coated slides, coverslipped with ProLong® Gold antifade reagent (Invitrogen) and stored at 20°C.
The primary antibodies used in the current study are listed in Table 1. The goat anti-CTb antibody recognizes the beta subunit of cholera toxin (manufacturer’s technical specifications) and preadsorption with 1 μg/ml and 5μg/ml of CTb abolished all immunostaining (Llewellyn-Smith et al., 1995). The rabbit anti-CTb antibody also recognizes the beta subunit of cholera toxin (manufacturer’s technical specifications). CTb labeling for both the goat anti-CTb and rabbit anti-CTb antibodies was found in discrete regions of Vc on the side ipsilateral of the abraded cornea that received CTb tracer. The regions displaying peak CTb labeling matched a previous study that used horseradish peroxidase (HRP) to label corneal afferents (Marfurt and Del Toro, 1987). Based on the discrete labeling pattern, it is unlikely that these antibodies bound nonspecifically to endogenous epitopes in Vc. The rat anti-SP antibody recognizes the five to eight amino acid residues of the C-terminal end of substance P (Cuello et al., 1979). The rat anti-SP antibody was tested for its specificity by radioimmunoassay and immunostaining for this antibody was abolished when it was preadsorbed with 200 μg/ml of the substance P peptide (Cuello et al., 1979). Immunostaining for the rabbit anti-CGRP antibody was completely abolished when preadsorbed with the rat alpha-CGRP peptide at a concentration of 10−5 M but was not abolished when pre-adsorbed with other peptides such as substance P (manufacturer’s technical information). The guinea pig anti-VGluT1 recognizes amino acid residues 542–560 of rat VGluT1 (Melone et al., 2005; Ramer, 2008). The guinea pig anti-VGluT2 antibody recognizes amino acid residues 565–582 of rat VGluT2 (Ramer, 2008; Schnell and Wessendorf, 2008). Immunostaining for these antibodies was completely abolished when they were preadsorbed with the immunogen peptides (manufacturer’s technical information). The specificity of the secondary antibodies was confirmed by incubating tissue with a particular primary antibody (e.g. guinea pig anti-VGluT1) followed by an incorrect secondary antibody (e.g. Alexa Fluor 488-conjugated donkey anti-goat). There was no immunolabeling in any of the mismatch combinations tested.
Table 1.
Primary antibodies
| Antibody | Source | Immunogen |
|---|---|---|
| Goat anti-CTb | List Biological Laboratories, Inc. | Purified cholera toxin beta subunit (manufacturer’s technical specifications) |
| Rabbit anti-CTb | Novus Biologicals | Purified choleragenoid |
| Rat anti-SP | Accurate Chemical and Scientific Corporation | Substance P peptide conjugated to bovine serum albumin |
| Rabbit anti-CGRP | Immunostar | Full length synthetic rat alpha-CGRP |
| Guinea pig anti-VGluT1 | Chemicon | Synthetic peptide corresponding to amino acid residues 542 – 560 of rat VGluT1 (GATHSTVQPPRPPPPVRDY) |
| Guinea pig anti-VGluT2 | Chemicon | Synthetic peptide corresponding to amino acid residues 565 – 582 of rat VGluT2 (VQEAQDAYSYKDRDDYS) |
Data analysis for confocal microscopy
Images were collected using a Zeiss LSM 510 META confocal microscope. Antibodies typically show limited penetration into vibratome sections, therefore, Z stacks bounded by the vertical extent of labeling into the tissue were collected (typically 10 μm). The ventrolateral region throughout Vc was examined. Corneal afferent labeling is most abundant at the transition zones at either extent of the ventrolateral subnucleus caudalis (Vc), thus samples from each region (rostral Vi/Vc and caudal Vc/C1) were collected and scanned in each animal. Images were captured as a Z-stack with a Plan-Apochromat 63x / 1.4 NA oil objective using the single pass, multi-tracking format of the Zeiss LSM 510 META software. Sections were chosen for analysis based on anatomical landmarks visible under darkfield illumination, such as fiber tracks and trigeminal laminae, as well as the presence of CTb-labeled corneal afferents. Only cases with optimal morphology that contained the labels of interest were included in the analyses. No adjustments were made to images used for analysis. Criteria for co-localization of CTb with either a peptide or VGluT marker were similar to our previous studies (Mitchell et al., 2004; Silverman et al., 2005). Specifically, the peptide or VGluT marker had to be included entirely within the CTb-labeled corneal afferent axonal varicosity in at least two consecutive optical sections. Also, co-localization analyses of CTb-labeled corneal afferent varicosities and the peptide or VGluT marker was assessed by two observers in which the first observer identified CTb-labeled varicosities in the channel used to detect the tract tracer, then the second channel was turned on to see the second marker and each varicosity was examined for content of the second marker. Twenty-five corneal afferent varicosities were counted in each Vc/C1 scan and 50 corneal afferent varicosities were counted in each Vi/Vc scan. Similar methods were used when analyzing for varicosities triple-labeled with CTb, CGRP and VGluT2. Confocal micrographs used for publication are projections of one or several optical sections that were adjusted for optimal brightness and contrast using Zeiss LSM 510 META software.
Statistics
The proportions of dual-labeled and triple-labeled varicosities to total varicosities in Vc/C1 and Vi/Vc were compared using the z-test in which high z values indicated that the proportions of the two groups were different and in which P values less than 0.05 were considered significant (SigmaStat; Systat Software, Inc., San Jose, CA).
