Abstract
Research on oxidative stress focused primarily on determining how reactive oxygen species (ROS) damage cells by indiscriminate reactions with their macromolecular machinery, particularly lipids, proteins, and DNA. However, many chronic diseases are not always a consequence of tissue necrosis, DNA, or protein damage, but rather to altered gene expression. Gene expression is highly regulated by the coordination of cell signaling systems that maintain tissue homeostasis. Therefore, much research has shifted to the understanding of how ROS reversibly control gene expression through cell signaling mechanisms. However, most research has focused on redox regulation of signal transduction within a cell, but we introduce a more comprehensive-systems biology approach to understanding oxidative signaling that includes gap junctional intercellular communication, which plays a role in coordinating gene expression between cells of a tissue needed to maintain tissue homeostasis. We propose a hypothesis that gap junctions are critical in modulating the levels of second messengers, such as low molecular weight reactive oxygen, needed in the transduction of an external signal to the nucleus in the expression of genes. Thus, any comprehensive-systems biology approach to understanding oxidative signaling must also include gap junctions, in which aberrant gap junctions have been clearly implicated in many human diseases. Antioxid. Redox Signal. 11, 297–307.
Oxidative Damage vs. Oxidative Signaling
Oxidative stress has long been affiliated with acute and chronic human diseases, and was believed to be primarily a consequence of indiscriminate, cumulative damage to proteins, lipids, and DNA from the vigorous production of reactive oxygen species (ROS) that overwhelm the cell's antioxidant defense system. However, pathological causes of many chronic diseases, particularly cancer, have also been linked with noncytotoxic nongenotoxic events, and the role of ROS and antioxidants in human diseases must also address epigenetic events (10, 15, 29, 76, 99).
Oxygen was first implicated in cancer as far back as the 1920s by Otto Warburg (111). Initially his theory of altered oxidative metabolism fell mostly on deaf ears. However, recently there has been a renewed interest in how cancer cells shift their energy production from oxidative phosphorylation to anaerobic glycolysis, the “Warburg Effect,” that is now considered a fundamental property of cancer cells and not just a consequence of malignant cell transformation (3, 54, 116). In contrast to this role of low oxygen tensions in cancer, the discovery of superoxide dismutase in 1968 by McCord and Fridovich (70) led to an explosion of research on the role of reactive oxygen, a product of high oxygen tensions, in the pathologies of biological organisms, and has been specifically connected with not only cancer but also many other human diseases (1, 41). For many years, research in oxidative stress at high oxygen tensions focused primarily on determining the mechanisms by which ROS damage cells by random reactions with the macromolecular machinery of a cell, particularly lipids, proteins, and DNA.
Over these last 4 decades, extensive research has determined many mechanisms on how ROS react with lipids, leading to the peroxidation of biological membranes resulting in necrotic lesions (31), how ROS react with the nucleotides of DNA leading to genetic instability and mutations (9, 31), and how ROS react with proteins leading to metabolic imbalances. Yet, many chronic diseases affiliated with oxidative stress, such as cancer and cardiovascular diseases, are not always a consequence of tissue necrosis, DNA damage, and genetic instability and mutations, or protein damage, but rather to altered gene expression through epigenetic mechanisms (98, 99, 104). Organic peroxides, for example, act as tumor promoters and not as initiators (34, 53, 73, 86), indicating that these oxidants are not mutagens, but rather epigenetic effectors. Hydrogen peroxide has also been confirmed to be a promoter, but not an initiator using two stage in vivo carcinogenesis model systems and transformation in vitro systems (53, 73, 74, 77). These results on the carcinogenicity of peroxides were only several of many experiments indicating that ROS contributed to chronic diseases at nongenotoxic, nondamaging levels. Consequently, in the past two decades, considerable research has shifted to the understanding of how ROS reversibly control the expression of genes at noncytotoxic doses (1, 28, 49, 89), with at least 127 genes and signal transducing proteins reported to be sensitive to reductive and oxidative (redox) states in the cell (1). This shift in thinking should start to shape both the design and the interpretation of experiments on the role of oxidative stress in human disease.
Oxidative Signaling Mechanisms in Mitogenesis and Cancer
Although many intracellular signaling pathways are known to be redox-sensitive, the mitogen-activated protein kinase (MAPK) and nuclear factor-kB (NF-kB) signal transduction pathways have been extensively studied (1, 30, 40, 89). These pathways, either directly or indirectly, are widely involved in many of the cells redox responses (1). Cancer is a disease that involves aberrant communication between and within cells (22, 99), and MAPK pathways are key signaling networks involved in the regulation of normal cell proliferation, survival, and differentiation (22, 79), thus redox regulation of MAPK pathways is one significant and probable link of oxidative stress and cancer. Numerous articles have been published that clearly link oxidative stress with hyperproliferation of cells within a tissue and has been clearly a focus of cancer research, thus understanding the mechanism of redox regulation of mitogenic signaling pathways such as MAPKs will offer more specific strategies of preventions and cures.
MAPK is not only activated by ROS (38, 71) but actually require the synthesis of H2O2 (14, 42, 52, 87, 88, 105, 106). Sundaresan et al. (88) were the first to demonstrate, using a vascular smooth muscle cell system, that a transient burst of H2O2 by an extracellular ligand, namely platelet derived growth factor (PDGF), is essential in the activation of extracellular receptor kinase-MAPK (ERK-MAPK). They demonstrated that the transfection of catalase, which dismutates H2O2, into these cells, prevented the activation of ERK-MAPK by PDGF (Fig. 1A and B). They also demonstrated that downstream events of MAPK activation, such as proliferation and increased cell motility, were also inhibited by catalase (Fig. 1E and F). This was one of many studies that demonstrated endogenous growth factors (extracellular ligands) generate ROS, which are then required downstream in intracellular signaling to successfully transmit their signals to the nucleus (58, 90). This seminal paper was one of the first to radically alter our traditional view of oxidative stress from an exclusive concept that reactive oxygen was always detrimental, via oxidative damage of cellular components, to one that reactive oxygen is also part of normal signaling mechanisms that can alter gene expression in an undamaging way. Thus, the pathologies of reactive oxygen can be linked to damage or cell signaling or both, depending on concentrations that range from being too low, to moderate or extremely high conditions.
FIG. 1.
