Abstract
Background
A high incidence of heart disease, especially idiopathic cardiomyopathy, is seen in chimpanzees (Pan troglodytes).
Methods
We reviewed clinical records and necropsy reports of 87 adult chimpanzees for possible causes of heart disease/idiopathic cardiomyopathy. We examined age, sex, cause of death, weight, diet, environment, infectious diseases, experimental uses, and clinical pathology.
Results
The overall prevalence of heart disease in chimpanzees was 67.81%; the prevalence of idiopathic cardiomyopathy was 51.72%. The prevalence of idiopathic cardiomyopathy was significantly higher in males (60.32%) than females (29.17%, p=0.009). The prevalence of other heart disease was higher in females (25%) than males (12.70%, p=0.165). Heart failure occurred in 47.13% of chimpanzees. Heart disease was the primary cause of death in 34.49% of chimpanzees; 29.88% died of unknown causes.
Conclusions
We found no evidence that diet, environment, viral agents, experimental use or disease exposure contributed to the deaths resulting from idiopathic cardiomyopathy in chimpanzees.
Keywords: ape, cardiomyopathy, atherosclerosis, arteriosclerosis, nonhuman primate
Introduction
Heart disease is the leading cause of death in the United States, and kills over 650,000 people annually [4, 26]. Cardiovascular diseases such as idiopathic cardiomyopathy, congestive heart failure, arteriosclerosis, and atherosclerosis have been documented in chimpanzees (Pan troglodytes) [8, 10, 17, 20] and gorillas[17, 22].
Idiopathic cardiomyopathy is of particular interest due to the high prevalence in chimpanzees [10]. Idiopathic dilated cardiomyopathy is the most common form of idiopathic cardiomyopathy in humans. It causes decreased ejection fraction, dilated chambers, and cardiomegaly, as well as fibrosis and hypertrophy of cardiac cells [21]. The first report of idiopathic cardiomyopathy in a captive chimpanzee was published in 1984 [8], and idiopathic cardiomyopathy has been identified with increasing frequency in our colony. Heart disease, with a high percentage of idiopathic cardiomyopathy, was the leading cause of death in chimpanzees at the Southwest National Primate Research Center (SNPRC) at the Southwest Foundation for Biomedical Research in San Antonio, Texas between 1982 and 1989 [10].
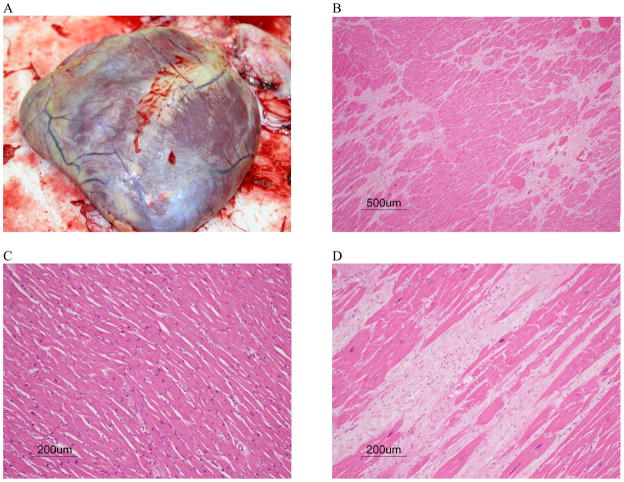
The first male chimpanzees diagnosed with idiopathic cardiomyopathy showed clinical signs of heart failure that included an abnormal electrocardiogram, and subcutaneous edema in the extremities. The necropsy and histology evaluations revealed hydropericardium, lung and spleen congestion, hemosiderosis in the lung and liver, as well as cardiomegaly, diffuse myocardial fibrosis, and atherosclerotic lesions of the coronary arteries [8, 21]. Figure 1 illustrates the gross and histological expression of idiopathic cardiomyopathy in chimpanzee heart tissue.
Figure 1. Gross and histological presentation of cardiomyopathy in the chimpanzee.
A, Cardiomyopathy. Note rounding of the heart and dilatation of the right ventricle; B and D, Heart, Cardiomyopathy. Note extensive fibrosis and degeneration of myofibers. H&E; C, Heart. Normal H&E.
