Abstract
This study examined the hypothesis that curcumin supplementation decreases blood levels of IL-6, MCP-1, TNF-α, hyperglycemia, and oxidative stress by using a cell-culture model and a diabetic rat model. U937 monocytes were cultured with control (7 mM) and high glucose (35 mM) in the absence or presence of curcumin (0.01–1 μM) at 37°C for 24 h. Diabetes was induced in Sprague–Dawley rats by injection of streptozotocin (STZ) (i.p., 65 mg/kg BW). Control buffer, olive oil, or curcumin (100 mg/kg BW) supplementation was administered by gavage daily for 7 weeks. Blood was collected by heart puncture with light anesthesia. Results show that the effect of high glucose on lipid peroxidation, IL-6, IL-8, MCP-1, and TNF-α secretion was inhibited by curcumin in cultured monocytes. In the rat model, diabetes caused a significant increase in blood levels of IL-6, MCP-1, TNF-α, glucose, HbA1, and oxidative stress, which was significantly decreased in curcumin-supplemented rats. Thus, curcumin can decrease markers of vascular inflammation and oxidative stress levels in both a cell-culture model and in the blood of diabetic rats. This suggests that curcumin supplementation can reduce glycemia and the risk of vascular inflammation in diabetes. Antioxid. Redox Signal. 11, 241–249.
Introduction
Vascular inflammation and cardiovascular disease (CVD) are the leading causes of morbidity and mortality in the diabetic population and remain major public-health issues (59). TNF-α, IL-6, IL-8, and MCP-1 are proinflammatory cytokines and widely recognized markers of vascular inflammation (55, 59). The levels of these cytokines are elevated in the blood of many diabetic patients (20, 40, 44). An increase in circulating levels of TNF-α, IL-6, IL-8, and MCP-1 can lead to increased insulin resistance, vascular inflammation, and the development of vascular disease (17, 59). Review of literature suggests that curcumin may be beneficial against many diseases, including diabetes (18, 56).
The molecular mechanisms by which curcumin supplementation may inhibit or delay diabetes-associated vascular inflammation and other complications are not known. Elevated blood levels of proinflammatory cytokines and increased glycosylation of proteins, enzymes, and insulin can reduce insulin sensitivity and are risk factors in the development of cataracts and vascular disease in diabetes (29, 55, 59). However, no previous study has examined the effect of curcumin supplementation on the levels of TNF-α, IL-6, or MCP-1 in diabetic patients or in animal models of diabetes. This study examined the hypothesis that curcumin supplementation decreases levels of TNF-α, IL-6, MCP-1, and hyperglycemia in diabetes. To examine this hypothesis, we studied the effect of curcumin and placebo supplementation on blood levels of TNF-α, IL-6, MCP-1, glucose, glycosylated hemoglobin, and oxidative stress in streptozotocin-treated diabetic rats and in a cell-culture model by using monocytes exposed to high glucose levels. We also examined the effects of curcumin and placebo on liver-function markers and red-cell indices in the blood of diabetic rats.
The results of this study demonstrate that curcumin supplementation decreases a diabetes-associated increase in proinflammatory cytokines, glycosylated hemoglobin, and oxidative stress in diabetic rats, and inhibits secretion of these cytokines and oxidative stress in cultured monocytes exposed to high levels of glucose.
Materials and Methods
Human pro-monocytic cell line
The U937 monocyte cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). These cells were maintained at 37°C in RPMI 1640 medium containing 7 mM glucose, 10% (vol/vol) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 12 mM sodium carbonate, 12 mM HEPES, and 2 mM glutamine in a humidified atmosphere containing 5% (vol/vol) CO2. For treatments, cells were washed once in plain RPMI 1640 before being suspended in fresh medium (complete) containing serum and other supplements (20).
Treatment with high glucose (HG) and curcumin
U937 (500,000 cells/ml) were treated with normal glucose (7 mM) and HG (35 mM) without and with curcumin (23). Curcumin was dissolved in alcohol, and a similar volume of alcohol was added to controls. Mannitol (35 mM) was used as an osmolarity control. For cytokine-secretion studies, cells were treated with lipopolysaccharide (LPS, 2 μg/ml) at 37°C for 24 h. LPS is used to stimulate the secretion of cytokines by HG in cell-culture studies. The literature contains many examples of a monocyte cell model and LPS being used (19) to understand the mechanisms of hyperglycemia-induced secretion and the expression of proinflammatory cytokine signaling pathways. In this study, cells were exposed to a high glucose concentration of 35 mM. Many previous studies have reported that glucose concentrations as high as 50 mM have been found in the blood of patients with uncontrolled diabetes (9, 34, 36). It is true that blood glucose levels in patients are not likely to stay as high as 35 mM for 24 h. However, tissue damage in diabetic patients occurs over many years of countless hyperglycemic and/or ketotic episodes. Thus, the glucose concentration of 35 mM used in this cell-culture study and by other investigators (42) does not seem unreasonable. Previous studies in the literature (3, 13) reported blood concentrations of curcumin as high as 1.75 μM in humans given 4–8 g of oral curcumin supplementation, which suggests that the concentrations of curcumin used in the cell-culture studies are reasonable.
