Abstract
Direct intercellular communication via gap junctions is critical in the control and coordination of vascular function. In the cardiovascular system, gap junctions are made up of one or more of four connexin proteins: Cx37, Cx40, Cx43, and Cx45. The expression of more than one gap-junction protein in the vasculature is not redundant. Rather, vascular connexins work in concert, first during the development of the cardiovascular system, and then in integrating smooth muscle and endothelial cell function, and in coordinating cell function along the length of the vessel wall. In addition, connexin-based channels have emerged as an important signaling pathway in the astrocyte-mediated neurovascular coupling. Direct electrical communication between endothelial cells and vascular smooth muscle cells via gap junctions is thought to play a relevant role in the control of vasomotor tone, providing the signaling pathway known as endothelium-derived hyperpolarizing factor (EDHF). Consistent with the importance of gap junctions in the regulation of vasomotor tone and arterial blood pressure, the expression of connexins is altered in diseases associated with vascular complications. In this review, we discuss the participation of connexin-based channels in the control of vascular function in physiologic and pathologic conditions, with a special emphasis on hypertension and diabetes. Antioxid. Redox Signal. 11, 251–266.
Introduction
Cell-to-cell communication is fundamental in vascular function, as blood vessels are complex, multicellular structures that must work as an unit, and thus, control of vasomotor tone depends on the fine synchronization of the smooth muscle and endothelial cell function along the length of the vessel wall. Synchronization and coordination is accomplished by an intricate system of radial and longitudinal cell-to-cell communication (10, 55, 170, 175). In addition, in the microcirculation, small arteries and arterioles form a complex network whose elements must work in concert to regulate blood-flow distribution and peripheral vascular resistance by precise, well-integrated changes in the luminal diameter of the vessels (55, 171, 173, 174, 177).
One mode of communication that plays a critical role in controlling the function of the vessel wall is the release of paracrine molecules such as nitric oxide (NO) and prostaglandins. This signaling pathway is widely recognized, and its role in vascular physiology has been extensively studied. A complementary mechanism of communication that has emerged as a key pathway to coordinate the vascular wall function is the direct cell-to-cell communication via gap junctions (Fig. 1). The expression of connexins has been reported to be altered in several animals models of pathologies associated with vascular complications (15), such as hypertension (56, 159) and diabetes (95, 215), which highlights the importance of the direct cell-to-cell interaction for vascular homeostasis.
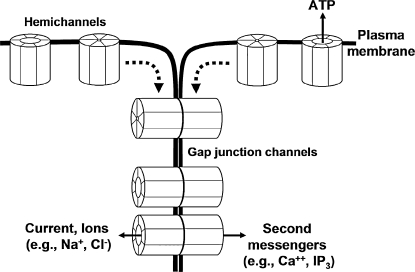
FIG. 1.
Gap-junction channels in the plasma membrane. The family of proteins known as connexins constitutes the structural and functional unit of gap-junctional intercellular communication. Oligomerization of six connexin proteins forms a connexon or hemichannel (49, 162). In response to stimuli such as phosphorylation or Ca2+, the associated six connexin subunits may coordinately change configuration to open the hemichannel and allow movement of paracrine signaling molecules such as ATP (70, 163). Hemichannels may diffuse laterally to the junctional membrane where they are in a position to dock with another hemichannel on the apposed membrane of the adjacent cell to complete a gap-junction channel (49, 162).
Gap junctions are intercellular channels that directly connect the cytoplasm of adjacent cells, allowing the passage of current and small signaling molecules (molecular mass <1,000 Da), such as Ca2+ and IP3 (49, 162) (Fig. 1). These intercellular channels comprise a protein family known as connexins (Cx), which are denoted according to their predicted molecular weight. Six connexins combine to make a connexon or hemichannel, and the association in the plasma membrane of two hemichannels provided by adjacent cells forms a functional gap-junction intercellular channel (49, 162) (Fig. 1). It is noteworthy that independent hemichannels may also remain unpaired and open to release paracrine signals such as ATP or NAD+ (70, 163). Although it has been suggested that monocytes and macrophages expressed Cx37 hemichannels that regulate cell adhesion (207), this mode of connexin-based signaling has not yet been demonstrated to participate directly in the control of the vascular wall function (Fig. 1).
At least twenty connexin isoforms have been described in mammals, and one cell type often expresses more than one connexin (162). However, the expression of several connexins in one cell is not redundant. Gap junctions are not just simple channels that offer a low-resistance intercellular pathway for exchange of small solutes. Rather, the connexins mediate specific cell-to-cell signaling pathways, and the molecular selectivity as well as subcellular localization differs among connexin (55, 56, 129, 162, 203). Thus, although these proteins may have some overlap in function, they work in concert (55, 56, 79, 182, 183) and, in many cases, the function of one connexin cannot be replaced by other connexin isoform (79, 203, 206, 216). In addition, hemichannels can be composed of one (homomeric channels) or a mixture (heteromeric channels) of connexin proteins. Homomeric channels formed by different connexin isoforms typically differ in unitary conductance, permeability, and regulation and, as expected, the mixture of connexins in a heteromeric channel results in unique communication properties (28, 84, 131, 141), which provide an additional mechanism for fine regulation of gap junction–mediated signaling processes (12, 129, 204).
Connexin in the Vasculature
The vascular gap junctions are assembled from one or more of four connexin proteins: Cx37, Cx40, Cx43, and Cx45 (49, 55, 81, 178). The expression of connexins in the vessel wall is not uniform and seems to vary with vessel size, vascular territory, and species (85, 191, 192). In most cases, Cx45 is observed only in the smooth muscle cells (109, 122, 158). Although Cx37 is typically confined to endothelial cells (64, 178, 191), it has also been detected in the smooth muscle cells (158). In contrast, Cx40 and Cx43 may be expressed in both cell types (64, 126, 178, 191), but Cx40 is located predominantly in the endothelial cells (64, 191), and Cx43 is the most prominent gap-junction protein found in the smooth muscle cells (191). It should be noted, however, that in the mouse, Cx40 has been detected exclusively in the endothelium (36, 57, 91, 115, 183).
Consistent with the importance of gap junctions for the vascular homeostasis, it has been found that selective ablation of connexin genes results in severe vascular malformations. Cx45 knockout mice die at early embryonic stages (E9.5 to E10.5) and exhibit major defects in remodeling and organization of blood vessels. In addition, these animals fail to form a smooth muscle layer surrounding the major arteries (109). Deletion of Cx43 modifies the expression of many genes known to be involved in the differentiation and function of vascular cells, and cell-signaling pathways important for the regulation of vasculogenesis and angiogenesis (197), which produces several alterations in the pattern of coronary artery development, and the embryos die at birth of blockage of the right ventricular outflow tract (25, 154). Although Cx40-deficient embryos exhibit small septational defects, deletion of one allele of Cx43 increases the cardiac malformations of Cx40-knockout mice and leads to neonatal death. In contrast, haploinsufficiency of Cx40 did not affect the Cx43-knockout phenotype (105). Deletion of Cx37 is not lethal and has not been shown to produce any particular vascular phenotype. However, simultaneous ablation of Cx37 and Cx40 results in severe vascular abnormalities in skin, testis, intestine, stomach, and lung, and the animals do not survive past the first postnatal day (183), which highlights the role of the endothelial cell–gap junction communication in the development of the vasculature. Taken together, these data indicate that individual connexin isoforms are differentially involved in vascular development and emphasize the notion that although these gap–junction proteins may be coexpressed, they basically work in concert.
Connexins in Vascular Physiology
Vascular smooth muscle
Synchronization of vasomotor tone among the smooth muscle cells is critical for the function of blood vessels. The contractile state of the smooth muscle cells is determined by the cytoplasmic Ca2+ concentration and the Ca2+ sensitivity of the contractile apparatus. Consequently, the smooth muscle cell-membrane potential plays a central role in the tonic control of intracellular Ca2+ concentration by modulating the influx of Ca2+ via L-type, voltage-dependent Ca2+ channels. Gap junctions play a central role integrating the smooth muscle cell function by coordinating changes in both membrane potential and intracellular Ca2+ between adjacent smooth muscle cells (22–24).
In addition, gap-junctional communication of vascular smooth muscle cells seems to be involved in the development of the myogenic vasomotor tone in resistance arteries (42, 118). Interestingly, the participation of the gap junction in this process is not related to synchronization of Ca2+ signaling, but rather to earlier signaling events such as coordination of the smooth muscle cell depolarization or, directly, the mechanosensitivity of the vascular smooth muscle. This hypothesis is supported by the fact that the gap-junction inhibitory peptide Gap27 or the gap-junction inhibitor 18α-gly-cyrrhetinic acid, in addition to blocking Ca2+ influx and vasoconstriction in mesenteric resistance arteries, also prevented pressure-induced smooth muscle cell depolarization (42). However, a nonspecific effect of the gap-junction blockers on ion channels cannot be discounted. In any case, the involvement of gap junctions in the myogenic response may be consistent with the finding that tensile stretch increased Cx43 expression as well as gap-junctional intercellular communication in vascular smooth muscle cells. Interestingly, this response was mediated by the formation of reactive oxygen species (29, 30, 190), which has been reported to contribute to the initiation of the myogenic constriction in mouse-tail arterioles (146).
In addition, Cx43 seems to be essential for coordination of cell proliferation and migration in the vasculature (55, 116, 151, 214), which was recently confirmed by the specific deletion of the Cx43 gene in the smooth muscle cell (124). In these animals, the injury to the carotid artery by vascular occlusion or wire injury resulted in an increase in the neointima and the adventitia formation (124), suggesting an accelerated growth of the smooth muscle cell with the Cx43 deletion, which was further confirmed by using cultured cells. In contrast to these findings, Chadjichristos et al. (19) showed that in heterozygous Cx43-knockout mice, the neointimal formation was reduced. However, in those animals, Cx43 was deleted from all cell types expressing Cx43, and the experiments included a high-fat diet, which may have influenced the result by either vascular adaptive responses to the diet or complex interactions between different cell types. Although the participation of Cx43 in neointimal formation demands further investigation, these data highlight the relevance of Cx43 in the feedback-control pathways necessary for vascular morphogenesis.
Endothelium
The endothelium plays a key role in the tonic control of blood pressure, and deletion of vascular connexin genes has disclosed that gap-junctional communication is essential in the coordination and integration of microvascular function by the endothelial cells in a very complex manner. Vascular endothelial cells–specific deletion of Cx43 (VEC Cx43−/−) results in hypotension (123) and, in contrast, ablation of Cx40 produces a hypertension associated with an irregular vasomotion (36, 37) and a dysregulation of renin production (107, 195, 196). Although deletion of Cx37 does not appear to alter vascular function or blood pressure, polymorphisms of this connexin have been associated with myocardial infarction, coronary artery disease, and atherosclerosis (13, 86, 211, 212). In mice, Cx40 and Cx37 are expressed primarily in the endothelium, which emphasizes the importance of the endothelial cell–gap junction communication in the control of cardiovascular homeostasis and the idea that vascular connexins work in concert coordinating specific signaling processes.