Even though there is a high prevalence of idiopathic cardiomyopathy in chimpanzees, few studies have been conducted to determine the causes. In humans, cardiovascular disease is linked to age, sex, diet, cholesterol, exercise, viral infections, stress, and genetic predisposition [5, 13, 18, 19, 21]. Infection with Trypanosoma cruzi, a protozoan spread by reduviid bugs (family Reduviidae), also contributes to heart disease in humans and animals in the southern United States and Central and South America [2, 3]. T. cruzi infection causes left ventricular dysfunction in humans with symptoms similar to dilated cardiomyopathy [3].
Since the cause of spontaneous heart disease in these chimpanzees is unknown, our goal was to retrospectively examine potential contributing factors and causes, and to identify some factors that might predict heart disease/idiopathic cardiomyopathy in chimpanzees. Despite the decline in the number of chimpanzees available for biomedical research in recent years, the high prevalence of heart disease-related mortality in captive colonies makes chimpanzees a potentially valuable animal model for studying heart disease.
Materials and Methods
Animals
The chimpanzee colony at the SNPRC during this evaluation period, 1982–2006, had an average census of 225. The chimpanzees were used in biomedical research involving hepatitis B and C and human immunodeficiency virus (HIV), as well as research with monoclonal antibody therapies. The chimpanzees were all maintained under similar conditions in indoor/outdoor metal and concrete cages. Standard commercial monkey chow (Tekladc, PMI Nutrition International, LLC, Brentwood, MO 63144) was fed and water was available ad libitum. Fresh fruits and vegetables were fed daily. All procedures and care were approved by the Institutional Animal Care and Use Committee.
Pathology
The pathology and clinical records of 87 deceased chimpanzees were retrospectively reviewed for this study. Male chimpanzees reach sexual maturity at approximately 156 months and females at 135 months [8]. The adult chimpanzees in this study were evaluated at 10 years of age or older [9]. We evaluated specific factors that may be related to the high rate of heart disease in chimpanzees; however, data for specific factors analyzed were not always available for comparison, so for some factors the sampled population was less than 87. The criteria evaluated were age, sex, cause of death, weight, diet, environment, history of infectious and experimental diseases, clinical pathology results, and extent of arteriosclerosis and atherosclerosis.
Chimpanzees with congestive heart failure were identified based on pathology records that specifically stated that congestive heart failure was present and/or indicated symptoms of congestive heart failure such as ascites, hydrothorax, hydropericardium, and the presence of hemosiderin in macrophages (heart failure cells) in the lungs [21]. Acute heart failure was assigned as the cause of death to chimpanzees that were specifically noted to have had acute heart failure in the clinical and/or pathology records.
The 87 chimpanzees evaluated were divided into three groups based on histologic evaluation of the hearts: idiopathic cardiomyopathy (IC), other heart disease (OHD), and no heart disease (NHD). Idiopathic cardiomyopathy was defined as the presence of multifocal to coalescing areas of fibrosis, necrosis, mineralization, and inflammation. The heart slides from chimpanzees with IC (n=45) were graded for severity and compared based on age, sex, and diagnosis of heart failure. Severity was based on a 0–5 rating scale: 0, normal; 1, minimal; 2, mild; 3, moderate; 4, moderately severe; 5, severe. The OHD group was not histologically diagnosed with idiopathic cardiomyopathy, but the cause of death was directly related to the heart and/or they exhibited chronic or acute heart failure as stated in pathology records. The NHD group had no history of heart disease with either normal heart tissue or only background histologic changes. Examples of background histologic changes would include the presence of few lymphocytes in the interstitum or mild fibrosis restricted to areas near the valves.
Clinical Pathology
Blood chemistry and hematology data for 63 chimpanzees for the year prior to death were available in the EZ Pro database (Glomed Systems Inc., North Richland Hills, TX 76180). The values of specific blood components related to heart failure averaged for each chimpanzee during its last year of life were: red blood cell count, white blood cell count, platelet count, hematocrit, hemoglobin, uric acid, glucose, blood urea nitrogen (BUN), creatinine, BUN/creatinine ratio, total protein, albumin, alanine aminotransferase (ALT/SGPT), aspartate aminotransferase (AST/SGOT), alkaline phosphate, sodium, potassium, creatine phosphokinase, iron, and any trace of blood or protein in urine. The normal ranges used of each blood component were species- and sex-specific for Pan troglodytes [7].
