Abstract
Gap junction channels interconnect several different types of cells in the lung, ranging from the alveolar epithelium to the pulmonary vasculature, each of which expresses a unique subset of gap junction proteins (connexins). Major lung functions regulated by gap junctional communication include coordination of ciliary beat frequency and inflammation. Gap junctions help enable the alveolus to regulate surfactant secretion as an integrated system, in which type I cells act as mechanical sensors that transmit calcium transients to type II cells. Thus, disruption of epithelial gap junctional communication, particularly during acute lung injury, can interfere with these processes and increase the severity of injury. Consistent with this, connexin expression is altered during lung injury, and connexin-deficiency has a negative impact on the injury response and lung-growth control. It has recently been shown that alcohol abuse is a significant risk factor associated with acute respiratory distress syndrome. Oxidant stress and hormone-signaling cascades in the lung induced by prolonged alcohol ingestion are discussed, as well as the effects of these pathways on connexin expression and function. Antioxid. Redox Signal. 11, 355—367.
Introduction
It has long been appreciated that gap junctions are ubiquitous in most mammalian tissues, and the lung is no exception (16, 60). Gap junctions are composed of proteins known as connexins, which form channels that enable neighboring cells to be interconnected (69, 107, 149). Gap junction channels serve multiple functions by enabling the diffusion of signaling molecules and metabolites throughout interconnected cells. This, in turn, enables cells in a tissue to function in a coordinated manner. The ability to share metabolites and antioxidant molecules through gap junctions enables the tissue to have a robust response to stress and injury.
A functional gap junction channel is composed of two connexin hexamers (or hemichannels) in two adjacent cells, which dock to form a complete channel (107, 149). Gap junction channels are typically arranged in semicrystalline arrays, known as plaques, at sites of cell–cell contact where intercellular communication occurs. However, free connexin hemichannels dispersed throughout the plasma membrane can also act as bona fide plasma membrane channels, enabling the exchange of aqueous molecules between the cytoplasm to the extracellular environment (59, 150).
Connexin Expression in Lung
Of the 20 mammalian connexins (161), several are differentially expressed throughout the lung. The pattern of expression depends on cell phenotype, which influences connexin transcription (Table 1). In normal lung, most epithelial cells express Cx32 and Cx43, whereas endothelial cells express predominantly Cx37, Cx40, and Cx43. The cells that line terminal airspaces, type I and type II alveolar epithelial cells, have been studied in considerable detail (2, 3, 12, 66, 80, 81, 92, 111, 165). The major connexins expressed by alveolar epithelial cells are Cx26, Cx32, Cx43, and Cx46. Others are expressed at low levels, such as Cx30.3 and Cx40. Expression of Cx37 by alveolar epithelial cells in situ is also low, but is consistently detectable by immunohistochemistry. Considerably more Cx37 is expressed by bronchiolar epithelium, but this is still less than the level observed for pulmonary endothelial cells (165).
Table 1.
Connexin Expression in the Lung
| Cell Type | Connexin expression | References |
|---|---|---|
| Airway epithelium | Cx26, Cx30, Cx31, Cx32, Cx37, Cx43 | 23, 75, 97, 147, 165, 186 |
| Trachea | Cx26, Cx43, Cx46 | 25, 31, 78 |
| Alveolar epithelium | 2, 3, 12, 66, 80, 81, 92, 111, 165 | |
| Type II | Cx26, Cx32, Cx37, Cx43, Cx46 | |
| Type I | Cx26, Cx37, Cx40, Cx43, Cx46 | |
| Pulmonary endothelium | Cx37, Cx40, Cx43 | 34, 134, 145, 165, 190 |
| Smooth muscle | Cx37, Cx40, Cx43 | 22, 92, 130 |
| Lung Fibroblasts | Cx43, Cx45 | 15, 92, 166, 194 |
Cx43 is fairly ubiquitous and is the major connexin functionally interconnecting type II and type I cells (3). By contrast, Cx32 is expressed exclusively by type II alveolar epithelial cells in normal adult rat lung. Interestingly, type I cells cannot form functional gap junctions with cells expressing only Cx32 (3). Type II cells form primarily heterocellular junctions in the normal lung (e.g., with type I cells). Thus, the role for Cx32 in alveolar epithelial physiology is not clear, because it is not likely to be participating in type I–type II cell gap junctions (3), and few type II–type II cell junctions exist. However, a hemichannel role for Cx32 expressed by type II cells is plausible (44). Studies from Cx32-deficient mice suggest a role for Cx32 in alveolar epithelial cell growth control, because these mice are more susceptible to benzene-induced lung toxicity and have a higher incidence of lung tumors (94, 95, 192). This is not unique to Cx32, though, because female Cx43-deficient mice are roughly twice as sensitive to urethane-induced tumor formation as are controls expressing normal Cx43 levels (13).
Connexins have a relatively rapid half-life of 1–5 h, suggesting that gap junction turnover is a constant process (107). Moreover, cells that express multiple connexins, including cells in the lung, have the potential to form heteromeric or mixed gap junction channels (38, 103). Whether connexins form heteromeric channels is determined by their biochemical compatibility. For instance, Cx32 and Cx43 are incompatible and cannot form heteromeric channels. However, cells also regulate the formation of mixed gap junctions by compatible connexins. For instance, two of the compatible connexins expressed by alveolar epithelial cells, Cx43 and Cx46, form heteromeric channels when expressed by type I alveolar epithelial cells, yet type II cells prevent Cx43 and Cx46 from interacting (2, 42, 82). Other examples of cells that regulate connexin assembly include endothelial cells that restrict formation of mixed gap junctions containing Cx37 and Cx40/Cx43 (79).
The mechanisms that regulate connexin hetero-oligomerization remain unknown. However, it is clear that by regulating connexin interactions, cells have the ability to form discrete functional zones of communication within the pulmonary system (100).
Intercellular Communication in the Lung
Gap junctions serve several functional roles in the lung (7, 34, 100, 102, 142) (Fig. 1). By extension, any condition that disrupts gap junctional coupling can have a deleterious effect on lung function. In the airways, gap junctional coupling can contribute to calcium signaling between ciliated epithelial cells to coordinate ciliary beating (151). Coordinated ciliary movement is needed to ensure the directional flow of mucus out of lungs to clear environmental toxicants and microorganisms. Mechanical stimulation of primary airway epithelial cells in culture induces an intercellular calcium wave (23, 80), which is transmitted from one cell to another by inositol 1,4,5-triphosphate (IP3) diffusing through gap junctions (24). However, in addition to the gap junction–mediated pathway, intercellular calcium transients between airway cells can be generated by extracellular nucleotide release and paracrine stimulation of purinergic receptors (73). In this case, connexins may help regulate ciliary beat frequency by acting as plasma membrane hemichannels to promote ATP secretion.
FIG. 1.
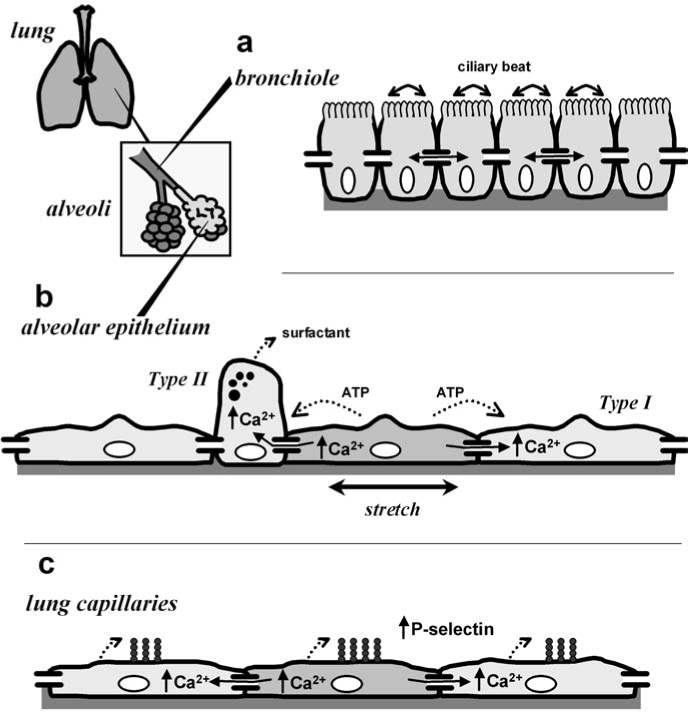
Intercellular communication in the lung. The lung consists of several distinct functional compartments. Shown in the inset are the terminal airspaces, alveoli, and bronchioles. (a) In the airways, including bronchioles, diffusion of IP3 through gap junctions enables the propagation of calcium waves, which help synchronize ciliary beating to allow directional transport of mucus. (b) The alveolar epithelium is a heterogeneous monolayer consisting of type II cells and type I cells. The alveolus acts as an integrated system in which type I cells respond to mechanical stimulation with an increase in intracellular calcium, which, in turn, is transmitted to type II cells via gap junctions to induce lamellar body fusion and secretion of pulmonary surfactant. Also shown is the alternative pathway, mediated by ATP secretion and paracrine stimulation via purinergic receptors. (c) In lung capillaries, transmission of calcium waves through pulmonary endothelial cell gap junctions upregulates the transport of P-selectin to the plasma membrane, thus transmitting a proinflammatory stimulus.