Although the mechanistic bases of the hypotension observed in VEC Cx43−/− are still unknown, the plasma levels of angiotensin I and II as well as NO were elevated in these animals (123), suggesting that a dysregulation of NO production may have been responsible for the hypotension, and the renin–angiotensin system was activated as a compensatory mechanism. It is interesting to note that shear stress upregulates the expression of Cx43 in cultured endothelial cells (6, 38) and in the endothelium of rat cardiac valves (93), which is consistent with the idea that Cx43 is involved in the sensitivity to mechanical stimuli.
Smooth muscle–endothelium communication via gap junction
The smooth muscle cells and the endothelial cells can also be electrically and metabolically connected by gap junctions located at discrete points of contact between the two cell types at the myoendothelial junction (MEJ) (11, 46, 127, 166, 179, 209). This heterocellular communication seems to play a pivotal role in the Ca2+-mediated responses induced by endothelium-dependent vasodilators, such as ACh (Fig. 2). These vasodilator responses are typically paralleled by hyperpolarization of the underlying smooth muscle cells (46, 71, 75). The relaxant pathway associated with smooth muscle hyperpolarization is thought to be independent on NO and prostacyclin production by the endothelial cells and has been attributed to the release of an endothelium-derived hyperpolarizing factor (EDHF) (50, 193). The identity of EDHF remains controversial, and several EDHF candidates have been proposed, such as K+ ions (43), epoxyeicosatrienoic acids (EETs) (4, 62), hydrogen peroxide (180), and C-type natriuretic peptide (CNP) (1, 20). However, the direct electrotonic transmission of a hyperpolarizing current from the endothelial cells to the smooth muscle cells via myoendothelial gap junctions (Fig. 2) has emerged as a most attractive hypothesis to explain the EDHF pathway (18, 40, 74). In this context, the increase in endothelial cell intracellular Ca2+ concentration activates the Ca2+-activated K+ channels (KCa) of small (SKCa) and intermediate conductance (IKCa), leading to the endothelium-dependent hyperpolarization of the smooth muscle cells via gap junctions located at the MEJ (18, 32, 44, 51, 148) (Fig. 2). Consistent with this hypothesis, the EDHF-dependent vasodilation is prevented by the connexinmimetic peptides that are thought to specifically block the gap junction (21, 35, 104), as well as endothelial cell–selective loading of antibodies directed against the carboxyl-terminal region of Cx40 (134).
FIG. 2.
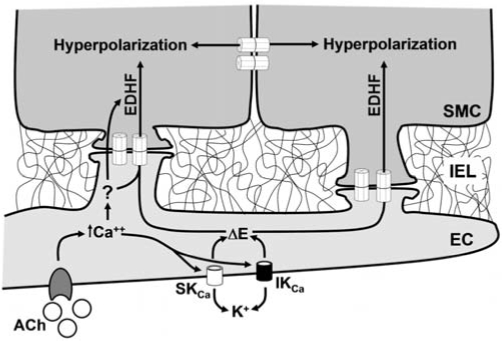
Control of vasomotor tone by the endothelium-derived hyperpolarizing factor. Extensions derived from either the smooth muscle cells or endothelial cells may penetrate the internal elastic lamina (IEL) to make close contact with the other cell type. These points of contact, known as myoendothelial junctions (MEJs), provide the structural organization to achieve direct heterocellular coupling between the two cell types via gap junctions (40, 55, 166), and thus, one basis for endothelium-mediated smooth muscle hyper-polarization (often referred to as endothelium-derived hyperpolarizing factor, EDHF). Endothelium-dependent vasodilators such as acetylcholine (ACh) induce an increase in endothelial cell intracellular Ca2+ concentration, which, in turn, activates the Ca2+-activated K+ channels (KCa) of small (SKCa) and intermediate conductance (IKCa). The endothelial cell hyperpolarization is transmitted electrotonically to the underlying smooth muscle cells via gap junctions located at the MEJ, contributing to the endothelium-dependent vasodilation (18, 44, 51). The question mark highlights that the participation of a diffusible EDHF has not been definitely discarded.
In addition to NO, shear stress has been reported to activate an EDHF-dependent vasodilator response (198), and the contribution of the EDHF-mediated responses seems to increase as the vessel size decreases (165, 181), which suggests that an EDHF pathway may be involved in the tonic control of peripheral vascular resistance. Consistent with this idea, intrarenal infusion of connexin-mimetic peptides homologous to the second extracellular loop of Cx43 (43Gap 27) or Cx40 (40Gap 27) not only decreased basal renal blood flow, but also increased mean arterial blood pressure of female rats, either in the presence or the absence of NO synthase (NOS) and cyclooxygenase (COX) blockade (35), suggesting that the connexin-mimetic peptides induced vasoconstriction by disrupting a tonic vasodilator signal.
Interestingly, in male animals, NO is the major endothelium-dependent vasodilator signal, but in female animals, EDHF prevails over NO or PGI2 (169), and estrogen appears to be responsible for this gender difference (90, 128, 143). The endothelial isoform of NOS (eNOS) is found in a inhibitory association with caveolin-1, a structural protein of caveolae (52, 65, 100, 139, 140), and estrogen modulates the expression of this negative regulator of eNOS, and the EDHF-positive regulators, Cx43 and Cx40 (35, 97, 128, 143) (Fig. 3). Thus, in ovariectomized rats, the level of caveolin-1, Cx43, and Cx40 decreases in parallel with an increase in the NO-mediated vasodilation and a reduction in the EDHF-mediated vasodilation sensitive to the gap-junction blockers 18β and 18α-glycyrrhetinic acid (128, 143). As expected, all the changes are recovered after estrogen replacement (128, 143).
FIG. 3.
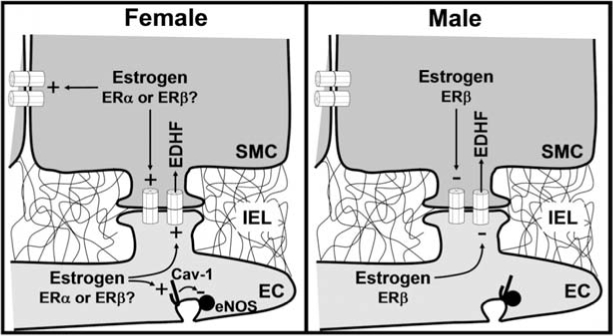
Possible sex differences in the regulation of the endothelium-derived hyperpolarizing factor (EDHF). Nitric oxide (NO) and EDHF are the major endothelium-derived vasodilator signals in resistance vessels. The involvement of these two vasodilator signals differs between male and female animals. NO prevails over EDHF in males, and the contrary is observed in females (130, 169). This gender difference may be explained by the estrogen modulation of the myoendothelial gap junction–mediated smooth muscle hyperpolarization. In female animals, estrogen upregulates the expression of caveolin-1 (Cav-1) (128, 143), a structural protein of caveolae that, in turn, inhibits the activity of eNOS (52, 65, 100). In addition, this hormone enhances the expression of Cx43 and Cx40 (128, 143), two gap-junction proteins found at the myoendothelial junctions (35, 97). As a result, the NO-dependent vasodilation is reduced, and the gap junction–mediated EDHF signaling is increased. The involvement of the estrogen receptor α (ERα) or β (ERβ) remains to be determined. In contrast, in male animals, the activation of ERβ reduces the gap junction–dependent smooth muscle hyperpolarization (130).
Estrogen also enhances the EDHF-mediated vasodilation in response to flow (90), which suggests that the myoendothelial gap-junction–dependent signaling pathway may be more important in the control of blood pressure in female than in male animals. Recently, this idea was confirmed by using an eNOS/COX-1 double knockout. Deletion of eNOS and COX-1 did not alter the mean arterial blood pressure in female mice, whereas the double knockout resulted in hypertension in male mice (169). The endothelium-dependent relaxation was intact in resistance vessels of female mice and was mediated by the smooth muscle hyperpolarization (169), strongly indicating that EDHF plays a predominant role in the tonic control of blood pressure in female animals. These data suggest that EDHF rather than NO may underlie the higher resistance of premenopausal women to cardiovascular diseases such as hypertension.
Two estrogen receptor (ER) subtypes, ERα and ERβ, are expressed in vascular endothelial cells as well as smooth muscle cells of both female and male animals (135, 136, 217). It is thought that ERα mediates most of the vascular effects of estrogen, but the importance of ERβ is emerging, and ERβ-deficient mice develop hypertension as they age (217). In addition, recently Luksha et al. (130) proposed that ERβ contributes to the sex differences observed in the endothelium-dependent control of vasomotor tone by reducing the gap junction–mediated EDHF signaling in the male mouse (Fig. 3).
Conduction of vasomotor responses
Longitudinal conduction of vasomotor responses provides an essential means of coordinating changes in diameter and flow distribution among vessels in a complex network. Vasomotor signals spread along the vessel length through gap junctions connecting cells of the vessel wall, and thereby, participate in the minute-to-minute coordination of vascular resistance by integrating function of proximal and distal vascular segments in the microcirculation (37, 55, 56). Although vasoconstrictor responses are thought to be conducted by the smooth muscle cells (8, 17, 202), the cellular pathway for conduction of vasodilator signals is more controversial and may be either exclusively the endothelium (47, 176) or both the smooth muscle and the endothelial cells (8, 17). The cellular pathway for conduction of vasomotor responses has been studied by selectively damaging a short segment of the endothelial cells or the smooth muscle cells with a light-dye (fluorescein-conjugated dextran) treatment. In feed arteries, selective damage of the endothelium completely blocked the ACh-induced conducted vasodilation (47, 176), but in arterioles, damage of either the endothelium or the smooth muscle did not affect the conduction of the response to ACh (8, 17), which led to the proposal that the cellular pathway for conducted vasodilations depends on the functional location of the vessel in the microvascular network (172). However, the cellular pathway of vasodilator signals may also depend on the stimulus that initiated the response, because, in contrast to ACh, selective damage of the endothelium blocked the vasodilation induced by bradykinin in arterioles (17, 202).
Direct measurements of membrane potential have shown that conducted vasomotor responses are associated with rapid propagation (milliseconds) of an electrical signal along the vessel length (47, 202, 208, 209). Because many observations have revealed an exponential decay of the conducted electrical signal, it was proposed that longitudinal spread of vasomotor responses reflects the passive, electrotonic conduction of changes in membrane potential via gap junctions connecting cells of the vessel wall (76, 150, 202). Therefore, the decay of the conducted vasomotor responses along the vessel length should be consistent with the length constant calculated from electrotonic potentials produced by current injection into the smooth muscle or endothelial cells of arterioles, which is between 0.9 and 1.6 mm (45, 87, 88).