Animal studies
All of the procedures were carried out in accordance with the ethical standards of the institution after approval by the institutional Animal Welfare Committee. Male Sprague–Dawley rats were purchased at 49–52 days of age (200–220 g) from Harlan (Indianapolis, IN) and allowed 2 days for environmental and trainer-handling acclimation. The rats were weighed and then fasted overnight before intraperitoneal injection of 65 mg/kg streptozotocin in citrate buffer (pH, 4.5). Control rats were injected with citrate buffer alone to serve as a normal control group (group 1). The rats were tested for hyperglycemia by measuring their blood glucose concentration at 3 and 7 days after the streptozotocin injections. Blood for the blood glucose was obtained via tail incision and measured by using an Advantage Accu-chek glucometer (Boehringer Mannheim Corp., Indianapolis, IN). The rats that became hyperglycemic (blood glucose, >300 mg/dl) were randomly divided into three groups (n = 6): group 2, diabetic controls; group 3, olive oil, 100 μL/100 g body weight; group 4, 100 mg curcumin/kg body weight. Curcumin was dissolved in olive oil. Each rat was supplemented with the appropriate dose of olive oil or curcumin daily for 7 weeks by oral gavage by using 20-gauge feeding needles (Popper and Sons, New Hyde Park, NY). The diabetic controls were supplemented with a buffer. Body weight and blood glucose concentrations were monitored weekly to determine the volume of curcumin or olive oil dosage supplementation. The rats were maintained under standard housing conditions at 22 ± 2°C with 12:12-h light/dark cycles with a standard 8640 lab chow diet (Harlan, Indianapolis, IN). At the end of 7 weeks, the rats were fasted overnight and then killed for analysis by exposure to halothane (2-bromo-2-chloro-1,1,1-trifluoroethane). At the time of death, eight rats were in the control; four, in the diabetic; four, in the olive oil; and four, in the curcumin group. Blood was collected via heart puncture with a 19.5-gauge needle into EDTA vacutainer tubes. EDTA-blood was centrifuged; the clear plasma and erythrocytes were saved, and buffy-coat layers were discarded. The cells were washed with cold 0.15 M sodium chloride solution 3 times after a 1:10 dilution.
Liver-function tests, blood cell count, and blood chemistry profile
A portion of blood from rats in each group was sent to the clinical laboratory of LSUHSC-Shreveport (located in the same building) for clinical tests to determine liver function and red blood cell counts.
TNF-α, IL-6, IL-8, MCP-1, insulin and cell viability assay
The cytokine levels were determined by the sandwich ELISA method with a commercially available kit from Fisher Thermo Scientific Co. (Rockford, Ill). These kits are apparently produced by the Endogen-Pierce company and distributed by Fisher Thermo Scientific Co. All appropriate controls and standards, as specified by the manufacturer's kit, were used. In the cytokine assay, controls (when referred to in the figures) are cells treated with LPS alone. Cells without LPS were also run in each experiment. The measured values of cells without LPS were considered a blank, and this value was subtracted from those of the other treatments. We did not assay levels of IL-8 in blood of diabetic rats because a rat-specific kit for IL-8 is not commercially available. Plasma insulin was determined by using a kit and reagents from ALPCO Diagnostics (Salem, NH). Cell viability was determined by using the Alamar Blue reduction bioassay (Alamar Biosciences, Sacramento, CA). This method is based on Alamar Blue dye reduction by live cells (20).
Lipid peroxidation and protein oxidation assay
Lipid peroxidation was assessed by measuring the thiobarbituric acid (TBA) reactivity of malondialdehyde (MDA), an end product of fatty acid peroxidation (16, 25). For this purpose, either 0.2 ml cells or plasma was suspended in 0.8 ml phosphate-buffered saline and 0.025 ml butylated hydroxytoluene (88 mg/10 ml absolute alcohol). Thirty percent trichloroacetic acid (0.5 ml) was then added. The tubes were vortexed and allowed to stand on ice for at least 2 h, and then centrifuged at 2,000 rpm for 15 min. For each sample, 1 ml supernatant was transferred to a new tube. To each of these was added 0.25 ml 1% TBA in 0.05N NaOH. The tubes were then mixed and kept in a boiling-water bath for 15 min. The concentration of the MDA–TBA complex was assessed as described earlier (16, 25). Protein oxidation was determined by measuring protein carbonyl as described by Yan et al. (61).
Measurement of glycosylated hemoglobin (HbA1) and glucose
The human erythrocyte is freely permeable to glucose, and within each erythrocyte, glycosylated hemoglobin is formed continuously from hemoglobin A at a rate dependent on the ambient glucose concentration. Glycosylated hemoglobin was determined by using Glyco-Tek Affinity column kits and reagents (cat no. 5351) purchased from Helena Laboratories (Beaumont, Texas). Glucose levels were determined by using glucose oxidase by Advantage Accu-check glucometer (Boehringer Mannheim Corporation, Indianapolis, IN).
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned. Data from both cell-culture studies and rats were analyzed statistically by using one-way ANOVA between different groups with Sigma Stat statistical software (Jandel Scientific, San Rafael, CA). When data passed a normality test, all groups were compared by using the Student–Newman–Keuls method. Kruskal–Wallis One ANOVA on Ranks (Dunn's method) was used for pairwise multiple comparisons when data failed a normality test. A p value of <0.05 was considered significant.