RESULTS
Corneal afferents project to two distinct regions of Vc
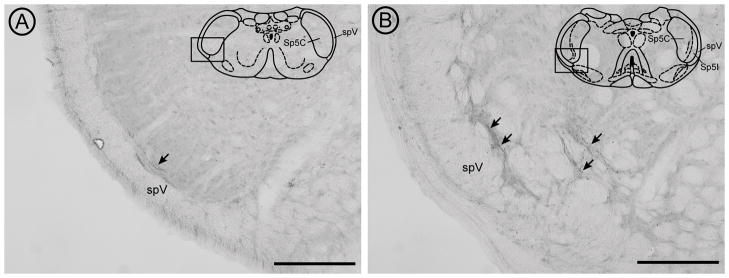
Previous studies have demonstrated that CTb traces myelinated afferents (LaMotte et al., 1991; Todd et al., 2003); although this has been disputed for non-cutaneous afferents (Corbett et al., 2005). In the present study, using CTb as an anterograde tracer, we found CTb labeling within both ventrolateral Vc/C1 [Figure 1A] and Vi/Vc [Figure 1B] as previously reported using HRP (Marfurt and Del Toro, 1987). CTb has the advantages of being a transganglionic tracer as well as providing clear labeling of afferent axons and varicosities using a stable reagent. Axons with punctate varicosites are seen in the outer lamina of ventrolateral Vc at the caudal transition region (Vc/C1) [Figure 1A]. Moving rostrally, the ventrolateral distribution of corneal afferents follows the bifurcation of the outer lamina of Vc as the interpolaris subnucleus develops and then the pattern is more diffuse at the transition with Vi [Figure 1B]. Regions of Vc between the Vc/C1 and Vi/Vc levels show lower densities of trigeminal corneal afferent labeling (not shown), consistent with previous reports that these afferents preferentially project to two distinct regions. Application of isolectin B4 (IB4), a marker of unmyelinated afferents, failed to yield labeling in Vc (data not shown; see Methodological Considerations portion of Discussion).
Figure 1.
Light micrographs show the distribution of peroxidase-labeled CTb-containing corneal afferents (arrows) at the caudal (A) and rostral (B) boundaries of ventrolateral Vc (diagram insets). The black box on each diagram delineates the ventrolateral regions of Vc/C1 (A) and Vi/Vc (B) that were imaged for analysis. Representative diagrams of the caudal and rostral Vc regions are modified from the digital atlas of Paxinos and Watson (Paxinos and Watson, 1998) and reproduced here with permission from the publisher. The tissue was incubated in goat anti-CTb primary antibody (1:25,000; List Biological Laboratories, Inc.) as described in the Methods section. Bound primary antibody was visualized by incubating tissue sections in biotinylated horse anti-goat secondary antibody (1:400; Vector Laboratories, Inc., Burlingame, CA) followed by incubation in Avidin-Biotin (Elite Vectastain ABC kit; Vector) and then in diaminobenzidine-hydrogen peroxide solution. Tissue was mounted on gelatin-coated slides, dehydrated and then coverslipped with DPX mounting medium (Sigma). Images were taken with on an Olympus BX51 microscope equipped with a DP71 camera (Olympus America, Inc, Center Valley, PA) and were adjusted for optimal brightness and contrast using Adobe Photoshop (Adobe Systems Inc., San Jose, CA). Sp5C = spinal trigeminal subnucleus caudalis, Sp5I = spinal trigeminal subnucleus interpolaris, spV = spinal trigeminal tract. Scale bars = 200 μm.
Rostrally projecting afferents are more likely to contain neuropeptides
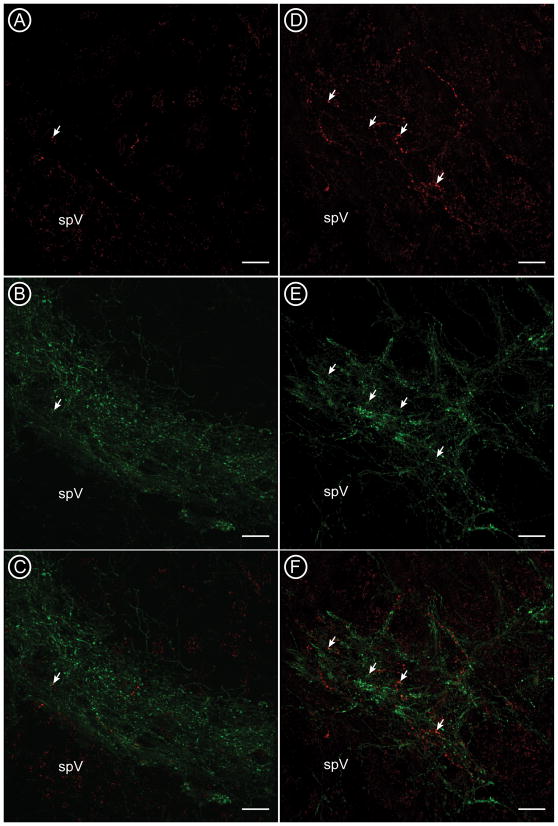
We combined tracing of CTb-labeled corneal afferents [Figure 2A and 2D] with detection of SP or CGRP. CGRP-immunoreactive (-ir) afferents are found in the outer laminae of caudal Vc [Figure 2B], and are more diffuse at the rostral transition between Vc and Vi [Figure 2E]. There was a similar pattern of SP labeling (not shown). Confocal analysis of CTb afferents and CGRP shows that a subset of CTb-labeled afferents contain CGRP [Figure 2C and 2F, arrows], but these are slightly more abundant in rostral Vi/Vc [Figure 2F] than in the caudal Vc/C1 region [Figure 2C]. used high magnification of these images to quantify the localization of peptides or VGluTs in individual CTb-labeled varicosities [Figure 3].
Figure 2.
Confocal micrographs show the distribution of corneal afferents labeled with CTb (A, D; red) and nociceptive CGRP-containing afferents (B, E; green) at the caudal (A-C) and rostral (D-F) boundaries of ventrolateral Vc. Some CTb-labeled varicosities also contained CGRP (arrows). spV = spinal trigeminal tract. Scale bars = 20 μm.
Figure 3.