Conduction of vasoconstrictor responses typically behaves as predicted by the electrotonic model. However, a simple electrotonic model often fails to predict conduction of vasodilator signals initiated by endothelium-dependent stimuli, such as ACh or bradykinin. These signals have been reported to propagate for many millimeters without showing noticeable decay in magnitude (36, 41, 47, 48) (Fig. 4). In addition, the electrical length constant of ACh-induced hyper-polarization has been shown to be longer than that measured for current injection (45), and the hyperpolarizing signal activated by ACh has been also reported to increase during the first 1,000 μm of longitudinal conduction (33). The lack of decay of these responses suggests that a regenerative, energy-dependent mechanism underlies the conduction process, similar to that described in neurons. Consistent with this idea, it was shown recently that electrical stimulation also activates a conducted, nondecremental endothelium-dependent vasodilation (Fig. 5) that is hypothesized to be mediated by a complex interplay between voltage-gated Na+ channels (Nav) and T-type, voltage-gated Ca2+ channels [T-Cav (54)], where Nav underlies the conduction of the signal and T-Cav mediates the vasodilation (Fig. 6). Interestingly, deletion of Cx40 selectively eliminates the regenerative component of the conducted vasodilation induced by ACh, bradykinin (36), or electrical stimulation (57), leaving a decaying component consistent with the electrotonic model, which demonstrates that gap junctions are the pathways for the intercellular propagation of these vasodilator signals and suggests that Cx40 may be functionally associated with the endothelial Nav (Fig. 6). Deletion of Cx37 did not affect conduction of vasodilator responses (Figueroa and Duling, unpublished observations), and replacement of Cx40 by Cx45 did not restore the nondecremental component of the conducted vasodilation activated by ACh or bradykinin (206), supporting the idea that individual connexins have different functions, but leaving much to be explained as to how such selectivity is conferred.
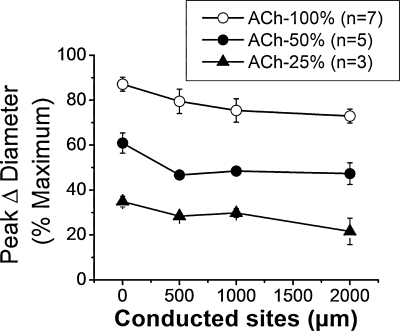
FIG. 4.
Conduction of vasodilator responses induced by the stimulation of cremasteric arterioles with a pulse of ACh. The microcirculation of the cremaster muscle was prepared as described previously (54, 57), and arterioles were stimulated focally with the ejection of ACh (10 μM) by a pressure-pulse from a micropipette (inner diameter, 3–4 μm). The changes in diameter were measured first at the stimulation site (local), and then, at locations 500, 1,000, and 2,000 μm upstream in four separate stimuli. Variations in diameter were expressed as percentage of the maximal dilation possible (% maximum). The duration of the pulse (300–700 ms) and the ejection pressure (10–20 psi) were set to induce a local vasodilation of ~100%, ~50%, or ~25%. Maximal diameter was estimated during superfusion of 1 mM adenosine. Note that the magnitude of the response decays only from the local site to the 500-μm conducted site and does not show a further reduction thereafter.
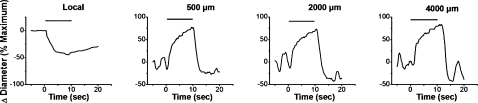
FIG. 5.
Representative tracings of the conduction of the vasomotor responses induced by focal electrical stimulation of cremasteric arterioles. The microcirculation of the cremaster muscle was prepared as described previously (54, 57). An Ag/AgCl reference electrode immersed in the superfusate was positioned symmetrically around the cremaster, and the arteriole was stimulated with a depolarizing train of pulses (30 Hz, 2 ms, 30 V) for 10 s by using a beveled micropipette (inner diameter, 3–4 μm) filled with 1 M NaCl. The stimulating pipette was inserted under the cremasteric mesothelium and positioned directly above the arteriole at a distance selected to evoke a local constriction of ~50% (54, 57). In separate stimuli, the changes in diameter were observed at the stimulation site (local), and at locations 500, 2,000, and 4,000 μm upstream. Variations in diameter were expressed as percentage of the maximal constriction or dilation possible (% maximum). Maximal diameter was estimated during superfusion of 1 mM adenosine. Focal electrical stimulation of the arteriole evoked a vasoconstriction that was restricted to a short vessel segment (~70–100 μm) at the stimulation site and, in addition, activated a rapid conducted vasodilation that spread along the length of the entire vessel without decay. Horizontal bars indicate the stimulation period.
FIG. 6.
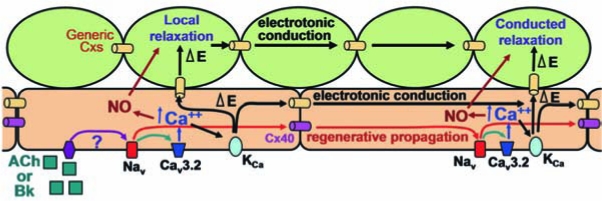
Hypothetical model of conducted vasodilator responses activated by endothelium-dependent vasodilators [based on data from (36, 54, 57, 206)]. Stimulation of the endothelial cells with ACh or bradykinin triggers a regenerative, conducted vasodilator signal that is mediated by the activation of voltage-dependent Na+ channels (Nav) and rapidly propagated along the endothelium selectively via Cx40 gap junctions (red lines). The Nav-mediated conducted electrical signal is transduced into vasodilation by activation of the T-type, voltage-dependent Ca2+ channels Cav3.2 and the subsequent initiation of the production of Ca2+-sensitive vasodilator signals such as NO and Ca2+-activated K+ channel (KCa)-mediated smooth muscle hyperpolarization (black lines). The KCa-dependent hyperpolarization may be conducted electrotonically along the longitudinal axis of the vessel by either the smooth muscle cells or the endothelial cells via Cx40 or other connexins present in the vascular wall, which, in this schematic, we designated “generic” Cxs. This hypothetical model is compatible with the evidence showing that deletion of Cx40 completely eliminates the regenerative component of the conducted vasodilator response (36, 57, 206). The decremental conducted vasodilation that remains in the absence of Cx40 may correspond to the electrotonic conduction of the KCa-dependent hyperpolarizing signal. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.lieberonline.com/ars)
The electrotonic and regenerative components of the conducted vasodilation activated by an endothelium-dependent vasodilator such as ACh are highlighted schematically in a hypothetical model shown in Fig. 6. The opening of inward-rectifier K+ channels (Kir) induced by the smooth muscle hyperpolarization may be an alternative hypothesis to explain the extended conduction of vasodilator responses. An intrinsic biophysical property of Kir channels is that they increase their activity on cell hyperpolarization, and it has been proposed that the activation of these K+ channels in the smooth muscle cells amplifies the hyperpolarizing current initiated by ACh, thereby facilitating the conduction of this signal (98). However, as mentioned earlier, current-induced hyperpolarization decays faster than the response induced by ACh (45), which argues against the participation of Kir alone in the nondecremental component of the conducted vasodilation and suggests that further investigation is needed to elucidate the mechanisms involved in the conduction of vasomotor responses.
Theoretic analysis of vascular adaptation to hemodynamic and metabolic stimuli indicate that conduction of vasodilator signals may also play a role in the long-term control of peripheral resistance by contributing to the maintenance of the structural homeostasis of the microvascular network (152). The vascular rarefaction observed during the development of hypertension may be an example of this. The phenomenon of rarefaction is manifested as a reduction in the number or density of microvessels, which occurs in two phases: a functional and an anatomic rarefaction (120). The functional rarefaction involves a reduction in the perfusion of microvessels that appears to reach a nadir at which they may then be disassembled, leading to the structural or anatomic rarefaction. Interestingly, mathematical simulations suggest that conduction of vasomotor responses induced by metabolic stimuli may be essential to preclude the disassembly of microvessels observed in the functional rarefaction (152, 153). Therefore, the functional longitudinal communication from capillaries up to arterioles via gap junctions (9, 185) provides pathways for both short-term modulation of diameter and for long-term regulation of the microvascular network architecture in physiologic and pathologic conditions.
Neurovascular coupling
Conduction of vasomotor signals is also involved in the control of cerebral microcirculation by neuronal activity, referred to as neurovascular coupling (3, 83, 121). In this case, however, vasomotor signals seem to be conducted by astrocytes, as opposed to smooth muscle or endothelium (2, 106, 137, 142, 189, 218). Tight spatial and temporal coupling between neuronal activity and blood flow is essential for the brain function (2, 83, 121, 161), and astrocytes are found in a strategic location between neurons and the microvasculature, with the astrocytic endfeet ensheathing the vessels (Fig. 7). This spatial organization places the astrocytes in a key position to orchestrate the neurovascular coupling, and an increasing body of evidence shows that the astrocyte transduces and conducts neuron-generated vasomotor signals to the local microvasculature (2, 138, 142, 189, 218). As a result, astrocytes couple neuronal activation to vasodilation of local parenchymal arterioles (Fig. 7), which, in turn, leads to an increase in blood-borne oxygen and glucose supply to satisfy the enhanced metabolic demand rapidly (2, 83, 121, 161).
FIG. 7.
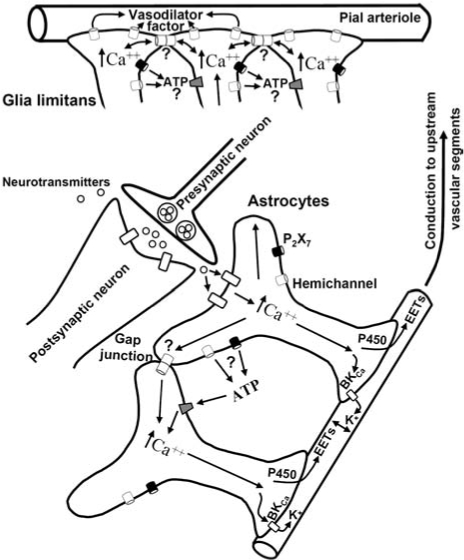
Connexin-based channels likely to be involved in neurovascular coupling. Neurotransmitters released on an increase in neuronal activity may exit the synaptic cleft and activate receptors on astrocytes (2, 218). These receptors cause an increase in astrocyte intracellular Ca2+ concentration that leads to the activation of large conductance Ca2+-activated K+ channels (BKCa) and/or cytochrome P450 epoxygenase (P450) at the astrocytic endfeet. The efflux of K+ through BKCa and/or production of epoxyeicosatrienoic acids (EETs) by P450 results in vasodilation of the parenchymal arterioles (2, 58, 59, 82, 187). The astrocyte-mediated vasodilator signal may be coordinated by the propagation of an interastrocyte Ca2+ signal via ATP release or directly by gap-junction communication. ATP may be released by either P2X7 receptors or unpaired hemichannels (70, 188). The potential role of interastrocyte Ca2+ waves in the coordination of the neurovascular coupling remains to be clearly defined. Local vasodilation of parenchymal cerebral arterioles must be communicated to upstream vascular segments to produce a functional increase of blood supply, and astrocytes may also communicate the vasodilator response to upstream vessels, such as the pial arterioles. These arterioles overlie a thick layer of astrocytic processes, called the glia limitans. The vasodilator signal triggered by neuronal activity reaches the glia limitans and induces a Cx43-based channel-dependent vasodilation (210). The mechanism by which Cx43 controls the astrocyte-mediated vasodilation has not been established, but coordination of the response between astrocytes, via gap-junction communication, or ATP release by hemichannels, is an interesting hypothesis that must be explored. An alternative hypothesis may be that a vasodilator factor is released at the endfeet via Cx43-based hemichannels.
