Abstract
OBJECTIVES:
The aim of this study was to examine the expression of the N-myc downstream-regulated gene 1 protein in benign and malignant lesions of the thyroid gland by immunohistochemistry.
INTRODUCTION:
N-myc downstream-regulated gene 1 encodes a protein whose expression is induced by various stimuli, including cell differentiation, exposure to heavy metals, hypoxia, and DNA damage. Increased N-myc downstream-regulated gene 1 expression has been detected in various types of tumors, but the role of N-myc downstream-regulated gene 1 expression in thyroid lesions remains to be determined.
METHODS:
A tissue microarray paraffin block containing 265 tissue fragments corresponding to normal thyroid, nodular goiter, follicular adenoma, papillary thyroid carcinoma (classical pattern and follicular variant), follicular carcinoma, and metastases of papillary and follicular thyroid carcinomas were analyzed by immunohistochemistry using a polyclonal anti- N-myc downstream-regulated gene 1 antibody.
RESULTS:
The immunohistochemical expression of N-myc downstream-regulated gene 1 was higher in carcinomas compared to normal thyroid glands and nodular goiters, with higher expression in classical papillary thyroid carcinomas and metastases of thyroid carcinomas (P < 0.001). A combined analysis showed higher immunohistochemical expression of NDRG1 in malignant lesions (classical pattern and follicular variant of papillary thyroid carcinomas, follicular carcinomas, and metastases of thyroid carcinomas) compared to benign thyroid lesions (goiter and follicular adenomas) (P = 0.043). In thyroid carcinomas, N-myc downstream-regulated gene 1 expression was significantly correlated with a more advanced TNM stage (P = 0.007) and age, metastasis, tumor extent, and size (AMES) high-risk group (P = 0.012).
CONCLUSIONS:
Thyroid carcinomas showed increased immunohistochemical N-myc downstream-regulated gene 1 expression compared to normal and benign thyroid lesions and is correlated with more advanced tumor stages.
Keywords: NDRG1, Thyroid Gland, Thyroid Carcinoma, Immunohistochemistry, Tissue Microarray
INTRODUCTION
Thyroid cancer is the most common malignant tumor of the endocrine system; approximately 37,200 new cases were expected to occur in the United States in 2009.1 In the city of São Paulo, Brazil, 1207 and 6004 cases of thyroid cancer in males and females, respectively, were identified from 1997 to 2003.2 According to some studies, the increase in the incidence of thyroid cancer can be explained by a higher frequency of early tumor detection due to the use of more sophisticated diagnostic techniques.3,4 Thyroid carcinomas that are derived from follicular epithelium can be classified into well-differentiated carcinomas (including papillary and follicular carcinomas), poorly differentiated carcinomas and undifferentiated (or anaplastic) carcinomas, based on well-established clinical findings and histological criteria.5 However, despite standardized criteria and nomenclature, variations exist in the histological classification of thyroid tumors among different observers. This disagreement is greater for encapsulated follicular thyroid tumors (adenoma, follicular carcinoma and some cases of the follicular variant of papillary carcinoma), in which the histological diagnosis is prone to subjective and discordant interpretations among pathologists.6-8 Histological findings may not be sufficient to establish a precise diagnosis and, consequently, to predict the clinical courses of these cases. Objective criteria and the identification of markers that permit better characterization of thyroid tumors are therefore required.9
N-myc downstream-regulated gene 1 (NDRG1) is a member of the NDRG gene family and encodes a 43-kD protein (NDRG1) with 394 amino acids.10 NDRG1 is located on human chromosome 8q24 and has been recognized as a gene whose mutation is associated with a demyelinating neuropathy called Charcot-Marie-Tooth disease, type 4D.11 NDRG1 is ubiquitously expressed in human tissues, and its messenger RNA has been detected in a variety of tissues, such as the digestive and respiratory tracts, liver, pancreas, kidneys, reproductive organs, placenta, skeletal muscle, heart, and brain.10,12 Immunohistochemical studies have shown that the NDRG1 protein is mainly expressed in the epithelial cells of different tissues, whereas no expression could be demonstrated in mesenchymal or endothelial cells.12 Other members of the NDRG family (NDRG2, NDRG3 and NDRG4) are homologous to the NDRG1 gene. However, in contrast to NDRG1, these genes most likely exert tissue-specific functions, as they are only expressed in certain types of tissues.13
