Abstract
Long-term exercise is associated with reduced atherosclerotic burden, inflammation, and enhanced endothelial progenitor cell (EPC) levels in mice. Infusion of progenitor cells in mice decreases atherosclerosis and suppresses inflammation. The aim of this study was to determine whether exercise-induced enhancement of EPCs is associated with reduced atherosclerosis and inflammation. To study this, 20-week old ApoE−/− mice with advanced atherosclerotic lesions (n = 12/group) were randomized to voluntary running or no running for 8 weeks. Exercise led to a potent suppression of elevated circulating proinflammatory cytokines without significant reduction of atherosclerotic lesions. When repeated in ApoE−/− mice with early atherosclerotic disease, exercise led to a 62% (p = 0.017) reduction in lesion thickness (intima-to-media ratio) at the aortic root. Interestingly, BM-EPC levels were significantly elevated under proinflammatory conditions seen in ApoE−/− mice and decreased in response to exercise, independent of the degree of atherosclerosis. Under early atherosclerotic conditions, long-term exercise reduces atherosclerotic plaque burden and is associated with reduced systemic inflammation. Elevated BM-EPCs seen in atherosclerotic conditions may be a marker of generalized vascular inflammation or injury, and decrease in response to exercise, along with other markers of inflammation. Antioxid. Redox Signal. 11, 15–23.
Introduction
Atherosclerosis is a complex vascular disease characterized by chronic inflammation within the arterial wall (3, 20). Studies in humans and animal models have provided evidence that regular exercise exerts beneficial effects on atherogenesis and coronary artery disease (CAD). Specifically, it has been shown that endurance exercise decreases atherosclerotic plaque formation (2, 5, 8, 12) and reduces neointima formation after carotid artery injury (17). Exercise has also been shown to reduce proinflammatory cytokines in patients with CAD (4) and metabolic syndrome (22). In ApoE−/− mice, exercise has been shown to improve endothelium-dependent vasorelaxation in isolated aortas and to decrease vascular oxidative stress in mice with atherosclerosis (7, 10). Endurance training also enhances the levels of EPCs in circulation and in the bone marrow (6, 8, 19), consistent with accumulating evidence that progenitor cells play a critical role in the maintenance of endothelial health and integrity (24) and in reducing cytokine levels under atherosclerotic conditions (18).
Our group previously published data showing that infusion of bone marrow progenitor cells from young ApoE−/− mice without atherosclerotic disease (but not cells from old diseased mice) into separate ApoE−/− mice attenuated the development of atherosclerosis and reduced levels of IL-6, a proinflammatory cytokine (18). Others have shown that infusion of progenitor cells attenuates neointima formation after carotid artery injury in mice (24). However, it remains unclear whether the enhancement of bone marrow or circulating endothelial progenitor cells is a mechanism by which exercise exerts its positive benefits on the cardiovascular system. In light of data that endurance exercise in a mouse model of atherogenesis led to increased EPC levels in the bone marrow and in circulation, in association with incorporation of these cells into neointima formation, we hypothesized that the enhancement of EPCs in response to exercise may mediate the beneficial impact of exercise on endothelial homeostasis, attenuation of atherosclerosis, and the suppression of systemic proinflammatory cytokines in atherosclerotic mice.
By using ApoE−/− mice, prone to developing atherosclerotic lesions accelerated by high-fat diet feeding, we studied the relation between exercise and bone marrow endothelial progenitor cells, and its impact on arteriosclerosis. We chose a voluntary running model to avoid the potential stressor of forced activity, taking advantage of the substantial capacity C57Bl6/J mice have for nocturnal running activity (23). We expected that long-term voluntary running under severe proinflammatory conditions would reduce atherosclerotic lesions and inflammatory cytokines in association with enhanced progenitor cell levels. Surprisingly, we found that sustained inflammatory cytokine suppression occurred without detectable reduction in atherosclerotic burden, in association with decreased BM-EPC levels, which are elevated in response to atherosclerosis.
Materials and Methods
Animals
C57Bl6/J and C57Bl6/J-ApoE−/− mice (Strain 2052) were obtained from Jackson Laboratories (Bar Harbor, ME). Each mouse was individually caged, handled, and used in compliance with the Institutional Animal Care and Use Committee guidelines. This includes ethical clearance for the study.