Calcium seems to be the intracellular vasomotor signal of the astrocyte-mediated neurovascular coupling. The increase in neuronal activity results in astrocytic calcium signaling that propagates through the astrocytic processes into the endfeet (2, 58, 142, 187, 218). The increase in cytosolic calcium concentration in the endfeet ultimately causes the release of vasoactive factors and arteriolar dilation (2, 58, 59, 187, 218) (Fig. 7). Interestingly, astrocytes express gap junctions (132, 133, 155, 162), and a calcium signal may propagate between neighboring astrocytes in a wavelike manner (27, 144, 145), coordinating the neurovascular coupling in the local cerebral microcirculation (2, 58, 142, 187, 218). However, the possible participation of gap junctions in the coordination of the astrocyte-mediated vasomotor signals remains to be established.
As described in the peripheral microcirculation (171, 177), local vasodilation of cerebral arterioles must be communicated to upstream vascular segments to produce a functional increase of blood-flow supply and to match the local metabolic demand (31, 92). Although vasomotor responses have been observed to be conducted in cerebral arterioles (39, 89), recently Xu et al. (210) demonstrated in vivo that astrocytes also play a central role in integrating the function of local arterioles with upstream cerebral vessels involved in the neurovascular coupling. Pial arterioles are important upstream vessels of the parenchymal cerebral arterioles. It is important to note that pial arterioles overlie a thick layer of astrocytic processes, known as the glia limitans, which isolate these arterioles from the neurons that are located right below (Fig. 7). Vasodilation of pial arterioles associated with neuronal activation was blocked by the selective elimination of astrocytes through treatment with l-α-aminoadipic acid, and, interestingly, this response was also sensitive to the inhibition of Cx43-based channels with the specific connexinmimetic peptide gap-27 (161, 210). The selectivity of the Cx43 inhibition was confirmed by using two connexin-blocking peptides: gap-27, which targets Cx43/37-based channels, and gap-26, which is a Cx40/37-selective peptide (210). Taken together, these data suggest that Cx43 is essential in the astrocytic signaling that mediates the neurovascular coupling. In astrocytes, Cx43 may be found forming unpaired hemichannels or gap-junction intercellular channels (155, 162, 186). Thus, astrocytic, Cx43-based channels could be involved in the coordination of calcium waves between astrocytes or in the release of vasoactive factors (Fig. 7), which is an interesting scientific challenge that requires further investigations.
Vascular Connexins in Pathology
Hypertension
Consistent with the participation of gap junctions in the control of vasomotor tone, the expression of vascular connexins is altered in hypertension (56). However, changes in several parameters associated with this pathology, such as mechanical load (30), bioavailability of NO (80, 101, 156, 213), shear stress (93, 99, 117), or angiotensin II (78, 103), may modulate the expression of connexins. In addition, gap junctions are involved in different and sometimes antagonistic (vasodilation vs. vasoconstriction) functions in the control of vascular function, and changes in connexin expression vary depending on the experimental model of hypertension or vascular territory studied (72, 77, 78, 80, 103, 157, 160, 199, 213). Therefore, a simple analysis of connexin expression does not allow one to determine whether connexins play a role in the genesis of hypertension or whether the changes observed are a consequence of the development of the pathology. Also, it is important to note that the heterocellular coupling between the smooth muscle and endothelial cells has been shown to be enhanced in spontaneously hypertensive rats (SHRs). However, in these animals, the vasodilation induced by ACh and the gap junction–mediated smooth muscle hyperpolarization were reduced, which was assumed to be the result of structural changes in the media, and that the greater heterocellular coupling was a compensatory mechanism to maintain the EDHF signaling in this model of hypertension (164). A recent review describes in detail the alterations of gap junctions in hypertension (56), and therefore, in this section of the review, we focus on the genetic manipulations of connexin expression that result in an altered control of arterial blood pressure.
Probably the clearest participation of gap junctions in hypertension is through the control of renin secretion. Cx37, −40, and −43 are expressed in the kidney and link the cells of the juxtaglomerular apparatus (JGA) (5, 114, 215). These three gap-junction proteins are expressed in the endothelial cells of afferent arterioles. In addition, Cx37 and Cx40 are found in renin-secreting cells and mesangial cells (5, 114, 215). Interestingly, endothelial cell communication via Cx43 appears to be a key in the control of renin secretion, because replacement of Cx43 by Cx32 (Cx43KI32) disrupted the regulation of renin synthesis and secretion (79). In Cx43KI32 mice, expression of renin was reduced, and the levels of this hormone were not downregulated by a high-salt diet or increased by clipping a renal artery in the two-kidney, one-clip (2K1C) model of renin-dependent renovascular hypertension (79).
In contrast to the inhibition of renin secretion observed in Cx43KI32 mice, deletion of Cx40 resulted in increased renin production and plasma renin concentration (Fig. 8), leading to the proposal that this connexin plays an essential role in the tonic inhibition of the renin system in the kidney (107, 196). Consistent with this idea, intrarenal pressure and angiotensin II failed to attenuate renin secretion in Cx40-deficient mice (196). Although Cx40 is the dominant gap-junction protein in renin-secreting cells, the dysregulation of the renin system observed in Cx40-knockout mice may depend, not on direct cell-to-cell signaling via Cx40 gap junctions, but on structural changes in the JGA architecture, as shown by Kurtz et al. (114). Ablation of Cx40 seems to affect the JGA development and, in the absence of Cx40, renin-expressing cells were not present in the terminal part of the afferent arteriole wall; instead, renin was found in cells of the extra-glomerular mesangium and periglomerular interstitium (114). Taken together, these studies highlight the importance of gap-junction communication in the control of JGA development and renin secretion. It is important to remember that Cx37 is also expressed in the JGA, and although deletion of Cx37 does not affect arterial blood pressure, the participation of this connexin in JGA development and control of the renin system remains to be determined.
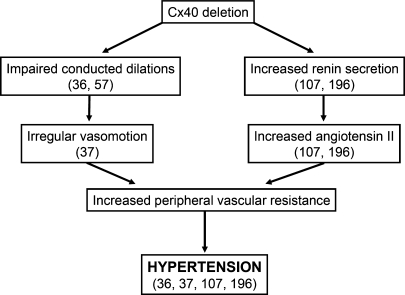
FIG. 8.
Mechanisms of the control of arterial blood pressure by Cx40. Cx40 seems to play a central role in the control of arterial blood pressure by the parallel coordination of the vasomotor tone of resistance vessels and by renin secretion in the juxtaglomerular apparatus (JGA). Deletion of Cx40 affects the endothelial cell–dependent longitudinal synchronization of the vessel wall function, which results in an impaired conduction of vasodilator responses (36, 57), an irregular vasomotion and segmental constrictions (37). At the same time, the absence of Cx40 in the renin-secreting cells at the JGA disrupts the tonic inhibition of renin system, leading to an increase in renin secretion with the consequent increase in angiotensin II levels (107, 196). The dysregulation in the longitudinal communication of microvessels and renin secretion converges to increase the peripheral vascular resistance and produce the hypertension observed in Cx40-knockout animals (36, 37, 107, 196).
Although it may be controversial (107), the increase in renin secretion does not fully account for the hypertension observed in Cx40-knockout mice because blockade of the angiotensin II receptor AT1 with candesartan (37) or the angiotensin I–converting enzyme (ACE) with enalapril (196) reduced, but did not revert the blood pressure of these animals to the normal values (Fig. 8). As mentioned earlier, deletion of Cx40 is also associated with an irregular vasomotion of microvessels and an impaired conduction of vasodilator signals (37). Therefore, the renin/angiotensin II–independent component of the hypertension triggered by the absence of Cx40 probably is caused by an interruption of the control and coordination of the cells of the vessel wall (Fig. 8). In support of the importance of coordination and integration of vasomotor tone in the control of arterial blood pressure (37, 55, 56) and structural adaptation of the microvascular network (152, 153), conduction of vasodilator responses has been found to be impaired in spontaneously hypertensive hamsters (110, 111) and SHRs (72).
The potential importance of endothelial cell Cx40 in the development of hypertension has also been noted in SHRs (103, 160). In the caudal artery of these animals, Rummery et al. (160) found decreased endothelial cell size and a reduction in the density of endothelial gap-junction plaques containing Cx40. Again, the heterogeneity in connexin participation arises because, in these experiments, no changes were found in the expression of Cx37 or Cx43.
Consistent with the key role played by Cx40 in the control of renin secretion and coordination of vasomotor tone, two closely related polymorphisms (−44A and +71G) within the regulatory region of the human Cx40 gene have been associated with increased risk of hypertension in men (61, 73). As expected, these polymorphisms seem to alter the activity of the Cx40 promoter, because in vitro reporter assays revealed that the Cx40 haplotype −44A/+71G reduced the expression of luciferase by 50%, and the −44A polymorphism negatively affects the promoter regulation by the transcription factors Sp1 and GATA4 (60, 61, 73).
Diabetes
As observed in hypertension, control and coordination of vasomotor tone play a critical role during the development of diabetes, and vascular complications are the leading causes of mortality in this pathology. Diabetes and hyperglycemia may lead to vascular dysfunction by several mechanisms, such as nonenzymatic glycosylation (16, 194), oxidative stress (69), polyol-myoinositol alteration (63), and activation of diacylglycerol (DAG)-protein kinase C (PKC) pathways (119, 205).
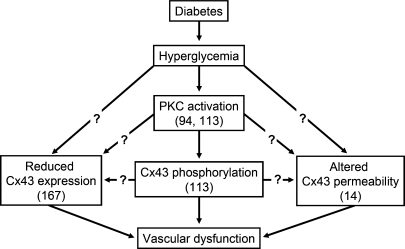
A large body of evidence shows that PKC-dependent phosphorylation of Cx43 affects the gap-junction intercellular communication (125, 162), and, in the vasculature, the direct activation of PKC with phorbol myristate acetate (PMA) reduces the Cx43-mediated dye coupling in primary cultures of human tonsil high endothelial cells (53). In accordance with these findings, high glucose concentrations inhibited gap-junction dye transfer through the activation of a PKC-dependent signaling pathway in cultured bovine aortic endothelial cells (94, 95). In addition, exposure to a high-glucose medium reduced gap-junction activity in rat microvascular endothelial cells (167), and the reduction in dye coupling observed in these cells was correlated with a decrease in Cx43 mRNA and protein levels (Fig. 9), whereas the expression of Cx37 and Cx40 was not affected (167). Together, these data suggest that inhibition of gap junction–endothelial cell communication by an increase in glucose concentration may be involved in the endothelial cell dysfunction typically observed in diabetes (Fig. 9).
FIG. 9.
Diabetes affects the Cx43-mediated communication in the vascular wall. The hyperglycemia associated with diabetes leads to a reduction in the gap-junction intercellular communication in the endothelial cells and smooth muscle cells through the activation of PKC (94, 113). The reduction in gap-junction communication induced by hyperglycemia is associated with a decrease in Cx43 expression (167), Cx43 phosphorylation (113), and changes in the permeability properties of the Cx43-based channels (14). Although the phosphorylation of Cx43 is mediated by PKC, the participation of this protein kinase in the changes of Cx43 expression and permeability remains to be established. Cx43 is an important gap-junction protein in the vasculature, and the disruption of Cx43-mediated gap-junction communication induced by hyperglycemia may contribute to the vascular dysfunction typically observed in diabetes.
