Abstract
OBJECTIVES
The aim of this work was to characterize patterns of late gadolinium enhancement (LGE) by cardiovascular magnetic resonance imaging in a hemodialysis population at high risk for cardiovascular events.
BACKGROUND
The prevalence and distribution of LGE and its relationship to left ventricular mass (LVM) and function in this population is unknown.
METHODS
Chronic hemodialysis patients at high risk for cardiovascular events—age >50 years, diabetes, or known cardiovascular disease—were enrolled prior to concerns regarding nephrogenic systemic fibrosis. Cardiovascular magnetic resonance imaging was performed in 24 patients (age, 59 ± 11 years; dialysis, 45 ± 38 months) and included steady-state free precession cine imaging and late gadolinium-enhanced, phase-sensitive, inversion-recovery gradient echo images. Left ventricular mass, volumes, and function were calculated and indexed to body surface area. A 16-segment analysis was performed to calculate percentage of LGE, LV wall thickness, and percentage of wall thickening.
RESULTS
Left ventricular ejection fraction was 48 ± 15%, and the LV mass index was 100 ± 52 g/m2. Late gadolinium enhancement was observed in 79% (19 of 24) of patients in 3 distinct patterns: infarct-related (32%, 6 of 19), diffuse (37%, 7 of 19), and focal noninfarct (37%, 7 of 19). Late gadolinium enhancement constituted 15 ± 18% of the LVM and correlated with LVM (r = 0.44, p = 0.03). A significant, inverse relationship existed between segmental LGE and the percentage of wall thickening (p > 0.0001). Excluding infarct-related segments, as end-diastolic wall thickness increased, so did LGE (p < 0.0001), and as LGE increased, the percentage of wall thickening decreased (p = 0.0012). After 23 ± 3 months of follow-up, 1 patient had developed nephrogenic systemic fibrosis. Seven of the patients (29%) had developed a hard cardiovascular event, 5 of 19 (26%) with LGE and 2 of 5 (40%) without.
CONCLUSIONS
Late gadolinium enhancement is prevalent in the hemodialysis population and its extent is related to LVM. Most cases of LGE are not infarct-related and are associated with hypertrophied, dysfunctional LV segments. Non-infarct-related LGE may signify fibrosis from LV hypertrophy and/or an infiltrative process. Further studies in this patient population will not be possible due to the risk of nephrogenic systemic fibrosis.
Keywords: magnetic resonance imaging, hemodialysis, gadolinium
According to the most recent U.S. Renal Data System report (1), nearly 336,000 Americans receive dialysis. In this rapidly increasing population, projected to be over 2 million patients by 2030, the prognosis is poor with cardiovascular disease, specifically sudden cardiac death, being the major contributor (2). Many factors may impact the susceptibility of the hemodialysis population to sudden cardiac death. Comorbidities such as coronary artery disease (CAD), cardiomyopathy, and particularly left ventricular hypertrophy (LVH) are prevalent, and they are recognized predictors of cardiovascular events (3,4). Noninvasive identification of markers of cardiovascular risk could have important clinical benefits.
Although the recent potential linkage between gadolinium contrast agents and nephrogenic systemic fibrosis (NSF) will limit its applicability (5–7), cardiovascular magnetic resonance (CMR) imaging is uniquely suited to characterize myocardial disease in the hemodialysis population. The CMR is a gold standard method for the assessment of left ventricular mass (LVM) with excellent intra-and interobserver reproducibility (8). Unlike echocardiography, CMR measures of LVM are not constrained by ventricular loading and are thus independent of hemodialysis sessions (9,10). Using late gadolinium enhancement (LGE), CMR has the ability to reliably detect myocardial fibrosis from multiple etiologies (11,12) and is a valuable indicator of cardiovascular prognosis (13–16). The objective of this study was to define the prevalence and distribution of LGE in a hemodialysis population at increased risk for cardiovascular events.