Gap junctions also play an important role in regulating secretion of pulmonary surfactant produced by type II alveolar epithelial cells. Pulmonary surfactant is a mixture of lipid and protein that performs the dual purposes of decreasing alveolar surface tension and regulating host defense in the lung (20, 183, 189), described in greater detail later. It is known that direct mechanical stimulation of type II cells can stimulate surfactant secretion through the calcium-dependent fusion of lamellar bodies to the plasma membrane (68, 185). Although this would imply that cell stretch induced by a deep breath could directly stimulate type II cell secretion, type II cells are localized to areas of the lung where they are shielded from direct mechanical stress, as compared with type I cells (136). This helps protect the alveolus, because type II cells are significantly more sensitive to mechanical stress than are type I cells (167).
Instead, the alveolus regulates surfactant secretion as an integrated system in which type I cells act as mechanical sensors that transmit calcium transients to type II cells via gap junctions. Strong evidence for this pathway comes from in situ fluorescence microscopy analysis of the intact lung (104). These calcium signals can be induced by inflation (10) or by changes in pulmonary vascular pressure (177) and require gap junctional communication between type I and type II cells (3, 66). Gap junctions also enable intercellular signals, which can propagate from one alveolus to the next (76). Again, it is important to note that intercellular calcium transients are not exclusively transmitted through gap junctions because alveolar calcium waves generated by extracellular ATP release and paracrine stimulation of purinergic receptors also contribute to mechanically regulated surfactant secretion (Fig. 1) (80, 82, 135, 146). However, given the ability of gap junction blockers to inhibit calcium waves in situ, whether the paracrine pathway can fully compensate for a loss of connexin function is not known.
Functions for gap junctional communication in the vasculature have been covered elsewhere in detail (51, 172). Of relevance to the pulmonary circulation, calcium waves that propagate along pulmonary vessels through gap junctions have been imaged in the intact perfused lung (134, 191). The requirement for Cx43 in pulmonary endothelial calcium waves was confirmed by using an endothelium-specific Cx43-deficient mouse model in which these waves were no longer present (134). One surprising result from in situ imaging was the discovery of spontaneous calcium signals generated from a subset of endothelial cells, referred to as “pacemaker cells,” localized to pulmonary branch points (191). Pacemaker cells are significantly more sensitive to mechanical stress, which results in a calcium wave of increased amplitude as compared with that of nonpacemaker cells. However, the frequency of the calcium oscillations in mechanically stressed vessels still matches the frequency in unstimulated vessels, underscoring the role of pacemaker cells in establishing signal-oscillation frequency (106).
Calcium waves induced by mechanical stimulation have been shown to increase pulmonary endothelial P-selection expression at the cell surface, suggesting a link to the inflammatory response (105, 134). This potentially injurious effect of endothelial Cx43 contrasts with pulmonary epithelium, where Cx43 is beneficial. Interestingly, the proinflammatory role for endothelial Cx43 is counterbalanced by an antiinflammatory role for Cx37 expressed by circulating monocytes (187). In this case, Cx37 inhibits inflammation by forming hemichannels that mediate ATP release and reduce monocyte adhesion to endothelial cells, as demonstrated in a mouse model of atherosclerosis. Although endothelial Cx37 had no effect in this system, whether Cx37 plays a role in modulating other pulmonary inflammatory responses remains to be determined. Pulmonary Cx40 also may have an antiinflammatory function in preventing lung injury and fibrosis (34).
Gap Junctions and Lung Injury
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) can develop in response to stresses as diverse as sepsis, trauma, gastric aspiration, and pneumonia (179). Hallmarks of ARDS include increased oxidant stress, lung inflammation, surfactant dysfunction, and disruption of the alveolar and endothelial barriers (39, 65, 115, 137). Because optimal lung function requires functional cell–cell contacts, even partial disruption of an epithelial monolayer can be a significant contributor to the severity of ARDS.
Significantly, >85% of patients with ARDS have at least a partial defect in lung-fluid clearance, which contributes to a high degree of patient morbidity and mortality (21, 193). Compromised barrier function is a particular concern, because patients with impaired lung fluid balance are 3 times more likely to die of ARDS than are patients with a maximal ability to clear lung fluid (164, 180). Alveolar flooding directly compromises gas exchange; unfortunately, the requirement for mechanical ventilation to improve tissue oxygenation causes direct alveolar cell injury, which further exacerbates lung function (114, 160, 168, 175).
Cross talk between connexins and tight junctions
Lung barrier function is controlled by tight junctions (101, 122, 155, 169, 171). Although gap junctions are not a direct structural component of tight junctions, they are frequently seen adjacent to tight-junction strands with freeze-fracture electron microscopy (154). However, connexins do not necessarily colocalize with tight junctions (61), and the gap junction content of some tight junctions is low (154).
Functionally, treatment of a rat lung endothelial cell line (RLE) or primary porcine brain endothelial cells with the gap junction inhibitors glycyrrhetinic acid or oleamide decreased barrier function by ~50 to 75% as compared with controls, based on transendothelial resistance and small-molecule flux measurements (129). Cx40 and Cx43 are biochemically associated with several tight-junction proteins, including occludin, claudin-5, and ZO-1, based on co-immunoprecipitation analysis. Because RLE cells are claudin deficient, experiments were done with cells in which claudin expression was reconstituted. Nonetheless, results with the RLE cells were consistent with primary brain endothelium, expressing endogenous claudins.
As an example from epithelia, expression of transfected Cx32 induced a ~25% increase in the barrier function of immortalized hepatocytes derived from Cx32-deficient mice (98). In this system, Cx32 expression enhanced localization of ZO-1 and JAM-A to the plasma membrane, suggesting an increase in tight-junction formation. Interestingly, Cx26-transfected hepatocytes show a reduction in the ability of ouabain to decrease barrier function; however, Cx26 had no effect on baseline transepithelial resistance (TER) (56). This underscores the notion of different connexins having specific roles for regulating tight junctions.
This specificity is due in part to differences in the ability of connexins to interact with different junction scaffold proteins (55). Because Cx40 and Cx43 interact with ZO-1, a protein that also directly interacts with claudins and occludin (45), any role for Cx40 or Cx43 in regulating tight junctions is likely to involve ZO-1, either as a cross-linking scaffold protein, via ZO-1 recruitment to cell junctions or via an effect on the distribution of the ZO-1 pool between gap and tight junctions. In contrast, whereas Cx32 can also co-immunoprecipitate with tight-junction proteins (96), it does not directly bind to ZO-1. Instead, interactions between Cx32 and tight junctions may be mediated by another scaffold protein, such as discs large homologue 1 (Dlgh1), which directly interacts with Cx32 (47). As another distinct mode of interaction, Cx26 binds directly to the hydrophilic surface of the coiled-coil C-terminal domain of occludin, which does not interact with Cx32 (131).
What role can gap junctions have in regulating other classes of cell junctions? One possibility is that gap junctions transmit intercellular signals to coordinate junction assembly. A related possibility is that gap junctions serve to balance the concentration of metabolites between cells (such as calcium or GTP), so that junction-regulatory proteins are exposed to comparable microenvironments. Alternatively, connexins may structurally regulate junctions through a direct interaction with other tight-junction proteins. Given the heterogeneity of gap junction and tight junction protein expression and assembly, the need for further work in this area is clear.
Connexins in lung injury
Connexin expression in the lung changes during the injury response (100, 102). In the injured lung, type II cell hyperplasia increases the frequency of type II cells in direct contact with other type II cells (116), both of which express Cx32 (4, 6, 81). Because type I–type II cell communication is mediated through Cx43-compatible connexins and is not mediated by Cx32 (3), this has the potential to provide type II cells with an independent pathway for communication that does not involve type I cells and may be used to regulate the injury response.
During the acute phase of lung injury, connexin expression in the alveolus is altered, where Cx43 and Cx46 expression is elevated (3, 92). Conversely, Cx40 expression at the whole-lung level decreases during the acute phase of injury (145). Some Cx46-expressing alveolar epithelial cells do not express typical type II cell markers and thus may represent a distinct subtype of cells proliferating in response to injury (58). Cx46 has relatively limited permeability, as compared with Cx32 and Cx43 (118, 123), suggesting a possible role for Cx46 in limiting metabolic depletion or intercellular transmission of toxic agents. Interestingly, lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have decreased Cx43 expression and function (166). The downstream effects of impaired fibroblast communication and whether epithelial Cx43 expression is decreased in late stages of fibrotic lung disease are not known.
Studies from connexin-deficient mice have also demonstrated a potential role for gap junctions in preventing lung injury. Isakson and co-workers (34) have shown that mice deficient in Cx40 and endothelial Cx43 spontaneously develop symptoms similar to pulmonary fibrosis (34). Knockout mice deficient for expression of either Cx40 alone (52, 158) or endothelial Cx43 alone (117) did not have an obvious pulmonary phenotype. However, as early as 8 weeks after birth, Cx40−/−:endothelial Cx43−/− mice showed deficiency in lung-barrier function, disorganized alveoli, and increased extracellular matrix deposition (34). Although the mechanistic basis for this phenotype is not known, these results suggest that intercellular communication between the vasculature and airspaces helps maintain lung morphology and function. Nonetheless, these results are difficult to reconcile with Cx43-mediated proinflammatory signaling in the pulmonary vasculature (134).