Glucose and diabetes may also alter vascular function by affecting gap-junction activity in vascular smooth muscle cells (102). In this regard, Kuroki et al. (95, 113) showed that high glucose levels reduce gap-junction intercellular communication of cultured aortic smooth muscle cells through the activation of a PKC-dependent pathway and the subsequent Cx43 phosphorylation (Fig. 9). In view of the importance of Cx43 gap junctions in smooth muscle cell proliferation and migration, as well as in vascular morphogenesis (55, 112, 124, 151, 214), the effect of high glucose on Cx43 gap-junction activity may contribute to the development of the diabetes-associated angiopathy. In addition, the permeability properties of the Cx43-derived gap-junction channel were reported to be altered in corporeal vascular smooth muscle cells isolated from streptozotocin-induced diabetic rats (14) (Fig. 9). As Cx43 appears to play a central role in the development of myogenic vasoconstriction, the alteration in the selectivity filter observed in the gap-junction communication of corporeal vascular myocytes may be involved in the diabetes-related erectile dysfunction.
The retina is a particularly sensitive tissue to be damaged by diabetes. Chronic hyperglycemia in diabetes is associated with the development and progression of pathologic changes in the retinal vasculature, and diabetic retinopathy is the leading cause of blindness in the working population (26). Retinal microvessels are well coupled via gap-junction channels, and, as may be expected, soon after the onset of streptozotocin-induced diabetes, the intercellular communication of the retinal microvascular network is disrupted (149). Therefore, the breakdown in the cell-to-cell organization via gap junctions may contribute to the early vascular dysfunction typically observed in diabetes (Fig. 9).
Concluding Remarks
Gap junctions play a multifaceted role in the vasculature that is essential in the control of gene expression, vascular development, and vascular function. However, the function of gap junctions in the vasculature does not depend only on the molecular selectivity or permeability of the different vascular connexin isoforms. It is now evident that connexins work in concert, and thus, the same connexin isoform may have distinct and sometimes antagonistic functions, depending on organization of the connexins and the cell type of the vessel wall in which they are expressed (e.g., the endothelial cells or the smooth muscle cells). In addition, the function of gap junctions may be influenced by the subcellular localization of the connexin-based channels. Gap junctions may be targeted to signaling microdomains such as tight junction, lipid raft, and caveolae (55, 147, 168). In accordance with that localization, connexins have been reported to be associated with cytoskeletal proteins and caveolin-1 (7, 56, 66–68, 147, 168). Such targeting positions the connexins in a strategic spatial relation with a variety of cell-signaling cascades in which they may participate in cell–cell signaling both by direct protein-to-protein interactions and by coordination of signaling pathways between two neighboring cells (34, 55, 147, 200, 201). Therefore, simple analysis of the level of connexin expression is not sufficient to understand fully the participation of the gap junction in cardiovascular function in normal and pathologic conditions. In addition, the cell-type distribution and, if it is possible, the subcellular localization of the specific connexin isoforms must be determined.
The apparent co-regulation of the connexin expression is another potentially important point in the study of vascular gap junctions. That is, deletion of one connexin isoform may affect the expression of another isoform (96, 108, 123, 184). Interestingly, this interaction may traverse the cell boundaries, and connexins present in one cell of the vascular wall may be linked to the expression of connexins in the functional associated cell type, as observed by Liao et al. (123), who reported a reduction in the Cx43 levels in the smooth muscle cells of VEC Cx43−/− mice. Consequently, changes in one connexin may be secondary to the alteration of a different connexin, which leads to a demand for parallel analysis of multiple connexin isoforms, especially during the study of gap junctions in chronic vascular diseases.
Although the participation of the gap junction in vascular function seems to be very complex, the development of connexin-knockout animals has been a great contribution to our understanding of how these proteins work in the vasculature. In addition, connexin-mimetic peptides have demonstrated to be an effective tool to dissect the participation of gap junctions in vascular function. However, the potential manipulation of connexin function and/or expression in therapeutic interventions must await the expected future development of selective pharmacologic tools that allow targeting gap junctions in a connexin-isoform and cell type–specific manner.
Acknowledgments
This work was supported by NIH grant HL53318 (to B.R.D.), grant BM14/2007 from Vicerrectoría Adjunta de Investigación y Doctorado–VRAID de la Pontificia Universidad Católica de Chile, and grant 11060289 from Fondo Nacional de Desarrollo Científico y Tecnológico–FONDECYT (to X.F.F.).
Abbreviations
ACE, angiotensin I–converting enzyme; Ach, acetylcholine; ATP, adenosine 5′-triphosphate; COX, cyclooxygenase; Cx, connexin; Cx43KI32, replacement of Cx43 by Cx32; DAG, diacylglycerol; EDHF, endothelium-derived hyperpolarizing factor; eNOS, endothelial nitric oxide synthase; ER, estrogen receptor; ERα, ɛstrogen receptor alpha; ERβ, estrogen receptor beta; IKCa, Ca2+-activated K+ channels of intermediate conductance; IP3, inositol 1, 4, 5-triphosphate; JGA, juxtaglomerular apparatus; KCa, Ca2+-activated K+ channels; 2K1C, two-kidney, one-clip model of hypertension; MEJ, myoendothelial junction; NAD+, nicotinamide adenine dinucleotide; Nav, voltage-gated Na+ channel; NO, nitric oxide; NOS, nitric oxide synthase; PGI2, prostacyclin; PKC, protein kinase C; PMA, phorbol 12- myristate 13-acetate; SHR, spontaneously hypertensive rat; SKCa, Ca2+-activated K+ channels of small conductance; T-Cav, T-type, voltage-gated Ca2+ channels; VEC Cx43−/−, vascular endothelial cell–specific deletion of Cx43.
References
- 1.Ahluwalia A. Hobbs AJ. Endothelium-derived C-type natriuretic peptide: more than just a hyperpolarizing factor. Trends Pharmacol Sci. 2005;26:162–167. doi: 10.1016/j.tips.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CM. Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 3.Andresen J. Shafi NI. Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL. Gragasin FS. Wu X. Wang S. McMurtry S. Kim DH. Platonov M. Koshal A. Hashimoto K. Campbell WB. Falck JR. Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 5.Arensbak B. Mikkelsen HB. Gustafsson F. Christensen T. Holstein-Rathlou NH. Expression of connexin 37, 40, and 43 mRNA and protein in renal preglomerular arterioles. Histochem Cell Biol. 2001;115:479–487. doi: 10.1007/s004180100275. [DOI] [PubMed] [Google Scholar]
- 6.Bao X. Clark CB. Frangos JA. Temporal gradient in shear-induced signaling pathway: involvement of MAP kinase, c-fos, and connexin43. Am J Physiol Heart Circ Physiol. 2000;278:H1598–H1605. doi: 10.1152/ajpheart.2000.278.5.H1598. [DOI] [PubMed] [Google Scholar]
- 7.Barth K. Gentsch M. Blasche R. Pfuller A. Parshyna I. Koslowski R. Barth G. Kasper M. Distribution of caveolin-1 and connexin43 in normal and injured alveolar epithelial R3/1 cells. Histochem Cell Biol. 2005;123:239–247. doi: 10.1007/s00418-004-0727-4. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett IS. Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H604–H612. doi: 10.1152/ajpheart.2000.278.2.H604. [DOI] [PubMed] [Google Scholar]
- 9.Beach JM. McGahren ED. Duling BR. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am J Physiol Heart Circ Physiol. 1998;44:H1489–H1496. doi: 10.1152/ajpheart.1998.275.4.H1489. [DOI] [PubMed] [Google Scholar]
- 10.Beny JL. Information networks in the arterial wall. News Physiol Sci. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- 11.Beny JL. Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol. 1994;266:H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- 12.Beyer EC. Gemel J. Seul KH. Larson DM. Banach K. Brink PR. Modulation of intercellular communication by differential regulation and heteromeric mixing of co-expressed connexins. Braz J Med Biol Res. 2000;33:391–397. doi: 10.1590/s0100-879x2000000400004. [DOI] [PubMed] [Google Scholar]
- 13.Boerma M. Forsberg L. Van Zeijl L. Morgenstern R. De Faire U. Lemne C. Erlinge D. Thulin T. Hong Y. Cotgreave IA. A genetic polymorphism in connexin 37 as a prognostic marker for atherosclerotic plaque development. J Intern Med. 1999;246:211–218. doi: 10.1046/j.1365-2796.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 14.Brink PR. Valiunas V. Wang HZ. Zhao W. Davies K. Christ GJ. Experimental diabetes alters connexin43 derived gap junction permeability in short-term cultures of rat corporeal vascular smooth muscle cells. J Urol. 2006;175:381–386. doi: 10.1016/S0022-5347(05)00007-8. [DOI] [PubMed] [Google Scholar]
- 15.Brisset AC. Isakson BE. Kwak BR. Connexin in vascular physiology and pathology. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucala R. Tracey KJ. Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budel S. Bartlett IS. Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res. 2003;93:61–68. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- 18.Busse R. Edwards G. Feletou M. Fleming I. Vanhoutte PM. Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 19.Chadjichristos CE. Matter CM. Roth I. Sutter E. Pelli G. Luscher TF. Chanson M. Kwak BR. Reduced connexin43 expression limits neointima formation after balloon distension injury in hypercholesterolemic mice. Circulation. 2006;113:2835–2843. doi: 10.1161/CIRCULATIONAHA.106.627703. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan SD. Nilsson H. Ahluwalia A. Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaytor AT. Bakker LM. Edwards DH. Griffith TM. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol. 2005;144:108–114. doi: 10.1038/sj.bjp.0706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christ GJ. Moreno AP. Melman A. Spray DC. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am J Physiol. 1992;263:C373–C383. doi: 10.1152/ajpcell.1992.263.2.C373. [DOI] [PubMed] [Google Scholar]
- 23.Christ GJ. Moreno AP. Parker M. Gondre CM. Valcic M. Melman A. Spray DC. Intercellular communication through gap junctions: a potential role in pharmacomechanical coupling and syncytial tissue contraction in vascular smooth muscle isolated from the human corpus cavernosum. Life Sci. 1991;49:PL195–PL200. doi: 10.1016/0024-3205(91)90489-x. [DOI] [PubMed] [Google Scholar]
- 24.Christ GJ. Spray DC. el-Sabban M. Moore LK. Brink PR. Gap junctions in vascular tissues: evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res. 1996;79:631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- 25.Clauss SB. Walker DL. Kirby ML. Schimel D. Lo CW. Patterning of coronary arteries in wildtype and connexin43 knockout mice. Dev Dyn. 2006;235:2786–2794. doi: 10.1002/dvdy.20887. [DOI] [PubMed] [Google Scholar]