Advanced lesions were developed by giving a high-fat diet (42% fat, 1.25% cholesterol) (Harlan-Teklad Madison, WI; diet 88137), whereas early lesions were induced by feeding a normal chow diet (3.5% fat, 0 cholesterol; Harlan-Teklad Madison; diet 2014S) starting from weaning and continued for the remainder of the study. Study animals were weaned at 3 weeks of age and were provided a normal chow diet, as described earlier for 5 weeks. At this point, the 8-week-old study animals were provided the study diet for a total of 12 weeks (high-fat diet to induce ApoE−/− mice with advanced lesions; normal-chow diet to induce ApoE−/− mice with early lesions, and also for wild-type controls).
To study the impact of exercise on ApoE−/− mice with advanced lesions, ApoE−/− mice subjected to the protocol, including high-fat diet feeding, were randomized to a voluntary running wheel or to sedentary cage activity for 8 weeks (n = 12/group). Wild-type mice subjected to a normal-chow diet from weaning were used as sedentary wild-type controls (n = 6). To study the impact of exercise on mice with early atherosclerotic lesions, ApoE−/− mice given a normal chow diet from weaning were randomized at age 20 weeks to voluntary exercise or sedentary cage activity (n = 6/group) and continued on this diet for the rest of the study protocol, as described earlier. A separate group of wild-type mice fed a normal chow diet served as sedentary controls (n = 6). The mice in each group (advanced and early lesions) were continued on their study diet throughout the exercise period.
The animals were sacrificed 24 h after the last bout of exercise. The animals were anesthesized with Nembutal (0.015 ml/g); at the point of complete anesthesia and analgesia (tested by tail pinch), the thoracic cavity was accessed, and the right ventricle was penetrated by a 20-gauge syringe, and the entire blood volume was collected into a syringe.
Exercise
Our model of exercise was the widely used voluntary running-wheel model, as described previously (23). Each mouse randomized to exercise was placed in a modified cage with a wheel attached to a magnetic sensing mechanism. This allowed the running activity of each mouse to be tracked by a computer, from which the corresponding distances and amount of time spent running were obtained. The mice ran predominantly at night for a total of 8 weeks. Individually caged sedentary controls were housed in the same room and handled as often as the trained mice.
Cytokine measurement
For cytokine measurement, blood samples were collected from anesthetized mice by right ventricular phlebotomy, as described earlier. Clotted blood was spun at 1,200 g for 10 min, and the serum was obtained and immediately stored at −80°C. At analysis, all the samples were thawed, and multiple cytokines were measured simultaneously by using the 10-plex cytokine/chemokines kit from Linco Research Systems (St. Charles, MO) by following the manufacturer's instructions. The following cytokines were measured: monocytes chemoattractant protein 1 (MCP-1), macrophage-inhibitory protein 1α (MIP-1α), tumor necrosis factor (TNF), interleukin 6 (IL-6), interleukin 12 (IL-12), interleukin 1α (IL-1α), and interleukin 1β (IL-1β).
Fluorescence-activated cell sorting
We measured endothelial progenitor cells (EPCs) as a fraction of total mononuclear cells. EPCs are defined as Sca-1 and VEGF-R2 double-positive mononuclear cells.
FACS analysis was performed on bone marrow cells (1 × 106 cells/100 μl) as previously described (18). Cells were stained with rat anti-mouse allophycocyanin (APC)-conjugated Sca-1 (IgG2Ak) and rat anti-mouse phycoerythrin (PE)-conjugated VEGFR2 (IgG2Ak) (eBioscience, San Diego, CA). The antibodies were titrated before use. IgG2Ak isotype controls were used for each antibody to estimate nonspecific binding of the κ side chain to Fc receptors on bone marrow cells, and the isotype controls were subtracted from each of the presented results. The stained cells were analyzed by using FACS Diva (Becton Dickinson, Franklin Lakes, NJ), and the data was analyzed by using Flow (Tree Star, Inc., Ashland, OR).
Pathology
Aortas were cut open to expose the endothelial surface for oil red O staining. Morphometric analyses were performed for plaque area and extent of lesion in a blinded fashion. Atherosclerotic burden was quantified by normalizing the area staining for oil red O to the area of the entire aortic area. For cross-sectional quantification, the aorta was cut at the root, proximal to the flexure of the arch. The cross-sections were stained with hematoxylin and eosin (H&E) and oil red O. These methods were previously published by our group (18).