Oxidant Stress and Signaling in the Alcoholic Lung
Although alcohol abuse is classically associated with liver disease (144), recent evidence has confirmed that chronic alcohol abuse is also a major risk factor contributing to the severity of ARDS (87, 125, 127). In a study of ICU patients, it was found that after adjusting for smoking and hepatic dysfunction, patients with a history of alcohol abuse were more than twice as susceptible to ARDS than were nonalcoholic patients (127). In large part, the increased susceptibility to ARDS caused by prolonged ethanol ingestion is due to a fundamental defect in lung-barrier function as a result of impaired tight-junction formation between type I alveolar epithelial cells (50, 64). As described earlier, decreased barrier function (e.g., a leaky lung) contributes to the severity of ARDS (179).
Dietary ethanol causes oxidant stress in the lung (126). The lung is particularly sensitive to oxidant stress, which is minimized by the antioxidant glutathione in the alveolar airspaces (85). Thus, one source of oxidant stress is from ethanol metabolism to acetaldehyde, which directly depletes the reduced glutathione pool (30, 126) (Fig. 2). The prominent role for oxidant stress and reactive oxygen species (ROS) in alcoholic lung suggests that antioxidant therapy could be a useful therapeutic approach. In animal models of alcohol ingestion, a diet enriched in the glutathione precursor procystine prevents the alcoholic lung phenotype (29, 62). However, complete reversal of the alcoholic lung phenotype requires several weeks of treatment and is not a suitable approach for an immediate treatment regimen for alcoholic lung.
FIG. 2.
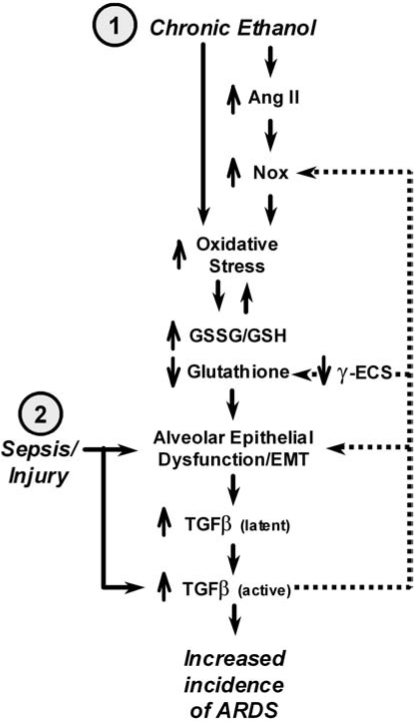
Oxidant and hormone stress responses in the alcoholic lung. Prolonged ethanol ingestion initiates and exacerbates oxidant stress via several pathways. Here is depicted a two-hit model for the role of alcoholic in ARDS. (1) Prolonged alcohol abuse causes direct oxidant stress because of the metabolism of ethanol to acetaldehyde. Ethanol also induces angiotensin II, which stimulates both the endothelium and epithelium to upregulate Nox activity. Oxidant stress depletes the alveolar epithelial glutathione pool, which induces cell damage and stimulates the cells to undergo an epithelial-to-mesenchyme transition (EMT) as a compensatory mechanism. Alveolar epithelial cells undergoing EMT increase production and secretion of TGF-β and have impaired alveolar barrier function, which adds further stress to the lung. (2) A second hit, such as direct trauma, infection, or sepsis, has an exaggerated effect on the alcoholic lung because of impaired alveolar epithelial function and the presence of a large pool of latent TGF-β, which is readily activated and exaggerates the normal injury response. Note the feedback loops in the diagram (dashed lines), indicating the potential for “runaway” activation of a deleterious injury response.
Three different studies have demonstrated that ethanol treatment of cells in vitro inhibits gap junctional communication (1, 26, 181). The ability of ethanol to inhibit gap junctions could be due to direct partitioning into cell membranes, analogous to the inhibitory effect of long-chain alcohols on connexins (86). Ethanol-induced depletion of the glutathione pool and increased oxidant stress can also inhibit gap junctional communication (170). The effect of ethanol on connexin expression is more variable, where ethanol was shown to inhibit Cx43 expression (26, 181), but had little effect on Cx26 or Cx32 (1, 181).
Whether ethanol has a comparable effect on other connexins or cell types remains to be determined. Specific alterations in gap junctional communication in response to oxidant stress may be a mechanism to decrease the intercellular transmission of toxic agents (14, 49), while also maintaining intercellular transfer of antioxidant compounds, including glutathione (57). Alternatively, complete shutdown of gap junctional communication can help preserve the bulk of the tissue at the expense of more extensive damage to isolated individual cells.
Hormone signaling in alcoholic lung
In addition to its metabolic effects on the antioxidant glutathione pool, ethanol also induces cell-signaling pathways that contribute to oxidant stress. In particular, ethanol stimulates angiotensin II activity (19) which, in turn, upregulates NADPH oxidase (Nox) (156). Interestingly, angiotensin II has also been shown to upregulate cardiovascular and epithelial Cx43 expression and function (26, 46, 89, 91) and can antagonize the effect of ethanol on gap junctional communication (26). In contrast, Cx40 appears to be less affected by angiotensin II (46). Given the role for endothelial Cx43 in inflammation, this is consistent with the notion that angiotensin II is proinflammatory as well (27).
Clearly, inflammation and the concomitant infiltration of neutrophils and activation of alveolar macrophages contribute to oxidant stress in response to acute lung injury (36). The intense oxidant load on alcoholic lung provides a condition in which the alveolar epithelium is prone to injury and apoptosis (29). As a response to oxidant stress, alveolar epithelial cells increase expression and secretion of TGF-β, the majority of which is the TGF-β1 isoform (19). In the otherwise healthy alcoholic lung, most of this TGF-β is inactive; however, a significant increase in active TGF-β is also found as compared with normal lung. This has influences on alveolar epithelial function by promoting the cells to undergo an epithelium-to-mesenchyme transition (EMT) (90, 93, 184) and is consistent with models in which TGF-β influences the acute phase of lung injury as well as the chronic phase (128, 138). Further, the large latent pool of TGF-β induced by prolonged ethanol ingestion plays an important role in exacerbating the influence of other insults on the lung (87). In essence, the alcoholic lung is primed to have an exaggerated response to the effects of subsequent insults that promote TGF-β activation (8, 88) (Fig. 2).
In addition to impairing the epithelial cell phenotype and compromising alveolar barrier function, EMT has been shown to decrease expression of Cx43 by embryonic carcinoma cells downstream of increased Snail expression and decreased cadherin expression (43). Whether this is the case for alveolar epithelial cell junctions remains to be determined. TGF-β can also increase oxidant stress by decreasing γ-glutamylcysteine synthetase expression (9, 84), thus reducing the antioxidant glutathione reserves of the lung. TGF-β also increases ROS production by increasing Nox expression (74, 163) and H2O2 production (176). In addition to ROS, reactive nitrogen species, including peroxynitrite, are generated during acute lung injury (67, 159), which can inhibit gap junctional communication (157).
Increased oxidant stress has the added potential to exacerbate alveolar injury and TGF-β expression by creating a positive-feedback loop, particularly if TGF-β expression and activation are driven by a second insult, such as sepsis or direct trauma (63). TGF-β can directly influence gap junctional communication; however, this effect varies depending on cell type. Studies have demonstrated that TGF-β1 increases (35, 141, 173), decreases (32, 108, 148), or has no effect (110) on gap junctional communication. TGF-β1 has also been found to simultaneously upregulate Cx43 and suppress Cx37 expression by endothelial cells (109), suggesting that differential regulation of connexins by TGF-β can provide a mechanism to alter intercellular communication.
Relevant to the lung injury response, we examined the effect of TGF-β on gap junctional communication between type I alveolar epithelial cells (Fig. 3). Primary rat alveolar epithelial cells were isolated and cultured for 6 days to generate a model type I cell monolayer (4, 135). The cells were then treated for 16 h with varying amounts of activated TGF-β1, and the level of intercellular communication was assessed by microinjecting calcein into individual cells and measuring the extent of dye transfer 5 min after microinjection. Consistent with previous reports, control cells were highly coupled and transferred calcein to nearly 20 cells through gap junctions (2). Treatment with increasing levels of TGF-β inhibited gap junctional communication by ~50%. Thus, alveolar epithelial cells decreased intercellular communication in response to TGF-β. This suggests that if a similar phenomenon occurs in situ, then one effect of TGF-β would be to dampen the intercellular communication required to regulate surfactant secretion and thus potentially further to promote acute lung injury.
FIG. 3.

TGF-β1 inhibits gap junctional communication between alveolar epithelial cells. Primary rat type II cells were isolated and cultured for 6 days in minimal essential medium to produce a model type I cell monolayer. The cells were then treated with varying amounts of TGF-β1 for 16 h; then the extent of gap junctional communication was determined by visualizing the intercellular transfer of calcein microinjected into individual cells with fluorescence microscopy. Dye transfer was quantified by counting the number of calcein-labeled cells per microinjection. Data were combined from two independent experiments counting ≥20 microinjections/treatment. Increasing concentrations of TGF-β1 significantly decreased intercellular communication, as determined by t test (*p < 0.05).