METHODS
Cardiovascular magnetic resonance studies were performed in consecutive subjects recruited from the University of Virginia Health System hemodialysis centers with the approval of the local Investigational Review Board, and all participants gave informed consent. Inclusion criteria included patients receiving chronic hemodialysis, defined as continuous hemodialysis for longer than 6 months, and the presence of increased risk for cardiovascular events. These included age >50 years, diabetes, or known cardiovascular disease. A contraindication to CMR was the only criteria for exclusion. Enrollment was conducted from October 28, 2005, to August 22, 2006. Enrollment was halted approximately 4 months before the December 2006 Food and Drug Administration warning regarding a possible relationship between gadolinium chelates and the development of NSF in patients with kidney failure (5,6).
Image acquisition
All CMR exams were performed on the day prior to a scheduled hemodialysis session. Studies were performed with an Avanto 1.5-T scanner (Siemens Healthcare, Erlangen, Germany) with a phased array chest coil. After scout imaging, left ventricular function was determined using steady-state free precession cine imaging (repetition time, 2.7 ms; echo time, 1.3 ms; flip angle, 73°; field of view, 300 to 350 mm; and resolution, 1.8 × 1.4 × 8.0 mm). Late gadolinium-enhanced imaging was performed 10 to 15 min following an injection of 0.15 mmol/kg gadolinium with a phase-sensitive, inversion-recovery sequence (repetition time, 700 ms; echo time, 4.2 ms; fixed inversion time, 300 ms; flip angle, 25°; field of view, 300 to 340 mm; resolution, 1.8 × 1.3 × 8 mm). Two different gadolinium-based contrast agents were used in the study: gadopentetate dimeglumine (Magnevist, Berlex, Montville, New Jersey) in 20 patients and gadodiamide (Omniscan, General Electric Healthcare, Princeton, New Jersey) in 4 patients. Both the cine and inversion-recovery sequences were performed in identical 2-, 3-, and 4-chamber long-axis views, as well as sequential short-axis acquisitions with a 20% interslice gap.
Image analysis
Dedicated analysis software (Argus, Siemens Healthcare) was used to calculate LVM, LV volumes, LV function, and LGE. Left ventricular mass and LV volume measurements were indexed to body surface area calculated from the measured height at the time of CMR and the goal dry or post-dialysis weight of the patients.
The presence of LGE required increased myocardial signal intensity 2 SD greater than that of remote myocardium (17,18) in at least 2 imaging planes. The signal intensity of enhanced and of nulled myocardium was determined from each short-axis image with LGE present. An American Heart Association 16-segment analysis (excluding the apex) (19) was performed to calculate the percentage of LGE, LV wall thickness, and the percentage of wall thickening using the same software. The total LGE mass was calculated from the LV short-axis images using Argus software. Total LGE and segmental LGE areas were manually traced and then divided by the total LVM and segmental LVM, respectively, to calculate total and segmental percent LGE. The determination of the LGE distribution pattern was made by a reader with over 10 years of experience, who was blinded to clinical data. Infarct-related LGE was determined by a characteristic subendocardial location in a coronary distribution. A noninfarct pattern of LGE in multiple territories was defined as diffuse LGE. Isolated midwall or subepicardial LGE was defined as a focal noninfarct LGE pattern.
Statistical analysis
Continuous variables, such as LGE mass, LVM, and hemodialysis duration between LGE subgroups, were analyzed by analysis of variance. Multiple comparisons were adjusted by the Bonferroni method. Segmental measurements were analyzed using a repeated measures model (20) for the effects of end-diastolic and end-systolic thickness and wall thickening (absolute and percentage) to the proportion of LGE per segment. Analyses were done using SAS software (SAS Institute, Cary, North Carolina) version 9.1 “PROC MIXED.” All data are presented as mean ± standard deviation and p < 0.05 was considered statistically significant.