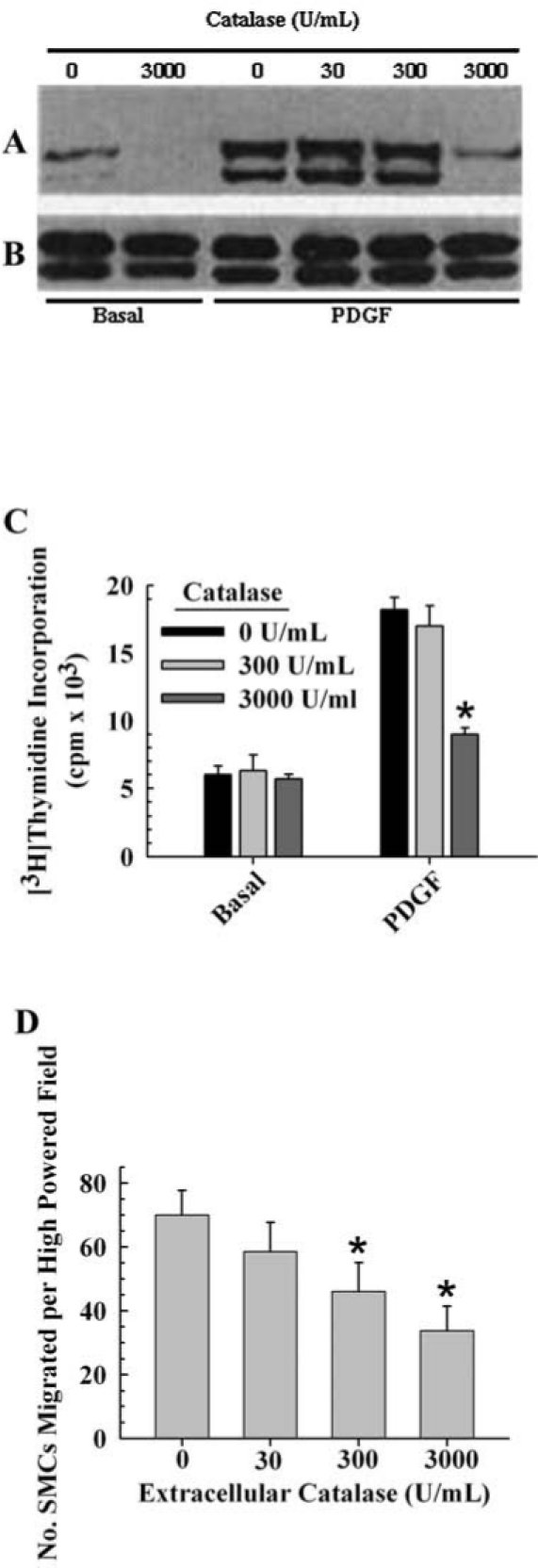
The involvement of H2O2 in growth factor-dependent activation of ERK-MAPK. Figure 1A and B reprinted, and the data in Fig. 1C and D replotted from ref. 88 with permission from Science. Vascular smooth muscle cells were used for all experiments. (A) shows a Western blot of the two ERK bands (p42 and p44) of samples precipitated with anti-phosphotyrosine antibodies and probed with anti phospho-ERK antibodies. (B) Western blot of total MAPK from whole cell lysates. (C) [3H]Thymidine incorporation in quadruplicate wells (mean SEM) after stimulation of cells with PDGF in the presence or absence of catalase. The data for the cells grown on basal medium were not significantly different from the control (ANOVA, p = 0.868). The data for the PDGF-treated cells were significantly different and the post-hoc test indicated that 3,000 U/mL, but not 300 U/mL, of catalase was significantly different from the control (ANOVA, p ≤ 0.002, F = 22.7; Holm–Sidak post-hoc test, p ≤ 0.05). Each value represents the average of three replicates ± one standard error. (D) Effect of intracellular catalase activity on PDGF-stimulated VSMC chemotaxis, as determined by the number of cells that migrated. The data were significantly different and the post-hoc test indicated that 300 and 3000 U/mL, but not 30 U/mL, of catalase was significantly different from the control (ANOVA, p ≤ 0.004, F = 10.175; Holm–Sidak post-hoc test, p ≤ 0.05). Each value represents the average of three replicates ± one standard error.
Gap Junctions, a Major Link in Systems Control of Mitogenesis and Cancer
The successful transmission of an extracellular signal from the membrane to the nucleus via intracellular signal transduction pathways in solid tissue cell types is also dependent upon intercellular signals through gap junctions (98, 99, 104). Gap junctions allow the passive transmission of intercellular signals within a tissue that ultimately modulate signal transduction (99). Gap junctions consist of two hexameric channels, termed connexons, docked to each other between adjacent cells. The family of proteins that form the hexameric connexon is termed connexins. Tissue homeostasis is maintained by opened gap junctional channels and, although the transient closure of channels is a normal response to growth factors, chronic inhibition of gap junctional intercellular communication (GJIC) by oncogenes, tumor promoters, or the chronic exposure to growth factors and cytokines via compensatory hyperplasia and chronic inflammation can lead to increases in proliferation and decreases in apoptosis and differentiation, leading to pathologies such as cancer and cardiovascular diseases (99).
The evidence for the role of GJIC in cancer has been extensively reviewed (25, 36, 50, 51, 55, 64, 72, 75, 81, 92) and the following are highlights of these papers: (a) exogenous and endogenous tumor promoters reversibly inhibit GJIC; (b) structure–activity relationship models showed a high concordance between inhibition of GJIC and the tumorigenicity of chemicals; (c) oncogenes inhibit GJIC; (d) tumor suppressor genes upregulate GJIC; (e) anti-tumor promoters upregulate GJIC; (f) restoration of GJIC in tumorigenic cells via transfection with gap junction genes results in normal growth and morphology of cells; (g) antisense gap junction genes transfected into cancer cells augment foci formation; (h) connexin 32 (Cx32) knockout mice administered with a single dose of an initiator, diethylnitrosamine, had 3.3 to 12.8 times increase of preneoplastic foci as compared to the wild type, while mice not treated with diethylnitrosamine did not exhibit preneoplastic lesions, indicating that the deletion of Cx32 promoted the carcinogenic effect of the initiator; (i) Cx32 knockout mice also exhibited increased levels of radiation and chemical-induced lung and liver tumor formation; and (j) the formation of heterotypic gap junctions between metastatic cells and cells of the target tissue leads to preferential metastasis. Intercellular communication through gap junction channels is a key component of the systems control of cellular events within a tissue that allows for the coordination of intracellular control of the metabolism and expression of genes between contiguous syncytium of cells into an organized hierarchal multicellular system. Interruption of this intercellular communication system allows for a change in the epigenome of the cells that permits aberrant cell growth and organization into tumorigenic tissues independent of the host organism's tissue.
Gap Junctions, Key Cell Structures in the Development of Multicellular Organisms and Tissue Homeostasis
Theodosius Dobzhansky wisely stated, “Nothing in biology makes sense except in the light of evolution” (23). In that context, it might be questioned as to why the gap junction and its associated family of highly conserved genes should be singled out as being more important in the evolution of the metazoan over the mitochondria, spindle fibers, nuclear membrane, tight junction, etc. Clearly, gap junctions are only one of the critical and vital structures/functions of a metazoan. What sets it apart is that it helps create and integrate extracellular phenotypes and functions that the individual cell does not possess, in particular, single cell organisms such as a bacterium. The evolutionary transition from the single cell organism to the first multicellular metazoan required the introduction of several new phenotypes, namely, growth control within this society of grouped cells via “contact-inhibition”; induction of specialized cells or “differentiation” through patterned expression of a large set of genes, not all of which can be expressed at the same time; and controlled cell suicide or apoptosis to allow cells that are useful at one stage of development to be replaced by a new group of cells needed at the next phase of development. The structure/function of specific gap junctions that allows new phenotypes, such as growth control and multiple types of gene patterns to be expressed in cells, all containing the same genome, to allow groups of contiguous, but not gap junction-coupled, cells to differentiate independently of each other, and to allow different phenotypes/functions to emerge when a collection of normal cells aggregates.
In the evolutionary-derived multicellular cellar organisms, three major cell types appeared in the various tissues and organs, namely, the stem cells (totipotent, pluripotent, multipotent, bipolar), the progenitor or transit cells with a finite life span, and the terminally differentiated cells. Homeostatic regulation of cell proliferation, differentiation, and apoptosis of these three cell types was facilitated via extra-(hormones, cytokines, growth factors), intra- (different intracellular signaling mechanisms, such as MAPK; NF-κB), and gap-junctional intercellular communication mechanisms needed to be delicately integrated, with the demonstration that stem cells seem to lack GJIC to restrict differentiation but still maintain growth control via negative secreted growth factors (96, 102). In addition, with experimental evidence linking gap junctions to growth control in progenitor cells (92, 94, 102), and apoptosis in solid tissues (112), the demonstration that cancer cells, characterized by being a disease of homeostasis, lacks growth control, can not terminally differentiate or apoptose properly, seems to support the role of gap junction genes as the “biological Rosetta Stone” that allows one to view them as allowing a systems mechanism to create higher order structures and functions. Without gap junctions, the higher order phenotypes and functions existing during different stages of embryonic/fetal/neonatal, adolescent, adult, and geriatric development could not exist. Endogenous and exogenous chemicals and genetic factors, that influence gap junction function, can cause a wide range of abnormal development and functional processes in many diseases. This illustrates that these gap junction functions are as vital to normal development and function as any other gene. Alteration of the many other non-gap junction genes, that can influence either survival or disease state, is probably affecting gap junctional intercellular communication indirectly.