Results
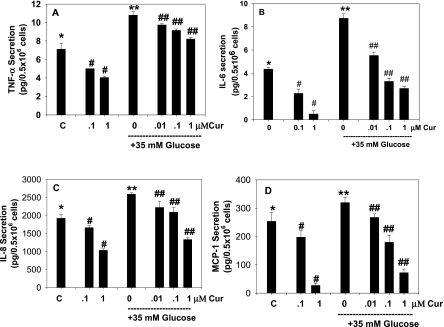
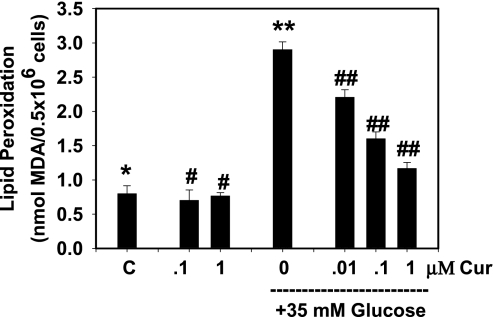
The effect of curcumin on HG-induced secretion of TNF-α is shown in Fig. 1A. High concentrations of glucose caused an increase in TNF-α. This increase in TNF-α was inhibited by supplementation with curcumin. The inhibitory effect of curcumin on TNF-α secretion was greater with increasing concentrations of curcumin, both in cells cultured with controls and in those exposed to high glucose concentrations. Similarly, data in Fig. 1B, C, and D illustrate a curcumin dose-dependent inhibition of IL-6, IL-8, and MCP-1 secretion in high glucose–treated monocytes. This shows that the inhibitory effect can be seen at curcumin concentrations of 0.01 μM. It is not clear why a difference was found in the extent of inhibitory effect in basal versus HG-treated cells at the same curcumin concentration. Severely diabetic patients do show blood glucose levels as high as 35 mM (9, 34, 36). Fig. 2 show that curcumin supplementation also prevented an increase in the lipid peroxidation caused by high glucose concentrations. Mannitol used as a control did not have any effect on TNF-α secretion or lipid peroxidation in monocytes (data not shown here).
FIG. 1.
Effect of curcumin on high glucose–induced secretion of TNF-α (A), IL-6 (B), IL-8 (C), and MCP-1 (D) in U937 monocytes. Values are expressed as mean ± SEM of four experiments. Differences between *vs#, *vs**, **vs## are significant (p < 0.05).
FIG. 2.
Effect of curcumin on high glucose–induced lipid peroxidation in U937 monocytes. Values are expressed as mean ± SEM of four experiments. Differences between *vs** and **vs## are significant (p < 0.05).
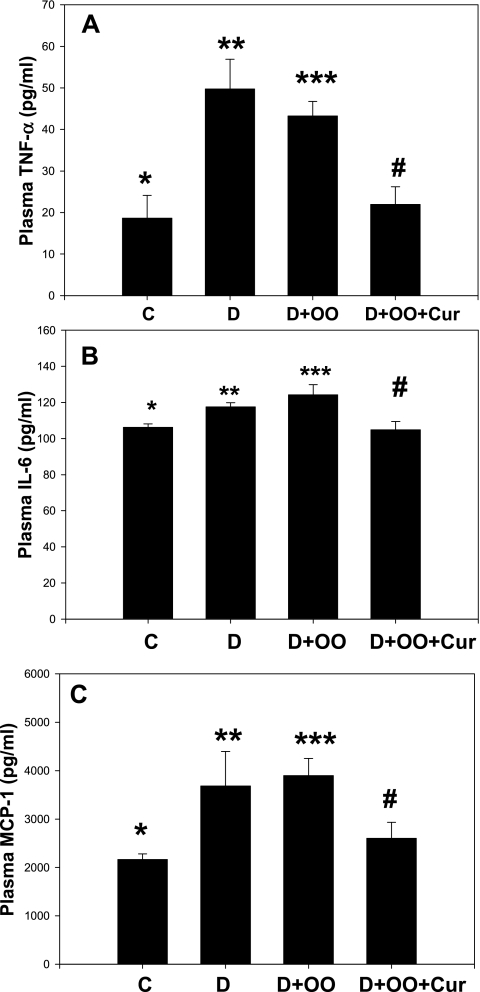
Figure 3A–C illustrates the effect of diabetes and curcumin supplementation on TNF-α, IL-6, and MCP-1 levels in the blood of diabetic rats. Significant increases in levels of TNF-α (p < 0.01), IL-6 (p < 0.05), and MCP-1 (p < 0.01) were found in the blood of diabetic rats (D) compared with that of control-normal rats (C). No difference was apparent in these cytokine levels between D and olive oil–control diabetic rats (D+OO). Curcumin supplementation to diabetic rats significantly reduced (p < 0.02) levels of TNF-α, IL-6, and MCP-1 in comparison to D+OO rats. This suggests that curcumin supplementation can reduce circulating levels of pro-inflammatory cytokines in diabetes.
FIG. 3.
Effect of curcumin supplementation on blood levels of TNF-α (A), IL-6 (B), and MCP-1 (C) in STZ-treated diabetic rats. Diabetic rats were orally supplemented with curcumin daily for 7 weeks. Values are expressed as mean ± SEM. Differences between *vs** (p < 0.01) and *** vs# are significant (p < 0.02). OO, olive oil; Cur, curcumin.
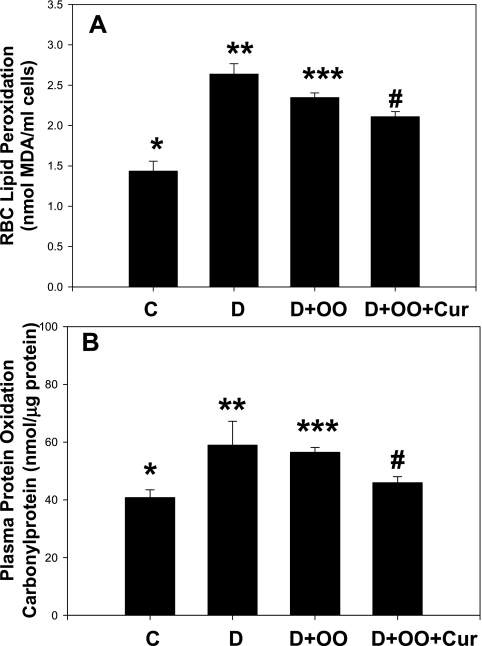
Figure 4A illustrates the effect of diabetes and curcumin supplementation on lipid peroxidation levels in the blood of diabetic rats. A significant increase was noted in levels of lipid peroxidation (p < 0.01) in the blood of diabetic rats (D) compared with that of control-normal rats (C). No difference in lipid peroxidation levels was seen between D and olive oil–control diabetic rats (D+OO). Curcumin supplementation to diabetic rats reduced levels of lipid peroxidation, but not significantly in comparison with D+OO rats. Protein-oxidation levels (Fig. 4B) in plasma were higher (p < 0.05) in the diabetic group compared with those of normal-controls. No difference in protein oxidation levels was found between diabetic rats and olive oil–control diabetic rats. Curcumin supplementation significantly reduced plasma protein oxidation levels in curcumin-supplemented (p < 0.05) diabetic rats in compared with those of the OO+D group.