Trypanosoma cruzi
Histopathologic examination has established that T. cruzi infection is endemic in the baboon, cynomolgus, and chimpanzee colonies at the SNPRC, which is located within the geographical range of the T. cruzi vectors [1, 12]. Only one chimpanzee has been diagnosed with T. cruzi-associated fatal myocarditis at the SNPRC (unpublished data). This chimpanzee is not included in this study because it died of acute myocarditis and was not in the time frame of this study.
Virology
Enteroviruses, such as the Coxsackie B viruses, have been detected in patients with dilated idiopathic cardiomyopathy and have been considered to be a potential etiologic agent of heart disease and heart failure [18, 21]. A screening panel for Coxsackie A and B was run on a 24-year-old male chimpanzee diagnosed with idiopathic cardiomyopathy after death. Serology for the Coxsackie A antibody panel included types 2, 4, 7, 9, 10, and 16; and the Coxsackie B antibody panel included types 1, 2, 3, 4, 5, and 6.
Another chimpanzee diagnosed with idiopathic cardiomyopathy had extensive lymphocytic myocardial inflammation associated with typical changes consistent with idiopathic cardiomyopathy. An extensive viral culture screen for this animal was run on samples from both the left and right ventricles which were placed on several cell lines including Vero, MDBK, CHO, MRC5, and A549 (VRL Laboratories, The Simian Diagnostic Laboratory, Ltd., San Antonio, TX 78229). Initially, cells were placed at a lower density so they would remain viable for a longer period. Cells were transferred to fresh monolayers after 3 weeks of incubation to determine any further cytopathic effect.
Experimental Use and Disease Exposure
The experimental history, disease exposure, and clinical history of each ape were compared with the assessment of heart disease. Positive and negative test results (whether they had become chronic carriers or not) for 58 of the chimpanzees that were exposed to HIV, hepatitis B and C, and/or blood products were analyzed in relation to heart disease.
Weight
Available documented weights for each animal were averaged per year and analyzed in 5-year intervals (±1 year).
Statistical Analysis
Data were analyzed using SPSS Viewer version 10.0. Student’s t test or Mann-Whitney test and ANOVA were used to compare quantitative variables between two or more than two groups, respectively. Chi-squared or Fisher’s exact test were used to compare qualitative variables. p-Values <0.05 were considered statistically significant.
Results
The 87 chimpanzees evaluated were classified into three groups: IC (n=45), OHD (n=14), and NHD (n=28).
Table 1 contains the incidence and percentages of heart disease based on sex. Heart disease (IC and OHD) was found in 59 of the chimpanzees (68%) and was more common in males (73%) than females (54%). Idiopathic cardiomyopathy was present in over half the chimpanzees (52%) and OHD was present in 16%, leaving only one-third (32%) of the chimpanzees without heart disease. Male chimpanzees had a higher prevalence of idiopathic cardiomyopathy(60%) than females (29%). Females had a higher prevalence of other heart disease (25%) than males (13%). Heart disease was the cause of death in 34% of the chimpanzees, and occurred predominantly in males (40%), compared to females (21%). Because 30% of the chimpanzees had unknown causes of death, the mortality rate caused by heart disease may be higher than reported.
Table 1.