Last, the connection of some new concepts, namely the role of the quality and quantity of adult stem cells (93) and of the expression and function of gap junctions, must be integrated into any “systems” approach to understand the higher order function of the epigenome that regulates the genomic information. After all, the genomic information is but a “blueprint.” It is the delicate and systematic differential expression of that genetic information that leads to normal development and function. This was beautifully stated by C. Markert (69): “Cells interact and communicate during embryonic development and through inductive stimuli mutually direct the divergent courses of their differentiation. Very little cell differentiation is truly autonomous in vertebrate organisms. The myriad cell phenotypes present in mammals, for example, must reflect a corresponding complexity in the timing, nature, and amount of inductive interactions. Whatever the nature of inductive stimuli may be, they emerge as a consequence of specific sequential interactions of cells during embryonic development. The first embryonic cells, blastomeres, of mice and other mammals are all totipotent. During cleavage and early morphogenesis, these cells come to occupy different positions in the three-dimensional embryo. Some cells are on the outside, some inside. The different environments of these cells cause the cells to express different patterns of metabolism in accordance with their own developing programs of gene function. These patterns of metabolism create new chemical environments for nearby cells and these changed environments induce yet new programs of gene function in responding cells. Thus, a progressive series of reciprocal interactions is established between the cellular environment and the genome of each cell. These interactions drive the cell along a specific path of differentiation until a stable equilibrium is reached in the adult. Thereafter, little change occurs in the specialized cells, and they become remarkably refractory to changes in the environment. They seem stably locked into the terminal patterns of gene function characteristic of adult cells. The genome seems no longer responsible to the signals that were effective earlier in development. Of course, changes can occur in adult cells that lead to renewed cell proliferation and altered differentiation as seen in neoplasms, both benign and malignant, but such changes are very rare indeed when one considers the number of cells potentially available for neoplastic transformation. Possibly mutations in regulatory DNA of dividing adult cells can occasionally lead to new and highly effective programs gene function that we recognize as neoplastic or malignant. However, most genetic changes in adult cells can probably lead to cell death since random changes in patterns of gene activity are not likely to be beneficial.”
Disruption of Gap Junction, a Central Function in Diseases
As noted in the “Oxidative signaling mechanisms in mitogenesis and cancer” section, the dysregulation of gap junctions is a common phenotype of cancer cells. However, altered gap junction functions are also linked to many other human diseases. Chronic exposure to epigenetic toxicants have been implicated in teratogenesis (95), reproductive dysfunction (32, 59, 114), altered muscle contractions in the heart and uterus (16, 17, 19–21, 46), and implicated in neurotoxic effects (97). More recently, the development of gap junction knockout mouse model systems and the identification of mutated gap junction genes in human diseases have greatly expanded our view of the role gap junctions play in various pathological conditions.
The first human disease specifically linked to a mutation of a gap junction gene, connexin32 (Cx32), was the X-linked form of Charcot–Marie–Tooth syndrome, which is a neuropathy that results from demyelination and axonal degeneration of peripheral nerves (7). The mutations of Cx32 have been implicated in the interruption of normal diffusion of metabolites between the Schwann cell body and its distal processes (27, 84). Since this discovery, many other mutations of various connexins have been linked to human diseases as reviewed by Kelsell et al. (47), such as hearing loss associated with mutations in Cx32, Cx26, Cx31, and Cx30; dominant epidermal diseases with Cx26; dominant skin diseases (erthrokeratoderma variablis) with Cx 31, and Cx 30.3; Clouston's hidrotic ectodermal dysplasia with Cx30, association with visceroatrial heterotaxy with Cx43; and dominant zonular pulverant cataract with Cx46 and Cx50.
Various knockout mouse model systems have produced surprising phenotypic results with some discrepancies between mouse knockout model systems and the human genetic connexin disorders (47). However, many of these studies indicated there is tissue-specific compensation between multiple connexins. The fact that not all connexin knockout model systems result in embryonic lethality and that some tissues that express a predominant form of connexin exhibited no tissue abnormalities while others did, lend credence to multiple connexins compensating for each other. As summarized by Kelsell et al. (47), knockouts for Cx32 resulted in hepatic abnormalities and susceptibility to hepatic tumors and mild peripheral neuropathies; for Cx 26 resulted in embryonic lethality at day 11; for Cx31 resulted in transient placental dysmorphogenesis; for Cx43 resulted in abnormal cardiac development and subsequent embryonic death; for Cx46 resulted in cataract formation; and for Cx 50 resulted in cataract formation and microphthalmia.
Oxidative Control of Gap Junctions
Considering that gap junctions play a critical role in maintaining the balance between proliferation, differentiation, and apoptosis of cells within a tissue, and many of the signal transduction pathways involved in cell proliferation is redox regulated, it is not surprising that gap junctions can also be regulated through redox mechanisms (44, 60–63, 67, 80, 103, 109). Some of the earliest evidence of oxidative stress in gap junction function are (a) oxidative-dependent hepatotoxic levels of carbon tetrachloride reversibly block GJIC between rat hepatocytes (81a); (b) antioxidants prevent tumor promoter-induced inhibition of GJIC in mouse hepatocytes (80a); (c) paraquat-generated ROS inhibit GJIC in mouse hepatocytes. More recently, we reported that hydrogen peroxide inhibited GJIC in a dose-dependent fashion (103). Free radical scavengers such as propylgallate and Trolox®, which do not affect H2O2 but scavenge its downstream products such as hydroxyl, alkoxyl, or peroxyl radicals, do not reverse the inhibitory effect of H2O2 (101, 103), which implicates that the redox regulation of gap junctions is by H2O2 and not its metabolites. Inhibition of GJIC by H2O2 is reversible (103) indicating that redox regulation of GJIC is not a consequent of irreversible protein damage.
Glutathione (GSH) in its reduced form is the major reductant of H2O2 in mammalian cells, and the depletion of this antioxidant commonly results in greater oxidative damage of macromolecules. This two-electron reduction of H2O2 by GSH to H2O is catalyzed by glutathione peroxidase, and clearly serves as a protective role against peroxide-dependent oxidative injury. A previous report determined the effect of depleting GSH on H2O2-dependent regulation of GJIC in a rat liver epithelial cell line (103). GSH depletion in this study was achieved by either the conjugation of GSH with diethylmaleate (DEM) or by inhibiting γ-glutamylcysteine synthetase, which catalyzes the rate-limiting step of the biosynthesis of GSH, with buthionine sulfoximine (BSO). When cells were treated with BSO for 24 h and DEM for 1 h, GSH level decreased by 72% and 95%, respectively (Fig. 2A and B, (103)). The depletion of GSH alone did not alter GJIC [Fig. 2A, (103)], but the addition of H2O2 to these GSH-depleted cells completely reversed the GJIC-inhibitory properties of H2O2, [Fig. 2B, (103)]. When cells were treated with BSO for only 1 h, no depletion of GSH was observed, and the addition of H2O2 to these GSH-sufficient cells containing BSO resulted in the inhibition of GJIC (Fig. 2B, (103)), which indicates that GSH depletion, and not the BSO, prevented H2O2 from inhibiting GJIC. This paradoxical reversal of the biological effect of H2O2 in the inhibition of GJIC contrasts with the traditional paradigm of oxidative damage where the removal of the primary antioxidative defense system results in increased damage.
FIG. 2.
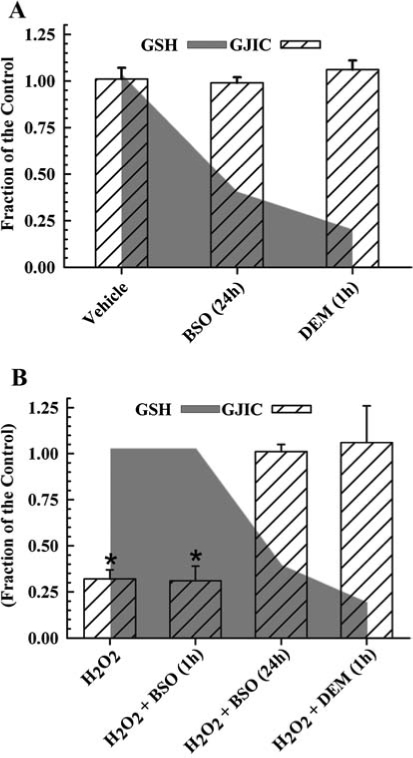
The role of GSH on H2O2-induced inhibition of GJIC. This figure was graphed with data obtained from Ref. 103 with permission from Acta Medica Nagasakiensia. (A) Averaged results of the scrape load-dye transfer data of GJIC from cell sufficient and deficient of GSH. (B) H2O2 effect on GSH depleted cells. The GSH levels are indicated in the shaded gray area, and GJIC activity in the hatched bars. The concentrations of H2O2, buthionine sulfoximine (BSO), and diethylmaleate (DEM) were 500, 100, and 1000 μM, respectively. The data are reported as a fraction of the control, and GJIC assessed using the scrape load/dye transfer technique. GSH was assessed with HPLC-electrochemical detection. The H2O2, H2O2 + BSO (1 h) treatments (GSH sufficient cells) were significantly different (*) from the control, however BSO (24 h) ± H2O2 or DEM (1 h) ± H2O2 (GSH deficient cells) were not significantly different from the control (ANOVA, p ≤ 0.001, F = 43.1; Holm–Sidak post-hoc test, p ≤ 0.05). Each value represents the average of three replicates ± one standard deviation. The cells used for these experiments is the established epithelial cell line F344 WB isolated from the livers of adult male Fischer 344 rats (100) generously donated from Drs. J. W. Grisham and M. S. Tsao, University of North Carolina (Chapel Hill, NC).