RESULTS
Patient demographics
Twenty-six patients fulfilled criteria for enrollment and signed informed consent. One subject withdrew due to obesity and another due to claustrophobia. The cohort was 59 ± 11 years of age, 54% male and 79% African American (Table 1). Diabetes mellitus and hypertension were the primary causes of renal failure: either alone or in combination, accounting for 83% of cases. The average duration of hemodialysis was 45 ± 38 months. X-ray coronary angiography had been previously performed in 75% (18 of 24) of all subjects. Comorbidities were prevalent and consisted of hypertension in all subjects, active or former smokers in 71% (17 of 24), diabetes mellitus in 71% (17 of 24), CAD (defined by a coronary stenosis ≥ 50% on previous X-ray coronary angiography) in 50% (12 of 24), a history of percutaneous coronary intervention or coronary artery bypass surgery in 29% (7 of 24), and stroke in 17% (4 of 24). One subject with pre-existing NSF was enrolled prior to the identification of a possible association with gadolinium chelates. The average pre-dialysis systolic and diastolic blood pressure and heart rate were 145 ± 30 mm Hg, 75 ± 17 mm Hg, and 85 ± 19 beats/min, respectively.
Table 1.
Baseline Characteristics for the Study Population and Presented by LGE Pattern Subgroups
| All Subjects (n = 24) | Infarct-Related LGE (n = 6) | Diffuse LGE (n = 7) | Focal Noninfarct LGE (n = 7) | No LGE (n = 5) | |
|---|---|---|---|---|---|
| Age, yrs | 59 ± 11 | 65 ± 12 | 59 ± 10 | 58 ± 15 | 59 ± 5 |
| Male, n (%) | 13 (54) | 2 (33) | 5 (71) | 5 (71) | 2 (40) |
| Comorbid conditions | |||||
| HTN, n (%) | 24 (100) | 6 (100) | 7 (100) | 7 (100) | 5 (100) |
| DM, n (%) | 17 (71) | 4 (67) | 4 (57) | 5 (71) | 4 (80) |
| Tobacco use, n (%) | 17 (71) | 4 (67) | 6 (86) | 5 (71) | 3 (60) |
| CAD, n (%) | 12 (50) | 6 (100) | 3 (43) | 2 (29) | 2 (40) |
| PCI or CABG, n (%) | 7 (29) | 5 (83) | 2 (29) | 1 (14) | 0 |
| Stroke, n (%) | 4 (17) | 1 (17) | 1 (14) | 0 | 2 (40) |
| Duration of HD, months | 45 ± 38 | 46 ± 40 | 66 ± 54 | 32 ± 11 | 28 ± 22 |
| Etiology of renal disease | |||||
| DM, n (%) | 10 (42) | 2 (33) | 0 | 5 (71) | 3 (60) |
| HTN, n (%) | 4 (17) | 2 (33) | 1 (14) | 1 (14) | 1 (20) |
| DM and HTN, n (%) | 6 (25) | 1 (17) | 4 (57) | 0 | 1 (20) |
| Cocaine and HTN, n (%) | 2 (8) | 0 | 2 (29) | 0 | 0 |
| PCKD, n (%) | 1 (4) | 0 | 0 | 1 (14) | 0 |
| Medicine toxicity, n (%) | 1 (4) | 1 (17) | 0 | 0 | 0 |
CABG = coronary artery bypass graft surgery; CAD = coronary artery disease; DM = diabetes mellitus; HD = hemodialysis; HTN = hypertension; LGE = late gadolinium enhancement; PCI = percutaneous coronary intervention; PCKD = polycystic kidney disease.
LGE
Late gadolinium enhancement was present in 79% of all patients (19 of 24). Subjects with LGE could be categorized into 1 of 3 groups based on the location and distribution of LGE (Fig. 1). An infarct-related pattern was present in 32% (6 of 19) of subjects with LGE (Fig. 1A). The diffuse pattern was demonstrated in 37% (7 of 19) of subjects, including the patient with pre-existent NSF (Fig. 1B). A single subject had LGE consistent with both an infarct-related and diffuse LGE pattern in different locations. The focal noninfarct pattern was seen in 37% (7 of 19) of subjects (Fig. 1C). None of the subjects in this subgroup had subendocardial LGE. The signal intensity of infarct-related, diffuse, and focal LGEs were 20 ± 4, 15 ± 8, and 15 ± 6 SD greater than that of nulled myocardium.
Figure 1. In Chronic Hemodialysis Patients, LGE by CMR Is Present in 3 Distinctive Patterns.