Lipid profile measurement
After animals were sacrificed as previously described, blood was collected into 1-ml capped tubes and allowed to coagulate. Serum was collected by centrifuging at 1,200 g for 10 min. The lipid-profile analysis was run by the Duke Clinical Core Laboratory on 200 μl of serum.
Statistical analysis
All results were presented as means ± SEM. One-way ANOVA was used, followed by a Newman–Keuls test to locate the differences. Differences with a value of p < 0.05 were considered statistically significant.
Results
Voluntary running in ApoE−/− mice had no impact on advanced atherosclerotic lesions
To examine the impact of exercise on atherosclerosis in this model, 8-week-old ApoE−/− mice were fed a high-fat diet for 12 weeks and subsequently randomized to a voluntary running wheel (A-KO-Ex, n = 12) or sedentary cage activity (A-KO-Sed, n = 12) for 8 weeks, with sedentary age-matched wild-type mice serving as controls (n = 6). Twenty-four hours after the last bout of exercise, we harvested aortas from sedentary and exercised ApoE−/− mice, as well as wild-type controls. The atherosclerotic lesion area was analyzed by en face staining of abdominal aortas; lesion thickness was quantified on cross-sectional aortic root staining with oil red O, as we previously described (18). In the sections of mice with advanced atherosclerotic lesions, exercise did not result in decreased atherosclerotic plaque burden in the abdominal aortas (Fig. 1A) or in the aortic root (Fig. 2, left graph). Exercise had no detectable impact on the lipid profile of these mice (Table 1).
FIG. 1.
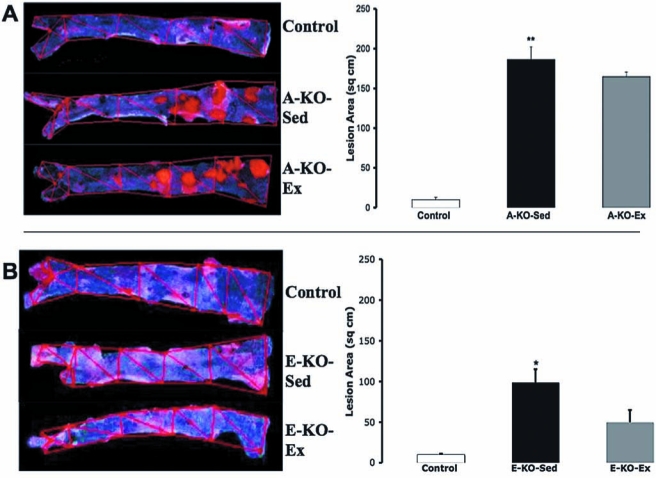
The effect of 8-week voluntary running on thoracoabdominal atherosclerotic lesions in ApoE−/− mice. (A) Representative images of oil red O–stained atherosclerotic lesions in the aortic trees from ApoE−/− mice on a high-fat diet without (A-KO-Sed, n = 12) and with (A-KO-Ex, n = 12) 8 weeks of voluntary running as compared with sedentary wild-type mice on a normal-chow diet (Control, n = 6) as described in Materials and Methods. Also shown is a graphic representation of the lesion area from these aortic trunks. (B) Representative images of oil red O staining of the trunks of the aortic trees from ApoE−/− mice on a normal-chow diet without (E-KO-Sed, n = 6) and with (E-KO-Ex, n = 6) 8 weeks of voluntary running as compared with sedentary wild-type mice on a normal-chow diet (Control, n = 6) as described in Materials and Methods; Adjacent is a graphic representation of the images. * and ** denote p < 0.05 and < 0.01, respectively, when comparing A-KO-Sed with Control.
FIG. 2.