Prevalence of heart disease in chimpanzees at the SNPRC (n=87)
| Males |
Females |
Total |
p | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Idiopathic cardiomyopathy | 38 | 60 | 7 | 29 | 45 | 52 | 0.009 |
| Other heart disease | 8 | 13 | 6 | 25 | 14 | 16 | 0.165 |
| No heart disease | 17 | 27 | 11 | 46 | 28 | 32 | 0.009 |
| Cause of death | |||||||
| Heart disease | 25 | 40 | 5 | 21 | 30 | 34 | 0.09 |
| Unknown | 18 | 29 | 8 | 33 | 26 | 30 | 0.66 |
| No heart disease | 20 | 32 | 11 | 46 | 31 | 36 | 0.22 |
| Total sampled | 63 | 100 | 24 | 100 | 87 | 100 | |
| % Total with heart disease | 46 | 73 | 13 | 54 | 59 | 67 | 0.09 |
p-values, males vs. females
The age of death for each sex and group is reported in Table 2. The mean death age was 27.80 years in the IC group, 28.91 years in the OHD group, and 24.87 years in the NHD group. Generally, male chimpanzees (IC=26.11 years, OHD=25.97 years, NHD=20.83 years) died at younger ages than females (IC=36.97 years, p=0.004, OHD=32.84 years, NHD=31.11 years, p=0.008). On average, males in the NHD group died the earliest. In chimpanzees with IC as a cause of death, males died earlier (24.85 years) than females (36.64 years, p=0.001). However, in chimpanzees with OHD as a cause of death, females died earlier (28.58 years) than males (34.07 years), but this difference was not statistically significant. The earliest case of heart disease as a cause of death was in a male that died of idiopathic cardiomyopathy at age 13.10 years. The earliest case of other heart disease as a cause of death was in a 10.10-year-old male.
Table 2.
Age of death of chimpanzees from all causes at SNPRC (n=87)
| Categories of heart disease | Males |
Females |
Total |
|||
|---|---|---|---|---|---|---|
| n | Average age of death | n | Average age of death | n | p | |
| Idiopathic cardiomyopathy | 38 | 26.11±1.30 | 7 | 36.96±2.56 | 45 | 0.004 |
| Other heart disease | 8 | 25.97±3.39 | 6 | 32.84±3.01 | 14 | 0.156 |
| No heart disease | 17 | 20.83±1.71 | 11 | 31.11±2.97 | 28 | 0.008 |
| Totals | 63 | 24.67±1.03 | 24 | 33.25±1.73 | 87 | 0.001 |
| Heart disease as the primary cause of chimpanzee death | ||||||
| Idiopathic cardiomyopathy | 22 | 24.85±3.56 | 2 | 36.64±3.20 | 24 | 0.001 |
| Other heart disease | 3 | 34.07±2.89 | 3 | 28.58±2.97 | 6 | 0.09 |
p-values = males vs. females
Table 3 shows the relationship between heart failure and cardiovascular disease. Congestive heart failure was the most common presentation in chimpanzees with both IC (40%) and OHD (57%). The remaining cases were generally evenly divided between acute heart failure or no heart failure. No heart failure was slightly more common in the OHD group, but the difference did not reach statistical significance.
Table 3.
Heart failure status in chimpanzees with cardiovascular disease
| Cardiomyopathy (n=45) |
Other heart disease (n=14) |
Chi- squared p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Congestive heart failure | 18 | 40 | 8 | 57 | 0.263 |
| Acute heart failure % | 13 | 29 | 2 | 14 | 0.233 |
| No heart failure % | 14 | 31 | 4 | 28 | 0.569 |
p-value, cardiomyopathy vs. other heart disease
The severity grading of chimpanzees with cardiomyopathy showed a significant correlation with red blood cell count (r=0.403, p=0.046), but no differences in sex (p=0.711), congestive heart failure (p=0.126), acute heart failure (p=0.580), atherosclerosis (p=0.570), or arteriosclerosis (p=0.595). No significant differences were seen in the incidence of arteriosclerosis or atherosclerosis. However, cases of arteriosclerosis or atherosclerosis were found in the IC and NHD groups but not in the OHD group. The group of chimpanzees with arteriosclerosis/atherosclerosis (n=8), were older (33.17±3.56 vs. 26.41±0.99, p=0.045) and had higher glucose levels (125.57±32.87 vs. 94.34±4.06, p=0.057) and lower potassium levels (2.85±0.29 vs 3.55±0.13, p=0.077) than those without arteriosclerosis. Arteriosclerosis/atherosclerosis lesions varied from mild to severe and involved the brain, basilar artery, and circle of Willis (n = 4), aorta (n = 3), heart (n = 1), kidney (n = 1), or adrenal gland (n = 1). Arteriosclerosis/atherosclerosis was related to the cause of death in only two chimpanzees: infarction of the brain associated with arteriosclerosis of the small arteries of the brain, and renal hemorrhage associated with arteriosclerosis of the renal arteries. No significant differences were seen comparing the severity grading of the histologic lesions in the IC group based on age, sex, or diagnosis of heart failure.