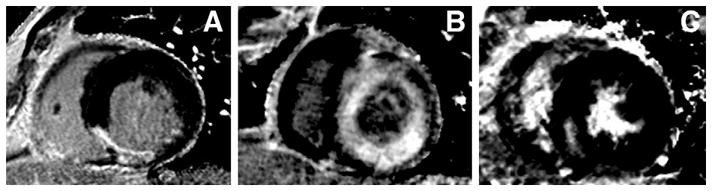
Panel A exhibits transmural late gadolinium enhancement (LGE) of the inferior septum consistent with an infarct pattern. The diffuse pattern is seen in panel B as circumferential LGE of the subendocardium and midwall, sparing the subepicardium. Panel C, with its focal inferior septal LGE in the midwall, is representative of the focal noninfarct LGE pattern. CMR = cardiovascular magnetic resonance.
Baseline characteristics such as age, the presence of hypertension, diabetes mellitus, and tobacco abuse for all LGE patterns were similar (Table 1). All subjects in the infarct-related LGE pattern group and 37% (7 of 19) of the remainder had at least a single coronary stenosis of ≥50%.
Clinical follow-up
Chart review at an average of 23 ± 3 months following the administration of gadolinium for this study revealed 1 newly diagnosed case of NSF. The CMR was performed 30 months after hemodialysis initiation, and the diagnosis was made 16 months after examination. Magnevist was the gadolinium chelate used, and a focal, subepicardial pattern of LGE was present in this subject. The patient had received 2 prior magnetic resonance angiograms (with type of gadolinium chelate unknown) at 13 and 21 months after hemodialysis initiation.
After 23 ± 3 months of follow-up, 7 of 24 (29%) of subjects have had hard cardiovascular events. Four subjects have died, 3 of whom had LGE (2 diffuse patterns and 1 focal pattern). Two patients with infarct-related LGE had nonfatal events: heart block requiring pacemaker placement and stroke. One subject without LGE had a resuscitated ventricular fibrillation arrest and underwent coronary artery bypass grafting and implantable cardioverter-defibrillator placement. Of note, 2 of 5 (40%) of patients without any LGE have had events.
LV global size and function
In the 24 patients, ejection fraction by CMR was 48 ± 15%, end-diastolic volume index was 88 ± 35 ml/m2, end-systolic volume index was 49 ± 29 ml/m2, LVM was 182 ± 81 g, and LVM index was 100 ± 52 g/m2 (Table 2). Average LGE mass was 31 ± 45 g, and LGE constituted 15 ± 18% of the LVM. The LGE mass correlated to LVM (r = 0.44, p = 0.03). A trend was noted toward a correlation between LGE mass and hemodialysis duration (p = 0.11).
Table 2.
CMR Examination Measures Calculated for the Study Population and Presented by LGE Pattern Subgroups
| All Subjects (n = 24) | Infarct-Related LGE (n = 6) | Diffuse LGE (n = 7) | Focal Noninfarct LGE (n = 7) | No LGE (n = 5) | |
|---|---|---|---|---|---|
| Volumetric measures | |||||
| Ejection fraction, % | 48 ± 15 | 45 ± 10 | 48 ± 16 | 49 ± 17 | 47 ± 21 |
| EDVI, ml/m2 | 88 ± 35 | 95 ± 25 | 77 ± 29 | 95 ± 51 | 88 ± 27 |
| ESVI, ml/m2 | 49 ± 29 | 53 ± 22 | 42 ± 24 | 54 ± 40 | 50 ± 33 |
| LVM, g | 182 ± 81 | 158 ± 67 | 212 ± 56 | 198 ± 114 | 149 ± 57 |
| LVMI, g/m2 | 100 ± 52 | 87 ± 25 | 113 ± 40 | 106 ± 85 | 89 ± 30 |
| LGE measures | |||||
| LGE mass, g | 31 ± 45 | 18 ± 14 | 82 ± 53* | 8 ± 7 | 0 |
| LGE/LVM, % | 15 ± 18 | 10 ± 6 | 37 ± 16* | 4 ± 2 | 0 |
p < 0.05 versus infarct-related and focal noninfarct LGE groups. Bonferroni method was used to adjust the multiple comparisons. The number of comparison here was 6.