Other examples of this phenomenon where depletion of GSH prevents an oxidative chemical-induced cellular event is in: (a) lindane-dependent inhibition of GJIC in myometrial smooth muscle cells (67), (b) peroxide induction of c-jun (56), and (c) the activation of NF-κB by H2O2 and O2·− (35). These results indicate that signaling pathways not only require H2O2 for activation but also GSH. The implications are that reductive scavenging systems, which typically protect against damage from high levels of oxidative stress, also are potentially involved in the normal oxidative signaling systems at lower levels of oxidative tensions.
Oxidative Integration of GJIC and Mitogenic Signal Transduction
A common response of a cell to growth factors is a transient production of H2O2 that is an essential coactivator of mitogen-activated protein kinases (MAPKs) (24, 90). NADPH oxidase, an enzyme that synthesizes H2O2, is a well-characterized enzyme known to play an antimicrobial role in phagocytic cell types (6, 57). More recently, various NADPH oxidases in nonphagocytic cells have been implicated in oxidative signaling and have been specifically linked to the activation of various MAPKs in response to different growth factors and cytokines (2, 5, 8, 11–14, 48, 65, 66, 68, 78, 82, 83, 85, 91, 107, 110, 115).
Diphenyleneiodonium (DPI) is a selective inhibitor of NADPH oxidase that has been extensively used to link the activity of this enzyme with specific cell functions and signaling mechanisms (90). Preincubation of DPI with the F344-WB cells prevented epidermal growth factor (EGF)-induced inhibition of GJIC (Fig. 3) (101), thus implicating NADPH oxidase in EGF-induced inhibition of GJIC. Inhibition of GJIC by EGF was also prevented by blocking MAPK/ERK kinase (MEK) activation with PD98054 (Fig. 3) (101), which agrees with previously published results (18, 43). These results are not unexpected considering that the MEK–ERK pathways are redox regulated, as cited above.
FIG. 3.
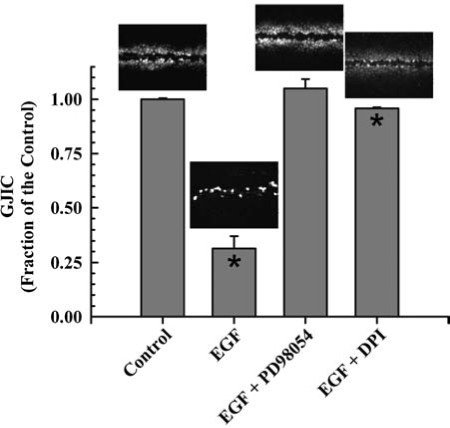
Involvement of Mek and NADPH oxidase in EGF-induced inhibition of GJIC. The figure was graphed with data obtained from ref. 101 with permission of Acta Medica Nagasakiensia. Averaged results of the scrape load-dye transfer data with a representative image of the cells at a magnification of 200X is placed above each bar. The concentrations of epidermal growth factor (EGF), diphenyleneiodonium (DPI), and PD98059 were 10 μg/l, 5 μM, and 20 μM respectively. Cells were incubated for 15 min with EGF and preincubated with PD98059 and DPI for 15 and 20 min, respectively. Quantitative results were from triplicate sets of data of SL/DT images presented. Only the EGF and EGF + DPI treated cells were significant (*) from the control (ANOVA, p < 0.001, F = 304; Tukey post-hoc p < 0.05). The cells used for these experiments is the established epithelial cell line F344 WB isolated from the livers of adult male Fischer 344 rats (100) generously donated from Drs. J. W. Grisham and M. S. Tsao, University of North Carolina (Chapel Hill, NC).
A major implication of these results is that gap junctions serve as a modulator of a signal transduction pathway, specifically MAPK (Fig. 4). Hypothetically, if gap junctions were not closed, then the generation of H2O2, an essential signaling cofactor of MAPK, in response to extracellular ligands could result in the dilution of H2O2 from the target cell into the neighboring cells, thereby decreasing the concentration of H2O2 to a nonthreshold level that would be insufficient for the activation of MAPK (Fig. 5). Superoxide and hydroxyl radicals are transient and unstable ROS that are unlikely to travel significant distances from the site of synthesis. However, H2O2 is a fairly stable intermediate capable of migrating from the site of production. Although there are no reports of H2O2 traversing the gap junction channels, this molecule, like H2O, is a very small polar compound and could conceivably traverse the channels similar to H2O. This dilution effect of a low molecular weight signaling cofactor would be quite prominent in an asymmetric environment that is typical of real tissues. Thus one significant role of gap junctions would be to maintain above threshold concentrations of positive signaling cofactors, which is consistent with closure of gap junction channels during mitogenesis. This hypothesis also allows for the prediction that the opened channel state, typically seen for differentiation and apoptosis, is needed to prevent threshold concentrations of negative signaling cofactors. These examples demonstrate how extra-, intra-, and intercellular signaling pathways might interact to coordinate the epigenetic expression of genes in response to ROS (Figs. 4 and 5).
FIG. 4.
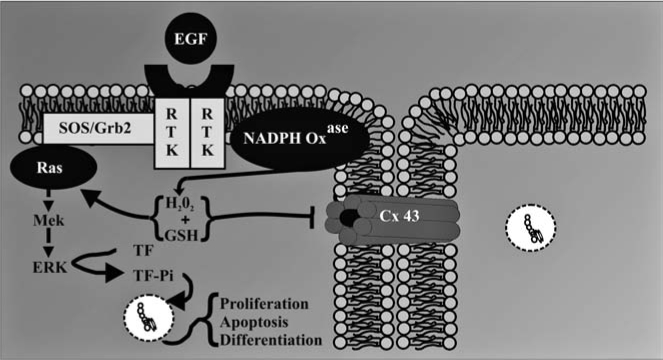
Gap junctions in cellular homeostasis. The figure was reproduced from ref. 101 with permission of Acta Medica Nagasakiensia. Extracellular signals, such as growth factors, interact with membrane receptors, which then activate intracellular signal transduction pathways that induce the transcription of genes via activated transcription factors. These intracellular pathways operate under cascade and scaffolding systems that cross-communicate with each other in controlling the expression of genes that direct the proliferation, differentiation, and apoptosis of cells within a tissue. These multiple checkpoints are further modulated by intercellular signals traversing gap junctions, thereby maintaining the homeostatic state of a tissue. Abnormal interruption of these integrated signaling pathways by food related and environmental toxicants results in diseased states, such as cancer.
FIG. 5.
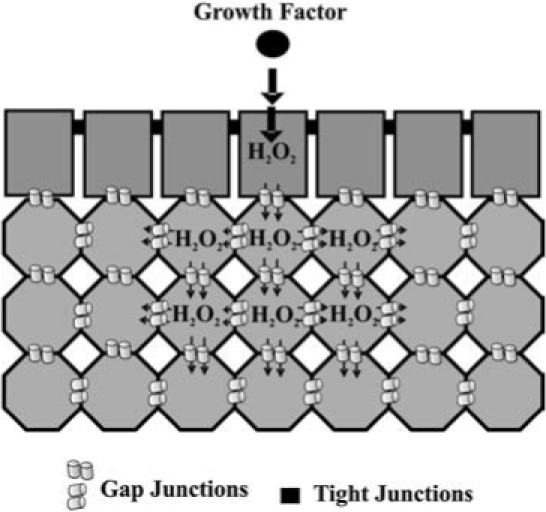
Gap junctional modulation of signal transduction. Signal transduction begins with a cell receptor binding to a ligand within an asymmetric tissue. Varying arrangements of cell types, as well as impermeable barriers to ligands created by tight junctions significantly contributes to tissue asymmetry, thus limiting the cell types that interact with soluble extracellular ligands. We hypothesize that these active ligand- receptor complexes recruit signal transduction proteins that generate small molecular weight (≤1000 Da) cofactors such as H2O2 produced in response to extracellular signals. Transient closure of gap junction channels during mitogenic initiation are, thus, needed to prevent a low molecular weight cofactor (i.e., H2O2) from diluting through the tissue to subthreshold levels needed for the activation of signal transduction pathways such as MAPK.