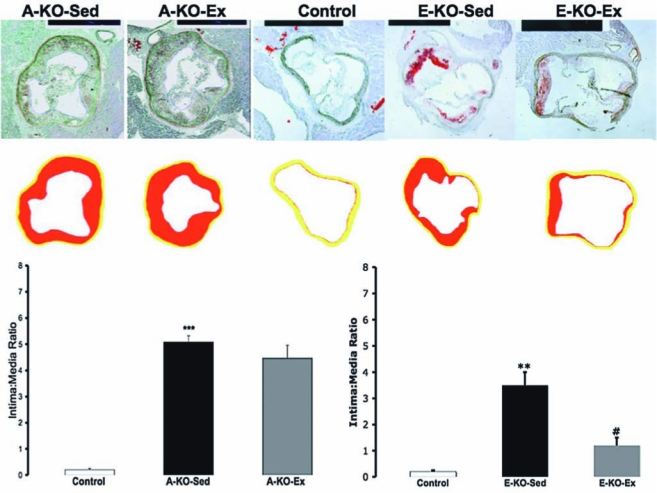
The effect of 8-week voluntary running on atherosclerotic lesions at the aortic root in ApoE−/− mice. Representative images of oil red O staining of aortic root cross-sections from ApoE−/− with advanced lesions randomized to no running or running (A-KO-Sed and A-KO-Ex, respectively), and ApoE−/− mice with early lesions randomized to sedentary activity or running (E-KO-Sed and E-KO-Ex) and sedentary wild-type control. Lower panels: schematic presentation of the atherosclerotic plaque intima (red) and media (yellow). The graphic depiction of the intima-to-media ratio is also shown for mice with advanced lesions (left graph) and early lesions (right graph). ** and *** denotes p < 0.01 and < 0.001, respectively, when comparing E-KO-Sed with Control. #p < 0.05, when comparing E-KO-Ex with E-KO-Sed.
Table 1.
Impact of Exercise on Lipid Profile of ApoE-/- Mice with Advanced Atherosclerosis
| Total cholesterol (mg/dl) | LDL-C (mg/dl) | HDL-C (mg/dl) | Triglycerides (mg/dl) | |
|---|---|---|---|---|
| Control | 274.6 ± 39 | 45.3 ± 18 | 215.3 ± 19.5 | 70.8 ± 23.3 |
| A-KO-Sed | 2,016 ± 129§ | 1,589 ± 129§ | 377.1 ± 13§ | 246 ± 63† |
| A-KO-Ex | 1,819.5 ± 185§ | 1,421.2 ± 136§ | 374.3 ± 43.6 | 120 ± 21 |
Lipid profile of ApoE−/− mice fed a high-fat diet and randomized to exercise (A-KO-Ex) or sedentary cage activity (A-KO-Sed), compared with wild-type sedentary controls (Control) fed a normal-chow diet, as described in Methods.
p < 0.05, †p < 0.01, ‡p < 0.001, §p < 0.0001 when compared with Control.
To confirm that our model of exercise could affect atherosclerosis to a similar degree as studied previously (8), we repeated the experiment with chow diet fed ApoE−/− mice, which develop less severe atherosclerotic lesions than those seen in age-matched high-fat diet–fed mice. Twenty-week-old normal chow-fed ApoE−/− mice were randomized to a voluntary running wheel (E-KO-Ex, n = 6) or sedentary cage activity (E-KO-Seed, n = 6) for 8 weeks, with a separate group of age-matched wild-type mice serving as sedentary controls (n = 6). At 28 weeks of age, normal chow diet–fed ApoE−/− mice had not developed significant atherosclerotic lesions in their thoracoabdominal aortas and appeared similar to WT mice. Exercise had a nonsignificant impact on reducing atherosclerosis in this area (Fig. 1B). The aortic roots of these mice, however, contained early atherosclerotic lesions. On examination of aortic root cross-sections, exercise exerted a 62% (p = 0.017) reduction in intima/media ratio (Fig. 2, right graph). This improvement was noted in the absence of significant change in the lipid profile of these mice (Table 2).
Table 2.
Impact of Exercise on Lipid Profile of ApoE-/- Mice with Early Atherosclerosis
| Total cholesterol (mg/dl) | LDL-C (mg/dl) | HDL-C (mg/dl) | Triglycerides (mg/dl) | |
|---|---|---|---|---|
| Control | 65.67 ± 1.74 | 0.933 ± 0.5 | 62.06 ± 1.21 | 16.5 ± 0.8 |
| E-KO-Sed | 480 ± 93.0† | 226.35 ± 69† | 219.2 ± 19.6† | 33 ± 3.5† |
| E-KO-Ex | 481 ± 41.7† | 241.97 ± 18† | 220.97 ± 21† | 89 ± 21†,‡ |
Lipid profile of ApoE−/− mice fed a normal chow diet and randomized to exercise (E-KO-Ex) or sedentary cage activity (E-KO-Sed), compared with wild-type sedentary controls (Control) fed a normal-chow diet, as described in Methods.
p < 0.05, †p < 0.01, when comparing E-KO-Sed with Control.
p < 0.001, when comparing E-KO-Ex with E-KO-Sed.