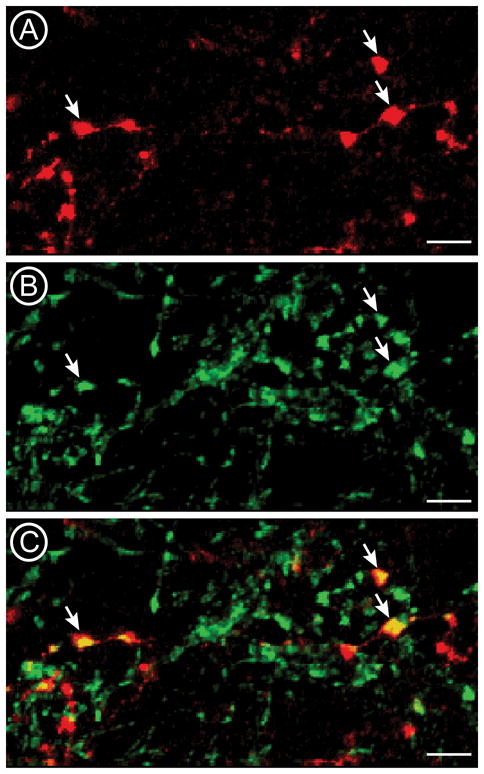
High magnification confocal micrographs show the distribution of corneal afferents labeled with CTb (A; red) and CGRP (B; green) in Vi/Vc. An overlay image (C) shows that a subset of CTb-labeled varicosities also contain CGRP-ir (arrows). Co-localized profiles appear yellow in the overlay. bars = 5 μm.
We found that overall, 15% of CTb-labeled varicosities contained CGRP, and 3% of CTb-labeled varicosities contained SP [Figure 4, Total]. The percentages of peptidergic corneal afferent fibers projecting to Vc are less than those found in previous rat and mouse studies looking at the peptidergic content of trigeminal ganglion neurons retrogradely labeled from the cornea (LaVail et al., 1993; De Felipe et al., 1999; Belmonte et al., 2004; Nakamura et al., 2007). This may be due to the fact that we used the transganglionic tracer CTb to label central terminals of thinly myelinated corneal afferents as compared to the retrograde Fluorogold tracer which does not distinguish among the different types of afferents (LaVail et al., 1993; De Felipe et al., 1999; Belmonte et al., 2004; Nakamura et al., 2007). However, similar to these previous studies, the percentage of corneal afferent varicosities containing CGRP was greater than that containing SP.
Figure 4.
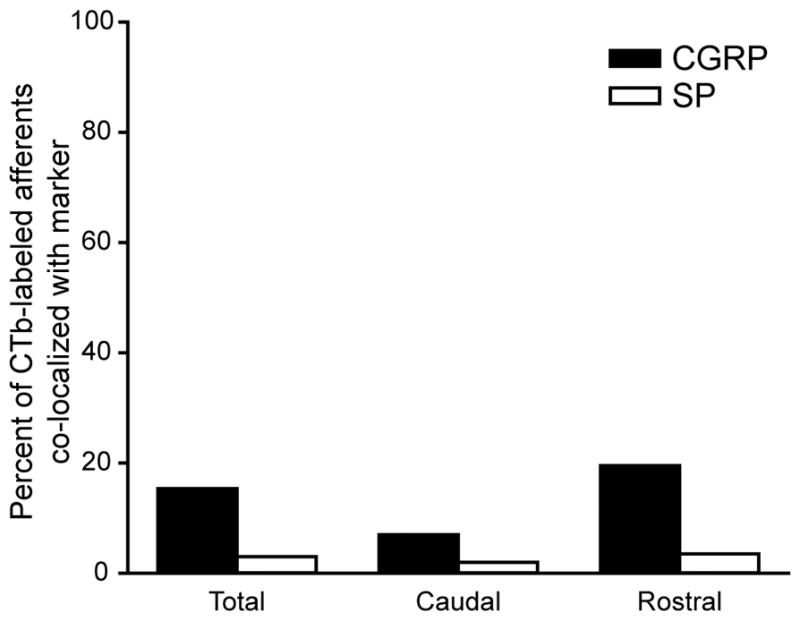
The summary graph shows the percentages of CTb-labeled corneal afferents that also contained either CGRP or SP immunoreactivity across four animals. Total percentages represent the sum of CGRP- or SP-labeled corneal afferents found caudally in Vc/C1 and rostrally in Vi/Vc. Statistics were used to compare the proportions of CGRP-containing varicosities to total CTb-labeled varicosities in Vc/C1 and Vi/Vc. There was a significant difference in this proportion between the two regions (z = 2.663, P = 0.008). There was no such difference when the proportion of SP-containing to total CTb-labeled varicosities in Vc/C1 and Vi/Vc were compared (z = 0.359, P = 0.720).
Interestingly, we found that the phenotypic peptide pattern was quite distinct if we examined the fibers projecting to Vc/C1 and Vi/Vc separately [Figure 4, Caudal and Rostral]. Here we found that CGRP-ir peptidergic fibers preferentially project to the rostral (20%) region compared to the caudal (7%) region. The proportion of CGRP-containing corneal afferents to total CTb-labeled varicosities in rostral Vi/Vc, as compared to the proportion in caudal Vc/C1 was statistically significant [Table 2; Figure 4]. A similar pattern also emerged for corneal afferents containing SP, but there was no significant difference between the proportion of SP-containing CTb-labeled corneal afferent varicosities to total CTb-labeled varicosities found rostrally and the proportion found caudally [Table 2; Figure 4]. Since CTb-labeled varicosities were more abundant rostrally, we counted more varicosities rostrally (50) than caudally (25) for each animal [Table 2]. However, the difference between the rostral and caudal distribution of CGRP-containing CTb-ir varicosities does not appear to be due to a lack of peptidergic fibers at the caudal level [Figure 2B]. These results suggest that specific types of corneal afferents project differentially to distinct regions of Vc, possibly underlying distinct functions (Bereiter et al., 1998; Bereiter et al., 2000; Belmonte et al., 2004). There were abundant peptidergic fibers in both regions, thus these studies demonstrate a different phenotype for corneal afferents, as opposed to other fibers, that project to the region.