NDRG1 protein expression is known to be induced by a variety of physiological and pathological stimuli. Expression of this protein is associated with cell growth arrest and terminal cell differentiation, as observed in squamous epithelial cells of the more superficial layers of the epidermis.12 During embryogenesis, NDRG1 expression is repressed by N-Myc and c-Myc during cell proliferation phases.14 Conversely, ligands such as vitamin D and retinoic acid, which bind to transcription factors involved in cell differentiation, inhibit growth and induce cell differentiation and NDRG1 expression.15 Other factors, such as an increase in intracellular calcium concentration, a reduction in glucose concentration, hypoxia, DNA damage and neoplasms, are also associated with NDRG1 expression.16 Heavy metals (such as nickel) induce cellular hypoxia and the expression of various genes that are responsive to hypoxia, including NDRG1.17,18
NDRG1 has also been characterized as a mediator of p53-dependent, anti-oncogenic functions. Stein et al. demonstrated that the p53-induced NDRG1 expression reduces cell proliferation in metastatic pulmonary cancer cells.19 However, the role of NDRG1 in tumor progression and the formation of metastases is controversial. Some studies have suggested that NDRG1 overexpression reduces the metastatic potential of tumor cells,20,21 whereas others have associated its increased expression with less differentiated or more metastatic aggressive tumors and a poor prognosis.22,23
In the present study, we analyzed the quantitative immunohistochemical expression of the NDRG1 protein in normal thyroid glands and benign and malignant thyroid lesions. We also investigated the correlation between NDRG1 protein expression and clinical-pathological variables in cases of primary thyroid carcinoma. To our knowledge, this is the first study to analyze NDRG1 protein expression in benign and malignant thyroid lesions.
MATERIALS AND METHODS
Samples
Paraffin blocks from 225 patients, including 62 cases of nodular goiter (NG), 53 cases of follicular adenoma (FA), 41 cases of classical papillary thyroid carcinoma (PTC), 31 cases of the follicular variant of papillary thyroid carcinoma (FV-PTC), and 36 cases of follicular carcinoma (FC) were selected from the archives of the Department of Pathology, University of São Paulo Medical School (comprising the period from 2000 to 2006) and the Department of Pathology, A. C. Camargo Hospital (comprising the period from 1984 to 2001). Histological diagnoses were made according to the classification of thyroid tumors proposed by the WHO.5 When available, paraffin blocks of lymph node metastases from thyroid carcinomas (M-TC) were also selected and included 17 cases of metastatic PTC, 5 cases of metastatic FV-PTC, and 1 case of metastatic FC. One case of bone metastasis from FC was also included in the study. The patient age ranged from 15 to 88 years (mean: 48.4 years old), and 184 (81.8%) patients were female.
Data regarding the TNM stage24 and age, metastasis, and extent and size (AMES) death risk criteria25 were obtained from the clinical records of patients with thyroid carcinoma (PTC, FV-PTC and FC). The median time of clinical follow-up and the interval for recurrence for patients with thyroid carcinoma was 53.5 months (range: 1 to 175 months) and 18.0 months (range: 2 to 48 months), respectively. No clinical follow-up data were available for eight patients (six with FC, one with PTC and one with FV-PTC). During this period, 16 cases of recurrence (4 followed by death) and 4 cases of death due to disseminated metastatic disease were observed. All deaths occurred in the group of patients with FC, and four patients were alive but still had diseases (two with PTC and two with FC).
This study was approved by the Ethics Committee for the Analysis of Research Projects of the University of São Paulo Medical School and the Research Ethics Committee of A. C. Camargo Hospital.
Construction of the tissue microarray
Slides were prepared for the tissue microarray (TMA) from the selected blocks, stained with hematoxylin-eosin and examined under a binocular light microscope (Nikon, Eclipse E100) for the selection of representative areas of the lesion. The areas of interest in the slides were stained with Indian ink and compared to the corresponding areas in the paraffin blocks, which were also stained with Indian ink.