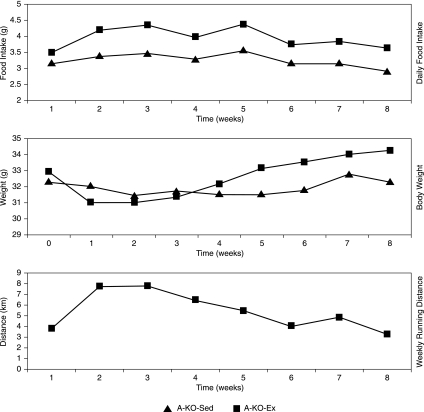
The mean food intake, body weight, and exercise activity of the A-KO-Ex mice is shown in Fig. 3 compared with those of A-KO-Sed mice fed a high-fat diet.
FIG. 3.
Mean food intake, body weight, and exercise activity of ApoE−/− with advanced lesions over time. Graph illustrates average food intake, body weight, and exercise activity as a function of time in ApoE−/− mice fed a high-fat diet and randomized to sedentary cage activity (A-KO-Sed) compared with mice randomized to exercise-wheel activity (A-KO-Ex).
Exercise potently suppresses circulating proinflammatory cytokine levels
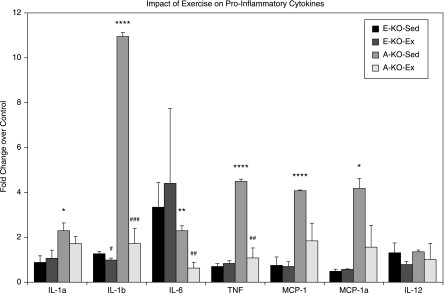
Because cytokines play important roles in the pathogenesis and complications of atherosclerosis, we examined the effects of voluntary running on circulating cytokines and chemokines from mice with early atherosclerosis (E-KO-Sed and E-KO-Ex, n = 6/group) and advanced atherosclerosis (A-KO-Sed and E-KO-Ex, n = 12/group), and wild-type controls (n = 6). We measured serum levels of cytokines and chemokines that have been previously implicated in the pathogenesis of atherosclerosis and CAD (1, 9, 11). The molecules measured included proinflammatory cytokines (TNF-α, IL-6, IL-1α, IL-1β, MCP-1, MIP-1α, and IL-12). In A-KO-Sed mice, all proinflammatory cytokines, except for IL-12, were significantly elevated compared with those in control mice (two- to 10-fold, p < 0.05) (Fig. 4). Exercise markedly normalized the proinflammatory cytokines, as seen in A-KO-Ex animals. In E-KO-Sed mice, levels of cytokines were not significantly different from those in the control mice, and exercise did not significantly change the profiles of these cytokines (Fig. 4).
FIG. 4.
Effects of exercise on circulating cytokines in mice with advanced and early atherosclerosis. Graphic representation of the impact of exercise on cytokines and chemokines in ApoE−/− mice with early lesions with and without exercise (E-KO-Ex and E-KO-Sed, respectively; n = 6/group) and mice with advanced lesions with and without exercise (A-KO-Ex and A-KO-Sed, respectively; n = 12/group) as noted in Materials and Methods. Data are expressed as fold change over sedentary wild-type controls as described. * and *** denote p < 0.05 and 0.001, respectively, when comparing sedentary mice with control. ## and ###, p < 0.01 and 0.001, respectively, when comparing exercised mice with sedentary mice.