Signaling Pathway-Specificity of Antioxidants: Implications in Prevention and Therapy of Diseases
Traditionally, oxidative stress was viewed as a consequence of too much reactive oxygen produced for the innate reductive scavenging system of a cell to handle, resulting in cellular damage. Certainly this is true for many acute oxygen toxicities that can lead to tissue necrosis, such as that seen in ischemia and reperfusion. But this is an overly simplistic view, considering that reactive oxygen as well as the innate scavenging systems is also involved in redox sensitive signaling. Considerable progress has been achieved in defining specific redox-dependent targets of intracellular oxidants (26) and the role of antioxidants similarly play specific roles, both redox sensitive and redox insensitive, in cell signaling and consequent gene expression profiles (4). Thus, understanding the mechanisms by which oxidative compounds promote and antioxidants prevent the development of tumors must begin integrating the different forms of cell signaling (102). To continue using the simple assumption that ROS are always detrimental to the organism and that high levels of antioxidants must be beneficial due to their scavenging properties of ROS is no longer acceptable. For example, previous intervention studies with antioxidant supplementation to human populations proved either ineffective, such as the N-acetylcysteine EUROSCAN studies in Europe (108, 113), or actually detrimental to human health, such as increased lung cancer of smokers in the β-carotene studies (CARET trials in USA and ABTC trials in Finland) (37, 113).
The complexity of oxidant and antioxidant interactions is apparent in a study of the effects of organic peroxides and antioxidants on gap junctions (102). As predicted, the tumor-promoting organic peroxides (33, 39), benzoylperoxide and dicumyl peroxide, inhibited GJIC and activated extracellular kinase (ERK)-MAPK, and the nonpromoting peroxide (33, 39), t-butylperoxide, had no effect on these two endpoints (102), yet t-butylperoxide caused considerably more oxidative DNA damage than BzOOH (45). Resveratrol, an antioxidant found in the products of grapes, such as red wine, and peanuts, prevented the inhibition of GJIC by dicumyl peroxide, which closed gap junction channel through a phosphatidylcholine-specific phospholipase C (PC-PLC) but not benzoylperoxide, which closed channels in a PC-PLC-independent way (102). The effect of resveratrol on ERK-MAPK was less specific where resveratrol blocked the activation of ERK by both benzoylperoxide and dicumylperoxide (102). The specificity of the antioxidant effect of resveratrol is further demonstrated by the lack of a protective effect of N-acetylcysteine (NAC) on preventing activation of ERK and the inhibition of GJIC by either tumor promoting organic peroxides. However, NAC did prevent the general oxidative events of BzOOH, such as the depletion of reduced glutathione (GSH) and the induction of cytotoxicity (102). These results clearly indicate diverse roles that antioxidants can play in cell signaling and the notion that by simply preventing general oxidative damaging events is not sufficient in protecting against oxidative promoters. The closing statement presented in the minireview of Azzi et al. (4) “Finally, the discrepancies in the outcome of the intervention studies may be understood if, instead of considering the simple paradigm of bad oxidants and good antioxidants, scientists will start to talk about the real molecular function of such compounds in each particular situation.” eloquently reflects the importance of studies focusing on the redox and nonredox mechanisms of antioxidants on signaling network systems.
Conclusions
Although oxidative damage is clearly a factor in many pathologic conditions affiliated with oxidative stress, oxidative signaling probably plays a greater role in chronic diseases. Thus, it will be crucial to understand how various oxidants and antioxidants specifically interact with cell signaling systems, and determine what levels are needed for gene expression controlling normal cellular tissue phenotypes. Considering that reactive oxygen is an essential participant in many normal cell signaling systems, then pathologic levels may not always result with an excess generation of reactive oxygen but also from deficient levels. Understanding the specific roles of antioxidants in cell signaling will better enable us to develop more effective and safer intervention strategies. Exposure to high levels of a single antioxidant could easily overwhelm a specific effect of a precise signaling protein or small group of signaling proteins, thus resulting in a toxic rather than beneficial effect. Cell signaling mechanisms affected by redox chemistries are not limited to only the signal transduction pathways of intracellular signaling but also involve intercellular signaling through gap junctions. Thus, any comprehensive-systems biology approach to understanding oxidative signaling must also include gap junctions, in which aberrant gap junctions have been clearly implicated in many human diseases. This is not surprising, considering that gene expression must be coordinated between cells of a tissue in order to maintain tissue homeostasis. We propose a hypothesis that one potential function of gap junctions is to modulate levels of second messengers that are either positive or negative cofactors needed in signal transduction. Several reactive oxygen species, such as superoxide, hydrogen peroxide, nitrous oxide, all have very low molecular weights not much different from that of water, thus can be predicted to readily traverse the gap junction channels, and can consequently serve as ideal second messengers in a network of signaling pathways that include gap junctions. Taking a more comprehensive-systems approach in understanding the redox mechanisms of cell signaling networks will greatly aid efforts in the prevention and treatment of diseases affected by oxidative stress.
Acknowledgments
This research was supported by NIEHS grants #R01 ES013268-01A2 to Upham. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS.
Abbreviations
BSO, buthionine sulfoximine; DEM, diethylmaleate; DPI, diphenyleneiodonium; EGF, epidermal growth factor; ERK, extracellular receptor kinase; GJIC, gap junctional intercellular communication; MEK, MAPK/ERK kinase; MAPK, mitogen activated protein kinase; ROS, reactive oxygen species.