Role of Pulmonary Surfactant in Acute Lung Injury
The alveolus acts as a coordinated system to regulate pulmonary surfactant secretion. Pulmonary surfactant is a mixture of lipids and proteins synthesized by type II cells, which lines the alveolar airspace to reduce surface tension at the air/liquid interface. The majority of surfactant lipid is phosphatidylcholine (PC), with phosphatidylglycerol (PG) and phosphatidylethanolamine (PE) as the other major phospholipids present (174). By and large, most mammalian surfactant lipid is saturated [e.g., dipalmitoyl-phosphatidylcholine (DPPC)], which can form a barrier to inhaled oxidants, such as ozone (37). Conversely, unsaturated lipids present in surfactant are reactive and can act as an antioxidant sink (162). Extensive lipid oxidation, including damage to cell-membrane lipids is deleterious (140). Oxidant damage to type II cells alters their ability to synthesize surfactant lipids, which further compromises the surface activity (40, 124). Consistent with this, overexpression of the antioxidant enzyme peroxiredoxin 6 is protective, because it can reduce phospholipid hydroperoxides in a glutathione-dependent reaction (53, 119, 178). Pulmonary surfactant also contains significant catalase and superoxide dismutase activity, which contributes to its ability to quench oxidant stress (121). In addition, surfactant lipids can have a direct role to help attenuate oxidant stress associated with inflammation by inserting into neutrophil membranes, which, in turn, inhibits Nox activity (33).
The other major components of pulmonary surfactant are four surfactant proteins, SP-A, SP-B, SP-C, and SP-D. SP-B and SP-C are hydrophobic and directly contribute to the biophysical properties of surfactant (20, 183). By contrast, SP-A and SP-D are members of the collectin protein family and are largely hydrophilic (41, 72). Although these proteins can bind to surfactant lipids and help organize them into higher-level structures, such as tubular myelin, they are largely dispensable from the standpoint of surfactant biophysics (28, 77, 99). Conversely, SP-A enhances surfactant lipid turnover, by enabling uptake of DPPC by type II cells (17). SP-D also helps regulate the surfactant lipid pool size, because surfactant degradation by macrophages and type II cells is impaired in SP-D–deficient mice (28), although the mechanistic basis for regulation of surfactant metabolism by SP-D remains unknown.
SP-A and SP-D play key roles in regulating lung inflammation, which can have downstream effects on oxidant stress and alveolar damage (188). Consistent with an immunoregulatory role for SP-A and SP-D, these proteins are required for efficient clearance of bacterial infections (54). Both SP-A and SP-D have carbohydrate-recognition domains that recognize bacterial polysaccharides, whereas the collagenous stalk region of the proteins binds to neutrophils, macrophages, and type II cells (70, 132, 152). SP-A and SP-D also bind viruses (71, 112). Thus, the collectin surfactant proteins act as co-receptors, or opsonizing agents, by coating pathogens and enabling them to be recognized and destroyed by the innate immune system in the lung.
A hyperreactive immune response and resultant oxidant stress is deleterious. One way that SP-A and SP-D can help keep this in check is to increase the efficiency of bacteriocidal activity so that the net inflammatory response is minimized (182). However, a comparison of SP-A– and SP-D–deficient mice indicates different roles for these proteins in regulating the macrophage respiratory burst (113). Alveolar macrophages from SP-A–deficient mice showed a dampened oxidative burst in response to phorbol esters, whereas macrophages from SP-D–deficient mice were hyperreactive (113). Note that macrophages from the SP-D–deficient mice are loaded with surfactant, which may shift them to a state more susceptible to activation.
Importantly, several examples show that the stressed and/or inflamed alveolus upregulates SP-A and SP-D while simultaneously decreasing SP-B and SP-C expression (11, 83). This has the dual effect of both increasing inflammation and depressing surfactant surface activity, which can further compromise lung function (139, 189). Consistent with a general role for surfactant in enhancing the antioxidant capacity of the lung, SP-D–knockout mice (in which the surfactant pool size is increased) resist hyperoxia (83). This, and the success of surfactant therapy for premature infants (5), has led to the notion that natural and pulmonary surfactants can be used as part of a treatment regimen for ARDS (115). However, treatment of adults with exogenous surfactant has had limited success. In part, this stems from a focus on using surfactant to recover the biophysical function of surfactant in adults, who have a large mature airspace as opposed to infants. Conversely, exploiting the immunomodulatory function of surfactant may ultimately be a more fruitful approach to treating adults with ARDS (115).
A net decrease in gap junctional communication will have a negative impact on surfactant and lamellar body secretion (76, 134). However, evidence suggests that type II cells secrete the majority of SP-A and SP-D through a distinct vesicular pathway from the hydrophobic surfactant proteins and lipids packaged into lamellar bodies (120, 133). Consistent with this, the SP-A content of lamellar bodies is low, and SP-D is largely undetectable (153). Although a complete lack of surfactant production and secretion is clearly deleterious, an imbalance in the regulation of surfactant secretion, which increases the relative level of SP-A and SP-D in the airspace at the expense of surface-active and antioxidant components of pulmonary surfactant can also compromise lung function. Whether this is due to decreased or altered intercellular communication in the alveolus during injury or infection remains to be determined.
Summary and Future Directions
The lung consists of several distinct functional zones, each of which is interconnected through gap junction channels. Gap junctions play several functional roles in the lung, and disruption of intercellular communication can have pathologic consequences. Although some tantalizing links exist between oxidant stress, lung injury, and gap junctional communication, more-direct evidence would help support this notion. It also is not clear whether gap junctions interconnect different pulmonary subcompartments, such as the alveolar epithelium and pulmonary circulation. Although several lines of evidence suggest that this is the case, the extent to which this occurs and which connexins mediate this type of cross-talk remains to be determined.
Although many different connexins clearly help assure proper pulmonary intercellular communication, Cx43 appears to play a central role in the regulation of alveolar function. However, this remains to be tested in transgenic mouse models, because Cx43-deficient mice have a neonatal lethal phenotype (143). Intriguingly, Cx43−/− newborn mice are cyanotic, suggesting pulmonary edema. Although this is due in large part to a defect in the cardiac outflow tract, compromised alveolar epithelial function could also contribute to the neonatal lethal phenotype. Lung-targeted Cx43 knockouts via the cre-lox system, analogous to endothelial targeted Cx43-deficient mice (117, 134), or dominant-negative connexins expressed in mice (18, 48), are expected to provide useful insights into Cx43 function in the lung.
Acknowledgments
This study was supported by National Institutes of Health Grants R01-HL083120, P50-AA013757 (M.K.) and T32-AA013528 (L.N.J.).
Abbreviations
ALI, acute lung injury; ARDS, acute respiratory distress syndrome; ATP, adenosine triphosphate; Cx, connexin; Dlgh1, discs large homologue 1; DPPC, dipalmitoyl-phosphatidylcholine; EMT, epithelial-to-mesenchyme transition; ICU, intensive care unit; IP3, inositol 1,4,5-triphosphate; JAM, junction adhesion molecule; Nox, nicotinamide adenine dinucleotide phosphate oxidase; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; RLE, rat lung endothelial; ROS, reactive oxygen species; TER, transepithelial resistance; TGF, transforming growth factor.