CMR = cardiovascular magnetic resonance; EDVI = end-diastolic volume index; ESVI = end-systolic volume index; LVM = left ventricular mass; LVMI = left ventricular mass index; other abbreviation as in Table 1.
The ejection fraction was similar among all LGE pattern groups (Table 2). The LVM index was elevated in all groups, but similar among groups. For comparison purposes, normal values are 65 ± 9 g/m2 for men and 52 ± 7 g/m2 for women (21). The LGE mass as a percentage of LVM was 4 ± 2% in the focal noninfarct LGE group, 10 ± 6% in the infarct-related, and 37 ± 16% in the diffuse LGE group (p < 0.05 vs. other 2 groups).
LV regional function
In the entire cohort, end-diastolic wall thickness was 10 ± 3 mm, end-systolic thickness was 15 ± 4 mm, absolute wall thickening was 5 ± 3 mm, and the percentage of wall thickening was 50 ± 32%. When all segments were evaluated, an inverse relationship was found between the proportion of LGE per segment and the percentage of wall thickening (p < 0.0001). When infarct-related segments were excluded, as end-diastolic thickness increased, so did LGE (p < 0.0001), and as LGE increased, the percentage of wall thickening decreased (p = 0.0012).
DISCUSSION
Chronic kidney failure portends a poor cardiovascular prognosis. The first 3 years from the initiation of hemodialysis brings the greatest risk of sudden cardiac death (1). In the last decade, a 10% increase in the mortality rate has been noted in the chronic dialysis population (1). As stated by the U.S. Renal Data System, “it is less clear how to identify patients at risk for sudden cardiac death” (1). Myocardial fibrosis due to LVH may be such a marker and LGE by CMR is a well-validated technique to identify myocardial fibrosis (11). Five of the 7 patients with events during the 23-month follow-up had LGE. Unfortunately, due to the concern regarding gadolinium and NSF, further studies to define the relationship between LGE and prognosis in this population will not be possible.
The location and distribution of LGE varies according to the underlying pathophysiologic state. Subendocardial or transmural LGE is most often a consequence of myocardial infarction (22). However, LGE is not exclusive to ischemic heart disease and can be seen in many cardiac conditions associated with myocardial fibrosis. Non-infarct-related LGE is often focal and present in the mid- or subepicardial myocardium in conditions such as myocarditis (23), sarcoidosis (24), and idiopathic-dilated (25) and hypertrophic cardiomyopathy (26).
Our investigation included 24 chronic hemodialysis patients at high risk for cardiovascular events. A high proportion (79%) of this population had LGE and most cases were non-infarct-related. Mark et al. (27) recently studied 134 end-stage renal failure patients in Scotland. In their study, only 28% (38 of 134) had LGE, and those cases were evenly composed of subendocardial and diffuse LGE. The lower prevalence of LGE, even in the face of higher CAD prevalence (59% in the study conducted by Mark et al. (27) vs. 50% in the present study), is likely due to differences between the study populations. Although subject age was similar, hemodialysis patients constituted only 69% of the cohort of Mark et al. (27) and only 55% of the LGE positive group. Hemodialysis itself may in some way contribute to myocardial fibrosis. The current study included a high proportion of African Americans, unlike the study conducted by Mark et al. (27), and this racial group may have a predisposition to myocardial fibrosis. Diabetics composed only 24% of the population of Mark et al. (27) compared with 71% of the current study. Advanced glycosylation end products in diabetes may also play a role in myocardial fibrosis (28). Measured LVM was similar between the 2 studies. Although LGE mass was only 9 ± 5 g in the LGE-positive patients in the study of Mark et al. (27) compared with 31 ± 45 g in the present study, the former study also found a significant relationship between LGE mass and LVM index (p < 0.01).