Exercise results in reduced bone marrow endothelial progenitor cell levels in ApoE−/− mice
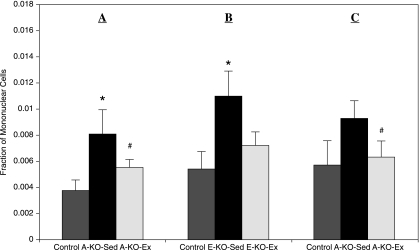
To determine how exercise affects bone marrow endothelial progenitor cell (EPC) levels in ApoE−/− mice, we measured EPCs previously defined as Sca-1/VEGF-R2 double-positive mononuclear cells (8) in the bone marrow of exercised or sedentary ApoE−/− mice. As described previously, high-fat diet–fed ApoE−/− mice were randomized to individual sedentary cages (A-KO-Sed) or running activity (A-KO-Ex) (n = 12/group), with sedentary wild-type mice as controls (n = 6). At baseline, a significant increase in Sca-1/VEGF-R2 double-positive bone marrow EPCs (104%; p = 0.039) was noted in A-KO-Sed when compared with age-matched WT controls. Furthermore, exercise resulted in the reduction of these EPCs by 34% (p = 0.018) in A-KO-Ex mice (Fig. 5A). To examine the impact of exercise on ApoE−/− mice with early lesions, this experiment was repeated with chow-fed mice, E-KO-Sed and E-KO-Ex (n = 6/group), as described. In this cohort, a 117% increase in baseline levels of Sca-1/VEGFR-2 double-positive EPCs (p = 0.025) was found in E-KO-Sed compared with controls. Eight weeks of voluntary exercise training resulted in a 32% reduction (p = 0.086) in E-KO-Ex compared with E-KO-Sed (Fig. 5B). To gain insight into the dynamics of bone marrow EPCs during a shorter period of exercise, we subjected a separate group of ApoE−/− mice with advanced lesions induced by the same protocol previously described to 1 week of voluntary aerobic exercise. In this group, sedentary ApoE−/− mice also had increased baseline BM-EPC levels compared with wild-type (64%; p = 0.066). Furthermore, exercise led to reduced levels of these progenitor cells (32%; p < 0.01) (Fig. 5C).
FIG. 5.
Impact of exercise on bone marrow endothelial progenitor cells (BM-EPCs) in ApoE−/− mice. BM-EPC content of (A) ApoE−/− mice with advanced atherosclerotic lesions after 8 weeks of exercise (A-KO-Sed and A-KO-Ex, n = 12/group); (B) ApoE−/− with early atherosclerotic lesions after 8 weeks of exercise (E-KO-Sed and E-KO-Ex; n = 6/group); (C) ApoE−/− mice with advanced atherosclerotic lesions after 1 week of exercise (n = 6, each group). WT-Sed, wild-type controls; KO-Sed, sedentary ApoE−/− mice; KO-Ex, exercised ApoE−/− mice. Values are expressed as mean ± SEM. *p < 0.05; **p < 0.01 when comparing KO-Sed with control, whereas #p < 0.05, ##p < 0.01 when comparing KO-Ex with KO-Sed.
Discussion
In this study, we showed that exercise is associated with reduced atherosclerotic burden, albeit in mice with early lesions. We also showed that exercise training exerts a strong suppressive effect on levels of cytokines and chemokines implicated in atherogenesis and plaque destabilization. However, the observed relation between exercise and bone marrow endothelial progenitor cells co-expressing Sca-1 and VEGF-R2 was surprising. Levels of these cells are elevated in mice with early and advanced atherosclerotic lesions, but decrease, along with proinflammatory cytokines, in response to exercise. Although it was previously shown that exercise reduces atherosclerosis in mice, the impact of exercise on early versus advanced lesions has not been studied. Our finding that mice with advanced atherosclerotic lesions subjected to aerobic exercise showed no improvement in atherosclerosis; whereas mice with early lesions benefited, suggests that the impact of exercise on atherogenesis is primarily to retard the progression to advanced lesions, rather than reversing advanced lesions
Evidence for the role of cytokines and inflammation in atherosclerosis, plaque destabilization, and rupture continues to accumulate (1, 9, 11). Our observation that these cytokines are significantly decreased in trained ApoE−/− mice is consistent with prior understanding that implicates proinflammatory cytokines in promoting atherogenesis (1). In the early atherosclerotic lesion group, levels of these proinflammatory cytokines were not as elevated as were those in mice with advanced lesions, and the fact that exercise did not reduce cytokine levels in this condition is not surprising. Exercise induces mild transient elevations in levels of these cytokines (15, 16), and in the setting of only mildly elevated cytokines compared with wild type, exercise is unlikely to have a large suppressive impact.
The effect of exercise on BM-EPCs under atherosclerotic conditions in mice was previously unknown. Interestingly, we found increased Sca-1/VEGF-R2 double-positive BM-EPCs in ApoE−/− mice with early and advanced lesions. Furthermore, exercise resulted in reduced levels of these cells in both groups of mice. Although unanticipated, the increase in BM-EPCs in atherosclerotic mice compared with controls may indicate response to an increased demand for such cells under conditions of widespread arterial disease or systemic inflammation (25). Alternatively, these high levels may suggest decreased mobilization from the bone marrow. Potential etiologies for the decrease in BM-EPCs in response to exercise include mobilization of these cells into circulation, differentiation of these cells, causing expression of another set of markers, decreased stimulus for production of EPCs, or a combination of these. Regardless of the actual mechanism, the decrease in inflammatory cytokines induced by exercise may be a trigger for this process (25).