- 26.Congdon NG. Friedman DS. Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 27.Cornell-Bell AH. Finkbeiner SM. Cooper MS. Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 28.Cottrell GT. Wu Y. Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am J Physiol Cell Physiol. 2002;282:C1469–C1482. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- 29.Cowan DB. Jones M. Garcia LM. Noria S. del Nido PJ. McGowan FX., Jr Hypoxia and stretch regulate intercellular communication in vascular smooth muscle cells through reactive oxygen species formation. Arterioscler Thromb Vasc Biol. 2003;23:1754–1760. doi: 10.1161/01.ATV.0000093546.10162.B2. [DOI] [PubMed] [Google Scholar]
- 30.Cowan DB. Lye SJ. Langille BL. Regulation of vascular connexin43 gene expression by mechanical loads. Circ Res. 1998;82:786–793. doi: 10.1161/01.res.82.7.786. [DOI] [PubMed] [Google Scholar]
- 31.Cox SB. Woolsey TA. Rovainen CM. Localized dynamic changes in cortical blood flow with whisker stimulation corresponds to matched vascular and neuronal architecture of rat barrels. J Cereb Blood Flow Metab. 1993;13:899–913. doi: 10.1038/jcbfm.1993.113. [DOI] [PubMed] [Google Scholar]
- 32.Crane GJ. Gallagher N. Dora KA. Garland CJ. Small-and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane GJ. Neild TO. Segal SS. Contribution of active membrane processes to conducted hyperpolarization in arterioles of hamster cheek pouch. Microcirculation. 2004;11:425–433. doi: 10.1080/10739680490457836. [DOI] [PubMed] [Google Scholar]
- 34.Dang X. Doble BW. Kardami E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem. 2003;242:35–38. [PubMed] [Google Scholar]
- 35.De Vriese AS. Van de Voorde J. Lameire NH. Effects of connexin-mimetic peptides on nitric oxide synthase- and cyclooxygenase-independent renal vasodilation. Kidney Int. 2002;61:177–185. doi: 10.1046/j.1523-1755.2002.00122.x. [DOI] [PubMed] [Google Scholar]
- 36.de Wit C. Roos F. Bolz SS. Kirchhoff S. Kruger O. Willecke K. Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 37.de Wit C. Roos F. Bolz SS. Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genom. 2003;13:169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 38.dePaola N. Davies PF. Pritchard WF., Jr Florez L. Harbeck N. Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci U S A. 1999;96:3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietrich HH. Kajita Y. Dacey RG., Jr Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am J Physiol. 1996;271:H1109–H1116. doi: 10.1152/ajpheart.1996.271.3.H1109. [DOI] [PubMed] [Google Scholar]
- 40.Dora KA. Sandow SL. Gallagher NT. Takano H. Rummery NM. Hill CE. Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 41.Doyle MP. Duling BR. Acetylcholine induces conducted vasodilation by nitric oxide-dependent and -independent mechanisms. Am J Physiol Heart Circ Physiol. 1997;41:H1364–H1371. doi: 10.1152/ajpheart.1997.272.3.H1364. [DOI] [PubMed] [Google Scholar]
- 42.Earley S. Resta TC. Walker BR. Disruption of smooth muscle gap junctions attenuates myogenic vasoconstriction of mesenteric resistance arteries. Am J Physiol Heart Circ Physiol. 2004;287:H2677–H2686. doi: 10.1152/ajpheart.00016.2004. [DOI] [PubMed] [Google Scholar]
- 43.Edwards G. Dora KA. Gardener MJ. Garland CJ. Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 44.Eichler I. Wibawa J. Grgic I. Knorr A. Brakemeier S. Pries AR. Hoyer J. Kohler R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emerson GG. Neild TO. Segal SS. Conduction of hyperpolarization along hamster feed arteries: augmentation by acetylcholine. Am J Physiol Heart Circ Physiol. 2002;283:H102–H109. doi: 10.1152/ajpheart.00038.2002. [DOI] [PubMed] [Google Scholar]
- 46.Emerson GG. Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 47.Emerson GG. Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 48.Emerson GG. Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–H167. doi: 10.1152/ajpheart.2001.280.1.H160. [DOI] [PubMed] [Google Scholar]
- 49.Evans WH. Martin PE. Gap junctions: structure and function (review) Mol Membr Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 50.Feletou M. Vanhoutte PM. Endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 1996;23:1082–1090. doi: 10.1111/j.1440-1681.1996.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 51.Feletou M. Vanhoutte PM. Weston AH. Edwards G. EDHF and endothelial potassium channels: IKCa and SKCa. Br J Pharmacol. 2003;140:225. doi: 10.1038/sj.bjp.0705425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feron O. Belhassen L. Kobzik L. Smith TW. Kelly RA. Michel T. Endothelial nitric oxide synthase targeting to caveolae: specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 53.Figueroa XF. Alvina K. Martinez AD. Garces G. Rosemblatt M. Boric MP. Saez JC. Histamine reduces gap junctional communication of human tonsil high endothelial cells in culture. Microvasc Res. 2004;68:247–257. doi: 10.1016/j.mvr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Figueroa XF. Chen CC. Campbell KP. Damon DN. Day KH. Ramos S. Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol. 2007;293:H1371–H1383. doi: 10.1152/ajpheart.01368.2006. [DOI] [PubMed] [Google Scholar]
- 55.Figueroa XF. Isakson BE. Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 2004;19:277–284. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- 56.Figueroa XF. Isakson BE. Duling BR. Vascular gap junctions in hypertension. Hypertension. 2006;48:804–811. doi: 10.1161/01.HYP.0000242483.03361.da. [DOI] [PubMed] [Google Scholar]
- 57.Figueroa XF. Paul DL. Simon AM. Goodenough DA. Day KH. Damon DN. Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 58.Filosa JA. Bonev AD. Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 59.Filosa JA. Bonev AD. Straub SV. Meredith AL. Wilkerson MK. Aldrich RW. Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 60.Firouzi M. Bierhuizen MF. Kok B. Teunissen BE. Jansen AT. Jongsma HJ. Groenewegen WA. The human Cx40 promoter polymorphism −44G–>A differentially affects transcriptional regulation by Sp1 and GATA4. Biochim Biophys Acta. 2006;1759:491–496. doi: 10.1016/j.bbaexp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Firouzi M. Kok B. Spiering W. Busjahn A. Bezzina CR. Ruijter JM. Koeleman BP. Schipper M. Groenewegen WA. Jongsma HJ. de Leeuw PW. Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens. 2006;24:325–330. doi: 10.1097/01.hjh.0000200512.40818.47. [DOI] [PubMed] [Google Scholar]
- 62.Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s) Pharmacol Res. 2004;49:525–533. doi: 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 63.Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973;288:831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- 64.Gabriels JE. Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Cardena G. Martasek P. Masters BS. Skidd PM. Couet J. Li S. Lisanti MP. Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin: functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 66.Giepmans BN. Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 67.Giepmans BN. Verlaan I. Hengeveld T. Janssen H. Calafat J. Falk MM. Moolenaar WH. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol. 2001;11:1364–1368. doi: 10.1016/s0960-9822(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 68.Giepmans BN. Verlaan I. Moolenaar WH. Connexin-43 interactions with ZO-1 and alpha- and beta-tubulin. Cell Commun Adhes. 2001;8:219–223. doi: 10.3109/15419060109080727. [DOI] [PubMed] [Google Scholar]
- 69.Giugliano D. Ceriello A. Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 70.Goodenough DA. Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 71.Goto K. Fujii K. Kansui Y. Abe I. Iida M. Critical role of gap junctions in endothelium-dependent hyperpolarization in rat mesenteric arteries. Clin Exp Pharmacol Physiol. 2002;29:595–602. doi: 10.1046/j.1440-1681.2002.03689.x. [DOI] [PubMed] [Google Scholar]
- 72.Goto K. Rummery NM. Grayson TH. Hill CE. Attenuation of conducted vasodilatation in rat mesenteric arteries during hypertension: role of inwardly rectifying potassium channels. J Physiol. 2004;561:215–231. doi: 10.1113/jphysiol.2004.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grayson TH. Is Cx40 a marker for hypertension? J Hypertens. 2006;24:279–280. doi: 10.1097/01.hjh.0000199804.95434.2b. [DOI] [PubMed] [Google Scholar]
- 74.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffith TM. Chaytor AT. Edwards DH. The obligatory link: role of gap junctional communication in endothelium-dependent smooth muscle hyperpolarization. Pharmacol Res. 2004;49:551–564. doi: 10.1016/j.phrs.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Gustafsson F. Holstein-Rathlou N. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand. 1999;167:11–21. doi: 10.1046/j.1365-201x.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- 77.Haefliger JA. Castillo E. Waeber G. Bergonzelli GE. Aubert JF. Sutter E. Nicod P. Waeber B. Meda P. Hypertension increases connexin43 in a tissue-specific manner. Circulation. 1997;95:1007–1014. doi: 10.1161/01.cir.95.4.1007. [DOI] [PubMed] [Google Scholar]
- 78.Haefliger JA. Demotz S. Braissant O. Suter E. Waeber B. Nicod P. Meda P. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int. 2001;60:190–201. doi: 10.1046/j.1523-1755.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 79.Haefliger JA. Krattinger N. Martin D. Pedrazzini T. Capponi A. Doring B. Plum A. Charollais A. Willecke K. Meda P. Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest. 2006;116:405–413. doi: 10.1172/JCI23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haefliger JA. Meda P. Formenton A. Wiesel P. Zanchi A. Brunner HR. Nicod P. Hayoz D. Aortic connexin43 is decreased during hypertension induced by inhibition of nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1999;19:1615–1622. doi: 10.1161/01.atv.19.7.1615. [DOI] [PubMed] [Google Scholar]
- 81.Haefliger JA. Nicod P. Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res. 2004;62:345–356. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 82.Harder DR. Alkayed NJ. Lange AR. Gebremedhin D. Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 83.Hawkins BT. Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 84.He DS. Jiang JX. Taffet SM. Burt JM. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1999;96:6495–6500. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hill CE. Rummery N. Hickey H. Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol. 2002;29:620–625. doi: 10.1046/j.1440-1681.2002.03699.x. [DOI] [PubMed] [Google Scholar]
- 86.Hirashiki A. Yamada Y. Murase Y. Suzuki Y. Kataoka H. Morimoto Y. Tajika T. Murohara T. Yokota M. Association of gene polymorphisms with coronary artery disease in low- or high-risk subjects defined by conventional risk factors. J Am Coll Cardiol. 2003;42:1429–1437. doi: 10.1016/s0735-1097(03)01062-3. [DOI] [PubMed] [Google Scholar]
- 87.Hirst GD. Edwards FR. Gould DJ. Sandow SL. Hill CE. Electrical properties of iridial arterioles of the rat. Am J Physiol. 1997;273:H2465–H2472. doi: 10.1152/ajpheart.1997.273.5.H2465. [DOI] [PubMed] [Google Scholar]
- 88.Hirst GD. Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Horiuchi T. Dietrich HH. Hongo K. Dacey RG., Jr Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33:2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- 90.Huang A. Wu Y. Sun D. Koller A. Kaley G. Effect of estrogen on flow-induced dilation in NO deficiency: role of prostaglandins and EDHF. J Appl Physiol. 2001;91:2561–2566. doi: 10.1152/jappl.2001.91.6.2561. [DOI] [PubMed] [Google Scholar]
- 91.Hwan SK. Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res. 2000;59:140–148. doi: 10.1006/mvre.1999.2216. [DOI] [PubMed] [Google Scholar]