Clinical Pathology
Table 4 shows the quantitative data and significant values for age, weight, and clinical pathology values based on sex and group. Males with IC were older than males with NHD (p=0.024). In females, the body weight at 35 years of age was higher in the IC group compared with the rest of the OHD (p=0.009) and NHD (p=0.002) groups and higher in the OHD group than in the NHD group (p=0.028). Cholesterol was lower in the OHD females than the NHD group (p=0.002). Males with IC had significantly lower levels of BUN (p=0.021) and lower BUN/Creatinine (p=0.007), ALT/SGPT (p=0.044), and AST/SGOT (p=0.023) ratios when compared with the OHD group, and lower levels of potassium (p=0.039) when compared with the NHD group. Males in the OHD group had lower albumin levels (IC, p=0.002; NHD, p=0.016) and higher uric acid levels (IC, p=0.001; NHD p=0.016) than in the other two groups. Males with OHD had higher levels of BUN, BUN/Creatinine, ALT/SGPT, AST/SGOT and lower potassium levels when compared with the NHD group, but the difference was not statistically significant. No significant differences were seen in the presence of blood or protein in the urinalysis results. Total protein (p=0.017) and albumin (p=0.009) levels were significantly lower in chimpanzees with congestive heart failure than those without congestive heart failure (data not shown).
Table 4.
Descriptive and significant data based on sex and group
| Normal Ranges 1 | Sex | Idiopathic Cardiomyopathy |
Other heart disease |
No heart disease |
p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean±SE | n | Mean±SE | n | Mean±SE | ||||
| Age | N/A | M | 38 | 26.11±1.4 a | 8 | 25.97±3.4 | 17 | 20.84±1.7 a | p=0.024 a |
| Weight at age 352 | Adult 45.0–47.4 kg | F | 2 | 61.98 a, b | 2 | 50.0 b, c | 3 | 43.5±1.3 a, c | p=0.002 a, p=0.009 b. p=0.028 c |
| Cholesterol | 152–314 mg/dl | F | 2 | 236.5 | 3 | 141.92±15.0 c | 6 | 224.46±9.6 c | p=0.002 c |
| Uric Acid | N/A | M | 10 | 3.12±0.3 b | 1 | 8.7 b, c | 4 | 3.85±0.4 c | p=0.001 b, p=0.016 c |
| BUN | 5–15 mg/dl | M | 23 | 18.27±3.0 b | 3 | 65.46±29.0 b | 7 | 31.44±17.5 | p=0.021 b |
| BUN/Creatinine | |||||||||
| Ratio | N/A | M | 23 | 10.45±1.1 b | 3 | 23.89±11.3 b | 7 | 11.22±2.0 | p=0.007 b |
| Albumin | 3.1–4.1 g/dl | M | 23 | 2.99±0.16 b | 3 | 2.49±0.06 b, c | 7 | 3.28±0.2 c | p=0.002 b, p=0.016 c |
| ALT/SGPT | 25–53 U/l | M | 23 | 63.89±8.6 b | 3 | 132.65±69.7 b | 7 | 47.96±7.7 | p=0.044 b |
| AST/SGOT | 2–48 U/l | M | 23 | 99.11±26.2 b | 3 | 424.32±365.3 b | 7 | 49.89±11.1 | p=0.023 b |
| Potassium | 3.3–4.9 mEq/l | M | 23 | 3.39±0.2 a | 3 | 3.38±0.3 | 7 | 4.18±0.3 a | p=0.039 a |
Normal range for clinical pathology values in chimpanzees was established by Hainsey et al. [7].
p<0.05 cardiomyopathy group vs. no heart disease group
p<0.05 cardiomyopathy group vs. other heart disease group
p<0.05 other heart disease group vs. no heart disease group
Weight
Females between the ages 20 and 40 years in the IC group weighed more than those in the OHD and NHD groups. Also, the females between the ages of 20 and 40 years in the IC group were 13.26–15.94 kg heavier than reported average female weights of 45.0 to 47.4 kg [1, 14]. Pathology records only indicated three obese chimpanzees in our study group, one of which was a 43-year-old female with no heart disease. One female in the IC group that had available weight data had congestive heart failure, but it was not the heaviest animal in the group. No other trends in weight for male or female chimpanzee groups were seen.