Using a tissue microarrayer (Beecher Instruments, Silver Springs, MD, USA), the areas of interest in each block were punctured with a 1-mm diameter needle, and cylindrical fragments were transferred in an ordered fashion to a recipient paraffin block (TMA block), as described previously.26 For the detailed identification of each cylindrical fragment, a map was constructed in an Excel spreadsheet to permit the exact localization of each case. The TMA block contained 265 thyroid cases (one core per case), corresponding to fragments of normal thyroid tissue (NT, n = 18), NG (n = 62), FA (n = 53), PTC (n = 41), FV-PTC (n = 31), FC (n = 36), and M-TC (n = 24, including 17 fragments of PTC metastases, 5 FV-PTC metastases, and 2 FC metastases).
A total of 120 histological 5-µm-thick sections were cut from each TMA block. The slides were covered with a layer of paraffin to prevent oxidation and stored in a freezer at −20°C. Two slides from different levels of the TMA block were used in this study.
Immunohistochemistry
Slides were left overnight in an oven at 56°C, followed by deparaffinization in xylene, graded alcohol, tap water and distilled water. Heat-induced antigen retrieval was performed using a domestic pressure cooker (Nigro, model Eterna 41/2 L, Brazil) with a boiled 0.01 M sodium citrate buffered solution (pH 6.0) for 4 min and cooled under running water, as described previously.27 Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 20 min. The antibody used was purchased from Santa Cruz Biotechnology (NDRG1 N-19, catalog sc-19464, Santa Cruz, CA, USA). The optimal dilution was defined using a well-known positive sample that was tested before it was used on TMA sections. The antibody was diluted 1∶1000 in 0.01 M phosphate-buffered saline (PBS), pH 7.4, with 0.1% sodium azide (Sigma, catalog S8032, St. Louis, MO, USA) and 1% bovine serum albumin (Sigma, catalog A9647, USA) to reduce background staining and incubated overnight at 4°C in a moist chamber. The staining procedure was performed using the labeled streptavidin-biotin method (LSAB+ System-HRP, DakoCytomation, catalog K0690, Carpinteria, CA, USA).28 Slides were incubated for 30 min at 37°C with the Link, washed with PBS and incubated for another 30 min at 37°C with streptavidin. The slides were developed with 60 mg 3,3′-diaminobenzidine tetrahydrochloride (Sigma, catalog D5637, St Louis, MO, USA), 1% dimethyl sulfoxide (Sigma, catalog D5879, St. Louis, MO, USA) and 0.06% hydrogen peroxide in PBS for 5 min and counterstained with Harris's hematoxylin, dehydrated and mounted with Entellan neu (Merck, catalog 1.07961, Damstadt, Germany). A sample of FC was used as a positive control. A negative control was performed by omitting the primary antibody.
Quantitative immunohistochemical evaluation
Cytoplasmic NDRG1 protein expression was analyzed quantitatively using a computer image capture system (Automated Cellular Imaging System - ACIS® III, K0690; Dako). The two slides were first scanned to capture and digitalization the image. Next, parameters were established regarding the staining intensity of NDRG1-immunostained thyroid follicular cells and unstained stromal (fibroblasts and endothelial cells) cells (“blue area”). Between two and five circular areas per core were analyzed for each of the 265 cases in both slides. First, we calculated the integrated optical density (IOD), which is related to the amount (density) of the antigen.29 A numerical value corresponding to the IOD for each circular area was obtained by multiplying the staining intensity by the area of positive staining (“brown area”). Then, the IOD was normalized to the tissue area: the numerical IOD value was divided by the total circular area (sum of the positively stained “brown area” and unstained “blue area”).30 For each core, the final numerical value corresponded to the mean value of the corrected IODs of the two to five circular areas analyzed. Cores presenting more than 50% tissue loss were excluded from the analysis. Finally, the mean of the final numerical values obtained for the cores present in each of the two slides was calculated for each case.