References
- 1.Abou Hashieh I. Mathieu S. Besson F. Gerolami A. Inhibition of gap junction intercellular communications of cultured rat hepatocytes by ethanol: role of ethanol metabolism. J Hepatol. 1996;24:360–367. doi: 10.1016/s0168-8278(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 2.Abraham V. Chou ML. DeBolt KM. Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol. 1999;276:L825–L834. doi: 10.1152/ajplung.1999.276.5.L825. [DOI] [PubMed] [Google Scholar]
- 3.Abraham V. Chou ML. George P. Pooler P. Zaman A. Savani RC. Koval M. Heterocellular gap junctional communication between alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1085–L1093. doi: 10.1152/ajplung.2001.280.6.L1085. [DOI] [PubMed] [Google Scholar]
- 4.Abraham V. DeBolt K. Savani R. Koval M. Regulation of gap junction proteins by alveolar epithelial cells in response to injury. Chest. 2002;116:35S. [PubMed] [Google Scholar]
- 5.Ainsworth SB. Milligan DW. Surfactant therapy for respiratory distress syndrome in premature neonates: a comparative review. Am J Respir Med. 1999;1:417–433. doi: 10.1007/BF03257169. [DOI] [PubMed] [Google Scholar]
- 6.Alford AI. Rannels DE. Extracellular matrix fibronectin alters connexin43 expression by alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L680–L688. doi: 10.1152/ajplung.2001.280.4.L680. [DOI] [PubMed] [Google Scholar]
- 7.Andreeva AV. Kutuzov MA. Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L259–L271. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 8.Araya J. Cambier S. Morris A. Finkbeiner W. Nishimura SL. Integrin-mediated transforming growth factor-beta activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am J Pathol. 2006;169:405–415. doi: 10.2353/ajpath.2006.060049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arsalane K. Dubois CM. Muanza T. Begin R. Boudreau F. Asselin C. Cantin AM. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 10.Ashino Y. Ying X. Dobbs LG. Bhattacharya J. [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am J Physiol Lung Cell Mol Physiol. 2000;279:L5–L13. doi: 10.1152/ajplung.2000.279.1.L5. [DOI] [PubMed] [Google Scholar]
- 11.Atochina EN. Beck JM. Preston AM. Haczku A. Tomer Y. Scanlon ST. Fusaro T. Casey J. Hawgood S. Gow AJ. Beers MF. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect Immun. 2004;72:6002–6011. doi: 10.1128/IAI.72.10.6002-6011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avanzo JL. Mennecier G. Mesnil M. Hernandez-Blazquez FJ. Fukumasu H. da Silva TC. Rao KV. Dagli ML. Deletion of a single allele of Cx43 is associated with a reduction in the gap junctional intercellular communication and increased cell proliferation of mouse lung pneumocytes type II. Cell Prolif. 2007;40:411–421. doi: 10.1111/j.1365-2184.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avanzo JL. Mesnil M. Hernandez-Blazquez FJ. Mackowiak II. Mori CM. da Silva TC. Oloris SC. Garate AP. Massironi SM. Yamasaki H. Dagli ML. Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis. 2004;25:1973–1982. doi: 10.1093/carcin/bgh193. [DOI] [PubMed] [Google Scholar]
- 14.Azzam EI. de Toledo SM. Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banoub RW. Fernstrom M. Malkinson AM. Ruch RJ. Enhancement of gap junctional intercellular communication by dibutyryl cyclic AMP in lung epithelial cells. Anticancer Res. 1996;16:3715–3719. [PubMed] [Google Scholar]
- 16.Bartels H. The air-blood barrier in the human lung: a freeze-fracture study. Cell Tissue Res. 1979;198:269–285. doi: 10.1007/BF00232010. [DOI] [PubMed] [Google Scholar]
- 17.Bates SR. Dodia C. Tao JQ. Fisher AB. Surfactant protein-A plays an important role in lung surfactant clearance: evidence using the surfactant protein-A gene-targeted mouse. Am J Physiol Lung Cell Mol Physiol. 2008;294:L325–L333. doi: 10.1152/ajplung.00341.2007. [DOI] [PubMed] [Google Scholar]
- 18.Beahm DL. Oshima A. Gaietta GM. Hand GM. Smock AE. Zucker SN. Toloue MM. Chandrasekhar A. Nicholson BJ. Sosinsky GE. Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem. 2006;281:7994–8009. doi: 10.1074/jbc.M506533200. [DOI] [PubMed] [Google Scholar]
- 19.Bechara RI. Pelaez A. Palacio A. Joshi PC. Hart CM. Brown LA. Raynor R. Guidot DM. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L363–L370. doi: 10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- 20.Beers MF. Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- 21.Berthiaume Y. Lesur O. Dagenais A. Treatment of adult respiratory distress syndrome: plea for rescue therapy of the alveolar epithelium. Thorax. 1999;54:150–160. doi: 10.1136/thx.54.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beyer EC. Reed KE. Westphale EM. Kanter HL. Larson DM. Molecular cloning and expression of rat connexin40, a gap junction protein expressed in vascular smooth muscle. J Membr Biol. 1992;127:69–76. doi: 10.1007/BF00232759. [DOI] [PubMed] [Google Scholar]
- 23.Boitano S. Dirksen ER. Evans WH. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium. 1998;23:1–9. doi: 10.1016/s0143-4160(98)90069-0. [DOI] [PubMed] [Google Scholar]
- 24.Boitano S. Dirksen ER. Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 25.Boitano S. Evans WH. Connexin mimetic peptides reversibly inhibit Ca(2+) signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L623–L630. doi: 10.1152/ajplung.2000.279.4.L623. [DOI] [PubMed] [Google Scholar]
- 26.Bokkala S. Reis HM. Rubin E. Joseph SK. Effect of angiotensin II and ethanol on the expression of connexin 43 in WB rat liver epithelial cells. Biochem J. 2001;357:769–777. doi: 10.1042/0264-6021:3570769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boos CJ. Lip GY. Is hypertension an inflammatory process? Curr Pharm Des. 2006;12:1623–1635. doi: 10.2174/138161206776843313. [DOI] [PubMed] [Google Scholar]
- 28.Botas C. Poulain F. Akiyama J. Brown C. Allen L. Goerke J. Clements J. Carlson E. Gillespie AM. Epstein C. Hawgood S. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci U S A. 1998;95:11869–11874. doi: 10.1073/pnas.95.20.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown LA. Harris FL. Guidot DM. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L377–L386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- 30.Brown LA. Harris FL. Ping XD. Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Carson JL. Reed W. Moats-Staats BM. Brighton LE. Gambling TM. Hu SC. Collier AM. Connexin 26 expression in human and ferret airways and lung during development. Am J Respir Cell Mol Biol. 1998;18:111–119. doi: 10.1165/ajrcmb.18.1.2789. [DOI] [PubMed] [Google Scholar]
- 32.Chandross KJ. Chanson M. Spray DC. Kessler JA. Transforming growth factor-beta 1 and forskolin modulate gap junctional communication and cellular phenotype of cultured Schwann cells. J Neurosci. 1995;15:262–273. doi: 10.1523/JNEUROSCI.15-01-00262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao W. Spragg RG. Smith RM. Inhibitory effect of porcine surfactant on the respiratory burst oxidase in human neutrophils: attenuation of p47phox and p67phox membrane translocation as the mechanism. J Clin Invest. 1995;96:2654–2660. doi: 10.1172/JCI118331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatterjee S. Baeter S. Bhattacharya J. Endothelial and epithelial signaling in the lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L517–L519. doi: 10.1152/ajplung.00202.2007. [DOI] [PubMed] [Google Scholar]
- 35.Chiba H. Sawada N. Oyamada M. Kojima T. Iba K. Ishii S. Mori M. Hormonal regulation of connexin 43 expression and gap junctional communication in human osteoblastic cells. Cell Struct Funct. 1994;19:173–177. doi: 10.1247/csf.19.173. [DOI] [PubMed] [Google Scholar]
- 36.Christofidou-Solomidou M. Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5:47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Connor LM. Ballinger CA. Albrecht TB. Postlethwait EM. Interfacial phospholipids inhibit ozone-reactive absorption-mediated cytotoxicity in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1169–L1178. doi: 10.1152/ajplung.00397.2003. [DOI] [PubMed] [Google Scholar]
- 38.Cottrell GT. Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta. 2005;1711:126–141. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Crandall ED. Matthay MA. Alveolar epithelial transport: basic science to clinical medicine. Am J Respir Crit Care Med. 2001;163:1021–1029. doi: 10.1164/ajrccm.163.4.2006116. [DOI] [PubMed] [Google Scholar]
- 40.Crim C. Longmore WJ. Sublethal hydrogen peroxide inhibits alveolar type II cell surfactant phospholipid biosynthetic enzymes. Am J Physiol. 1995;268:L129–L135. doi: 10.1152/ajplung.1995.268.1.L129. [DOI] [PubMed] [Google Scholar]
- 41.Crouch E. Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 42.Das Sarma J. Meyer RA. Wang F. Abraham V. Lo CW. Koval M. Multimeric connexin interactions prior to the trans-Golgi network. J Cell Sci. 2001;114:4013–4024. doi: 10.1242/jcs.114.22.4013. [DOI] [PubMed] [Google Scholar]
- 43.de Boer TP. van Veen TA. Bierhuizen MF. Kok B. Rook MB. Boonen KJ. Vos MA. Doevendans PA. de Bakker JM. van der Heyden MA. Connexin43 repression following epithelium-to-mesenchyme transition in embryonal carcinoma cells requires Snail1 transcription factor. Differentiation. 2007;75:208–218. doi: 10.1111/j.1432-0436.2006.00133.x. [DOI] [PubMed] [Google Scholar]