Virology
The result of the Coxsackie B virus screening for the 23-year-old male with cardiomyopathy showed some level of reactivity (1:8 for Coxsackie A type 2, 4, 7, 9, 10, 16). However, this chimpanzee could not be considered positive because it was within reasonable antibody reference range (<1:8). The viral culture for the other chimpanzee in the IC group failed to reveal any infections agents using standard cell culture methodology.
Experimental and Disease Exposure
There were no obvious differences in experimental use or natural disease among the three groups (n=54). Positive test results for HIV, hepatitis B and C, and blood product exposure was found in only one male with idiopathic cardiomyopathy (n=58). Therefore, natural and experimental disease exposure was essentially ruled out as a cause of cardiovascular disease in chimpanzees.
Diet and Environment
All the chimpanzees lived in essentially identical environments and ate essentially the same food. Therefore, these two criteria were considered as neutral or nondiscriminatory in regard to the development of heart disease in this population.
Discussion
The prevalence of heart disease in necropsied chimpanzees at SNPRC in this 23-year period was 73% (46/63) for male and 54% (13/24) for female chimpanzees. The mortality rate attributable to heart disease was 40% (25/63) for males and 21% (5/24) for females. However, the mortality rate for chimpanzees with heart disease may be higher because 30% (26/87) of the population died of unknown causes. Spontaneous idiopathic cardiomyopathy was found in over half of the population (n=45). Because age was not correlated with the prevalence of heart disease (except arteriosclerosis), it is difficult to imagine strategies to minimize disease.
Sex plays a major role in the occurrence of heart disease in chimpanzees. More male chimpanzees were diagnosed with heart disease (73%) than females (54%). This prevalence of heart disease in chimpanzees is considerably higher than humans. The prevalence of heart disease in American men (ages 20 and older) is 34.40% compared to 33.96% in females; however, females with cardiovascular disease have a higher mortality rate than men [21]. Possible reasons for this difference include protective properties of estrogen on the heart combined with genetic and environmental factors, as well as the expression of some genes on the Y chromosome that play roles in blood pressure and stress response [15, 25]. Chronic stress has been shown to have serious adverse effects on the heart, especially in males, because glucocorticoids lower levels of testosterone creating a high cortisol:testosterone ratio [6, 16].
Common risk factors for heart disease in humans do not seem to affect heart disease in chimpanzees at SNPRC. There was a trend in female weights, with females in the IC group between the ages 20–40 years weighing more than those in the OHD and NHD groups. Also, females in the IC group weighed more (by 13.26 to 15.94 kg) than the reported average weights for age-related female chimpanzees [11, 14]. Pathology records indicated only three obese chimpanzees, one of which was a 43-year-old female with no heart disease. However, body mass index would be a better measure of obesity.
The results suggest that, on average, females live longer than males in every group. In human models, the risk of developing heart disease increases with age; a high prevalence in men occurs about 10 years before women and continues to increase [24]. This is also true in chimpanzees. Of the 24 females in the population, 12 of them died at age 32 or above, and all had a form of cardiovascular disease. Male chimpanzees showed a higher prevalence of heart disease than females at a younger age. Also, all male chimpanzees over the age of 30 (n=18) had some form of heart disease, with nine having heart disease as cause of death and six having an unknown cause of death. Interestingly, this prevalence data shows similar trends with human heart disease.
Clinical indications of heart failure can be seen in the blood chemistry values of the chimpanzees with heart disease. The data for chimpanzees with congestive heart failure showed significantly lower levels of total protein (p=0.017) and albumin (p=0.009). This indicates hypoproteinemia, a common clinical indication of heart failure in both humans and animals. Also, males in the IC and OHD groups had lower levels of albumin and higher ALT/SGPT and AST/SGOT values compared to the normal reported ranges. Both low serum protein and increased liver enzymes can be evidence of abnormal heart conditions, congestive heart failure, and inflammatory diseases such as myocarditis [21, 27, 28]. Furthermore, the potassium level of males with cardiomyopathy was significantly lower than males without heart disease (p=0.039), but within normal ranges. This difference may indicate decreased heart function and contractility during the last year of life for males with idiopathic cardiomyopathy.