Table 2.
Regional differences in CGRP or SP immunoreactivity of CTb-labeled corneal afferents*
| Total | Caudal – Vc/C1 | Rostral – Vi/Vc | |
|---|---|---|---|
| CGRP-ir | 46 | 7 | 39 |
| Not CGRP-ir | 254 | 93 | 161 |
| SP-ir | 9 | 2 | 7 |
| Not SP-ir | 291 | 98 | 193 |
The Total column indicates the raw numbers of CTb-labeled varicosities that were present in both regions (Caudal and Rostral). The Caudal-Vc/C1 and Rostral-Vi/Vc columns indicate the raw numbers of dual-labeled varicosities in that region for each peptide. Total number of CTb-labeled varicosities were 100 (caudal) and 200 (rostral) from 4 animals.
Vesicular glutamate transporters 1 and 2 are more abundant in rostrally projecting corneal afferents
Since corneal afferents are thought to use glutamate as a neurotransmitter, we examined the content of these fibers for vesicular glutamate transporters (VGluTs). There are three known isoforms, VGluT1, VGluT2, and VGluT3 that have been identified. VGluT1 and VGluT2 are often found in complementary distributions within the brain and are thought to account for most glutamate-releasing terminals in the brain (Renick et al., 1999; Todd et al., 2003; Li et al., 2003b; Corbett et al., 2005). VGluT1 and VGluT2 are thought to be excellent markers for axon terminals that release glutamate as a neurotransmitter (Takamori, 2006; Liguz-Lecznar and Skangiel-Kramska, 2007). VGluT3 is also found in the brain, but is thought to be located primarily in cholinergic and serotonergic fibers (Gras et al., 2002). The distribution of VGluT1 [Figure 5] and VGluT2 labeling in Vc was similar to previous studies (Li et al., 2003a).
Figure 5.
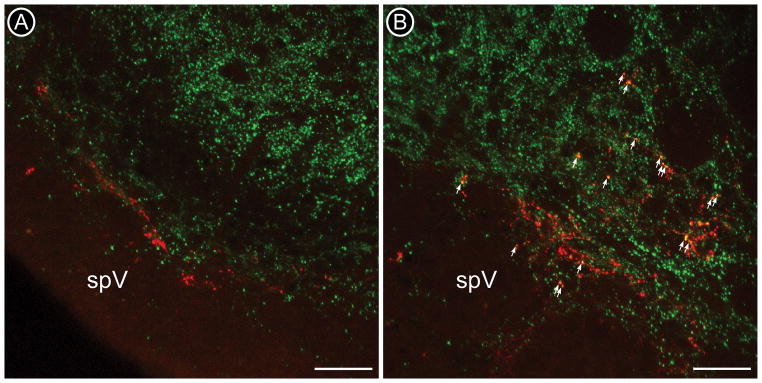
Confocal micrographs of corneal afferents labeled with CTb-ir (red) and VGluT1-ir (green) at the ventrolateral Vc/C1 (A) and Vi/Vc (B) transition regions. CTb-labeled corneal afferent fibers (red) are found in lamina I and II in Vc/C1 and are more dispersed at the rostral Vi/Vc boundary. VGluT1 labeling (green) is found in lamina I and II inner (IIi) in Vc/C1 (A) and diffusely throughout the ventrolateral Vi/Vc (B). CTb-labeled varicosities containing VGluT1-ir are more abundant in Vi/Vc (B). Co-localized profiles appear yellow and a few are indicated by arrows. spV = spinal trigeminal tract. Scale bars = 50 μm.
We used a similar method as described above to examine VGluT content in CTb-labeled corneal afferents. We found that overall, 33% of CTb-labeled corneal afferents in Vc contained VGluT1 and 28% contained VGluT2 [Figure 6, Total]. Similar to CGRP-containing afferents, we found that VGluT1 was preferentially in CTb-labeled corneal afferents in the rostral Vi/Vc region rather than the caudal Vc/C1 region [Table 3; Figure 5, arrows; Figure 6, Caudal and Rostral]. VGluT2-containing corneal afferents were also found to preferentially project to Vi/Vc [Table 3; Figure 6, Caudal and Rostral], although it appears that this difference is not as robust as the pattern demonstrated by VGluT1-ir corneal afferents. Even if these are completely distinct populations, we found that about 61% of corneal afferents contained a VGluT, leaving open the possibility that some corneal afferents contain peptides but not a VGluT. Functionally this suggests that these primary afferents may release peptides but not glutamate. While initially surprising to us based on previous studies (De Biasi S. and Rustioni, 1988; Broman et al., 1993), a simliar conclusion has been reached by other laboratories (Todd et al., 2003; Morris et al., 2005).
Figure 6.