- 44.De Vuyst E. Decrock E. Cabooter L. Dubyak GR. Naus CC. Evans WH. Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denker BM. Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 46.Dhein S. Polontchouk L. Salameh A. Haefliger JA. Pharmacological modulation and differential regulation of the cardiac gap junction proteins connexin 43 and connexin 40. Biol Cell. 2002;94:409–422. doi: 10.1016/s0248-4900(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 47.Duffy HS. Iacobas I. Hotchkiss K. Hirst-Jensen BJ. Bosco A. Dandachi N. Dermietzel R. Sorgen PL. Spray DC. The gap junction protein connexin32 interacts with the Src homology 3/hook domain of discs large homolog 1. J Biol Chem. 2007;282:9789–9796. doi: 10.1074/jbc.M605261200. [DOI] [PubMed] [Google Scholar]
- 48.Elias LA. Wang DD. Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 49.Elshami AA. Saavedra A. Zhang H. Kucharczuk JC. Spray DC. Fishman GI. Amin KM. Kaiser LR. Albelda SM. Gap junctions play a role in the “bystander effect” of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther. 1996;3:85–92. [PubMed] [Google Scholar]
- 50.Fernandez AL. Koval M. Fan X. Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol. 2007;41:371–379. doi: 10.1016/j.alcohol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Figueroa XF. Isakson BE. Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 2004;19:277–284. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- 52.Figueroa XF. Paul DL. Simon AM. Goodenough DA. Day KH. Damon DN. Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 53.Fisher AB. Dodia C. Yu K. Manevich Y. Feinstein SI. Lung phospholipid metabolism in transgenic mice overexpressing peroxiredoxin 6. Biochim Biophys Acta. 2006;1761:785–792. doi: 10.1016/j.bbalip.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Giannoni E. Sawa T. Allen L. Wiener-Kronish J. Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giepmans BN. Role of connexin43-interacting proteins at gap junctions. Adv Cardiol. 2006;42:41–56. doi: 10.1159/000092561. [DOI] [PubMed] [Google Scholar]
- 56.Go M. Kojima T. Takano K. Murata M. Koizumi J. Kurose M. Kamekura R. Osanai M. Chiba H. Spray DC, et al. Connexin 26 expression prevents down-regulation of barrier and fence functions of tight junctions by Na+/K+-ATPase inhibitor ouabain in human airway epithelial cell line Calu-3. Exp Cell Res. 2006;312:3847–3856. doi: 10.1016/j.yexcr.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Goldberg GS. Valiunas V. Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez R. Yang YH. Griffin C. Allen L. Tigue Z. Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol. 2005;288:L179–L189. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- 59.Goodenough DA. Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 60.Gordon RE. Heller RF. Del Valle JR. Heller RF. Membrane perturbations and mediation of gap junction formation in response to taurine treatment in normal and injured alveolar epithelia. Exp Lung Res. 1989;15:895–908. doi: 10.3109/01902148909069634. [DOI] [PubMed] [Google Scholar]
- 61.Guerrier A. Fonlupt P. Morand I. Rabilloud R. Audebet C. Krutovskikh V. Gros D. Rousset B. Munari-Silem Y. Gap junctions and cell polarity: connexin32 and connexin43 expressed in polarized thyroid epithelial cells assemble into separate gap junctions, which are located in distinct regions of the lateral plasma membrane domain. J Cell Sci. 1995;108:2609–2617. doi: 10.1242/jcs.108.7.2609. [DOI] [PubMed] [Google Scholar]
- 62.Guidot DM. Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res. 2000;24:1070–1076. [PubMed] [Google Scholar]
- 63.Guidot DM. Folkesson HG. Jain L. Sznajder JI. Pittet JF. Matthay MA. Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2006;291:L301–L306. doi: 10.1152/ajplung.00153.2006. [DOI] [PubMed] [Google Scholar]
- 64.Guidot DM. Modelska K. Lois M. Jain L. Moss IM. Pittet JF. Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L127–L135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- 65.Guo RF. Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal. 2007;9:1991–2002. doi: 10.1089/ars.2007.1785. [DOI] [PubMed] [Google Scholar]
- 66.Guo Y. Martinez-Williams C. Yellowley CE. Donahue HJ. Rannels DE. Connexin expression by alveolar epithelial cells is regulated by extracellular matrix. Am J Physiol Lung Cell Mol Physiol. 2001;280:L191–L202. doi: 10.1152/ajplung.2001.280.2.L191. [DOI] [PubMed] [Google Scholar]
- 67.Haddad IY. Pataki G. Hu P. Galliani C. Beckman JS. Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haller T. Dietl P. Pfaller K. Frick M. Mair N. Paulmichl M. Hess MW. Furst J. Maly K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J Cell Biol. 2001;155:279–289. doi: 10.1083/jcb.200102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 70.Hartshorn KL. Crouch E. White MR. Colamussi ML. Kakkanatt A. Tauber B. Shepherd V. Sastry KN. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am J Physiol. 1998;274:L958–L969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 71.Hartshorn KL. White MR. Voelker DR. Coburn J. Zaner K. Crouch EC. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem J. 2000;351:449–458. [PMC free article] [PubMed] [Google Scholar]
- 72.Hawgood S. Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol. 2001;63:495–519. doi: 10.1146/annurev.physiol.63.1.495. [DOI] [PubMed] [Google Scholar]
- 73.Homolya L. Steinberg TH. Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu YC. Wang LF. Chien YW. Nitric oxide in the pathogenesis of diffuse pulmonary fibrosis. Free Radic Biol Med. 2007;42:599–607. doi: 10.1016/j.freeradbiomed.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 75.Huang S. Dudez T. Scerri I. Thomas MA. Giepmans BN. Suter S. Chanson M. Defective activation of c-Src in cystic fibrosis airway epithelial cells results in loss of tumor necrosis factor-alpha-induced gap junction regulation. J Biol Chem. 2003;278:8326–8332. doi: 10.1074/jbc.M208264200. [DOI] [PubMed] [Google Scholar]
- 76.Ichimura H. Parthasarathi K. Lindert J. Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol. 2006;291:L596–L601. doi: 10.1152/ajplung.00036.2006. [DOI] [PubMed] [Google Scholar]
- 77.Ikegami M. Korfhagen TR. Whitsett JA. Bruno MD. Wert SE. Wada K. Jobe AH. Characteristics of surfactant from SP-A-deficient mice. Am J Physiol. 1998;275:L247–L254. doi: 10.1152/ajplung.1998.275.2.L247. [DOI] [PubMed] [Google Scholar]
- 78.Isakson BE. Damon DN. Day KH. Liao Y. Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol. 2006;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 79.Isakson BE. Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 80.Isakson BE. Evans WH. Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol. 2001;280:L221–L228. doi: 10.1152/ajplung.2001.280.2.L221. [DOI] [PubMed] [Google Scholar]
- 81.Isakson BE. Lubman RL. Seedorf GJ. Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am J Physiol Cell Physiol. 2001;281:C1291–C1299. doi: 10.1152/ajpcell.2001.281.4.C1291. [DOI] [PubMed] [Google Scholar]
- 82.Isakson BE. Seedorf GJ. Lubman RL. Evans WH. Boitano S. Cell-cell communication in heterocellular cultures of alveolar epithelial cells. Am. J. Respir Cell Mol Biol. 2003;29:552–561. doi: 10.1165/rcmb.2002-0281OC. [DOI] [PubMed] [Google Scholar]
- 83.Jain D. Atochina-Vasserman E. Kadire H. Tomer Y. Inch A. Scott P. Savani RC. Gow AJ. Beers MF. SP-D-deficient mice are resistant to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L861–L871. doi: 10.1152/ajplung.00145.2006. [DOI] [PubMed] [Google Scholar]
- 84.Jardine H. MacNee W. Donaldson K. Rahman I. Molecular mechanism of transforming growth factor (TGF)-beta1-induced glutathione depletion in alveolar epithelial cells: involvement of AP-1/ARE and Fra-1. J Biol Chem. 2002;277:21158–21166. doi: 10.1074/jbc.M112145200. [DOI] [PubMed] [Google Scholar]
- 85.Jean JC. Liu Y. Brown LA. Marc RE. Klings E. Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L766–L776. doi: 10.1152/ajplung.00250.2000. [DOI] [PubMed] [Google Scholar]
- 86.Johnston MF. Simon SA. Ramon F. Interaction of anaesthetics with electrical synapses. Nature. 1980;286:498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- 87.Joshi PC. Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol. 2007;292:L813–L823. doi: 10.1152/ajplung.00348.2006. [DOI] [PubMed] [Google Scholar]
- 88.Kang HR. Cho SJ. Lee CG. Homer RJ. Elias JA. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem. 2007;282:7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- 89.Kansui Y. Fujii K. Nakamura K. Goto K. Oniki H. Abe I. Shibata Y. Iida M. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol. 2004;287:H216–H224. doi: 10.1152/ajpheart.00915.2003. [DOI] [PubMed] [Google Scholar]
- 90.Kasai H. Allen JT. Mason RM. Kamimura T. Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2004;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kasi VS. Xiao HD. Shang LL. Iravanian S. Langberg J. Witham EA. Jiao Z. Gallego CJ. Bernstein KE. Dudley SC., Jr Cardiac-restricted angiotensin-converting enzyme overexpression causes conduction defects and connexin dysregulation. Am J Physiol Heart Circ Physiol. 2007;293:H182–H192. doi: 10.1152/ajpheart.00684.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kasper M. Traub O. Reimann T. Bjermer L. Grossmann H. Muller M. Wenzel KW. Upregulation of gap junction protein connexin43 in alveolar epithelial cells of rats with radiation-induced pulmonary fibrosis. Histochem Cell Biol. 1996;106:419–424. doi: 10.1007/BF02473301. [DOI] [PubMed] [Google Scholar]
- 93.Kim KK. Kugler MC. Wolters PJ. Robillard L. Galvez MG. Brumwell AN. Sheppard D. Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.King TJ. Gurley KE. Prunty J. Shin JL. Kemp CJ. Lampe PD. Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene. 2005;24:1718–1726. doi: 10.1038/sj.onc.1208355. [DOI] [PubMed] [Google Scholar]