FIG. 4.
Effect of curcumin supplementation on red cell lipid peroxidation (A) and plasma protein oxidation levels (B) of STZ-treated diabetic rats. Diabetic rats were orally supplemented with curcumin, daily, for 7 weeks. Values are expressed as mean ± SEM. Differences between *vs** (p < 0.01) in red cell lipid peroxidation, and for plasma protein oxidation between *vs** (p < 0.05) and ***vs# are significant (p < 0.05). OO, olive oil; Cur, curcumin.
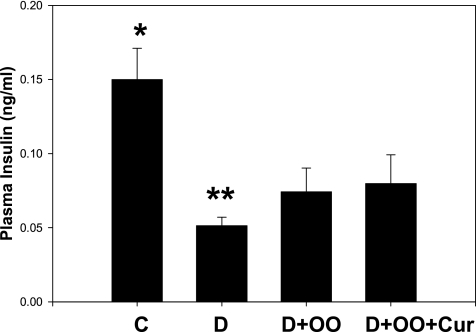
Figure 5 demonstrates that circulating insulin levels were significantly low (p < 0.02) in diabetic rats. Olive oil alone or curcumin supplementation did not significantly influence the plasma insulin concentrations in diabetic rats. Insulin levels were similar between olive oil– and curcumin-supplementation D groups.
FIG. 5.
Effect of curcumin supplementation on plasma insulin levels. Diabetic rats were orally supplemented with curcumin daily for 7 weeks. Values are expressed as mean ± SEM. Differences between *vs** are significant (p < 0.02). OO, olive oil; Cur, curcumin.
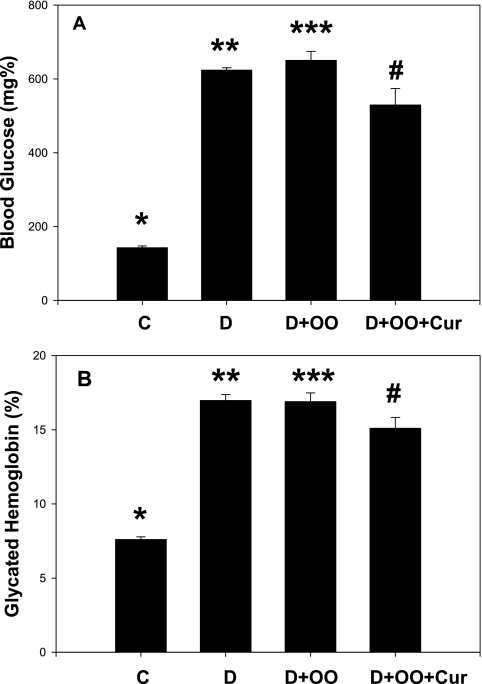
The effect of curcumin supplementation on blood glucose and glycated hemoglobin levels is shown in Fig. 6A and B. A significant increase in both blood glucose (p < 0.01) and glycated hemoglobin (p < 0.01) occurred in diabetic rats. Olive oil supplementation alone did not show any effect on blood fasting glucose or glycosylated hemoglobin. Figure 6A shows that there was a modest decrease in fasting blood glucose levels in curcumin-supplemented diabetic rats (p = 0.047) in comparison with OO+D rats. Curcumin supplementation also showed a significant effect on lowering glycated hemoglobin levels (p < 0.04) in curcumin-supplemented diabetic rats when compared with OO+D rats (Fig. 6B).
FIG. 6.
Effect of curcumin supplementation on blood levels of glucose (A) and glycated hemoglobin (B) in STZ-treated diabetic rats. Diabetic rats were orally supplemented with curcumin daily for 7 weeks. Values are expressed as mean ± SEM. Differences between *vs** (p < 0.01) and *** and # (p < 0.047) are significant. Cur, curcumin; OO, olive oil.
Curcumin supplementation did not show any effect on hemoglobin levels, hematocrit, or RBC counts in diabetic rats (data not given here). This rules out any role of altered red cell survival on lower glycosylated hemoglobin levels in curcumin-supplemented diabetic rats.
We also determined blood levels of alanine aminotransferase (ALT), alkaline phosphatase (AP), aspartate aminotransferase (AST), and total and conjugated bilirubin levels in the blood of control, diabetic, and curcumin-supplemented diabetic rats. The data indicate that diabetes per se is associated with liver damage; however, curcumin supplementation does not seem to cause any additional toxicity, as assessed by liver-function tests (data not shown here).
Discussion
Curcumin is the substance that gives the spice turmeric, which is used extensively in Indian cuisine as a component of curry powder, its yellow color (2). Curcumin is extracted from the roots of the Curcuma longa plant (turmeric) (2). Turmeric extract consists of three different curcuminoids: curcumin, demethoxycurcumin, and bis-demethoxycurcumin (2). Curcumin (diferuloylmethane) is the most active component of turmeric. It is believed that curcumin is a potent antioxidant and antiinflammatory agent (28, 39).