Limitations of our study include the absence of any data showing the impact of exercise on circulating EPCs under early and advanced atherosclerotic conditions. As a result, we are unable to conclude whether the decrease in BM-EPCs is due to mobilization of these cells into circulation, or differentiation into cells expressing other markers (and not Sca-1/VEGF-R2), or decreased proliferation of these cells. Further, we do not have functional data on NO signaling or oxidative stress to support prior findings that the absence or antagonizing of NO signaling attenuates the response of EPCs to exercise (7, 14).
Taken together, our results provide evidence that, consistent with prior data, exercise is able to retard atherogenesis. In our mouse model, this appears to be true for mice with early lesions but not those with advanced lesions. Further, under advanced atherosclerotic conditions, significant systemic inflammation, and elevated BM-EPCs are present (25, 26). The impact of exercise under these conditions is reduction in proinflammatory cytokines, rather than reduced end-stage atherosclerotic plaque burden. With thorough understanding of the effects of cytokines on cardiovascular destabilization, inflammatory cytokine antagonism could represent a potent target for palliation of atherosclerotic disease and death.
Acknowledgments
This work was supported by the National Institutes of Health (AG023073, HL71536, and HL73042) to P.J.G.-C.; (AG023073-01A1S1) and a Howard Hughes Medical Institute Medical Research Fellowship to O.A.A. We thank W. Carl Stone for excellent technical assistance, as well as Mythreye Karthikayan, Ph.D., and Gregory Lam, M.D., for assistance with the project.
Abbreviations
A-KO-Ex, ApoE−/− mice with advanced atherosclerosis randomized to exercise; A-KO-Sed, ApoE−/− mice with advanced atherosclerosis randomized to sedentary activity; ANOVA, analysis of variance; ApoE, apolipoprotein E; BM-EPC(s), bone marrow endothelial progenitor cell(s); C57BL6/J, C57Black6/J Wild-type mouse strain; CAD, coronary artery disease; E-KO-Ex, ApoE−/− mice with early atherosclerosis randomized to exercise; E-KO-Sed, ApoE−/− mice with early atherosclerosis randomized to sedentary activity; FACS, Fluorescence-activated cell sorter; Fc, fixed-chain portion of immunoglobulin; EPC(s), endothelial progenitor cell(s); H&E, hematoxylin and eosin; HDL-C, high-density lipoprotein cholesterol; IgG, immunoglobulin G; IL-1α, interleukin 1 alpha; IL-1β, interleukin 1 beta; IL-12, interleukin 12; IL-6, interleukin 6, LDL-C, low-density lipoprotein cholesterol; MCP-1, monocyte chemoattractant protein; MIP-1, macrophage-inhibitory protein; NO, nitric oxide; PE, phycoerythrin; Sca-1, stem cell antigen 1; TNF, tumor necrosis factor; VEGF-R2, vascular endothelial growth factor receptor 2; WT, wild type.
References
- 1.Charo I. Taubman M. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 2.Gielen S. Schuler G. Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation. 2001;103:E1–E6. doi: 10.1161/01.cir.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 3.Hansson G. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ. Shin YO. Bae JS. Lee JB. Ham JH. Son YJ. Kim JK. Kim C. Lee BK. Oh JK. Othman T. Min YK. Yang HM. Beneficial effects of cardiac rehabilitation and exercise after percutaneous coronary intervention on hsCRP and inflammatory cytokines in CAD patients. Pflugers Arch. 2008;455:1081–1088. doi: 10.1007/s00424-007-0356-6. [DOI] [PubMed] [Google Scholar]
- 5.Kramsch DM. Aspen AJ. Abramowitz BM. Kreimendahl T. Hood WB., Jr Reduction of coronary atherosclerosis by moderate conditioning exercise in monkeys on a pro-inflammatory diet. N Engl J Med. 1981;305:1483–1489. doi: 10.1056/NEJM198112173052501. [DOI] [PubMed] [Google Scholar]