- 92.Iadecola C. Yang G. Ebner TJ. Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- 93.Inai T. Mancuso MR. McDonald DM. Kobayashi J. Nakamura K. Shibata Y. Shear stress-induced upregulation of connexin 43 expression in endothelial cells on upstream surfaces of rat cardiac valves. Histochem Cell Biol. 2004;122:477–483. doi: 10.1007/s00418-004-0717-6. [DOI] [PubMed] [Google Scholar]
- 94.Inoguchi T. Ueda F. Umeda F. Yamashita T. Nawata H. Inhibition of intercellular communication via gap junction in cultured aortic endothelial cells by elevated. Biochem Biophys Res Commun. 1995;208:492–497. doi: 10.1006/bbrc.1995.1365. [DOI] [PubMed] [Google Scholar]
- 95.Inoguchi T. Yu HY. Imamura M. Kakimoto M. Kuroki T. Maruyama T. Nawata H. Altered gap junction activity in cardiovascular tissues of diabetes. Med Electron Microsc. 2001;34:86–91. doi: 10.1007/s007950170002. [DOI] [PubMed] [Google Scholar]
- 96.Isakson BE. Damon DN. Day KH. Liao Y. Duling BR. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol. 2006;290:H1199–H1205. doi: 10.1152/ajpheart.00945.2005. [DOI] [PubMed] [Google Scholar]
- 97.Isakson BE. Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 98.Jantzi MC. Brett SE. Jackson WF. Corteling R. Vigmond EJ. Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291:H1319–H1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- 99.Johnson TL. Nerem RM. Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium. 2007;14:215–226. doi: 10.1080/10623320701617233. [DOI] [PubMed] [Google Scholar]
- 100.Ju H. Zou R. Venema VJ. Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 101.Kameritsch P. Hoffmann A. Pohl U. Opposing effects of nitric oxide on different connexins expressed in the vascular system. Cell Commun Adhes. 2003;10:305–309. doi: 10.1080/cac.10.4-6.305.309. [DOI] [PubMed] [Google Scholar]
- 102.Kang N. Alexander G. Park JK. Maasch C. Buchwalow I. Luft FC. Haller H. Differential expression of protein kinase C isoforms in streptozotocin-induced diabetic rats. Kidney Int. 1999;56:1737–1750. doi: 10.1046/j.1523-1755.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 103.Kansui Y. Fujii K. Nakamura K. Goto K. Oniki H. Abe I. Shibata Y. Iida M. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol. 2004;287:H216–H224. doi: 10.1152/ajpheart.00915.2003. [DOI] [PubMed] [Google Scholar]
- 104.Karagiannis J. Rand M. Li CG. Role of gap junctions in endothelium-derived hyperpolarizing factor-mediated vasodilatation in rat renal artery. Acta Pharmacol Sin. 2004;25:1031–1037. [PubMed] [Google Scholar]
- 105.Kirchhoff S. Kim JS. Hagendorff A. Thonnissen E. Kruger O. Lamers WH. Willecke K. Abnormal cardiac conduction and morphogenesis in connexin40 and connexin43 double-deficient mice. [see comments] Circulation Research. 2000;87:399–405. doi: 10.1161/01.res.87.5.399. [DOI] [PubMed] [Google Scholar]
- 106.Koehler RC. Gebremedhin D. Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krattinger N. Capponi A. Mazzolai L. Aubert JF. Caille D. Nicod P. Waeber G. Meda P. Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 108.Kruger O. Beny JL. Chabaud F. Traub O. Theis M. Brix K. Kirchhoff S. Willecke K. Altered dye diffusion and up-regulation of connexin37 in mouse aortic endothelium deficient in connexin40. J Vasc Res. 2002;39:160–172. doi: 10.1159/000057764. [DOI] [PubMed] [Google Scholar]
- 109.Kruger O. Plum A. Kim JS. Winterhager E. Maxeiner S. Hallas G. Kirchhoff S. Traub O. Lamers WH. Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 110.Kurjiaka DT. The conduction of dilation along an arteriole is diminished in the cremaster muscle of hypertensive hamsters. J Vasc Res. 2004;41:517–524. doi: 10.1159/000081808. [DOI] [PubMed] [Google Scholar]
- 111.Kurjiaka DT. Bender SB. Nye DD. Wiehler WB. Welsh DG. Hypertension attenuates cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2005;288:H861–H870. doi: 10.1152/ajpheart.00729.2004. [DOI] [PubMed] [Google Scholar]
- 112.Kurjiaka DT. Steele TD. Olsen MV. Burt JM. Gap junction permeability is diminished in proliferating vascular smooth muscle cells. Am J Physiol. 1998;275:C1674–C1682. doi: 10.1152/ajpcell.1998.275.6.C1674. [DOI] [PubMed] [Google Scholar]
- 113.Kuroki T. Inoguchi T. Umeda F. Ueda F. Nawata H. High glucose induces alteration of gap junction permeability and phosphorylation of connexin-43 in cultured aortic smooth muscle cells. Diabetes. 1998;47:931–936. doi: 10.2337/diabetes.47.6.931. [DOI] [PubMed] [Google Scholar]
- 114.Kurtz L. Schweda F. de Wit C. Kriz W. Witzgall R. Warth R. Sauter A. Kurtz A. Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 115.Kwak BR. Mulhaupt F. Veillard N. Gros DB. Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- 116.Kwak BR. Pepper MS. Gros DB. Meda P. Inhibition of endothelial wound repair by dominant negative connexin inhibitors. Mol Biol Cell. 2001;12:831–845. doi: 10.1091/mbc.12.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwak BR. Silacci P. Stergiopulos N. Hayoz D. Meda P. Shear stress and cyclic circumferential stretch, but not pressure, alter connexin43 expression in endothelial cells. Cell Commun Adhes. 2005;12:261–270. doi: 10.1080/15419060500514119. [DOI] [PubMed] [Google Scholar]
- 118.Lagaud G. Karicheti V. Knot HJ. Christ GJ. Laher I. Inhibitors of gap junctions attenuate myogenic tone in cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2177–H2186. doi: 10.1152/ajpheart.00605.2001. [DOI] [PubMed] [Google Scholar]
- 119.Lee TS. Saltsman KA. Ohashi H. King GL. Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci U S A. 1989;86:5141–5145. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 120.Levy BI. Ambrosio G. Pries AR. Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 121.Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- 122.Li X. Simard JM. Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;281:H1890–H1898. doi: 10.1152/ajpheart.2001.281.5.H1890. [DOI] [PubMed] [Google Scholar]
- 123.Liao Y. Day KH. Damon DN. Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A. 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liao Y. Regan CP. Manabe I. Owens GK. Day KH. Damon DN. Duling BR. Smooth muscle-targeted knockout of connexin43 enhances neointimal formation in response to vascular injury. Arterioscler Thromb Vasc Biol. 2007;27:1037–1042. doi: 10.1161/ATVBAHA.106.137182. [DOI] [PubMed] [Google Scholar]
- 125.Lin D. Takemoto DJ. Oxidative activation of protein kinase Cgamma through the C1 domain: effects on gap junctions. J Biol Chem. 2005;280:13682–13693. doi: 10.1074/jbc.M407762200. [DOI] [PubMed] [Google Scholar]
- 126.Little TL. Beyer EC. Duling BR. Connexin-43 and connexin-40 gap junctional proteins are present in arteriolar smooth-muscle and endothelium in-vivo. Am J Physiol. 1995;37:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 127.Little TL. Xia J. Duling BR. Dye tracers define differential endothelial and smooth-muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- 128.Liu MY. Hattori Y. Sato A. Ichikawa R. Zhang XH. Sakuma I. Ovariectomy attenuates hyperpolarization and relaxation mediated by endothelium-derived hyperpolarizing factor in female rat mesenteric artery: a concomitant decrease in connexin-43 expression. J Cardiovasc Pharmacol. 2002;40:938–948. doi: 10.1097/00005344-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 129.Locke D. Liu J. Harris AL. Lipid rafts prepared by different methods contain different connexin channels, but gap junctions are not lipid rafts. Biochemistry. 2005;44:13027–13042. doi: 10.1021/bi050495a. [DOI] [PubMed] [Google Scholar]
- 130.Luksha L. Poston L. Gustafsson JA. Hultenby K. Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol. 2006;577:945–955. doi: 10.1113/jphysiol.2006.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martinez AD. Hayrapetyan V. Moreno AP. Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- 132.Martinez AD. Saez JC. Arachidonic acid-induced dye uncoupling in rat cortical astrocytes is mediated by arachidonic acid byproducts. Brain Res. 1999;816:411–423. doi: 10.1016/s0006-8993(98)01016-6. [DOI] [PubMed] [Google Scholar]
- 133.Martinez AD. Saez JC. Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res Brain Res Rev. 2000;32:250–258. doi: 10.1016/s0165-0173(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 134.Mather S. Dora KA. Sandow SL. Winter P. Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 135.McDonnell DP. The molecular pharmacology of SERMs. Trends Endocrinol Metab. 1999;10:301–311. doi: 10.1016/s1043-2760(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 136.Mendelsohn ME. Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 137.Metea MR. Newman EA. Calcium signaling in specialized glial cells. Glia. 2006;54:650–655. doi: 10.1002/glia.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Metea MR. Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Michel JB. Feron O. Sacks D. Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 140.Michel JB. Feron O. Sase K. Prabhakar P. Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem. 1997;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 141.Moreno AP. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res. 2004;62:276–286. doi: 10.1016/j.cardiores.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 142.Mulligan SJ. MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 143.Nawate S. Fukao M. Sakuma I. Soma T. Nagai K. Takikawa O. Miwa S. Kitabatake A. Reciprocal changes in endothelium-derived hyperpolarizing factor and nitric oxide system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol. 2005;144:178–189. doi: 10.1038/sj.bjp.0706091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 145.Nedergaard M. Ransom B. Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 146.Nowicki PT. Flavahan S. Hassanain H. Mitra S. Holland S. Goldschmidt-Clermont PJ. Flavahan NA. Redox signaling of the arteriolar myogenic response. Circ Res. 2001;89:114–116. doi: 10.1161/hh1401.094367. [DOI] [PubMed] [Google Scholar]
- 147.Nusrat A. Parkos CA. Verkade P. Foley CS. Liang TW. Innis-Whitehouse W. Eastburn KK. Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 148.Ohashi M. Satoh K. Itoh T. Acetylcholine-induced membrane potential changes in endothelial cells of rabbit aortic valve. Br J Pharmacol. 1999;126:19–26. doi: 10.1038/sj.bjp.0702262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oku H. Kodama T. Sakagami K. Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci. 2001;42:1915–1920. [PubMed] [Google Scholar]
- 150.Pacicca C. Schaad O. Beny JL. Electrotonic propagation of kinin-induced, endothelium-dependent hyperpolarizations in pig coronary smooth muscles. J Vasc Res. 1996;33:380–385. doi: 10.1159/000159166. [DOI] [PubMed] [Google Scholar]
- 151.Polacek D. Bech F. McKinsey JF. Davies PF. Connexin43 gene expression in the rabbit arterial wall: effects of hypercholesterolemia, balloon injury and their combination. J Vasc Res. 1997;34:19–30. doi: 10.1159/000159198. [DOI] [PubMed] [Google Scholar]
- 152.Pries AR. Secomb TW. Gaehtgens P. Structural adaptation and stability of microvascular networks: theory and simulations. Am J Physiol. 1998;275:H349–H360. doi: 10.1152/ajpheart.1998.275.2.H349. [DOI] [PubMed] [Google Scholar]
- 153.Pries AR. Secomb TW. Gaehtgens P. Structural autoregulation of terminal vascular beds: vascular adaptation and development of hypertension. Hypertension. 1999;33:153–161. doi: 10.1161/01.hyp.33.1.153. [DOI] [PubMed] [Google Scholar]