In experimental studies, curcumin has been shown to reduce hyperlipidemia (5), delay the development of cataracts (57), ameliorate renal lesions (4), and reduce the cross-linking of collagen (48) and nephropathy (52, 53) in a streptozotocin-treated diabetic animal model. Curcumin has also been shown to lower blood glucose levels in type 2 diabetic KK-Ay mice (41) and streptozotocin-treated rats (34). Curcumin supplementation promoted wound healing in streptozotocin-treated diabetic rats and genetically diabetic mice (54) and attenuated the phenylephrin-induced increase in vascular reactivity in the aortae of streptozotocin-treated diabetic rats (35).
This study demonstrates that diabetic rats had elevated blood levels of TNF-α, IL-6, and MCP-1, and oxidative stress similar to those observed in diabetic patients (19–22, 26, 44). The effect of diabetes on elevated TNF-α, IL-6, and MCP-1 levels was abolished in diabetic rats supplemented daily with curcumin but not in those supplemented with placebo. This is a novel finding. Diabetic rats supplemented with curcumin also had a modest but significantly lower blood glycated hemoglobin level, which suggests an improvement in blood glucose levels in curcumin-supplemented rats compared with placebo-supplemented diabetic rats. This suggests an overall improvement in markers of vascular inflammation and glycemia control in curcumin-supplemented diabetic rats compared with diabetic rats not supplemented with curcumin.
TNF-α reduces insulin-stimulated receptor tyrosine kinase activity at low concentrations and can also decrease the expression of the insulin receptors IRS-1 and Glut-4 at higher concentrations, as well as increasing the phosphorylation of serine 307 in IRS-1, thus impairing its ability to bind to the insulin receptor and initiate downstream signaling (17). Thus, TNF-α plays an important role in insulin resistance and the vascular inflammation process through its multiple actions (17, 59). The role of IL-6 in vascular inflammation has been shown in studies with IL-6 knockout mice who exhibit resistance to splanchnic artery occlusion shock. Anti–IL-6 therapy significantly prevents the inflammatory process in mice (14), and other studies have shown increased levels of lipid peroxidation and inflammation in mice that overexpress IL-6 (10). Circulating IL-6, IL-8, and MCP-1 levels are elevated in insulin-resistant states such as obesity, impaired glucose tolerance, and type 1 and 2 diabetes (27, 55, 59). Studies using knockout mice lacking MCP-1 or IL-8 or their corresponding receptors show a significantly reduced progression of atherosclerosis (26). Overexpression of MCP-1 causes inhibition of AKT and tyrosine phosphorylation in liver and skeletal muscle, macrophage recruitment, and insulin resistance in aP2-MCP-1 mice (27). IL-8 and MCP-1 play important roles in the vascular inflammation process through its multiple actions, including recruitment of neutrophils and T lymphocytes into the subendothelial space, monocyte adhesion to endothelium, and migration of vascular smooth muscle cells. Our observation of decreased secretion of TNF-α, IL-6, IL-8, and MCP-1, as a result of curcumin supplementation in cultured monocytes, and decreased levels of circulating TNF-α, IL-6, and MCP-1 in the blood of diabetic rats, is novel.
The monocyte infiltration into subendothelial space that occurs through the combined actions of locally produced chemotactic cytokines and adhesion molecules expressed on the injured endothelial surface is critical in the vascular inflammation and the development of atherosclerosis. MCP-1 is produced constitutively, or after induction of oxidative stress or growth factors by a variety of cell types, including monocytes, smooth muscle cells and endothelial cells (47, 60). MCP-1 is a potent chemotactic factor for monocytes (47). Increased expression of MCP-1 mRNA or proteins has been observed in animals and humans with arteriosclerosis or atherosclerosis (58, 62). Studies using in vitro monocyte-endothelial adhesion assay and in vivo studies using knockout mice for CCR2 gene (receptor of MCP-1) and antibodies for MCP-1 have demonstrated that MCP-1 plays an important role in the vascular inflammation and atherosclerotic lesion formation (15, 32, 33). This suggests that the monocyte cell-culture model used in this study is physiologically relevant in the understanding of various mechanisms that contribute to the development of vascular inflammation and atherosclerosis.
This study did not observe any significant effect of supplementation with olive oil alone on any of the parameters studied, such as blood levels of cytokines, lipid peroxidation, fasting glucose, or glycated hemoglobin levels, in diabetic rats. Curcumin was mixed in olive oil as a vehicle for its administration via gavage to rats, as done in previous studies (29). However, olive oil itself can also have an antioxidant effect (45). Therefore, olive oil–supplemented rats were used as a control for analyses of data obtained with curcumin-supplemented diabetic rats. No differences were found between the blood levels of hemoglobin, RBC counts, or hematocrits in diabetic rats receiving placebo or curcumin supplementation, which suggests that the lower glycated hemoglobin levels observed in curcumin-supplemented rats is not due to any change in the life span of RBCs. No changes in liver-function tests were observed in curcumin-supplemented compared with placebo-supplemented diabetic rats, which suggests that curcumin supplementation does not cause any toxicity.
Previous studies have also shown that curcumin inhibits oxygen radical production in PMA-treated lymphocytes (6). The complete mechanisms by which curcumin inhibits cellular oxidative stress are not known. It is also not known whether some or all of the antioxidant effect of curcumin is due to its chelating property. Curcumin supplementation caused reduction in blood glucose levels, which in turn can lower glucose-induced oxidative stress and the levels of proinflammatory cytokines (19, 20, 23–26). Oxidative stress can also influence the expression of multiple genes in monocytes and other cells, including signaling molecules such as PKC, NF-κB, and ERK (19, 26, 51); overexpression of these genes stimulates the secretion of proinflammatory cytokines. Curcumin has been shown to activate defensive genes and protects against oxidative stress in neurons and brain cells (43, 50).