Statistical analysis
Box plots were constructed for the different thyroid lesions using the results of the quantitative analysis of NDRG1 immunohistochemical expression. These groups were compared using the Kruskal-Wallis and Mann-Whitney tests. For cases with a thyroid carcinoma diagnosis (PTC, FV-PTC or FC), the association between the quantitative immunohistochemical expression of NDRG1 and the clinical-pathological variables (age, tumor size, TNM stage and AMES risk classification) was determined using a Student's t-test. A P-value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software, version 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
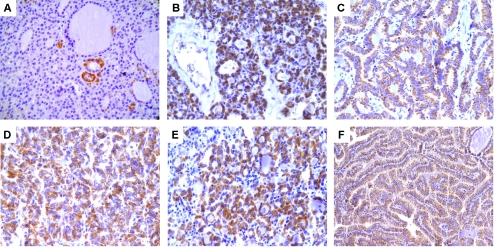
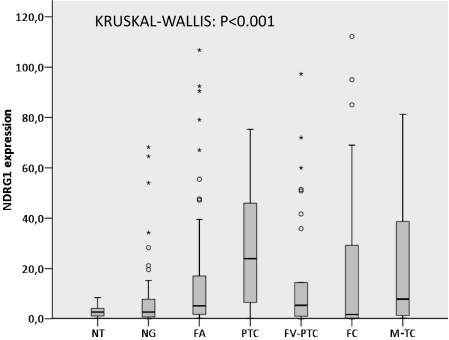
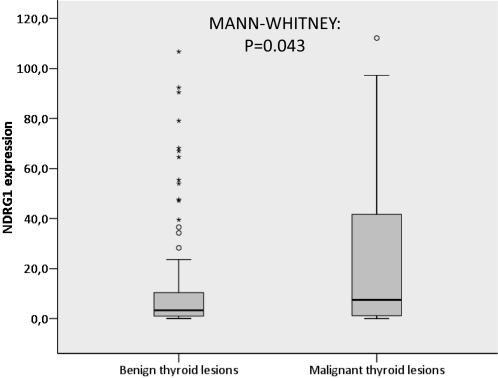
NDRG1 protein expression was observed in epithelial (follicular) cells, with no staining in stromal or endothelial cells. The positive cases showed moderate-to-strong cytoplasmic NDRG1 immunoreactivity (Figure 1). For each case with a thyroid lesion and NT, the numerical values obtained by the quantitative analysis of the immunohistochemical expression of NDRG1 are reported as means and standard deviations (Table 1). The quantitative expression of NDRG1 was higher in carcinomas compared to NTs and NGs, with higher expression levels in PTC and M-TC (P < 0.001) (Figure 2). When analyzed together, the quantitative expression of NDRG1 was higher in the group of malignant lesions (PTC, FV-PTC, FC, and M-TC) compared to the group of benign thyroid lesions (NG and AF) (P = 0.043) (Figure 3).
Figure 1.
Immunohistochemical expression of NDRG1 in thyroid lesions. A. Nodular goiter; original magnification, 400×. B. Follicular adenoma; original magnification, 400×. C. Classical papillary thyroid carcinoma; original magnification, 400×. D. Follicular variant of papillary thyroid carcinoma; original magnification, 400×. E. Follicular carcinoma; original magnification, 400×. F. Metastatic classical papillary thyroid carcinoma; original magnification, 200×.
Table 1.
Quantitative analysis of NDRG1 immunohistochemical expression in thyroid lesions.
| Diagnosis | N | Mean | Standard deviation |
| Normal thyroid | 15 | 3.13 | 2.67 |
| Nodular goiter | 55 | 8.33 | 14.89 |
| Follicular adenoma | 51 | 17.30 | 27.11 |
| Classical papillary thyroid carcinoma | 39 | 29.22 | 25.82 |
| Follicular variant of papillary carcinoma | 30 | 16.86 | 25.38 |
| Follicular carcinoma | 34 | 18.79 | 31.88 |
| Thyroid carcinoma metastases1 | 23 | 21.34 | 25.07 |
Eighteen cases were excluded from the analysis because of tissue loss (normal thyroid, three cases; nodular goiter, seven cases; follicular adenoma, two cases; classical papillary thyroid carcinoma, two cases; follicular variant of papillary thyroid carcinoma, one case; follicular carcinoma, two cases; and papillary thyroid carcinoma metastases, one case).
Includes 16 cases of classical papillary thyroid carcinoma metastasis, 5 of follicular variant of papillary thyroid carcinoma, and 2 of follicular carcinoma.
Kruskal-Wallis test, P < 0.001.
Figure 2.