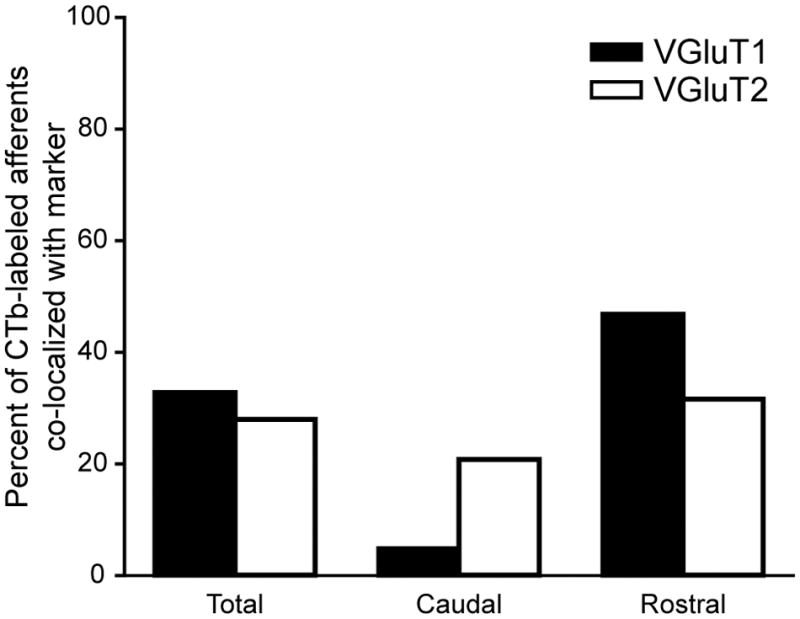
The summary graph shows the percentages of CTb-labeled corneal afferents that also contained one of the VGluTs across five animals. The percentages in the Total columns represent the sum of VGluT1- or VGluT2-labeled corneal afferents in both caudal Vc/C1 and rostral Vi/Vc. We compared the proportions of VGluT1-containing to total CTb-labeled varicosities in Vc/C1 and Vi/Vc and found that there was a significant difference in this proportion between these two regions (z = 8.050, P < 0.001). There was also a significant difference in the proportions of VGluT2-containing to total CTb-labeled varicosites in Vc/C1 and Vi/Vc (z = 2.074, P = 0.038).
Table 3.
Regional differences in VGluT1 or VGluT2 immunoreactivity of CTb-labeled corneal afferents*
| Total | Caudal – Vc/C1 | Rostral – Vi/Vc | |
|---|---|---|---|
| VGluT1-ir | 123 | 6 | 117 |
| Not VGluT1-ir | 252 | 119 | 133 |
| VGluT2-ir | 105 | 26 | 79 |
| Not VGluT2-ir | 270 | 99 | 171 |
The Total column indicates the raw number of varicosities that were present in both regions analyzed (Caudal and Rostral). The Caudal-Vc/C1 and Rostral-Vi/Vc columns indicate the raw numbers of varicosities in that region for each VGluT. Total number of CTb-labeled varicosities were 125 (caudal) and 250 (rostral) from 5 animals.
Very few corneal afferents are both peptidergic and glutamatergic
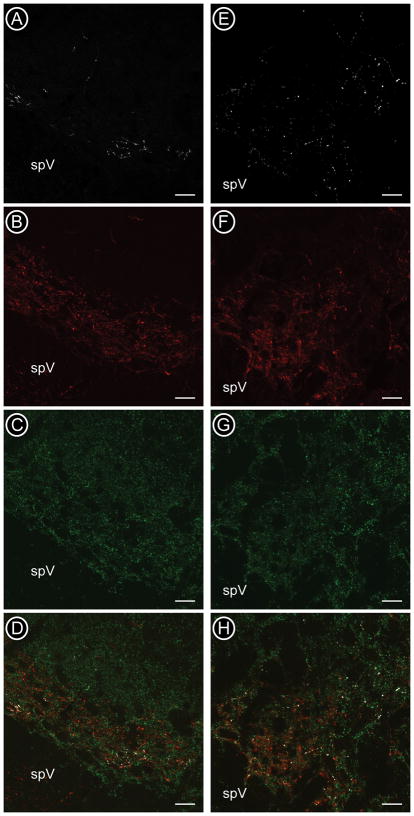
In order to directly determine if peptides and VGluTs are co-localized in corneal afferents, we conducted triple labeling studies using the most abundant peptidergic marker (CGRP) and the glutamatergic marker VGluT2 which was most evenly distributed in both caudal and rostral Vc. The distribution of CGRP and VGluT2 has also been shown to be similar in rodent spinal cord dorsal horn (Todd et al., 2003; Morris et al., 2005). We quantified the degree of co-localization in corneal afferents identified by CTb traced from the corneal surface [Figure 7]. These studies showed that, as predicted from the dual-labeling studies, CGRP and VGluT2 were individually both more abundant in rostrally-projecting CTb-labeled corneal afferents as compared to those projecting caudally [Table 4]. However, very few CTb-labeled varicosities in the caudal (1%) and rostral (2%) regions contained both CGRP and VGluT2 [Table 4]. These results are in agreement with previous studies in rodents looking at sensory afferents in the spinal cord dorsal horn (Todd et al., 2003; Morris et al., 2005). The results of the present study suggest that the population of corneal afferents projecting to the rostral and caudal regions of Vc are largely heterogeneous in terms of their neurotransmitter content, with very few afferents in either region defined as both peptidergic and glutamatergic.
Figure 7.
Confocal micrographs of CTb (A, E, white), CGRP (B, F, red) and VGluT2 (C, G, green) at the caudal (Vc/C1) (A - D) and rostral (Vi/Vc) (E - H) boundaries of trigeminal subnucleus caudalis. All three markers were present in the superficial laminae of ventrolateral Vc/C1 (D) and were more dispersed in the ventrolateral region of Vi/Vc (H). spV = spinal trigeminal tract. Scale bars = 20μm.
Table 4.
Few CTb-labeled corneal afferents contained both CGRP and VGluT2 immunoreactivity *
| Region | CGRP-ir + VGluT2-ir | CGRP-ir | VGluT2-ir | Neither |
|---|---|---|---|---|
| Caudal – Vc/C1 | 2 (1%) | 5 (3%) | 7 (4%) | 161 (92%) |
| Rostral – Vi/Vc | 7 (2%) | 32 (9%) | 39 (11%) | 272 (78%) |
Each column in the table indicates the raw number of varicosities in that category for each region. Percentages indicate the breakdown of CTb-labeled varicosities within that region. Total number of CTb-labeled varicosities were 175 (caudal) and 350 (rostral) from 7 animals. There was a higher proportion of CGRP-containing to total CTb-labeled varicosities rostrally than caudally (z = 2.359, P = 0.018). We found the same was true for VGluT2-containing varicosities (z = 2.523, P = 0.018). There was no difference in the proportion of CTb-labeled varicosities that contained both CGRP and VGluT2 in rostral Vi/Vc as compared to caudal Vc/C1 (z = 0.482; P = 0.630).