Our study demonstrates that curcumin supplementation results in inhibition of oxidative stress and proinflammatory cytokines in diabetic rats and a cell-culture model. The degree of effect of curcumin is different for different cytokines because different cytokines are regulated by a number of complex signal-transduction pathways. It is difficult to provide any specific explanation for varying effect of curcumin on different cytokines. The inhibitory effect of curcumin on proinflammatory cytokine inhibition may be mediated either by oxidative stress–dependent or –independent pathways (27, 31, 49). It is possible that different cytokines are influenced to different degrees by oxidative stress or glycosylation of proteins caused by high glucose or diabetes, which may explain why the magnitude of the protective effect of curcumin was quite different among the various cytokines in both cell-culture and rat studies.
Begum et al. (7) examined the efficacy of curcumin versus tetrahydrocurcumin supplementation on markers of vascular inflammation and in reducing amyloid plaque burden in a model of neuroinflammation and Alzheimer disease in mice. This study found that only curcumin administration prevented protein oxidation and amyloid plaque formation; however, both curcumin and tetrahydrocurcumin caused reduction in nitrotyrosine, F2 isoprostane, and IL-1β. This study suggested that the dienone bridge present in curcumin may be necessary for some of the beneficial effects of curcumin (7). Curcumin supplementation prevents the TNF-α–induced overexpression of signaling molecules such as PKC, NF-κB, JNK, and ERK (2, 7, 18). Regulation of heme oxygenase-1 expression by curcuminoids through Nrf2 by a PI3-kinase/Akt-mediated pathway has been observed in mouse β cells (46). Whether a similar inhibition of regulatory genes and signaling pathways occurs in the tissues of curcumin-treated diabetic rats is not known and must be investigated. Nevertheless, inhibition of circulating levels of proinflammatory cytokines can potentially increase insulin sensitivity and glucose metabolism in curcumin-supplemented diabetic rats.
The concentration of curcumin used in our rat study was 100 mg/kg body weight. A similar dose of curcumin was shown to be effective in preventing nephropathy in animal studies (30). This is equivalent to 6 g per adult, assuming 60 kg BW per adult. This dose of curcumin is similar to that used in humans (3, 13). Blood concentrations of curcumin as high as 1.75 μM have been observed in humans given 4 to 8 gm of oral curcumin supplementation (3, 13), which suggests that the concentrations of curcumin used in the cell-culture studies are physiologically relevant. More investigations are needed to determine the lowest effective dose of curcumin supplementation needed to decrease levels of oxidative stress and vascular inflammation markers in diabetic rats.
Many factors, including proinflammatory cytokines, glycosylation of proteins, and oxidative stress, are thought to contribute to the development of vascular inflammation in diabetes (17, 24, 44, 55, 59). This study suggests that curcumin supplementation has the potential to decrease cellular oxidative stress, reduce the blood levels of proinflammatory cytokines, and thereby inhibit the pathogenesis of vascular inflammation in diabetes. No clinical trial has been done to determine whether curcumin supplementation can indeed delay or prevent diabetes-associated complications. The evidence that curcumin can inhibit markers of vascular inflammation must be explored at the clinical level to see whether curcumin can reduce levels of proinflammatory cytokines in the diabetic patient population. If so, then curcumin supplementation could be used as an adjuvant therapy for reduction of vascular inflammation and CVD in diabetes.
Acknowledgments
The author (S.K.J.) is supported by a grant from NIDDK and the Office of Dietary Supplements of the National Institutes of Health (RO1 DK064797 and RO1 DK072433). We thank Ms. Georgia Morgan for excellent editing of this manuscript.
Abbreviations
ANOVA, Analyses of variance statistical test; CVD, cardiovascular disease; IL-6, interleukin-6; IL-8, interleukin-8; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; OO, olive oil; STZ, streptozotocin; SD rats, Sprague–Dawley rats; TNF-α, tumor necrosis factor-alpha.
Disclosure Statement
No competing financial interests exist.