- 95.King TJ. Lampe PD. The gap junction protein connexin32 is a mouse lung tumor suppressor. Cancer Res. 2004;64:7191–7196. doi: 10.1158/0008-5472.CAN-04-0624. [DOI] [PubMed] [Google Scholar]
- 96.Kojima T. Kokai Y. Chiba H. Yamamoto M. Mochizuki Y. Sawada N. Cx32 but not Cx26 is associated with tight junctions in primary cultures of rat hepatocytes. Exp Cell Res. 2001;263:193–201. doi: 10.1006/excr.2000.5103. [DOI] [PubMed] [Google Scholar]
- 97.Kojima T. Murata M. Go M. Spray DC. Sawada N. Connexins maintain tight junctions in epithelial cells. J Membr Biol. 2007;217:13–19. doi: 10.1007/s00232-007-9021-4. [DOI] [PubMed] [Google Scholar]
- 98.Kojima T. Spray DC. Kokai Y. Chiba H. Mochizuki Y. Sawada N. Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp Cell Res. 2002;276:40–51. doi: 10.1006/excr.2002.5511. [DOI] [PubMed] [Google Scholar]
- 99.Korfhagen TR. Bruno MD. Ross GF. Huelsman KM. Ikegami M. Jobe AH. Wert SE. Stripp BR. Morris RE. Glasser SW. Bachurski CJ. Iwamoto HS. Whitsett JA. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci U S A. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koval M. Sharing signals: connecting lung epithelial cells with gap junction channels. Am J Physiol Lung Cell Mol Physiol. 2002;283:L875–L893. doi: 10.1152/ajplung.00078.2002. [DOI] [PubMed] [Google Scholar]
- 101.Koval M. Claudins: key pieces in the tight junction puzzle. Cell Commun Adhes. 2006;13:127–138. doi: 10.1080/15419060600726209. [DOI] [PubMed] [Google Scholar]
- 102.Koval M. Connexins, tissue expression. In: Laurent GJ, editor; Shapiro SD, editor. Encyclopedia of respiratory medicine. Oxford: Elsevier; 2006. pp. 558–560. [Google Scholar]
- 103.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16:159–166. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuebler WM. Parthasarathi K. Lindert J. Bhattacharya J. Real-time lung microscopy. J Appl Physiol. 2007;102:1255–1264. doi: 10.1152/japplphysiol.00786.2006. [DOI] [PubMed] [Google Scholar]
- 105.Kuebler WM. Parthasarathi K. Wang PM. Bhattacharya J. A novel signaling mechanism between gas and blood compartments of the lung. J Clin Invest. 2000;105:905–913. doi: 10.1172/JCI8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuebler WM. Ying X. Bhattacharya J. Pressure-induced endothelial Ca(2+) oscillations in lung capillaries. Am J Physiol Lung Cell Mol Physiol. 2002;282:L917–L923. doi: 10.1152/ajplung.00275.2001. [DOI] [PubMed] [Google Scholar]
- 107.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Larson DM. Christensen TG. Sagar GD. Beyer EC. TGF-beta1 induces an accumulation of connexin43 in a lysosomal compartment in endothelial cells. Endothelium. 2001;8:255–260. doi: 10.3109/10623320109090802. [DOI] [PubMed] [Google Scholar]
- 109.Larson DM. Wrobleski MJ. Sagar GD. Westphale EM. Beyer EC. Differential regulation of connexin43 and connexin37 in endothelial cells by cell density, growth, and TGF-beta1. Am J Physiol. 1997;272:C405–C415. doi: 10.1152/ajpcell.1997.272.2.C405. [DOI] [PubMed] [Google Scholar]
- 110.Lee SW. Tomasetto C. Paul D. Keyomarsi K. Sager R. Transcriptional downregulation of gap junction proteins blocks junctional communication in human mammary tumor cell lines. J Cell Biol. 1992;118:1213–1221. doi: 10.1083/jcb.118.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee YC. Yellowley CE. Li Z. Donahue HJ. Rannels DE. Expression of functional gap junctions in cultured pulmonary alveolar epithelial cells. Am J Physiol. 1997;272:L1105–L1114. doi: 10.1152/ajplung.1997.272.6.L1105. [DOI] [PubMed] [Google Scholar]
- 112.LeVine AM. Gwozdz J. Stark J. Bruno M. Whitsett J. Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest. 1999;103:1015–1021. doi: 10.1172/JCI5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.LeVine AM. Whitsett JA. Gwozdz JA. Richardson TR. Fisher JH. Burhans MS. Korfhagen TR. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol. 165:3934–3940. doi: 10.4049/jimmunol.165.7.3934. [DOI] [PubMed] [Google Scholar]
- 114.Levine GK. Deutschman CS. Helfaer MA. Margulies SS. Sepsis-induced lung injury in rats increases alveolar epithelial vulnerability to stretch. Crit Care Med. 2006;34:1746–1751. doi: 10.1097/01.CCM.0000218813.77367.E2. [DOI] [PubMed] [Google Scholar]
- 115.Lewis JF. Veldhuizen RA. The future of surfactant therapy during ALI/ARDS. Semin Respir Crit Care Med. 2006;27:377–388. doi: 10.1055/s-2006-948291. [DOI] [PubMed] [Google Scholar]
- 116.Li X. Shu R. Filippatos G. Uhal BD. Apoptosis in lung injury and remodeling. J Appl Physiol. 2004;97:1535–1542. doi: 10.1152/japplphysiol.00519.2004. [DOI] [PubMed] [Google Scholar]
- 117.Liao Y. Day KH. Damon DN. Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A. 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin GC. Rurangirwa JK. Koval M. Steinberg TH. Gap junctional communication modulates agonist-induced calcium oscillations in transfected HeLa cells. J Cell Sci. 2004;117:881–887. doi: 10.1242/jcs.00942. [DOI] [PubMed] [Google Scholar]
- 119.Manevich Y. Sweitzer T. Pak JH. Feinstein SI. Muzykantov V. Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci U S A. 2002;99:11599–11604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mason RJ. Lewis MC. Edeen KE. McCormick-Shannon K. Nielsen LD. Shannon JM. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2002;282:L249–L258. doi: 10.1152/ajplung.00027.2001. [DOI] [PubMed] [Google Scholar]
- 121.Matalon S. Holm BA. Baker RR. Whitfield MK. Freeman BA. Characterization of antioxidant activities of pulmonary surfactant mixtures. Biochim Biophys Acta. 1990;1035:121–127. doi: 10.1016/0304-4165(90)90105-6. [DOI] [PubMed] [Google Scholar]
- 122.Matter K. Aijaz S. Tsapara A. Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 123.Minogue PJ. Liu X. Ebihara L. Beyer EC. Berthoud VM. An aberrant sequence in a connexin46 mutant underlies congenital cataracts. J Biol Chem. 2005;280:40788–40795. doi: 10.1074/jbc.M504765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Minoo P. King RJ. Coalson JJ. Surfactant proteins and lipids are regulated independently during hyperoxia. Am J Physiol. 1992;263:L291–L298. doi: 10.1152/ajplung.1992.263.2.L291. [DOI] [PubMed] [Google Scholar]
- 125.Moss M. Bucher B. Moore FA. Moore EE. Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- 126.Moss M. Guidot DM. Wong-Lambertina M. Ten Hoor T. Perez RL. Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 127.Moss M. Parsons PE. Steinberg KP. Hudson LD. Guidot DM. Burnham EL. Eaton S. Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 128.Munger JS. Huang X. Kawakatsu H. Griffiths MJ. Dalton SL. Wu J. Pittet JF. Kaminski N. Garat C. Matthay MA. Rifkin DB. Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 129.Nagasawa K. Chiba H. Fujita H. Kojima T. Saito T. Endo T. Sawada N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J Cell Physiol. 2006;208:123–132. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- 130.Nakamura K. Inai T. Nakamura K. Shibata Y. Distribution of gap junction protein connexin 37 in smooth muscle cells of the rat trachea and pulmonary artery. Arch Histol Cytol. 1999;62:27–37. doi: 10.1679/aohc.62.27. [DOI] [PubMed] [Google Scholar]
- 131.Nusrat A. Chen JA. Foley CS. Liang TW. Tom J. Cromwell M. Quan C. Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816–29822. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- 132.O'Riordan DM. Standing JE. Kwon KY. Chang D. Crouch EC. Limper AH. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J Clin Invest. 1995;95:2699–2710. doi: 10.1172/JCI117972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Osanai K. Mason RJ. Voelker DR. Trafficking of newly synthesized surfactant protein A in isolated rat alveolar type II cells. Am J Respir Cell Mol Biol. 1998;19:929–935. doi: 10.1165/ajrcmb.19.6.3292. [DOI] [PubMed] [Google Scholar]
- 134.Parthasarathi K. Ichimura H. Monma E. Lindert J. Quadri S. Issekutz A. Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest. 2006;116:2193–2200. doi: 10.1172/JCI26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patel AS. Reigada D. Mitchell CH. Bates SR. Margulies SS. Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L489–L496. doi: 10.1152/ajplung.00074.2005. [DOI] [PubMed] [Google Scholar]
- 136.Perlman CE. Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol. 2007;103:1037–1044. doi: 10.1152/japplphysiol.00160.2007. [DOI] [PubMed] [Google Scholar]
- 137.Piantadosi CA. Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004;141:460–470. doi: 10.7326/0003-4819-141-6-200409210-00012. [DOI] [PubMed] [Google Scholar]
- 138.Pittet JF. Griffiths MJ. Geiser T. Kaminski N. Dalton SL. Huang X. Brown LA. Gotwals PJ. Koteliansky VE. Matthay MA. Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Postle AD. Mander A. Reid KB. Wang JY. Wright SM. Moustaki M. Warner JO. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol. 1999;20:1–98. doi: 10.1165/ajrcmb.20.1.3253. [DOI] [PubMed] [Google Scholar]
- 140.Putman E. van Golde LM. Haagsman HP. Toxic oxidant species and their impact on the pulmonary surfactant system. Lung. 1997;175:75–103. doi: 10.1007/pl00007561. [DOI] [PubMed] [Google Scholar]
- 141.Rama A. Matsushita T. Charolidi N. Rothery S. Dupont E. Severs NJ. Up-regulation of connexin43 correlates with increased synthetic activity and enhanced contractile differentiation in TGF-beta-treated human aortic smooth muscle cells. Eur J Cell Biol. 2006;85:375–386. doi: 10.1016/j.ejcb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 142.Rannels DE. Gap junction communication in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1083–L1084. doi: 10.1152/ajplung.2001.280.6.L1083. [DOI] [PubMed] [Google Scholar]