DISCUSSION
Corneal afferent fibers project to the brainstem to mediate nociception and to initiate blink responses (Belmonte et al., 2004; Henriquez and Evinger, 2007). In spite of the enormous importance of these afferents in maintaining the integrity of the surface of the eye, little is known about the anatomy of their projections into the brainstem. Our studies are the first to specifically quantify neurotransmitter and transporter content of thinly myelinated corneal afferents traced into the trigeminal subnucleus caudalis from the cornea. By examining the central terminals of corneal afferents, we show significant differences in the peptide and vesicular glutamate transporter content of afferents projecting to rostral and caudal regions of Vc. These studies demonstrate that thinly myelinated corneal afferents are not homogenous and the differences in neuropeptide and glutamate transporters may be important for what type of information is relayed via these fibers as well as their central targets.
Previous studies in the trigeminal ganglion (LaVail et al., 1993; Nakamura et al., 2007), and Vi/Vc (Murata and Masuko, 2006) have shown that a subpopulation of corneal afferents contain peptides and our findings support those results. By quantifying central terminals of thinly myelinated corneal afferents, we found that 15% of these afferents contained CGRP and 3% contained SP, proportions that are lower than that found in other studies (27% CGRP and 8% SP reported by (Nakamura et al., 2007); 41% CGRP and 31% SP in the mouse (LaVail et al., 1993)) that quantified the peptidergic content of trigeminal ganglion cells projecting to the cornea. However, the previous studies used the retrograde tracer Fluorogold that traces afferents indiscriminately and is not transported transganglionically. Therefore, the smaller percentages of peptide-containing afferents in the present study may reflect the specificity of the CTb tracer to predominantly identify thinly myelinated afferents that innervate the cornea.
We found that the CGRP-containing corneal afferents were preferentially targeted to distinct areas in Vc, with 20% of CTb-labeled corneal afferents in rostral Vc containing CGRP while only 7% of caudal CTb-labeled afferents contained the peptide. There were very few CTb-labeled corneal afferents that contained SP, and those that were SP-ir did not preferentially target a specific region of Vc. The proportions previously described in the trigeminal ganglion are an average of all afferent fibers that cannot differentiate the divergent central projections of the peptidergic and non-peptidergic or myelinated and unmyelinated afferents. It has been argued that these neuropeptides may distinguish nociceptive afferents from non-nociceptive afferents, but this interpretation is not consistent with data suggesting that the vast majority of corneal afferents, both unmyelinated and lightly myelinated are nociceptive. The present anatomical study was not designed to measure functional differences between the corneal afferents projecting to Vc/C1 and those projecting to Vi/Vc. However, it is possible that the non-peptidgeric afferents projecting to Vi/Vc may be more involved in mediating the blink reflex and other mechanisms involved in signalling the need to maintain or restore the corneal epithelium rather than frank nociceptive processing (Hirata et al., 2004).
Since it has been suggested that all primary afferents release glutamate (De Biasi S. and Rustioni, 1988; Broman et al., 1993) we sought to examine the vesicular glutamate transporter (VGluT) content of thinly myelinated corneal afferents. VGluTs are necessary for transport of glutamate into synaptic vesicles and are thus thought to be the best markers available of excitatory glutamatergic axon terminals (Takamori, 2006; Liguz-Lecznar and Skangiel-Kramska, 2007) and a better marker than glutamate itself (Li et al., 2003a). Although the functional significance of the two VGluT isoforms is not clear (Takamori, 2006), VGluT1 and VGluT2 have often been reported to be found in different, complementary populations of primary afferent fibers (Todd et al., 2003; Fujiyama et al., 2003; Corbett et al., 2005; Lachamp et al., 2006). In contrast to most studies, one recent study has also demonstrated that VGluT1 and VGluT2 are extensively co-localized in trigeminal ganglion neurons and axon terminals of lamina I and II in the trigeminal dorsal horn, although the peripheral area of innervation of these neurons was not determined (Li et al., 2003b).
In the present study, we examined the distribution of VGluT1 and VGluT2 individually in CTb-labeled corneal afferents. We were unable to examine the co-localization of VGluT1 and VGluT2 in CTb-labeled corneal afferents since the antibodies for both markers were made in the same species. We found that within thinly myelinated corneal afferents, VGluT1 and VGluT2 were both more abundant in rostrally-projecting afferents as compared to caudally-projecting afferents, supporting the notion that these afferents may underlie different functions in different regions of Vc. However, even assuming VGluT1 and VGluT2 are contained in non-overlapping populations of corneal afferents, our combined percentage of afferents that contained one of the VGluTs is 61%. Thus, there is a portion of corneal afferents in which we were not able to detect one of these transporters. This maybe be due to the conservative nature of our analysis, or it may indicate the existence of corneal afferents that do not contain one of the transporters, and thus suggests these afferents may not release glutamate as a transmitter. Overall, our findings do show that there are clear anatomical distinctions between corneal afferents projecting to the caudal versus rostral regions of Vc.
In the triple-labeling studies, we analyzed the co-localization of CGRP and VGluT2 in CTb-labeled corneal afferents in order to determine whether these afferents were both peptidergic and glutamatergic and if there were any differences in the distribution of these afferents in Vc. Previous studies have suggested that glutamate and neuropeptides are released from the same primary afferent terminals (De Biasi S. and Rustioni, 1988). However, we found that very few corneal afferents contain both VGluT2 and CGRP, supporting studies in the mouse (Morris et al., 2005) and rat (Todd et al., 2003; Landry et al., 2004) spinal cord dorsal horn which demonstrated that primary sensory afferents may be peptidergic or glutamatergic but are rarely both. One interpretation of this finding is that these primary sensory afferents must contain a different vesicular glutamate transporter (Todd et al., 2003) or an alternative way to store and release glutamate (Keast and Stephensen, 2000; Morris et al., 2005). An alternative interpretation is that the vesicular glutamate transporter and exocytotic proteins required for glutamate storage and release from peptidergic primary sensory afferents are not present or at detectable levels in these afferents under normal, non-pathological conditions (Morris et al., 2005).