References
- 1.Aggarwal BB. Shishodia S. Takada Y. Banerjee S. Newman RA. Bueso-Ramos CE. Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB. Sundram C. Malani N. Ichikawa H. Curcumin: the Indian gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Anand P. Kunnumakkara AB. Newman RA. Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 4.Babu P. Srinivasan K. Amelioration of renal lesions associated with diabetes by dietary curcumin in streptozotocin diabetic rats. Mol Cell Biochem. 1998;181:87–96. doi: 10.1023/a:1006821828706. [DOI] [PubMed] [Google Scholar]
- 5.Babu P. Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol Cell Biochem. 1997;166:169–175. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanyam M. Koteswari A. Kumar R. Monickaraj S. Maheswari J. Mohan V. Curcumin-induced inhibition of cellular reactive oxygen species generation: novel therapeutic implications. J Biosci. 2003;28:715–721. doi: 10.1007/BF02708432. [DOI] [PubMed] [Google Scholar]
- 7.Begum AN. Jones MR. Lim GP. Morihara T. Kim P. Heath DD. Rock CL. Pruitt MA. Yang F. Hudspeth B. Hu S. Faull KF. Teter B. Cole GM. Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer disease. J Pharmacol Exp Ther. 2008;326:195–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boring L. Gosling J. Cleary M. Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 9.Candiloros H. Muller S. Zeghari N. Donner M. Drouin P. Ziegler O. Decreased erythrocyte membrane fluidity in poorly controlled IDDM: influence of ketone bodies. Diabetes Care. 1995;18:549–551. doi: 10.2337/diacare.18.4.549. [DOI] [PubMed] [Google Scholar]
- 10.Castelnau PA. Garrett RS. Palinski W. Witztum JL. Campbell IL. Powell HC. Abnormal iron deposition associated with lipid peroxidation in transgenic mice expressing interleukin-6 in the brain. J Neuropathol Exp Neurol. 1998;57:268–282. doi: 10.1097/00005072-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Chen A. Xu J. Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 12.Chen A. Xu J. Activation of PPARγ by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288:G447–G456. doi: 10.1152/ajpgi.00209.2004. [DOI] [PubMed] [Google Scholar]
- 13.Cheng AL. Hsu CH. Lin JK. Hsu MM. Ho YF. Shen TS. Ko JY. Lin JT. Lin BR. Ming-Shiang W. Yu HS. Jee SH. Chen GS. Chen TM. Chen CA. Lai MK. Pu YS. Pan MH. Wang YJ. Tsai CC. Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 14.Cuzzocrea S. De Sarro G. Costantino G. Ciliberto G. Mazzon E. De Sarro A. Caputi AP. IL-6 knockout mice exhibit resistance to splanchnic artery occlusion shock. J Leukoc Biol. 1999;66:471–480. doi: 10.1002/jlb.66.3.471. [DOI] [PubMed] [Google Scholar]
- 15.Egashira K. Molecular mechanisms mediating inflammation in vascular disease: special reference to monocyte chemoattractant protein-1. Hypertension. 2003;41:834–841. doi: 10.1161/01.HYP.0000051642.65283.36. [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H. Lang J. Zadravec S. Slater T. Detection of malonaldehyde by high-performance liquid chromatography. Methods Enzymol. 1984;105:319–328. doi: 10.1016/s0076-6879(84)05041-2. [DOI] [PubMed] [Google Scholar]
- 17.Evans JL. Maddux BA. Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxidant Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 18.Goel A. Kunnumakkara AB. Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Guha M. Bai W. Nadler JL. Natarajan R. Molecular mechanisms of tumor necrosis factor a gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem. 2000;275:17728–17739. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]
- 20.Jain SK. Kannan K. Lim G. McVie R. Bocchini JA. Hyperketonemia increases TNF-α secretion in cultured U937 monocytes and type-1 diabetic patients. Diabetes. 2002;51:2287–2293. doi: 10.2337/diabetes.51.7.2287. [DOI] [PubMed] [Google Scholar]
- 21.Jain SK. McVie R. Duett J. Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38:1539–1543. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- 22.Jain SK. McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes. 1999;48:1850–1555. doi: 10.2337/diabetes.48.9.1850. [DOI] [PubMed] [Google Scholar]
- 23.Jain SK. Rains J. Jones K. Effect of curcumin on protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels. Free Radic Biol Med. 2006;41:92–96. doi: 10.1016/j.freeradbiomed.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Jain SK. Rains JL. Croad JL. High glucose and ketosis (acetoacetate) increases, and chromium niacinate decreases, IL-6, IL-8, and MCP-1 secretion and oxidative stress in U937 monocytes. Antioxid Redox Signal. 2007;9:1581–1590. doi: 10.1089/ars.2007.1577. [DOI] [PubMed] [Google Scholar]
- 25.Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem. 1989;264:21340–21345. [PubMed] [Google Scholar]
- 26.Jay D. Hitomi H. Griendling KK. Oxidative stress, diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Kamei N. Tobe K. Suzuki R. Ohsugi M. Watanabe T. Kubota N. Ohtsuka-Kowatari N. Kumagai K. Sakamoto K. Kobayashi M. Yamauchi T. Ueki K. Oishi Y. Nishimura S. Manabe I. Hashimoto H. Ohnishi Y. Ogata H. Tokuyama K. Tsunoda M. Ide T. Murakami K. Nagai R. Kadowaki R. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 28.Khan N. Afaq F. Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 29.Kunnumakkara AB. Guha S. Krishnan S. Diagaradjane P. Gelovani J. Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 30.Kuwabara N. Tamada S. Iwai T. Teramoto K. Kaneda N. Yokimura T. Nakatani T. Miura K. Attenuation of renal fibrosis by curcumin in rat obstructive nephropathy. Urology. 2006;67:440–446. doi: 10.1016/j.urology.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Kowluru RA. Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab. 2007;16:4–8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyanagi M. Egashira K. Kitamoto S. Ni W. Shimokawa H. Takeya M. Yoshimura T. Takeshita A. Role of monocyte chemoattractant protein-1 in cardiovascular remodelling induced by chronic blockade of nitric oxide synthesis. Circulation. 2000;102:2243–2248. doi: 10.1161/01.cir.102.18.2243. [DOI] [PubMed] [Google Scholar]