References
- 1.Allen RG. Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 2.Ammendola R. Ruocchio MR. Chirico G. Russo L. De Felice C. Esposito F. Russo T. Cimino F. Inhibition of NADH/NADPH oxidase affects signal transduction by growth factor receptors in normal fibroblasts. Arch Biochem Biophys. 2002;397:253–257. doi: 10.1006/abbi.2001.2641. [DOI] [PubMed] [Google Scholar]
- 3.Anghileri LJ. Warburg's cancer theory revisited: A fundamentally new approach. Arch Geschwulstforsch. 1983;53:1–8. [PubMed] [Google Scholar]
- 4.Azzi A. Davies KJ. Kelly F. Free radical biology–-terminology and critical thinking. FEBS Lett. 2004;558:3–6. doi: 10.1016/s0014-5793(03)01526-6. [DOI] [PubMed] [Google Scholar]
- 5.Bae YS. Sung JY. Kim OS. Kim YJ. Hur KC. Kazlauskas A. Rhee SG. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:10527–10531. doi: 10.1074/jbc.275.14.10527. [DOI] [PubMed] [Google Scholar]
- 6.Behe P. Segal AW. The function of the NADPH oxidase of phagocytes, and its relationship to other NOXs. Biochem Soc Trans. 2007;35:1100–1103. doi: 10.1042/BST0351100. [DOI] [PubMed] [Google Scholar]
- 7.Bergoffen J. Scherer SS. Wang S. Scott MO. Bone LJ. Paul DL. Chen K. Lensch MW. Chance PF. Fischbeck KH. Connexin mutations in X-linked Charcot–Marie–Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- 8.Brar SS. Kennedy TP. Sturrock AB. Huecksteadt TP. Quinn MT. Murphy TM. Chitano P. Hoidal JR. NADPH oxidase promotes NF-kappaB activation and proliferation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;282:L782–L795. doi: 10.1152/ajplung.00206.2001. [DOI] [PubMed] [Google Scholar]
- 9.Cadet J. Douki T. Ravanat JL. Artifacts associated with the measurement of oxidized DNA bases. Environ Health Perspect. 1997;105:1034–1039. doi: 10.1289/ehp.105-1470384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerda S. Weitzman SA. Influence of oxygen radical injury on DNA methylation. Mutat Res. 1997;386:141–152. doi: 10.1016/s1383-5742(96)00050-6. [DOI] [PubMed] [Google Scholar]
- 11.Chan SH. Hsu KS. Huang CC. Wang LL. Ou CC. Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 12.Chen JX. Zheng H. Lawrence ML. Blackwell TS. Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;291:H1563–H1572. doi: 10.1152/ajpheart.01081.2005. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q. Li W. Quan Z. Sumpio BE. Modulation of vascular smooth muscle cell alignment by cyclic strain is dependent on reactive oxygen species and P38 mitogen-activated protein kinase. J Vascular Surg. 2003;37:660–668. doi: 10.1067/mva.2003.95. [DOI] [PubMed] [Google Scholar]
- 14.Cheng–Hsien C. Yung–Ho H. Yuh–Mou S. Chun–Cheng H. Horng–Mo L. Huei–Mei H. Tso–Hsiao C. Src homology 2-containing phosphotyrosine phosphatase regulates endothelin-1-induced epidermal growth factor receptor transactivation in rat renal tubular cell NRK-52E. Pflugers Archiv: Eur J Physiol. 2006;452:16–24. doi: 10.1007/s00424-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 15.Colavitti R. Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–281. doi: 10.1080/15216540500091890. [DOI] [PubMed] [Google Scholar]
- 16.Cole WC. Garfield RE. Evidence for physiological regulation of myometrial gap junction permeability. Am J Physiol. 1986;251:C411–C420. doi: 10.1152/ajpcell.1986.251.3.C411. [DOI] [PubMed] [Google Scholar]
- 17.Cole WC. Picone JB. Sperelakis N. Gap junction uncoupling and discontinuous propagation in the heart. A comparison of experimental data with computer simulations. Biophys J. 1988;53:809–818. doi: 10.1016/S0006-3495(88)83160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cottrell GT. Lin R. Warn–Cramer BJ. Lau AF. Burt JM. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am J Physiol Cell Physiol. 2003;284:C511–C520. doi: 10.1152/ajpcell.00214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Mello WC. Cell-to-cell communication in heart and other tissues. Prog Biophys Mole Biol. 1982;39:147–182. doi: 10.1016/0079-6107(83)90016-0. [DOI] [PubMed] [Google Scholar]
- 20.De Mello WC. Influence of intracellular renin on heart cell communication. Hypertension. 1995;25:1172–1177. doi: 10.1161/01.hyp.25.6.1172. [DOI] [PubMed] [Google Scholar]
- 21.De Mello WC. Impaired cell communication in the diabetic heart. The role of the renin angiotensin system. Mol Cell Biochem. 2007;296:53–58. doi: 10.1007/s11010-006-9297-1. [DOI] [PubMed] [Google Scholar]
- 22.Dhillon AS. Hagan S. Rath O. Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 23.Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Amer Biol Teacher. 1973;35:125–129. [Google Scholar]
- 24.Esposito F. Ammendola R. Faraonio R. Russo T. Cimino F. Redox control of signal transduction, gene expression and cellular senescence. Neurochem Res. 2004;29:617–628. doi: 10.1023/b:nere.0000014832.78725.1a. [DOI] [PubMed] [Google Scholar]
- 25.Evert M. Morphology and morphometric investigation of hepatocellular preneoplastic lesions and neoplasms in connexin32-deficient mice. Carcinogenesis. 2002;23:697–703. doi: 10.1093/carcin/23.5.697. [DOI] [PubMed] [Google Scholar]
- 26.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 27.Fischbeck KH. Abel A. Lin GS. Scherer SS. X-linked Charcot–Marie–Tooth disease and connexin32. Ann NY Acad Sci. 1999;883:36–41. [PubMed] [Google Scholar]
- 28.Frein D. Schildknecht S. Bachschmid M. Ullrich V. Redox regulation: a new challenge for pharmacology. Biochem Pharmacol. 2005;70:811–823. doi: 10.1016/j.bcp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima S. Kinoshita A. Puatanachokchai R. Kushida M. Wainbuchi H. Morimura K. Hormesis and dose-response-mediated mechanisms in carcinogenesis: Evidence for a threshold in carcinogenicity of nongenotoxic carcinogens. Carcinogenesis. 2005;26:1835–1845. doi: 10.1093/carcin/bgi160. [DOI] [PubMed] [Google Scholar]
- 30.Gabbita SP. Robinson KA. Stewart CA. Floyd RA. Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys. 2000;376:1–13. doi: 10.1006/abbi.1999.1685. [DOI] [PubMed] [Google Scholar]
- 31.Gille G. Sigler K. Oxidative stress and living cells. Folia-Microbiologica. 1995;40:131–152. doi: 10.1007/BF02815413. [DOI] [PubMed] [Google Scholar]
- 32.Gilula NB. Fawcett DW. Aoki A. The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev Biol. 1976;50:142–168. doi: 10.1016/0012-1606(76)90074-9. [DOI] [PubMed] [Google Scholar]
- 33.Gimenez Conti IB. Binder RL. Johnston D. Slaga TJ. Comparison of the skin tumor-promoting potential of different organic peroxides in SENCAR mice. Toxicol Appl Pharmacol. 1998;149:73–79. doi: 10.1006/taap.1997.8355. [DOI] [PubMed] [Google Scholar]
- 34.Gimenez–Conti IB. Binder RL. Johnston D. Slaga TJ. Comparison of the skin tumor-promoting potential of different organic peroxides in SENCAR mice. Toxicol Appl Pharmacol. 1998;149:73–79. doi: 10.1006/taap.1997.8355. [DOI] [PubMed] [Google Scholar]
- 35.Ginn–Pease ME. Whisler RL. Optimal NF kappa B mediated transcriptional responses in Jurkat T cells exposed to oxidative stress are dependent on intracellular glutathione and costimulatory signals. Biochem Biophys Res Commun. 1996;226:695–702. doi: 10.1006/bbrc.1996.1416. [DOI] [PubMed] [Google Scholar]
- 36.Glick AB. Yuspa SH. Tissue homeostasis and the control of the neoplastic phenotype in epithelial cancers. Semin Cancer Biol. 2005;15:75–83. doi: 10.1016/j.semcancer.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Goodman GE. Prevention of lung cancer. Crit Rev Oncol Hematol. 2000;33:187–197. doi: 10.1016/s1040-8428(99)00074-8. [DOI] [PubMed] [Google Scholar]
- 38.Guyton KZ. Liu Y. Gorospe M. Xu Q. Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 39.Hanausek M. Walaszek Z. Viaje A. LaBate M. Spears E. Farrell D. Henrich R. Tveit A. Walborg EFJ. Slaga TJ. Exposure of mouse skin to organic peroxides: subchronic effects related to carcinogenic potential. Carcinogenesis. 2004;25:431–437. doi: 10.1093/carcin/bgh022. [DOI] [PubMed] [Google Scholar]