Prior histologic studies may provide valuable insight into the potential meaning of the noninfarct pattern of LGE seen in the present study. Mall et al. (29) conducted autopsies on 60 patients on hemodialysis for >6 months, excluding subjects with obstructive CAD. They demonstrated that up to 91% of chronically uremic patients have diffuse noncoronary intermyocardiocytic fibrosis of the LV free wall that correlates to hemodialysis duration and is not present in controls (29). They noted that the fibrosis was not similar in appearance to that found in hypertension, myocarditis, or myocardial infarction. Mall et al. (29) were able to relate intermyocardiocytic fibrosis to diabetes and uremia, independent of hemodialysis. The independent effect of diabetes mellitus may help explain the discrepancy between the prevalence of fibrosis discovered as LGE in the present study and that of Mark et al. (27). At autopsy, Mall et al. (29) established a relationship between the severity of fibrosis and duration of dialysis. In our smaller patient sample, we found a trend toward such a relationship.
Circumferential, subendocardial LGE that does not correspond to coronary vascular beds has been described in amyloidosis (30).The diffuse noninfarct pattern of LGE in the present study appears similar to that described in amyloidosis (30). Amyloidosis due to beta-2-microglobulin deposition is a known but rare complication of hemodialysis (31). The true prevalence of dialysis-related amyloidosis is unknown and thought to be decreasing with the advent and use of high flux dialyzing membranes (32). The presence of intermyocardiocytic fibrosis in an autopsy study was not due to amyloidosis as specific histological staining (Congo red) failed to identify beta-2 microglobulin (29). Although histological confirmation was not performed in the present study, the possibility of amyloidosis in the patients with diffuse LGE remains.
A significant relationship was noted between LVH and LGE, and an inverse relationship was found between LGE and segmental LV dysfunction. The fibrosis or infiltrative disease identified by LGE likely contributes to the dysfunction associated with LVH. A similar relationship has been demonstrated by Choudhury et al. (26) in hypertrophic cardiomyopathy. Left ventricular hypertrophy is present in 60% to 80% of patients upon initiation of dialysis (33) and has been shown to be an independent predictor of cardiovascular events (4,34,35). Further investigation into the time course and risk factors underlying this process may prove to be of clinical value.
Limitations and clinical implications
Our study population size is relatively small. Future studies will be limited by concern regarding the potential link between gadolinium chelates and NSF in renal failure patients (5,6). In our small cohort of 24 subjects, 1 new case of NSF was noted after 23 ± 3 months of follow-up in a patient who had received gadolinium chelates previously. The incidence of this disorder is unknown, but in high-risk individuals, it may be as high as 5% (7).
The lack of histologic correlation allows for only speculation as to the etiology of the nonischemic LGE identified in this cohort. Although the numbers remain small and the follow-up is preliminary, the presence of LGE did not appear to predict events (5 of 19 patients) in this population as events were equally or even more prevalent (2 of 5 patients) in patients without LGE. However, LGE is known to have prognostic value in a number of clinical settings, especially as it relates to risk of ventricular arrhythmia (15,16).
CONCLUSIONS
This study pertains to a chronic hemodialysis population at high risk for cardiovascular events and establishes that LGE is prevalent and related to the degree of LVH. The majority of LGE in this population is not infarct-related and may represent fibrosis due to LVH and/or an infiltrative process. Non-infarct-related LGE is more prevalent in hypertrophied, dysfunctional LV segments. Hemodialysis patients are at high risk for sudden cardiac death and although risk factors such as LVH have been recognized, no mechanism or specific markers have been identified. The distribution and extent of LGE may be such a marker, although further studies using gadolinium chelates in this population will no longer be possible.