NDRG1 expression in normal, benign and malignant thyroid gland lesions. Box plot shows the data of the quantitative immunohistochemical expression of NDRG1 for normal thyroid (NT), nodular goiter (NG), follicular adenoma (FA), classical papillary thyroid carcinoma (PTC), follicular variant of papillary thyroid carcinoma (FV-PTC), follicular carcinoma (FC), and metastases of thyroid carcinoma (M-TC) (P < 0.001, Kruskal-Wallis test). The “o” and “*” symbols indicate outlier and extreme outlier values, respectively.
Figure 3.
NDRG1 expression in benign and malignant thyroid lesions. Box plot shows the data of the quantitative immunohistochemical expression of NDRG1 for benign (nodular goiter and follicular adenoma) and malignant (classical papillary thyroid carcinoma, follicular variant of papillary thyroid carcinoma, follicular carcinoma, and metastases of thyroid carcinoma) thyroid lesions (P = 0.043, Mann-Whitney test). The “o” and “*” symbols indicate outlier and extreme outlier values, respectively.
The association between the quantitative immunohistochemical expression of NDRG1 and the clinical-pathological variables was analyzed in cases of primary thyroid carcinoma (PTC, FV-PTC and FC). In this group of tumors, the quantitative expression of NDRG1 was significantly higher in patients who were aged 45 years or older (P = 0.019), at an advanced TNM stage (stages III and IV) (P = 0.007), and in an AMES high-risk group (P = 0.012) (Table 2).
Table 2.
NDRG1 protein expression and clinical-pathological variables in patients with thyroid carcinoma1.
| Variable | N | NDRG1 (mean) | Standard deviation | P |
| Age | ||||
| <45 years | 38 | 13.7 | 23.4 | |
| ≥45 years | 62 | 27.3 | 29.7 | 0.019 |
| Tumor size | ||||
| T1-T22 | 41 | 17.0 | 25.4 | |
| T3-T43 | 53 | 27.5 | 30.1 | 0.076 |
| TNM stage | ||||
| Stages I and II | 51 | 17.7 | 26.9 | |
| Stages III and IV | 36 | 34.3 | 29.0 | 0.007 |
| AMES classification | ||||
| Low risk | 56 | 18.3 | 26.3 | |
| High risk | 33 | 34.0 | 30.2 | 0.012 |
Classical papillary thyroid carcinoma, follicular variant of papillary thyroid carcinoma and follicular carcinoma.
T1 refers to tumors with a largest dimension of 2 cm or less, and T2 refers to tumors with a largest dimension of more than 2 cm but no more than 4 cm.
T3 refers to tumors with a largest dimension of more than 4 cm, confined to the thyroid gland or any tumor with minimal extrathyroid extension, and T4 refers to tumors that extend beyond the thyroid capsule.
DISCUSSION
To our knowledge, this is the first study to analyze the immunohistochemical expression of the NDRG1 protein in a large number of benign (NG and FA) and malignant (PTC, FV-PTC, FC, and M-TC) thyroid lesions. We demonstrated increased expression of NDRG1 in thyroid tumors compared to NGs and NTs. Cangul also studied the immunohistochemical expression of NDRG1 in a variety of human tumors (including breast, kidney, lung and prostate carcinomas, and melanoma and glioblastoma multiforme) and reported higher NDRG1 protein expression in tumors compared to normal tissue.18 In the colon, Wang et al. observed increased NDRG1 expression in carcinomas compared to adenomas and normal colon mucosa.31 Reis et al. analyzed 213,636 transcripts that were derived from normal and tumor tissues of the oral cavity, larynx, pharynx and thyroid using the open reading frame expressed sequence tags (ORESTES) technique. Three (ZRF1, RAP140 and NDRG1) of the 250 potential markers selected in that study were analyzed by quantitative RT-PCR in oral cavity and laryngeal samples. The authors detected an increase in the levels of NDRG1 mRNA in 50% of oral cavity tumors.32 In contrast, Bandyopadhyay et al. observed lower NDRG1 immunohistochemical expression in breast carcinoma but strong expression in normal mammary lobules.33