- 6.Laufs U. Urhausen A. Werner N. Scharhag J. Heitz A. Kissner G. Bohm M. Kindermann W. Nickenig G. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil. 2005;12:407–414. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 7.Laufs U. Wassmann S. Czech T. Münzel T. Eisenhauer M. Böhm M. Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 8.Laufs U. Werner N. Link A. Endres M. Wassmann S. Jurgens K. Miche E. Bohm M. Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 9.Linton M. Fazio S. Macrophages, inflammation and atherosclerosis. Int J Obes Relat Metab Disord. 2003;27:535–540. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- 10.Meilhac O. Ramachandran S. Chiang K. Santanam N. Parthasarathy S. Role of arterial wall antioxidant defense in beneficial effects of exercise on atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2001;21:1681–1688. doi: 10.1161/hq1001.097106. [DOI] [PubMed] [Google Scholar]
- 11.Moreno PR. Falk E. Palacios IF. Newell JB. Fuster V. Fallon JT. Macrophage infiltration in acute coronary syndromes: implications for plaque rupture. Circulation. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 12.Napoli C. Williams-Ignarro S. de Nigris F. Lerman LO. D'Armiento FP. Crimi E. Byrns RE. Casamassimi A. Lanza A. Gombos F. Sica V. Ignarro LJ. Physical training and metabolic supplementation reduce spontaneous atherosclerotic plaque rupture and prolong survival in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2006;103:10479–10484. doi: 10.1073/pnas.0602774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niebauer J. Maxwell AJ. Lin PS. Wang D. Tsao PS. Cooke JP. NOS inhibition accelerates atherogenesis: reversal by exercise. Am J Physiol Heart Circ Physiol. 2003;285:H535–H540. doi: 10.1152/ajpheart.00360.2001. [DOI] [PubMed] [Google Scholar]
- 14.Paul JD. Powell TM. Thompson M. Benjamin M. Rodrigo M. Carlow A. Annavajjhala V. Shiva S. Dejam A. Gladwin MT. McCoy JP. Zalos G. Press B. Murphy M. Hill JM. Csako G. Waclawiw MA. Cannon RO. Endothelial progenitor cell mobilization and increased intravascular nitric oxide in patients undergoing cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2007;27:65–73. doi: 10.1097/01.HCR.0000265031.10145.50. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen BK. Bruunsgaard H. Ostrowski K. Krabbe K. Hansen H. Krzywkowski K. Toft A. Sondergaard SR. Petersen EW. Ibfelt T. Schjerling P. Cytokines in aging and exercise. Int J Sports Med. 2000;21(suppl 1):S4–S9. doi: 10.1055/s-2000-1444. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen BK. Ostrowski K. Rohde T. Bruunsgaard H. The cytokine response to strenuous exercise. Can J Physiol Pharmacol. 1998;76:505–511. doi: 10.1139/cjpp-76-5-505. [DOI] [PubMed] [Google Scholar]
- 17.Pynn M. Schafer K. Konstantinides S. Halle M. Exercise training reduces neointimal growth and stabilizes vascular lesions developing after injury in apolipoprotein E-deficient mice. Circulation. 2004;109:386–392. doi: 10.1161/01.CIR.0000109500.03050.7C. [DOI] [PubMed] [Google Scholar]
- 18.Rauscher FM. Goldschmidt-Clermont PJ. Davis BH. Wang T. Gregg D. Ramaswami P. Pippen AM. Annex BH. Dong C. Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 19.Rehman J. Li J. Parvathaneni L. Karlsson G. Panchal VR. Temm CJ. Mahenthiran J. March KL. Exercise acutely increases circulating endothelial progenitor cells and monocyte/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004;43:2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 20.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 21.Shimada K. Kishimoto C. Okabe TA. Hattori M. Murayama T. Yokode M. Kita T. Exercise training reduces severity of atherosclerosis in apolipoprotein E knockout mice via nitric oxide. Circ J. 2007;71:1147–1151. doi: 10.1253/circj.71.1147. [DOI] [PubMed] [Google Scholar]
- 22.Troseid M. Lappegard KT. Claudi T. Damas JK. Morkrid L. Brendberg R. Mollnes TE. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25:349–355. doi: 10.1016/j.ehj.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Waters RE. Rotevatn S. Li P. Annex BH. Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1342–C1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- 24.Werner N. Junk S. Laufs U. Link A. Walenta K. Bohm M. Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 25.Goldschmidt-Clermont PJ. Craeger MA. Losordo DW. Lam GK. Wassef M. Dzau VJ. Atherosclerosis 2005: Recent discoveries and novel hypotheses. Circulation. 2005;112:3348–3353. doi: 10.1161/CIRCULATIONAHA.105.577460. [DOI] [PubMed] [Google Scholar]
- 26.Dimmeler S. Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]