- 33.Lefer DJ. Granger DN. Monocyte rolling in early atherogenesis: vital role in lesion development. Circ Res. 1999;84:1353–1355. doi: 10.1161/01.res.84.11.1353. [DOI] [PubMed] [Google Scholar]
- 34.Mahesh T. Balasubashini M. Menon V. Effect of photo-irradiated curcumin treatment against oxidative stress in streptozotocin-induced diabetic rats. J Med Food. 2005;8:251–255. doi: 10.1089/jmf.2005.8.251. [DOI] [PubMed] [Google Scholar]
- 35.Majithiya JB. Balaraman R. Time-dependent changes in antioxidant enzymes and vascular reactivity of aorta in streptozotocin-induced diabetic rats treated with curcumin. J Cardiovasc Pharmacol. 2005;46:697–705. doi: 10.1097/01.fjc.0000183720.85014.24. [DOI] [PubMed] [Google Scholar]
- 36.McDonnell CM. Pedreira CC. Vadamalayan B. Cameron FJ. Werther GA. Diabetic ketoacidosis, hyperosmolarity and hypernatremia: Are high carbohydrate drinks worsening initial presentation? Pediatr Diabetes. 2005;6:90–94. doi: 10.1111/j.1399-543X.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 37.Motterlini R. Foresti R. Bassi R. Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;7:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 38.Murthy K. Harrington JT. Siegel RD. Profound hypokalemia in diabetic ketoacidosis: a therapeutic challenge. Endocr Pract. 2005;5:331–334. doi: 10.4158/EP.11.5.331. [DOI] [PubMed] [Google Scholar]
- 39.Mythri RB. Jagatha B. Pradhan N. Andersen J. Bharath MM. Mitochondrial complex I inhibition in Parkinson's disease: How can curcumin protect mitochondria? Antioxid Redox Signal. 2007;9:399–408. doi: 10.1089/ars.2006.1479. [DOI] [PubMed] [Google Scholar]
- 40.Nicolls MR. Haskins K. Flores SC. Oxidant stress, immune dysregulation, and vascular function in type I diabetes. Antioxid Redox Signal. 2007;9:879–889. doi: 10.1089/ars.2007.1631. [DOI] [PubMed] [Google Scholar]
- 41.Nishiyama T. Mae T. Kishida H. Tsukagawa M. Mimaki Y. Kuroda M. Sashida Y. Takahashi K. Kawada T. Nakagawa K. Kitahara M. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L) suppress and increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem. 2005;53:959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- 42.Noh H. Ha H. Yu MR. Kim YO. Kim JH. Lee HB. Angiotension II mediates high glucose-induced TGF-beta1 and fibronectin upregulation in HPMC through reactive oxygen species. Periton Dial Int. 2005;25:38–47. [PubMed] [Google Scholar]
- 43.Pari L. Murugan P. Tetrahydrocurcumin prevents brain lipid peroxidation in streptozotocin-induced diabetic rats. J Med Food. 2007;10:323–329. doi: 10.1089/jmf.2006.058. [DOI] [PubMed] [Google Scholar]
- 44.Pennathur S. Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 45.Peroz-Jimenez F. Ruano J. Perez-Martinez P. Lopez-Segura F. Lopez-Miranda J. The influence of olive oil on human health: not a question of fat alone. Mol Nutr Food Res. 2007;51:1199–1208. doi: 10.1002/mnfr.200600273. [DOI] [PubMed] [Google Scholar]
- 46.Pugazhenthi S. Akhov L. Selvaraj G. Wang M. Alam J. Regulation of heme oxygenase-1 expression by demethoxy curcuminoids through Nrf2 by a PI3-kinase/Akt-mediated pathway in mouse β-cells. Am J Physiol Endocrinol Metab. 2007;293:E645–E655. doi: 10.1152/ajpendo.00111.2007. [DOI] [PubMed] [Google Scholar]
- 47.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 48.Sajithlal G. Chithra P. Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998;56:1607–1614. doi: 10.1016/s0006-2952(98)00237-8. [DOI] [PubMed] [Google Scholar]
- 49.Sandur SK. Pandey MK. Sung B. Ahn KS. Marakami A. Sethi G. Limtrakul P. Badbaev V. Aggarwal BB. Curcumin demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin, and turmerones differentially regulate anti-inflammatory and antiproliferative responses through a ROS-independent mechanism. Carcinogensis. 2007;43:568–580. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 50.Scapagnini G. Colombrita C. Amadio M. D'Agata V. Arcelli E. Sapienza M. Quattrone A. Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 51.Shah S. Iqbal M. Karam J. Salifu M. McFarlane SI. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: pathophysiological insights. Antioxid Redox Signal. 2007;9:911–929. doi: 10.1089/ars.2007.1629. [DOI] [PubMed] [Google Scholar]
- 52.Sharma S. Chopra K. Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res. 2007;21:278–283. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- 53.Sharma S. Kulkarni SK. Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetes nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 54.Sidhu G. Mani H. Gaddipati J. Singh A. Seth P. Banaudha K. Patnaik G. Maheshwari R. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Rep Reg. 1999;7:362–374. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 55.Singh U. Devraj S. Jialal I. Vitamin E, oxidative stress and inflammation. Annu Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 56.Strimpakos AS. Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 57.Suryanarayana P. Saraswat M. Mrudula T. Krishna P. Krishnaswamy K. Reddy G. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci. 2005;46:2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- 58.Takeya M. Yoshimura T. Leonard EJ. Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993;24:534–539. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- 59.Wellen KE. Hotamisligil GS. Inflammation, stress, and oxidative stress. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing L. Remick DG. Promoter elements responsible for antioxidant regulation of MCP-1 gene expression. Antioxid Redox Signal. 2007;9:1979–1989. doi: 10.1089/ars.2007.1921. [DOI] [PubMed] [Google Scholar]
- 61.Yan LJ. Traber MG. Kobuchi H. Matsugo S. Trischler HJ. Packer L. Efficacy of hypochlorous acid scavengers in the prevention of protein carbonyl formation. Arch Biochem Biophys. 1996;327:330–334. doi: 10.1006/abbi.1996.0130. [DOI] [PubMed] [Google Scholar]
- 62.Ylä-Herttuala S. Lipton BA. Rosenfeld ME. Särkioja T. Yoshimura T. Leonard EJ. Witztum JL. Steinberg D. Expression of monocyte chemoattractant protein-1 in macrophage rich areas of human and rabbit atherosclerosis lesions. Proc Natl Acad Sci U S A. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]