The role of the NDRG1 protein in tumor progression and metastasis formation is still controversial and is likely to be tissue-specific. Several studies have shown that a decrease in NDRG1 protein expression is associated with a more advanced tumor stage and lower survival rates. In prostate carcinomas, lower immunohistochemical expression of NDRG1 was associated with a Gleason grade >7 and poorly differentiated tumors. According to the authors, 70% of localized prostate tumors expressed NDRG1, whereas only 25% of metastatic tumors were positive for this marker.21 In breast cancer, 60% of patients with bone metastases presented with reduced NDRG1 expression, with survival being lower in patients whose tumors did not express NDRG1.33 In esophageal squamous cell carcinoma, reduced NDRG1 expression was associated with more invasive tumors and a more advanced TNM stage. In addition, a lower survival rate was observed in patients with low NDRG1 expression compared to those with high expression.34 A less differentiated histological grade, more advanced pathological stage and lower overall survival were correlated with reduced immunohistochemical expression of NDRG1 in pancreatic ductal adenocarcinoma.35
We found that increased immunohistochemical expression of NDRG1 was correlated with a more advanced TNM stage (stages III and IV) and an AMES high-risk category in patients with thyroid carcinoma (PTC, FV-PTC, and FC). An association between increased NDRG1 expression and more aggressive tumors and poor prognosis has been reported in other studies. Chua et al. observed that increased NDRG1 expression in hepatocellular carcinoma was associated with a more advanced tumor stage (stages III and IV), larger tumors and poorly differentiated tumors, according to the Edmondson-Steiner histological classification. In their study, patients whose tumors presented increased NDRG1 protein expression showed lower overall survival compared to those with low NDRG1 levels.22 In cervical adenocarcinoma, increased immunohistochemical expression of NDRG1 was correlated with a larger tumor diameter, greater depth of stromal invasion, the presence of angiolymphatic invasion, a larger number of lymph node metastases, poorly or moderately differentiated tumors and poor prognosis, as demonstrated by lower progression-free and overall survival rates.23
Some studies have suggested that NDRG1 is a metastasis suppressor gene. Guan et al. observed lower NDRG1 expression in cell lines derived from colon cancer metastases compared to cell lines derived from primary colon cancer. Inducing NDRG1 expression in metastatic cell lines, the authors observed that neoplastic cells expressed markers related to cell differentiation of the colon epithelium, such as CEA and E-cadherin.20 In vivo studies have shown that cell lines derived from colon and prostate cancers that were transfected with NDRG1 and injected into mice produced a smaller number of hepatic and pulmonary metastases, respectively, than control lines.20,21 However, in human colorectal carcinoma samples, Wang et al. demonstrated increased NDRG1 protein expression in primary tumors with metastases to the lymph nodes compared to those without metastases (non-metastatic carcinoma). The authors concluded that higher NDRG1 expression was associated with a higher probability of lymph node metastasis. Within this context, according to the authors, NDRG1 could be a gene that is involved in tumor progression and the promotion of metastases of colorectal carcinoma.31
CONCLUSIONS
The present study demonstrated an increase in the immunohistochemical expression of NDRG1 in primary and metastatic thyroid carcinomas compared to NT, NG, and FA. We also observed an association between NDRG1 expression and a more advanced TNM stage and an AMES high-risk category in patients with thyroid carcinoma (PTC, FV-PTC, and FC). These findings suggest a role for NDRG1 in thyroid tumor progression. More detailed studies are necessary to better define the role of NDRG1 in tumorigenesis and thyroid tumor progression, including the investigation of this protein in poorly differentiated or anaplastic thyroid carcinomas.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. 10.3322/CA.2007.0010 [DOI] [PubMed] [Google Scholar]