Acknowledgments
Supported in part by T32 EB003841–03 (to Dr. Schietinger) and R01 HL075792 (to Dr. Kramer). Dr. Kramer receives research equipment support from Siemens Healthcare. This manuscript was guest edited by Warren J. Manning, MD.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CMR
cardiovascular magnetic resonance
- LGE
late gadolinium enhancement
- LVH
left ventricular hypertrophy
- LVM
left ventricular mass
- NSF
nephrogenic systemic fibrosis
References
- 1.U.S. Renal Data System. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 2.Robin J, Weinberg K, Tiongson J, et al. Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm. 2006;3:1196–201. doi: 10.1016/j.hrthm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Beto JA, Coronado BE, et al. on behalf of National Kidney Foundation Task Force on Cardiovascular Disease. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 4.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277–85. [PubMed] [Google Scholar]
- 5.Food and Drug Administration. Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI): Omniscan, OptiMARK, Magnevist, ProHance, and MultiHance. Dec 22, 2006. [Google Scholar]
- 6.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–8. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 7.Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148–57. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]
- 8.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750–5. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 9.Stewart GA, Foster J, Cowan M, et al. Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int. 1999;56:2248–53. doi: 10.1046/j.1523-1755.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 10.Hunold P, Vogt FM, Heemann UW, Zimmermann U, Barkhausen J. Myocardial mass and volume measurement of hypertrophic left ventricles by MRI—study in dialysis patients examined before and after dialysis. J Cardiovasc Magn Reson. 2003;5:553–61. doi: 10.1081/jcmr-120025230. [DOI] [PubMed] [Google Scholar]
- 11.Isbell DC, Kramer CM. Cardiovascular magnetic resonance: structure, function, perfusion, and viability. J Nucl Cardiol. 2005;12:324–36. doi: 10.1016/j.nuclcard.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–74. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 13.Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561–7. doi: 10.1016/s0735-1097(03)00189-x. [DOI] [PubMed] [Google Scholar]
- 14.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 15.Bello D, Fieno DS, Kim RJ, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–8. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 17.Fieno DS, Hillenbrand HB, Rehwald WG, et al. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol. 2004;43:2124–31. doi: 10.1016/j.jacc.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 18.Mahrholdt H, Wagner A, Holly TA, et al. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322–7. doi: 10.1161/01.cir.0000036368.63317.1c. [DOI] [PubMed] [Google Scholar]
- 19.Cerqueira MD, Weissman NJ, Dilsizian V, et al. on behalf of Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 20.Crowder M, Hand D. Analysis of repeated measures. Boca Raton, FL: Chapman and Hall; 1990. [Google Scholar]
- 21.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–9. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 22.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non–Q-wave myocardial infarction. Lancet. 2001;357:21–8. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 23.Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–8. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 24.Smedema JP, Snoep G, van Kroonen-burgh MP, et al. The additional value of gadolinium-enhanced MRI to standard assessment for cardiac involvement in patients with pulmonary sarcoidosis. Chest. 2005;128:1629–37. doi: 10.1378/chest.128.3.1629. [DOI] [PubMed] [Google Scholar]
- 25.McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury L, Mahrholdt H, Wagner A, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–64. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 27.Mark PB, Johnston N, Groenning BA, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69:1839–45. doi: 10.1038/sj.ki.5000249. [DOI] [PubMed] [Google Scholar]
- 28.Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004;17:S31–8. doi: 10.1016/j.amjhyper.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Mall G, Huther W, Schneider J, Lundin P, Ritz E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant. 1990;5:39–44. doi: 10.1093/ndt/5.1.39. [DOI] [PubMed] [Google Scholar]
- 30.Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–93. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 31.Gejyo F, Odani S, Yamada T, et al. Beta 2-microglobulin: a new form of amyloid protein associated with chronic hemodialysis. Kidney Int. 1986;30:385–90. doi: 10.1038/ki.1986.196. [DOI] [PubMed] [Google Scholar]
- 32.Floege J, Koch KM. Beta 2-microglobulin associated amyloidosis and therapy with high flux hemodialysis membranes. Clin Nephrol. 1994;42 (Suppl 1):S52–6. [PubMed] [Google Scholar]
- 33.Amann K, Rychlik I, Miltenberger-Milteny G, Ritz E. Left ventricular hypertrophy in renal failure. Kidney Int Suppl. 1998;68:S78–85. doi: 10.1046/j.1523-1755.1998.06818.x. [DOI] [PubMed] [Google Scholar]
- 34.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–90. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 35.Zoccali C, Benedetto FA, Mallamaci F, et al. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65:1492–8. doi: 10.1111/j.1523-1755.2004.00530.x. [DOI] [PubMed] [Google Scholar]