- 40.Hensley K. Robinson KA. Gabbita SP. Salsman S. Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;15:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 41.Hippeli S. Heiser I. Elstner EF. Activated oxygen and free oxygen radicals in pathology: New insights and analogies between animals and plants. Plant Physiol Biochem. 1999;37:167–178. [Google Scholar]
- 42.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 43.Huang RP. Peng A. Hossain MZ. Fan Y. Jagdale A. Boynton AL. Tumor promotion by hydrogen peroxide in rat liver epithelial cells. Carcinogenesis. 1999;20:485–492. doi: 10.1093/carcin/20.3.485. [DOI] [PubMed] [Google Scholar]
- 44.Hwang JW. Park JS. Jo EH. Kim SJ. Yoon BS. Kim SH. Lee YS. Kang KS. Chinese cabbage extracts and sulforaphane can protect H2O2-induced inhibition of gap junctional intercellular communication through the inactivation of ERK1/2 and p38 MAP kinases. J Agricult Food Chem. 2005;53:8205–8210. doi: 10.1021/jf051747h. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal M. Sharma SD. Mizote A. Fujisawa M. Okada S. Differential role of hydrogen peroxide and organic hydroperoxides in augmenting ferric nitrilotriacetate (FeNTA)-mediated DNA damage: implications for carcinogenesis. Teratog Carcinog Mutagen. 2003;(Suppl 1):13–21. doi: 10.1002/tcm.10045. [DOI] [PubMed] [Google Scholar]
- 46.Kamrin MA. Carney EW. Chou K. Cummings A. Dostal LA. Harris C. Henck JW. Loch-Caruso R. Miller RK. Female reproductive and developmental toxicology: Overview and current approaches. Toxicol Lett. 1994;74:99–119. doi: 10.1016/0378-4274(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 47.Kelsell DP. Dunlop J. Hodgins MB. Human diseases: Clues to cracking the connexin code? Trends Cell Biol. 2001;11:2–6. doi: 10.1016/s0962-8924(00)01866-3. [DOI] [PubMed] [Google Scholar]
- 48.Kim BC. Kim HG. Lee SA. Lim S. Park EH. Kim SJ. Lim CJ. Genipin-induced apoptosis in hepatoma cells is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of mitochondrial pathway. Biochem Pharmacol. 2005;70:1398–1407. doi: 10.1016/j.bcp.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 49.Kimura H. Sawada T. Oshima S. Kozawa K. Ishioka T. Kato M. Toxicity and roles of reactive oxygen species. Curr Drug Targets Inflamm Allergy. 2005;4:489–495. doi: 10.2174/1568010054526287. [DOI] [PubMed] [Google Scholar]
- 50.King TJ. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis. 2004;25:669–680. doi: 10.1093/carcin/bgh071. [DOI] [PubMed] [Google Scholar]
- 51.King TJ. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene. 2005;24:1718–1726. doi: 10.1038/sj.onc.1208355. [DOI] [PubMed] [Google Scholar]
- 52.Kishida KT. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein SA. Slaga TJ. Effects of peroxides on rodent skin: Epidermal hyperplasia and tumor promotion. J Invest Dermatol. 1982;79:30–34. doi: 10.1111/1523-1747.ep12510444. [DOI] [PubMed] [Google Scholar]
- 54.Koike T. Kimura N. Miyazaki K. Yabuta T. Kumamoto K. Takenoshita S. Chen J. Kobayashi M. Hosokawa M. Taniguchi A. Kojima T. Ishida N. Kawakita M. Yamamoto H. Takematsu H. Suzuki A. Kozutsumi Y. Kannagi R. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci USA. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krutovskikh V. Yamasaki H. The role of gap junctional intercellular communication (GJIC) disorders in experimental and human carcinogenesis. Histol Histopathol. 1997;12:761–768. [PubMed] [Google Scholar]
- 56.Kuo ML. Meng TC. Lin JK. Involvement of glutathione in induction of c-jun proto-oncogene by methylmethane-sulfonate in NIH 3T3 cells. Carcinogenesis. 1996;17:815–820. doi: 10.1093/carcin/17.4.815. [DOI] [PubMed] [Google Scholar]
- 57.Lambeth JD. Kawahara T. Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 59.Larsen WJ. Wert SE. Brunner GD. A dramatic loss of cumulus cell gap junctions is correlated with germinal vesicle breakdown in rat oocytes. Dev Biol. 1986;113:517–521. doi: 10.1016/0012-1606(86)90187-9. [DOI] [PubMed] [Google Scholar]
- 60.Lee KW. Hur HJ. Lee HJ. Lee CY. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J Agric Food Chem. 2005;53:1990–1995. doi: 10.1021/jf0486040. [DOI] [PubMed] [Google Scholar]
- 61.Lee KW. Jung JW. Kang KS. Lee HJ. p38 is a key signaling molecule for H-ras-induced inhibition of gap junction intercellular communication in rat liver epithelial cells. Ann NY Acad Sci. 2004;1030:258–263. doi: 10.1196/annals.1329.032. [DOI] [PubMed] [Google Scholar]
- 62.Lee KW. Lee SJ. Kang NJ. Lee CY. Lee HJ. Effects of phenolics in Empire apples on hydrogen peroxide-induced inhibition of gap-junctional intercellular communication. BioFactors. 2004;21:361–365. doi: 10.1002/biof.552210169. [DOI] [PubMed] [Google Scholar]
- 63.Lee SJ. Lee KW. Lee HJ. Abies nephrolepis leaf phenolics prevent the inhibition of gap junction intercellular communication by hydrogen peroxide in rat liver epithelial cells. BioFactors. 2004;21:357–360. doi: 10.1002/biof.552210168. [DOI] [PubMed] [Google Scholar]
- 64.Leithe E. Sirnes S. Omori Y. Rivedal E. Downregulation of gap junctions in cancer cells. Crit Rev Oncog. 2006;12:225–256. doi: 10.1615/critrevoncog.v12.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 65.Li JM. Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 66.Lin FY. Chen YH. Tasi JS. Chen JW. Yang TL. Wang HJ. Li CY. Chen YL. Lin SJ. Endotoxin induces toll-like receptor 4 expression in vascular smooth muscle cells via NADPH oxidase activation and mitogen-activated protein kinase signaling pathways. Arterioscler Thromb Vasc Biol. 2006;26:2630–2637. doi: 10.1161/01.ATV.0000247259.01257.b3. [DOI] [PubMed] [Google Scholar]
- 67.Loch–Caruso R. Upham BL. Harris C. Trosko JE. Divergent roles for glutathione in lindane-induced acute and delayed-onset inhibition of rat myometrial gap junctions. Toxicol Sci. 2005;85:694–702. doi: 10.1093/toxsci/kfi123. [DOI] [PubMed] [Google Scholar]
- 68.Lu Q. Qiu TQ. Yang H. Ligustilide inhibits vascular smooth muscle cells proliferation. Eur J Pharmacol. 2006;542:136–140. doi: 10.1016/j.ejphar.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 69.Markert CL. Genetic control of cell interactions in chimeras. Develop Genetics. 1984;4:267–279. [Google Scholar]
- 70.McCord JM. Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 71.McCubrey JA. Lahair MM. Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 72.Mesnil M. Crespin S. Avanzo JL. Zaidan–Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Mitchel RE. Morrison DP. Gragtmans NJ. Tumorigenesis and carcinogenesis in mouse skin treated with hyperthermia during stage I or stage II of tumor promotion. Carcinogenesis. 1987;8:1875–1879. doi: 10.1093/carcin/8.12.1875. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura Y. Gindhart TD. Winterstein D. Tomita I. Seed JL. Colburn NH. Early superoxide dismutase-sensitive event promotes neoplastic transformation in mouse epidermal JB6 cells. Carcinogenesis. 1988;9:203–207. doi: 10.1093/carcin/9.2.203. [DOI] [PubMed] [Google Scholar]
- 75.Pointis G. Fiorini C. Gilleron J. Carette D. Segretain D. Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis. Curr Med Chem. 2007;14:2288–2303. doi: 10.2174/092986707781696564. [DOI] [PubMed] [Google Scholar]
- 76.Prasad KN. Cole WC. Hovland AR. Prasad KC. Nahreini P. Kumar B. Edwards–Prasad J. Andreatta CP. Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biologic rationale. Curr Opin Neurol. 1999;12:761–770. doi: 10.1097/00019052-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 77.Radosevich CA. Weitzman SA. Hydrogen peroxide induces squamous metaplasia in a hamster tracheal organ explant culture model. Carcinogenesis. 1989;10:1943–1946. doi: 10.1093/carcin/10.10.1943. [DOI] [PubMed] [Google Scholar]
- 78.Reich H. Tritchler D. Herzenberg AM. Kassiri Z. Zhou X. Gao W. Scholey JW. Albumin activates ERK via EGF receptor in human renal epithelial cells. J Am Soc Nephrol. 2005;16:1266–1278. doi: 10.1681/ASN.2004030222. [DOI] [PubMed] [Google Scholar]