Trypanosoma cruzi as a cause of cardiomyopathy deserves further investigation in the chimpanzees at SNPRC because the vector is endemic to this area. T. cruzi is responsible for Chagas heart disease, which causes left ventricular dysfunction, similar to dilated cardiomyopathy [3]. This protozoan is transferred in the reduviid bug feces, which enter the blood stream of the host after scratching of the bite site or by oral ingestion by specific hosts [1, 3, 23]. Further T. cruzi testing in chimpanzees is essential to assess whether the protozoan causes cardiomyopathy in chimpanzees.
The cause of spontaneous heart disease, especially idiopathic cardiomyopathy, in chimpanzees at SNPRC remains unknown. We have essentially eliminated possible causes that can affect both males and females at an equal probability. Those factors that did not affect the prevalence of heart disease in chimpanzees include diet, environment, weight, natural disease, experimental use, and experimental disease exposure to HIV, hepatitis B and C, and blood products. Coxsackie virus A and B and other viruses were not found in the chimpanzees with idiopathic cardiomyopathy that we tested, indicating that viruses are not contributing to heart disease; however, more viral screenings should be carried out. Chimpanzees do not exhibit a high prevalence of arteriosclerosis or atherosclerosis, which indicates that vascular disease was not a major cause of death in chimpanzees.
Acknowledgments
This research was funded in part by National Institutes of Health/National Center for Research Resources (NIH/NCRR) grant P51 RR013986 to the Southwest National Primate Research Center. Chimpanzees were housed in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR016228 from the NIH/NCRR. This research was supported in part by National Institutes of Health grants R01 RR016347.
We thank the members of the clinical pathology and histology laboratory at SFBR, especially Cathy Snider, Marie Silva, Rita Sholund, Maurine Robbins, Michaelle Hohmann, Denise Trejo, and Rosy Cordova.
References
- 1.Argañaraz ER, Hubbard GB, Ramos LA, Ford AL, Nitz N, Leland MM, VandeBerg JL, Teixeira ARL. Blood-sucking lice may disseminate Trypanosoma cruzi infection in baboons. Rev Inst Med Trop S Paulo. 2001;43:271–276. doi: 10.1590/s0036-46652001000500007. [DOI] [PubMed] [Google Scholar]
- 2.Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Townsend Peterson A, Ramsey J, Wirtz RA, Robinson LE. Chagas disease in a domestic transmission cycle, Southern Texas, USA. Emerg Infect Dis. 2003;9:103–105. doi: 10.3201/eid0901.020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bestetti RB, Muccillo G. Clinical course of Chagas’ heart disease: a comparison with dilated cardiomyopathy. Int J Cardiol. 1997;60:187–193. doi: 10.1016/s0167-5273(97)00083-1. [DOI] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention, National Center for Health Statistics. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2003. Vital Health Stat. 2005;10(225) Ser. [PubMed] [Google Scholar]
- 5.Esch T, Stefano GB, Fricchione GL, Benson H. Stress in cardiovascular disease. Med Sci Monit. 2002;8:RA93–RA101. [PubMed] [Google Scholar]
- 6.Filaire E, Bernain X, Sagnol M, Lac G. Preliminary results on mood state, salivary testosterone: cortisol ratio and team performance in a professional soccer team. Eur J Appl Physiol. 2001;86:179–184. doi: 10.1007/s004210100512. [DOI] [PubMed] [Google Scholar]
- 7.Hainsey BM, Hubbard GB, Leland MM, Brasky KM. Clinical parameters of the normal baboons (Papio species) and chimpanzees (Pan troglodytes) Lab Anim Sci. 1993;43:236–243. [PubMed] [Google Scholar]
- 8.Hansen JF, Alford PL, Keeling ME. Diffuse myocardial fibrosis and congestive heart failure in an adult male chimpanzee. Vet Pathol. 1984;21:529–531. doi: 10.1177/030098588402100514. [DOI] [PubMed] [Google Scholar]