Methodological considerations
Numerous studies have successfully labeled neurons in the trigeminal ganglion that project to the cornea using a variety of tract tracers and viruses (LaVail et al., 1993; Nakamura et al., 2007), but few studies have successfully traced corneal afferent terminals transganglionically to the brainstem Tract tracing of corneal afferent fibers to ventrolateral Vc/C1 and Vi/Vc has been reported using HRP as a tracer (Marfurt and Del Toro, 1987). In the present studies we describe a method to label corneal afferents that is compatible with both confocal and electron microscopic analyses. We use a brief application of 1-heptanol to produce a corneal abrasion of uniform depth, only removing the tear film and outer epithelial layer (De Felipe et al., 1999), a treatment that does not induce immediate early gene expression in Vc, suggesting that it does not produce overt injury (De Felipe and Belmonte, 1999). It has been suggested that removal and replacement of the outer epithelial layers of the cornea represents normal wear of this tissue, as seen during daily exposure to the environment and abrasion by blinking. We found that the 1-heptanol application also produces more consistent tracer labeling than mechanical abrasion.
Similar to the previous studies using HRP, we found that cholera toxin subunit B (CTb) consistently produce labeling at two distinct regions of trigeminal dorsal horn following corneal application. CTb consistently labeled afferents to both rostral and caudal regions of Vc, but the neurochemical phenotype of these afferents is quite distinct. Previous studies of trigeminal ganglion cells suggested some heterogeneity between corneal-projecting neurons that might underlie differences in responses to different stimuli or that might underlie different functions, ranging from sensory perception to supporting renewal of the epithelium (LaVail et al., 1993). Our anatomical findings also emphasize dramatic differences based on where these fibers project in the brainstem which may be related to their function as well. We recognize that immunocytochemical methods may not label every structure that contains the antigen or antigens of interest, potentially leading to an underestimation of the co-localization of the markers in this study. We also recognize that CTb may not label the entire population of nociceptive corneal afferent fibers. For example, we would suspect that isolectin B4 (IB4) might be a useful tracer as this is present in many populations of nociceptive fibers and we have used it successfully to label unmyelinated afferent fibers in other brain regions. However, we were unable to produce labeling of corneal afferents using IB4. Interestingly, this finding is consisent with a previous study showing that IB4 was not present in trigeminal ganglia traced from the cornea using FluoroGold (LaVail et al., 1993).
Acknowledgments
Support: This work was supported by grants from the National Institutes of Health: R01 DE056301 (SAA); T32 NS045553 (DMH); and a shared instrumentation grant, RR016858. KT was support by the OHSU/NSI summer internship program, and HH was supported by the Saturday Academy Apprenticeships in Science and Engineering program.
Other acknowledgements: The authors are grateful to Jessica L. Martin for her assistance in refining the corneal afferent labeling methods and Kelsey Whittier for technical assistance.
LITERATURE CITED
- Aicher SA, Mitchell JL, Swanson KC, Zadina JE. Endomorphin-2 axon terminals contact mu-opioid receptor-containing dendrites in trigeminal dorsal horn. Brain Res. 2003;977(2):190–8. doi: 10.1016/s0006-8993(03)02678-7. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF. N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor antagonism reduces Fos-like immunoreactivity in central trigeminal neurons after corneal stimulation in the rat. Neuroscience. 1996;73(1):249–58. doi: 10.1016/0306-4522(96)00038-3. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Bereiter DF, Tonnessen BH, Maclean DB. Selective blockade of substance P or neurokinin A receptors reduces the expression of c-fos in trigeminal subnucleus caudalis after corneal stimulation in the rat. Neuroscience. 1998;83(2):525–34. doi: 10.1016/s0306-4522(97)00433-8. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88(3):221–4. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Broman J, Anderson S, Ottersen OP. Enrichment of glutamate-like immunoreactivity in primary afferent terminals throughout the spinal cord dorsal horn. Eur J Neurosci. 1993;5(8):1050–61. doi: 10.1111/j.1460-9568.1993.tb00958.x. [DOI] [PubMed] [Google Scholar]
- Corbett EK, Sinfield JK, McWilliam PN, Deuchars J, Batten TF. Differential expression of vesicular glutamate transporters by vagal afferent terminals in rat nucleus of the solitary tract: projections from the heart preferentially express vesicular glutamate transporter 1. Neuroscience. 2005;135(1):133–45. doi: 10.1016/j.neuroscience.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Galfre G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979;76(7):3532–6. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi S, Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc Natl Acad Sci U S A. 1988;85(20):7820–4. doi: 10.1073/pnas.85.20.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe C, Belmonte C. c-Jun expression after axotomy of corneal trigeminal ganglion neurons is dependent on the site of injury. Eur J Neurosci. 1999;11(3):899–906. doi: 10.1046/j.1460-9568.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Gonzalez GG, Gallar J, Belmonte C. Quantification and immunocytochemical characteristics of trigeminal ganglion neurons projecting to the cornea: effect of corneal wounding. Eur J Pain. 1999;3(1):31–9. doi: 10.1053/eujp.1998.0100. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Hioki H, Tomioka R, Taki K, Tamamaki N, Nomura S, Okamoto K, Kaneko T. Changes of immunocytochemical localization of vesicular glutamate transporters in the rat visual system after the retinofugal denervation. J Comp Neurol. 2003;465(2):234–49. doi: 10.1002/cne.10848. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El MS. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22(13):5442–51. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007;179(4):691–702. doi: 10.1007/s00221-006-0826-7. [DOI] [PubMed] [Google Scholar]
- Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. 2004;24(17):4224–32. doi: 10.1523/JNEUROSCI.0381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR, Stephensen TM. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424(4):577–87. [PubMed] [Google Scholar]
- Lachamp P, Crest M, Kessler JP. Vesicular glutamate transporters type 1 and 2 expression in axon terminals of the rat nucleus of the solitary tract. Neuroscience. 2006;137(1):73–81. doi: 10.1016/j.neuroscience.2005.08.048. [DOI] [PubMed] [Google Scholar]
- LaMotte CC, Kapadia SE, Shapiro CM. Central projections of the sciatic, saphenous, median, and ulnar nerves of the rat demonstrated by transganglionic transport of choleragenoid-HRP (B-HRP) and wheat germ agglutinin-HRP (WGA-HRP) J Comp Neurol. 1991;311(4):546–62. doi: 10.1002/cne.903110409. [DOI] [PubMed] [Google Scholar]
- Landry M, Bouali-Benazzouz R, El MS, Ravassard P, Nagy F. Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J Comp Neurol. 2004;468(3):380–94. doi: 10.1002/cne.10988. [DOI] [PubMed] [Google Scholar]
- LaVail JH, Johnson WE, Spencer LC. Immunohistochemical identification of trigeminal ganglion neurons that innervate the mouse cornea: relevance to intercellular spread of herpes simplex virus. J Comp Neurol. 1993;327(1):133–40. doi: 10.1002/cne.903270111. [DOI] [PubMed] [Google Scholar]
- Li JL, Fujiyama F, Kaneko T, Mizuno N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J Comp Neurol. 2003a;457(3):236–49. doi: 10.1002/cne.10556. [DOI] [PubMed] [Google Scholar]
- Li JL, Xiong KH, Dong YL, Fujiyama F, Kaneko T, Mizuno N. Vesicular glutamate transporters, VGluT1 and VGluT2, in the trigeminal ganglion neurons of the rat, with special reference to coexpression. J Comp Neurol. 2003b;463(2):212–20. doi: 10.1002/cne.10755. [DOI] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters (VGLUTs): the three musketeers of glutamatergic system. Acta Neurobiol Exp (Wars ) 2007;67(3):207–18. doi: 10.55782/ane-2007-1649. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Pilowsky P, Minson JB, Chalmers J. Synapses on axons of sympathetic preganglionic neurons in rat and rabbit thoracic spinal cord. J Comp Neurol. 1995;354(2):193–208. doi: 10.1002/cne.903540204. [DOI] [PubMed] [Google Scholar]
- MacIver MB, Tanelian DL. Structural and functional specialization of A delta and C fiber free nerve endings innervating rabbit corneal epithelium. J Neurosci. 1993;13(10):4511–24. doi: 10.1523/JNEUROSCI.13-10-04511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol. 1987;261(3):450–9. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- Martinez S, Belmonte C. C-Fos expression in trigeminal nucleus neurons after chemical irritation of the cornea: reduction by selective blockade of nociceptor chemosensitivity. Exp Brain Res. 1996;109(1):56–62. doi: 10.1007/BF00228626. [DOI] [PubMed] [Google Scholar]
- Melone M, Burette A, Weinberg RJ. Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J Comp Neurol. 2005;492(4):495–509. doi: 10.1002/cne.20743. [DOI] [PubMed] [Google Scholar]
- Meng ID, Bereiter DA. Differential distribution of Fos-like immunoreactivity in the spinal trigeminal nucleus after noxious and innocuous thermal and chemical stimulation of rat cornea. Neuroscience. 1996;72(1):243–54. doi: 10.1016/0306-4522(95)00541-2. [DOI] [PubMed] [Google Scholar]
- Mitchell JL, Silverman MB, Aicher SA. Rat trigeminal lamina I neurons that project to thalamic or parabrachial nuclei contain the mu-opioid receptor. Neuroscience. 2004;128(3):571–82. doi: 10.1016/j.neuroscience.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Morris JL, Konig P, Shimizu T, Jobling P, Gibbins IL. Most peptide-containing sensory neurons lack proteins for exocytotic release and vesicular transport of glutamate. J Comp Neurol. 2005;483(1):1–16. doi: 10.1002/cne.20399. [DOI] [PubMed] [Google Scholar]
- Murata Y, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006;1085(1):87–94. doi: 10.1016/j.brainres.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M, Mimura O. Morphological and immunohistochemical characterization of the trigeminal ganglion neurons innervating the cornea and upper eyelid of the rat. J Chem Neuroanat. 2007;34(3–4):95–101. doi: 10.1016/j.jchemneu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Elsevier/Academic Press; London: 1998. [Google Scholar]
- Ramer MS. Anatomical and functional characterization of neuropil in the gracile fasciculus. J Comp Neurol. 2008;510(3):283–96. doi: 10.1002/cne.21785. [DOI] [PubMed] [Google Scholar]
- Renick SE, Kleven DT, Chan J, Stenius K, Milner TA, Pickel VM, Fremeau RT., Jr The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain. J Neurosci. 1999;19(1):21–33. doi: 10.1523/JNEUROSCI.19-01-00021.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell SA, Wessendorf MW. Coexpression of the mu-opioid receptor splice variant MOR1C and the vesicular glutamate transporter 2 (VGLUT2) in rat central nervous system. J Comp Neurol. 2008;508(4):542–64. doi: 10.1002/cne.21712. [DOI] [PubMed] [Google Scholar]
- Silverman MB, Hermes SM, Zadina JE, Aicher SA. Mu-opioid receptor is present in dendritic targets of Endomorphin-2 axon terminals in the nuclei of the solitary tract. Neuroscience. 2005;135(3):887–96. doi: 10.1016/j.neuroscience.2005.06.072. [DOI] [PubMed] [Google Scholar]
- Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55(4):343–51. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17(1):13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol. 2006;96(6):3465–73. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]