- 143.Reaume AG. de Sousa PA. Kulkarni S. Langille BL. Zhu D. Davies TC. Juneja SC. Kidder GM. Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 144.Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2007;23:283–291. doi: 10.1097/MOG.0b013e3280f27582. [DOI] [PubMed] [Google Scholar]
- 145.Rignault S. Haefliger JA. Waeber B. Liaudet L. Feihl F. Acute inflammation decreases the expression of connexin 40 in mouse lung. Shock. 2007;28:78–85. doi: 10.1097/shk.0b013e3180310bd1. [DOI] [PubMed] [Google Scholar]
- 146.Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:233–243. doi: 10.1016/s1095-6433(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 147.Ruch RJ. Porter S. Koffler LD. Dwyer-Nield LD. Malkinson AM. Defective gap junctional intercellular communication in lung cancer: loss of an important mediator of tissue homeostasis and phenotypic regulation. Exp Lung Res. 2001;27:231–243. doi: 10.1080/019021401300053984. [DOI] [PubMed] [Google Scholar]
- 148.Rudkin GH. Yamaguchi DT. Ishida K. Peterson WJ. Bahadosingh F. Thye D. Miller TA. Transforming growth factor-beta, osteogenin, and bone morphogenetic protein-2 inhibit intercellular communication and alter cell proliferation in MC3T3-E1 cells. J Cell Physiol. 1996;168:433–441. doi: 10.1002/(SICI)1097-4652(199608)168:2<433::AID-JCP22>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 149.Saez JC. Berthoud VM. Branes MC. Martinez AD. Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 150.Saez JC. Retamal MA. Basilio D. Bukauskas FF. Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sanderson MJ. Chow I. Dirksen ER. Intercellular communication between ciliated cells in culture. Am J Physiol. 1988;254:C63–74. doi: 10.1152/ajpcell.1988.254.1.C63. [DOI] [PubMed] [Google Scholar]
- 152.Sano H. Kuroki Y. Honma T. Ogasawara Y. Sohma H. Voelker DR. Akino T. Analysis of chimeric proteins identifies the regions in the carbohydrate recognition domains of rat lung collectins that are essential for interactions with phospholipids, glycolipids, and alveolar type II cells. J Biol Chem. 1998;273:4783–4789. doi: 10.1074/jbc.273.8.4783. [DOI] [PubMed] [Google Scholar]
- 153.Schmiedl A. Ochs M. Muhlfeld C. Johnen G. Brasch F. Distribution of surfactant proteins in type II pneumocytes of newborn, 14-day old, and adult rats: an immunoelectron microscopic and stereological study. Histochem Cell Biol. 2005;124:465–476. doi: 10.1007/s00418-005-0066-0. [DOI] [PubMed] [Google Scholar]
- 154.Schneeberger EE. Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- 155.Schneeberger EE. Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 156.Seshiah PN. Weber DS. Rocic P. Valppu L. Taniyama Y. Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 157.Sharov VS. Briviba K. Sies H. Peroxynitrite diminishes gap junctional communication: protection by selenite supplementation. IUBMB Life. 1999;48:379–384. doi: 10.1080/713803538. [DOI] [PubMed] [Google Scholar]
- 158.Simon AM. McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 159.Sittipunt C. Steinberg KP. Ruzinski JT. Myles C. Zhu S. Goodman RB. Hudson LD. Matalon S. Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 160.Slutsky AS. Ventilator-induced lung injury: from barotrauma to biotrauma. Respir Care. 2005;50:646–659. [PubMed] [Google Scholar]
- 161.Sohl G. Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 162.Sosenko IR. Innis SM. Frank L. Polyunsaturated fatty acids and protection of newborn rats from oxygen toxicity. J Pediatr. 1988;112:630–637. doi: 10.1016/s0022-3476(88)80186-0. [DOI] [PubMed] [Google Scholar]
- 163.Sturrock A. Cahill B. Norman K. Huecksteadt TP. Hill K. Sanders K. Karwande SV. Stringham JC. Bull DA. Gleich M. Kennedy TP. Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 164.Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am J Respir Crit Care Med. 2001;163:1293–1294. doi: 10.1164/ajrccm.163.6.ed1801d. [DOI] [PubMed] [Google Scholar]
- 165.Traub O. Hertlein B. Kasper M. Eckert R. Krisciukaitis A. Hulser D. Willecke K. Characterization of the gap junction protein connexin37 in murine endothelium, respiratory epithelium, and after transfection in human HeLa cells. Eur J Cell Biol. 1998;77:313–322. doi: 10.1016/S0171-9335(98)80090-3. [DOI] [PubMed] [Google Scholar]
- 166.Trovato-Salinaro A. Trovato-Salinaro E. Failla M. Mastruzzo C. Tomaselli V. Gili E. Crimi N. Condorelli DF. Vancheri C. Altered intercellular communication in lung fibroblast cultures from patients with idiopathic pulmonary fibrosis. Respir Res. 2006;7:122. doi: 10.1186/1465-9921-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tschumperlin DJ. Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol. 1998;275:L1173–L1183. doi: 10.1152/ajplung.1998.275.6.L1173. [DOI] [PubMed] [Google Scholar]
- 168.Tschumperlin DJ. Oswari J. Margulies AS. Deformation-induced injury of alveolar epithelial cells: effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 169.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Upham BL. Kang KS. Cho HY. Trosko JE. Hydrogen peroxide inhibits gap junctional intercellular communication in glutathione sufficient but not glutathione deficient cells. Carcinogenesis. 1997;18:37–42. doi: 10.1093/carcin/18.1.37. [DOI] [PubMed] [Google Scholar]
- 171.Van Itallie CM. Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 172.van Veen TA. van Rijen HV. Jongsma HJ. Physiology of cardiovascular gap junctions. Adv Cardiol. 2006;42:18–40. doi: 10.1159/000092560. [DOI] [PubMed] [Google Scholar]
- 173.van Zoelen EJ. Tertoolen LG. Transforming growth factor-beta enhances the extent of intercellular communication between normal rat kidney cells. J Biol Chem. 1991;266:12075–12081. [PubMed] [Google Scholar]
- 174.Veldhuizen R. Nag K. Orgeig S. Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 175.Vlahakis NE. Hubmayr RD. Response of alveolar cells to mechanical stress. Curr Opin Crit Care. 2003;9:2–8. doi: 10.1097/00075198-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 176.Waghray M. Cui Z. Horowitz JC. Subramanian IM. Martinez FJ. Toews GB. Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 177.Wang PM. Fujita E. Bhattacharya J. Mechanotransduction in the lung: vascular regulation of type II cell exocytosis. Am J Physiol Lung Cell Mol Physiol. 2001;282:L912–916. doi: 10.1152/ajplung.00303.2001. [DOI] [PubMed] [Google Scholar]
- 178.Wang Y. Phelan SA. Manevich Y. Feinstein SI. Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol. 2006;34:481–486. doi: 10.1165/rcmb.2005-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ware LB. Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 180.Ware LB. Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 181.Wentlandt K. Kushnir M. Naus CC. Carlen PL. Ethanol inhibits gap junctional coupling between P19 cells. Alcohol Clin Exp Res. 2004;28:1284–1290. doi: 10.1097/01.alc.0000139705.17646.ba. [DOI] [PubMed] [Google Scholar]
- 182.Wert SE. Yoshida M. LeVine AM. Ikegami M. Jones T. Ross GF. Fisher JH. Korfhagen TR. Whitsett JA. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene-inactivated mice. Proc Natl Acad Sci U S A. 2000;97:5972–5977. doi: 10.1073/pnas.100448997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Whitsett JA. Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. doi: 10.1056/NEJMra022387. [DOI] [PubMed] [Google Scholar]
- 184.Willis BC. Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 185.Wirtz HR. Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science. 1990;250:1266–1269. doi: 10.1126/science.2173861. [DOI] [PubMed] [Google Scholar]
- 186.Wiszniewski L. Sanz J. Scerri I. Gasparotto E. Dudez T. Lacroix JS. Suter S. Gallati S. Chanson M. Functional expression of connexin30 and connexin31 in the polarized human airway epithelium. Differentiation. 2007;75:382–392. doi: 10.1111/j.1432-0436.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 187.Wong CW. Christen T. Roth I. Chadjichristos CE. Derouette JP. Foglia BF. Chanson M. Goodenough DA. Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 188.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 189.Wright JR. The “wisdom” of lung surfactant: balancing host defense and surface tension-reducing functions. Am J Physiol Lung Cell Mol Physiol. 2006;291:L847–L850. doi: 10.1152/ajplung.00261.2006. [DOI] [PubMed] [Google Scholar]
- 190.Yeh HI. Rothery S. Dupont E. Coppen SR. Severs NJ. Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res. 1998;83:1248–1263. doi: 10.1161/01.res.83.12.1248. [DOI] [PubMed] [Google Scholar]
- 191.Ying X. Minamiya Y. Fu C. Bhattacharya J. Ca2+ waves in lung capillary endothelium. Circ Res. 1996;79:898–908. doi: 10.1161/01.res.79.4.898. [DOI] [PubMed] [Google Scholar]
- 192.Yoon BI. Hirabayashi Y. Kawasaki Y. Tsuboi I. Ott T. Kodama Y. Kanno J. Kim DY. Willecke K. Inoue T. Exacerbation of benzene pneumotoxicity in connexin 32 knockout mice: enhanced proliferation of CYP2E1-immunoreactive alveolar epithelial cells. Toxicology. 2004;195:19–29. doi: 10.1016/j.tox.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 193.Zemans RL. Matthay MA. Bench-to-bedside review: the role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury. Crit Care. 2004;8:469–477. doi: 10.1186/cc2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang ZQ. Hu Y. Wang BJ. Lin ZX. Naus CC. Nicholson BJ. Effective asymmetry in gap junctional intercellular communication between populations of human normal lung fibroblasts and lung carcinoma cells. Carcinogenesis. 2004;25:473–482. doi: 10.1093/carcin/bgh036. [DOI] [PubMed] [Google Scholar]