- 154.Reaume AG. de Sousa PA. Kulkarni S. Langille BL. Zhu D. Davies TC. Juneja SC. Kidder GM. Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 155.Retamal MA. Cortes CJ. Reuss L. Bennett MV. Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci U S A. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roh CR. Heo JH. Yang SH. Bae DS. Regulation of connexin 43 by nitric oxide in primary uterine myocytes from term pregnant women. Am J Obstet Gynecol. 2002;187:434–440. doi: 10.1067/mob.2002.123600. [DOI] [PubMed] [Google Scholar]
- 157.Rummery NM. Grayson TH. Hill CE. Angiotensin-converting enzyme inhibition restores endothelial but not medial connexin expression in hypertensive rats. J Hypertens. 2005;23:317–328. doi: 10.1097/00004872-200502000-00014. [DOI] [PubMed] [Google Scholar]
- 158.Rummery NM. Hickey H. McGurk G. Hill CE. Connexin37 is the major connexin expressed in the media of caudal artery. Arterioscler Thromb Vasc Biol. 2002;22:1427–1432. doi: 10.1161/01.atv.0000028814.45706.e5. [DOI] [PubMed] [Google Scholar]
- 159.Rummery NM. Hill CE. Vascular gap junctions and implications for hypertension. Clin Exp Pharmacol Physiol. 2004;31:659–667. doi: 10.1111/j.1440-1681.2004.04071.x. [DOI] [PubMed] [Google Scholar]
- 160.Rummery NM. McKenzie KU. Whitworth JA. Hill CE. Decreased endothelial size and connexin expression in rat caudal arteries during hypertension. J Hypertens. 2002;20:247–253. doi: 10.1097/00004872-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 161.Saez JC. Astrocytes as connexin-dependent signaling cells for local blood flow regulation. Am J Physiol Heart Circ Physiol. 2008;294:H586–H587. doi: 10.1152/ajpheart.91445.2007. [DOI] [PubMed] [Google Scholar]
- 162.Saez JC. Berthoud VM. Branes MC. Martinez AD. Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 163.Saez JC. Retamal MA. Basilio D. Bukauskas FF. Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sandow SL. Bramich NJ. Bandi HP. Rummery NM. Hill CE. Structure, function, and endothelium-derived hyperpolarizing factor in the caudal artery of the SHR and WKY rat. Arterioscler Thromb Vasc Biol. 2003;23:822–828. doi: 10.1161/01.ATV.0000067425.06019.D7. [DOI] [PubMed] [Google Scholar]
- 165.Sandow SL. Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 166.Sandow SL. Looft-Wilson R. Doran B. Grayson TH. Segal SS. Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res. 2003;60:643–653. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 167.Sato T. Haimovici R. Kao R. Li AF. Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002;51:1565–1571. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- 168.Schubert AL. Schubert W. Spray DC. Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 169.Scotland RS. Madhani M. Chauhan S. Moncada S. Andresen J. Nilsson H. Hobbs AJ. Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796–803. doi: 10.1161/01.CIR.0000155238.70797.4E. [DOI] [PubMed] [Google Scholar]
- 170.Segal SS. Cell-to-cell communication coordinates blood flow control. Hypertension. 1994;23:1113–1120. doi: 10.1161/01.hyp.23.6.1113. [DOI] [PubMed] [Google Scholar]
- 171.Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand. 2000;168:511–518. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- 172.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 173.Segal SS. Damon DN. Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol. 1989;256:H832–H837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- 174.Segal SS. Duling BR. Communication between feed arteries and microvessels in hamster striated muscle: segmental vascular responses are functionally coordinated. Circ Res. 1986;59:283–290. doi: 10.1161/01.res.59.3.283. [DOI] [PubMed] [Google Scholar]
- 175.Segal SS. Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling. Am J Physiol. 1989;256:H838–H845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- 176.Segal SS. Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Segal SS. Kurjiaka DT. Coordination of blood flow control in the resistance vasculature of skeletal muscle. Med Sci Sports Exerc. 1995;27:1158–1164. [PubMed] [Google Scholar]
- 178.Severs NJ. Rothery S. Dupont E. Coppen SR. Yeh HI. Ko YS. Matsushita T. Kaba R. Halliday D. Immunocyto-chemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech. 2001;52:301–322. doi: 10.1002/1097-0029(20010201)52:3<301::AID-JEMT1015>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 179.Sheridan JD. Dye transfer in small vessels from rat omentum: homologous and heterologous junctions. J Cell Biol. 1980;87:61a. [Google Scholar]
- 180.Shimokawa H. Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol. 2005;39:725–732. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 181.Shimokawa H. Yasutake H. Fujii K. Owada MK. Nakaike R. Fukumoto Y. Takayanagi T. Nagao T. Egashira K. Fujishima M. Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 182.Simon AM. Goodenough DA. Diverse functions of vertebrate gap junctions. Trends Cell Biol. 1998;8:477–483. doi: 10.1016/s0962-8924(98)01372-5. [DOI] [PubMed] [Google Scholar]
- 183.Simon AM. McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251:206–220. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- 184.Simon AM. McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J Cell Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- 185.Song H. Tyml K. Evidence for sensing and integration of biological signals by the capillary network. Am J Physiol. 1993;265:H1235–H1242. doi: 10.1152/ajpheart.1993.265.4.H1235. [DOI] [PubMed] [Google Scholar]
- 186.Stout CE. Costantin JL. Naus CC. Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 187.Straub SV. Bonev AD. Wilkerson MK. Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol. 2006;128:659–669. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Suadicani SO. Brosnan CF. Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Takano T. Tian GF. Peng W. Lou N. Libionka W. Han X. Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 190.Tulenko TN. Regulating cross-talk between vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:1707–1709. doi: 10.1161/01.ATV.0000093820.81258.5F. [DOI] [PubMed] [Google Scholar]
- 191.van Kempen MJ. Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–486. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- 192.van Kempen MJ. ten Velde I. Wessels A. Oosthoek PW. Gros D. Jongsma HJ. Moorman AF. Lamers WH. Differential connexin distribution accommodates cardiac function in different species. Microsc Res Tech. 1995;31:420–436. doi: 10.1002/jemt.1070310511. [DOI] [PubMed] [Google Scholar]
- 193.Vanhoutte PM. Endothelium-dependent hyperpolarizations: the history. Pharmacol Res. 2004;49:503–508. doi: 10.1016/j.phrs.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 194.Vlassara H. Fuh H. Makita Z. Krungkrai S. Cerami A. Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci U S A. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wagner C. de Wit C. Gerl M. Kurtz A. Hocherl K. Increased expression of cyclooxygenase 2 contributes to aberrant renin production in connexin 40-deficient kidneys. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1781–R1786. doi: 10.1152/ajpregu.00439.2007. [DOI] [PubMed] [Google Scholar]
- 196.Wagner C. de Wit C. Kurtz L. Grunberger C. Kurtz A. Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 197.Walker DL. Vacha SJ. Kirby ML. Lo CW. Connexin43 deficiency causes dysregulation of coronary vasculogenesis. Dev Biol. 2005;284:479–498. doi: 10.1016/j.ydbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 198.Watanabe S. Yashiro Y. Mizuno R. Ohhashi T. Involvement of NO and EDHF in flow-induced vasodilation in isolated hamster cremasteric arterioles. J Vasc Res. 2005;42:137–147. doi: 10.1159/000083652. [DOI] [PubMed] [Google Scholar]
- 199.Watts SW. Webb RC. Vascular gap junctional communication is increased in mineralocorticoid-salt hypertension. Hypertension. 1996;28:888–893. doi: 10.1161/01.hyp.28.5.888. [DOI] [PubMed] [Google Scholar]
- 200.Wei CJ. Francis R. Xu X. Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 201.Wei CJ. Xu X. Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20:811–838. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 202.Welsh DG. Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 203.White TW. Nonredundant gap junction functions. News Physiol Sci. 2003;18:95–99. doi: 10.1152/nips.01430.2002. [DOI] [PubMed] [Google Scholar]
- 204.White TW. Bruzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J Bioenerg Biomembr. 1996;28:339–350. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- 205.Williams B. Schrier RW. Glucose-induced protein kinase C activity regulates arachidonic acid release and eicosanoid production by cultured glomerular mesangial cells. J Clin Invest. 1993;92:2889–2896. doi: 10.1172/JCI116911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wolfle SE. Schmidt VJ. Hoepfl B. Gebert A. Alcolea S. Gros D. de Wit C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res. 2007;101:1292–1299. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- 207.Wong CW. Christen T. Roth I. Chadjichristos CE. Derouette JP. Foglia BF. Chanson M. Goodenough DA. Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 208.Xia J. Duling BR. Electromechanical coupling and the conducted vasomotor response. Am J Physiol. 1995;38:H2022–H2030. doi: 10.1152/ajpheart.1995.269.6.H2022. [DOI] [PubMed] [Google Scholar]
- 209.Xia J. Little TL. Duling BR. Cellular pathways of the conducted electrical response in arterioles of hamster cheek pouch in vitro. Am J Physiol Heart Circ Physiol. 1995;38:H2031–H2038. doi: 10.1152/ajpheart.1995.269.6.H2031. [DOI] [PubMed] [Google Scholar]
- 210.Xu HL. Mao L. Ye S. Paisansathan C. Vetri F. Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2007;294:H622–H632. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- 211.Yamada Y. Ichihara S. Izawa H. Tanaka M. Yokota M. Genetic risk for coronary artery disease in individuals with or without type 2 diabetes. Mol Genet Metab. 2004;81:282–290. doi: 10.1016/j.ymgme.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 212.Yamada Y. Izawa H. Ichihara S. Takatsu F. Ishihara H. Hirayama H. Sone T. Tanaka M. Yokota M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916–1923. doi: 10.1056/NEJMoa021445. [DOI] [PubMed] [Google Scholar]
- 213.Yeh HI. Lee PY. Su CH. Tian TY. Ko YS. Tsai CH. Reduced expression of endothelial connexins 43 and 37 in hypertensive rats is rectified after 7-day carvedilol treatment. Am J Hypertens. 2006;19:129–135. doi: 10.1016/j.amjhyper.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 214.Yeh HI. Lupu F. Dupont E. Severs NJ. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler Thromb Vasc Biol. 1997;17:3174–3184. doi: 10.1161/01.atv.17.11.3174. [DOI] [PubMed] [Google Scholar]
- 215.Zhang J. Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int. 2005;68:1171–1185. doi: 10.1111/j.1523-1755.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- 216.Zheng-Fischhofer Q. Ghanem A. Kim JS. Kibschull M. Schwarz G. Schwab JO. Nagy J. Winterhager E. Tiemann K. Willecke K. Connexin31 cannot functionally replace connexin43 during cardiac morphogenesis in mice. J Cell Sci. 2006;119:693–701. doi: 10.1242/jcs.02800. [DOI] [PubMed] [Google Scholar]
- 217.Zhu Y. Bian Z. Lu P. Karas RH. Bao L. Cox D. Hodgin J. Shaul PW. Thoren P. Smithies O. Gustafsson JA. Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 218.Zonta M. Angulo MC. Gobbo S. Rosengarten B. Hossmann KA. Pozzan T. Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]