- 2.Mirra AP, Latorre MRDO, Veneziano DB. Incidência de câncer no município de São Paulo, Brasil, 1997–2003. In: Mirra AP, Latorre MRDO, Veneziano DB, editors. Registro de Câncer de São Paulo; 2007. pp. 9–21. In: Incidência de câncer no município de São Paulo, Brasil, 1997–2003; Tendência no período 1969–2003; Mortalidade por câncer no município de São Paulo, Brasil, 1997–2003. [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. 10.1001/jama.295.18.2164 [DOI] [PubMed] [Google Scholar]
- 4.Kent WD, Hall SF, Isolato PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177:1357–61. doi: 10.1503/cmaj.061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLellis RA, Williams ED. Thyroid and parathyroid tumors: introduction. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press; 2004. pp. 51–6. [Google Scholar]
- 6.Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002;26:1508–14. doi: 10.1097/00000478-200211000-00014. 10.1097/00000478-200211000-00014 [DOI] [PubMed] [Google Scholar]
- 7.Franc B, de la Salmonière P, Lange F, Hoang C, Louvel A, de Roquancourt A, et al. Interobserver and intraobserver reproducibility in the histopathology of follicular thyroid carcinoma. Hum Pathol. 2003;34:1092–100. doi: 10.1016/s0046-8177(03)00403-9. 10.1016/S0046-8177(03)00403-9 [DOI] [PubMed] [Google Scholar]
- 8.Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336–40. doi: 10.1097/01.pas.0000135519.34847.f6. 10.1097/01.pas.0000135519.34847.f6 [DOI] [PubMed] [Google Scholar]
- 9.Asa SL. The role of immunohistochemical markers in the diagnosis of follicular-patterned lesions of the thyroid. Endocr Pathol. 2005;16:295–309. doi: 10.1385/ep:16:4:295. 10.1385/EP:16:4:295 [DOI] [PubMed] [Google Scholar]
- 10.Kokame K, Kato H, Miyata T. Homocysteine-respondent genes in vascular endothelial cells identified by differential display analysis. J Biol Chem. 1996;271:29659–65. doi: 10.1074/jbc.271.47.29659. 10.1074/jbc.271.47.29659 [DOI] [PubMed] [Google Scholar]
- 11.Kalaydjieva L, Hallmayer J, Chandler D, Savov A, Nikolova A, Angelicheva D, et al. Gene mapping in Gypsies identifies a novel demyelinating neuropathy on chromosome 8q24. Nat Genet. 1996;14:214–17. doi: 10.1038/ng1096-214. 10.1038/ng1096-214 [DOI] [PubMed] [Google Scholar]
- 12.Lachat P, Shaw P, Gebhard S, van Belzen N, Chaubert P, Bosman FT. Expression of NDRG1, a differentiation-related gene, in human tissues. Histochem Cell Biol. 2002;118:399–408. doi: 10.1007/s00418-002-0460-9. 10.1007/s00418-002-0460-9 [DOI] [PubMed] [Google Scholar]
- 13.Qu X, Zhai Y, Wei H, Zhang C, Xing G, Yu Y, et al. Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol Cell Biochem. 2002;229:35–44. doi: 10.1023/a:1017934810825. 10.1023/A:1017934810825 [DOI] [PubMed] [Google Scholar]
- 14.Shimono A, Okuda T, Kondoh H. N-myc-dependent repression of ndr1, a gene identified by direct subtraction of whole mouse embryo cDNAs between wild type and N-myc mutant. Mech Dev. 1999;83:39–52. doi: 10.1016/s0925-4773(99)00025-8. 10.1016/S0925-4773(99)00025-8 [DOI] [PubMed] [Google Scholar]
- 15.Piquemal D, Joulia D, Balaguer P, Basset A, Marti J, Commes T. Differential expression of the RTP/Drg/Ndr1 gene product in proliferating and growth arrested cells. Biochim Biophys Acta. 1999;1450:364–73. doi: 10.1016/s0167-4889(99)00056-7. 10.1016/S0167-4889(99)00056-7 [DOI] [PubMed] [Google Scholar]
- 16.Ellen TP, Ke Q, Zhang P, Costa M. NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis. 2008;29:2–8. doi: 10.1093/carcin/bgm200. 10.1093/carcin/bgm200 [DOI] [PubMed] [Google Scholar]
- 17.Salnikow K, An WG, Melillo G, Blagosklonny MK, Costa M. Nickel-induced transformation shifts the balance between HIF-1 and p53 transcription factors. Carcinogenesis. 1999;20:1819–23. doi: 10.1093/carcin/20.9.1819. 10.1093/carcin/20.9.1819 [DOI] [PubMed] [Google Scholar]