- 79.Roberts PJ. Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 80.Rouach N. Calvo CF. Duquennoy H. Glowinski J. Giaume C. Hydrogen peroxide increases gap junctional communication and induces astrocyte toxicity: regulation by brain macrophages. Glia. 2004;45:28–38. doi: 10.1002/glia.10300. [DOI] [PubMed] [Google Scholar]
- 80a.Ruch RJ and Kiaunig JE. Antioxidant prevention of tumor promoter induced inhibition of mouse hepatocyte intercellular communication. Cancer Lett. 1986;33:137–150. doi: 10.1016/0304-3835(86)90018-2. [DOI] [PubMed] [Google Scholar]
- 81.Ruch RJ. Trosko JE. DC Gap-junction communication in chemical carcinogenesis. Drug Metab Rev. 2001;33:117–124. doi: 10.1081/dmr-100000137. [DOI] [PubMed] [Google Scholar]
- 81a.Saez JL and Spray DC. Gap junctional communication between hepatocytes is reversibly blocked by carbon-tetrachloride. Hepatology. 1986;6:1197. [Google Scholar]
- 82.Sano M. Fukuda K. Sato T. Kawaguchi H. Suematsu M. Matsuda S. Koyasu H. Matsui H. Yamauchi–Takihara K. Harada M. Saito Y. Ogawa S. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;89:661–669. doi: 10.1161/hh2001.098873. [DOI] [PubMed] [Google Scholar]
- 83.Saran M. To what end does nature produce superoxide? NADPH oxidase as an autocrine modifier of membrane phospholipids generating paracrine lipid messengers. Free Radic Res. 2003;37:1045–1059. doi: 10.1080/10715760310001594631. [DOI] [PubMed] [Google Scholar]
- 84.Scherer SS. Bone LJ. Deschenes SM. Abel A. Balice GR. Fischbeck KH. The role of the gap junction protein connexin32 in the pathogenesis of X-linked Charcot–Marie–Tooth disease. Novartis Found Symp. 1999;219:175–185. doi: 10.1002/9780470515587.ch11. [DOI] [PubMed] [Google Scholar]
- 85.Shen WL. Gao PJ. Che ZQ. Ji KD. Yin M. Yan C. Berk BC. Zhu DL. NAD(P)H oxidase-derived reactive oxygen species regulate angiotensin-II induced adventitial fibroblast phenotypic differentiation. Biochem Biophys Res Commun. 2006;339:337–343. doi: 10.1016/j.bbrc.2005.10.207. [DOI] [PubMed] [Google Scholar]
- 86.Slaga TJ. Klein SA. Triplett LL. Yotti LP. Trosko KE. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981;213:1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- 87.Song HJ. Kim JS. Lee MJ. Nam YS. Sohn UD. Reactive oxygen species mediate ET-1-induced activation of ERK1/2 signaling in cultured feline esophageal smooth muscle cells. Arch Pharm Res. 2007;30:1080–1087. doi: 10.1007/BF02980241. [DOI] [PubMed] [Google Scholar]
- 88.Sundaresan M. Yu ZX. Ferrans VJ. Irani K. Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 89.Thannickal VJ. Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 90.Torres M. Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 91.Touyz RM. Yao G. Viel E. Amiri F. Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22:1141–1149. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 92.Trosko JE. The role of stem cells and gap junctional intercellular communication in carcinogenesis. J Biochem Mol Biol. 2003;36:43–48. doi: 10.5483/bmbrep.2003.36.1.043. [DOI] [PubMed] [Google Scholar]
- 93.Trosko JE. Human adult stem cells as targets for cancer stem cells: Evolution; Oct-4 gene, and cell to cell communication. In: Dittmar T, editor; Zaenkar K, editor. Stem Cells and Cancer. New York: Nova Publishers; 2008. [Google Scholar]
- 94.Trosko JE. Chang CC. Role of stem cells and gap junctional intercellular communication in human carcinogenesis. Radiat Res. 2001;155:175–180. doi: 10.1667/0033-7587(2001)155[0175:roscag]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 95.Trosko JE. Chang CC. Netzloff M. The role of inhibited cell-cell communication in teratogenesis. Teratog Carcinog Mutagen. 1982;2:31–45. doi: 10.1002/1520-6866(1990)2:1<31::aid-tcm1770020105>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 96.Trosko JE. Chang CC. Wilson MR. Upham B. Hayashi T. Wade M. Gap junctions and the regulation of cellular functions of stem cells during development and differentiation. Methods. 2000;20:245–264. doi: 10.1006/meth.1999.0941. [DOI] [PubMed] [Google Scholar]
- 97.Trosko JE. Jone C. Chang CC. Inhibition of gap junctional-mediated intercellular communication in vitro by aldrin, dieldrin, and toxaphene: A possible cellular mechanism for their tumor-promoting and neurotoxic effects. Molec Toxicol. 1987;1:83–93. [PubMed] [Google Scholar]
- 98.Trosko JE. Ruch RJ. Cell–cell communication in carcinogenesis. Front Biosci. 1998;3:208–236. doi: 10.2741/a275. [DOI] [PubMed] [Google Scholar]
- 99.Trosko JE. Upham BL. The emperor wears no clothes in the field of carcinogen risk assessment: ignored concepts in cancer risk assessment. Mutagenesis. 2005;20:81–92. doi: 10.1093/mutage/gei017. [DOI] [PubMed] [Google Scholar]
- 100.Tsao MS. Smith JD. Nelson KG. Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of ‘oval’ cells. Exp Cell Res. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 101.Upham BL. Trosko JE. A paradigm shift in the understanding of oxidative stress and it's implications to exposure of low-level ionizing radiation. Acta Medica Nagasakiensia. 2006;50:63–68. [Google Scholar]
- 102.Upham BL. Guzvic M. Scott J. Carbone JM. Blaha L. Coe C. Li LL. Rummel AM. Trosko JE. Inhibition of gap junctional intercellular communication and activation of mitogen-activated protein kinase by tumor-promoting organic peroxides and protection by resveratrol. Nutr Cancer. 2007;57:38–47. doi: 10.1080/01635580701268188. [DOI] [PubMed] [Google Scholar]
- 103.Upham BL. Kang KS. Cho HY. Trosko JE. Hydrogen peroxide inhibits gap junctional intercellular communication in glutathione sufficient but not glutathione deficient cells. Carcinogenesis. 1997;18:37–42. doi: 10.1093/carcin/18.1.37. [DOI] [PubMed] [Google Scholar]
- 104.Upham BL. Weis LM. Trosko JE. Modulated gap junctional intercellular communication as a biomarker of PAH epigenetic toxicity: structure-function relationship. Environ Health Perspect. 1998;106:975–981. doi: 10.1289/ehp.98106s4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ushio–Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 106.Ushio–Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 107.Van Heerebeek L. Meischl C. Stooker W. Meijer CJ. Niessen HW. Roos D. NADPH oxidase(s): new source(s) of reactive oxygen species in the vascular system? J Clin Pathol. 2002;55:561–568. doi: 10.1136/jcp.55.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Zandwijk N. Dalesio O. Pastorino U. de Vries N. van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 109.VanSlyke JK. Musil LS. Cytosolic stress reduces degradation of connexin43 internalized from the cell surface and enhances gap junction formation and function. Mol Biol Cell. 2005;16:5247–5257. doi: 10.1091/mbc.E05-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Z. Castresana MR. Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 111.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 112.Wilson MR. Close TW. Trosko JE. Cell population dynamics (apoptosis, mitosis, and cell-cell communication) during disruption of homeostasis. Exp Cell Res. 2000;254:257–268. doi: 10.1006/excr.1999.4771. [DOI] [PubMed] [Google Scholar]
- 113.Wright GS. Gruidl ME. Early detection and prevention of lung cancer. Curr Opin Oncol. 2000;12:143–148. doi: 10.1097/00001622-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 114.Ye YX. Bombick D. Hirst K. Zhang GX. Chang CC. Trosko JE. Akera T. The modulation of gap junctional communication by gossypol in various mammalian cell lines in vitro. Fund Appl Toxicol. 1990;14:817–832. doi: 10.1016/0272-0590(90)90306-5. [DOI] [PubMed] [Google Scholar]
- 115.Zhang X. Shan P. Sasidhar M. Chupp GL. Flavell RA. Choi AM. Lee PJ. Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am J Respir Cell Mol Biol. 2003;28:305–315. doi: 10.1165/rcmb.2002-0156OC. [DOI] [PubMed] [Google Scholar]
- 116.Zong WX. Ditsworth D. Bauer DE. Wang ZQ. Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]