- 9.Harvey PH, Martin RD, Clutton-Brock TH. Life histories in comparative perspective. In: Smuts, Cheney, Seyfarth, Wrangham, Struhsaker, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 181–196. [Google Scholar]
- 10.Hubbard GB, Rick Lee D, Eichberg JW. Diseases and pathology of chimpanzees at the Southwest Foundation for Biomedical Research. Am J Primatol. 1991;24:273–282. [Google Scholar]
- 11.Jungers WL, Susman RL. Body size and allometry in African apes. In: Susman, editor. The Pygmy Chimpanzee. New York: Plenum Press; 1984. pp. 131–78. [Google Scholar]
- 12.Kasa TJ, Lathrop GD, Dupuy HJ, Bonney CH, Toft JD. An endemic focus of Trypanosoma cruzi infection in a subhuman primate research colony. J Am Vet Med Assoc. 1977;171:850–854. [PubMed] [Google Scholar]
- 13.Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 14.Leigh SR, Shea BT. Ontogeny of body size variation in African apes. Am J Phys Anthropol. 1996;99:43–64. doi: 10.1002/(SICI)1096-8644(199601)99:1<43::AID-AJPA3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest. 2003;112:302–307. doi: 10.1172/JCI19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacAdams MR, White RH, Chipps BE. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann Intern Med. 1986;104:648–651. doi: 10.7326/0003-4819-104-5-648. [DOI] [PubMed] [Google Scholar]
- 17.McNamara T, Dolensek EP, Lui S-K, Dierenfeld E. Cardiomyopathy associated with vitamin E deficiency in two mountain lowland gorillas. Proceedings of the First International Conference on Zoological and Avian Medicine; East Northport, NY: AAV/AAZV; 1987. p. 493. [Google Scholar]
- 18.Pauschinger M, Doerner A, Kuehl U, Schwimmbeck PL, Poller W, Kandolf R, Schultheiss HP. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation. 1999;99:889–895. doi: 10.1161/01.cir.99.7.889. [DOI] [PubMed] [Google Scholar]
- 19.Pilote L, Dasgupta K, Guru V, Humphries KH, McGrath J, Norris C, Rabi D, Tremblay J, Alamian A, Barnett T, Cox J, Ghali WA, Grace S, Hamet P, Ho T, Kirkland S, Lambert M, Libersan D, O’Loughlin J, Paradis G, Petrovich M, Tagalakis V. A comprehensive view of sex-specific issues related to cardiovascular disease. Can Med Assoc J. 2007;176:S1–S44. doi: 10.1503/cmaj.051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt RE. Systematic pathology of chimpanzees. J Med Primatol. 1978;7:274–318. doi: 10.1159/000459914. [DOI] [PubMed] [Google Scholar]
- 21.Schoen FJ. The heart. In: Robbins, Cotran, editors. Pathologic Basis of Disease. 7. Elsevier; New York: 2005. pp. 555–618. [Google Scholar]
- 22.Schulman FY, Farb A, Virmani R, Montali RJ. Fibrosing cardiomyopathy in captive Western Lowland gorillas (Gorilla, gorilla, gorilla) in the United Sates: A retrospective study. J Zoo Wildlife Med. 1995;26:43–51. [Google Scholar]
- 23.Teixeira ARL. The stercorarian trypanosomes. In: Soulsby, editor. Immune Responses in Parasitic Infections: Immunology, Immunopathology, and Immunoprophylaxis. CRC Press; Boca Raton: 1987. pp. 25–115. [Google Scholar]
- 24.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay J, Petrovich M, Hamet P. Genetic and sex determinants of hypertension and CVD. Can Med Assoc J. 2007;176:S1–S44. [Google Scholar]
- 26.World Health Organization. World Heath Statistics 2006. Geneva: World Heath Organization; 2006. [Google Scholar]
- 27.Wu AH. General clinical tests, albumin. In: Wilson, Wurm, editors. The Clinical Guide to Laboratory Tests. 4. Elsevier; New York: 2006. pp. 66–71. [Google Scholar]
- 28.Wu AH. General clinical tests, aspartate aminotransferase. In: Wilson, Wurm, editors. The Clinical Guide to Laboratory Tests. 4. Elsevier; New York: 2006. pp. 154–158. [Google Scholar]