- 18.Cangul H. Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC Genet. 2004;5:27. doi: 10.1186/1471-2156-5-27. 10.1186/1471-2156-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein S, Thomas EK, Herzog B, Westfall MD, Rocheleau V, Jackson RS, II, et al. NDRG1 is necessary for p53-dependent apoptosis. J Biol Chem. 2004;279:48930–40. doi: 10.1074/jbc.M400386200. 10.1074/jbc.M400386200 [DOI] [PubMed] [Google Scholar]
- 20.Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM, Pardee AB. DRG-1 as a differentiation-related, putative metastatic suppressor gene in human colon cancer. Cancer Res. 2000;60:749–55. [PubMed] [Google Scholar]
- 21.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 2003;63:1731–6. [PubMed] [Google Scholar]
- 22.Chua M-S, Sun H, Cheung ST, Mason V, Higgins J, Ross DT, et al. Overexpression of NDRG1 is an indicator of poor prognosis in hepatocellular carcinoma. Mod Pathol. 2007;20:76–83. doi: 10.1038/modpathol.3800711. 10.1038/modpathol.3800711 [DOI] [PubMed] [Google Scholar]
- 23.Nishio S, Ushijima K, Tsuda N, Takemoto S, Kawano K, Yamaguchi T, et al. Cap43/NDRG1/Drg-1 is a molecular target for angiogenesis and a prognostic indicator in cervical adenocarcinoma. Cancer Lett. 2008;264:36–43. doi: 10.1016/j.canlet.2008.01.020. 10.1016/j.canlet.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 24.Sobin LH, Wittekind Ch. TNM classification of malignant tumours. New York: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 25.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 26.Simon R, Mirlacher M, Sauter G. Tissue microarrays in cancer diagnosis. Expert Rev Mol Diagn. 2003;3:421–30. doi: 10.1586/14737159.3.4.421. 10.1586/14737159.3.4.421 [DOI] [PubMed] [Google Scholar]
- 27.Norton AJ, Jordan S, Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994;173:371–9. doi: 10.1002/path.1711730413. 10.1002/path.1711730413 [DOI] [PubMed] [Google Scholar]
- 28.Guesdon JL, Ternynck T, Avrameas S. The use of avidin-biotin interaction in immunoenzimatic techniques. J Histochem Cytochem. 1979;27:1131–9. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- 29.Kraan MC, Haringman JJ, Ahern MJ, Breedveld FC, Smith MD, Tak PP. Quantification of the cell infiltrate in synovial tissue by digital image analysis. Rheumatology. 2000;39:43–9. doi: 10.1093/rheumatology/39.1.43. 10.1093/rheumatology/39.1.43 [DOI] [PubMed] [Google Scholar]
- 30.Wicht H, Maronde E, Olcese J, Korf H-W. A semiquantitative image-analytical method for the recovering of dose-response curves in immunocytochemical preparations. J Histochem Cytochem. 1999;47:411–9. doi: 10.1177/002215549904700315. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Wang F, Wang W-Q, Gao Q, Wei W-L, Yang Y, et al. Correlation of N-myc downstream-related gene 1 overexpression with progressive growth of colorectal neoplasm. World J Gastroenterol. 2004;10:550–4. doi: 10.3748/wjg.v10.i4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis EM, Ojopi EP, Alberto FL, Rahal P, Tsukumo F, Mancini UM, et al. Large-scale transcriptome analyses reveal new genetic marker candidates of head, neck and thyroid cancer. Cancer Res. 2005;65:1693–9. doi: 10.1158/0008-5472.CAN-04-3506. 10.1158/0008-5472.CAN-04-3506 [DOI] [PubMed] [Google Scholar]
- 33.Bandyopadhyay S, Pai SK, Hirota S, Hosobe S, Takano Y, Saito K, et al. Role of the putative tumor metastasis suppressor gene Drg-1 in breast cancer progression. Oncogene. 2004;23:5675–81. doi: 10.1038/sj.onc.1207734. 10.1038/sj.onc.1207734 [DOI] [PubMed] [Google Scholar]
- 34.Ando T, Ishiguro H, Kimura M, Mitsui A, Kurehara H, Sugito N, et al. Decreased expression of NDRG1 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2006;19:454–8. doi: 10.1111/j.1442-2050.2006.00618.x. 10.1111/j.1442-2050.2006.00618.x [DOI] [PubMed] [Google Scholar]
- 35.Maruyama Y, Ono M, Kawahara A, Yokoyama T, Basaki Y, Kage M, et al. Tumor growth suppression in pancreatic cancer by a putative metastasis suppressor gene Cap43/NDRG1/Drg-1 through modulation of angiogenesis. Cancer Res. 2006;66:6233–42. doi: 10.1158/0008-5472.CAN-06-0183. 10.1158/0008-5472.CAN-06-0183 [DOI] [PubMed] [Google Scholar]