It has been reported that hepatitis C virus (HCV) may be lymphotropic in the setting of human immunodeficiency virus type 1 (HIV-1) coinfection. In HIV-infected subjects, HCV may replicate in the same cells as HIV-1, which increases the opportunity for direct interactions between these pathogens. Without any antiviral-therapy pressure, both viruses could establish a competitive relation for this particular cellular scenario.1
The HCV 5′ untranslated region (5′UTR), responsible for the initiation of viral translation via an internal ribosome entry site (IRES), has been previously described to contain specific nucleotide substitutions when cultured in infected lymphoid cells.2 Sequence variability in this region has important implications for structural organization and the function of the IRES element and could correlate with HCV RNA concentration.3
Because of the increased mortality and reduced treatment-response rates in coinfected subjects, understanding the selection pressures underlying the evolution of HCV is important for the development of strategies to control both viruses. Previous studies based on different HCV coding-genomic regions provided evidence of decreased genetic diversity in HIV/HCV coinfection compared with those conducted on monoinfection, suggestive of reduced immune selective pressure,4,5 although this finding is controversial.6–8 After initiation of highly active antiretroviral therapy (HAART), the genetic diversity of HCV was shown to increase,5 at least in some genomic regions.9 This increase in diversity seems to reflect an improved selective pressure, requires time to develop,10 and is seen mostly in patients with virologic and CD4 T-cell responses to HAART.17,19 With successful immune response, it would be reasonable to predict that enhanced control of HCV would result. As a consequence, a greater recognition of virus-infected hepatocytes and consequent alanine aminotransferase (ALT) flares are expected to occur.
Although protein-coding regions may be under significant selection pressure after immune reconstitution, this could not be the case of the 5′UTR, highlighting secondary structural constraints within the UTR that are likely to be critical for viral replication and translation.
We report in a comprehensive assessing longitudinal study, the nucleotide diversity of HCV at 5′UTR in a group of HIV/HCV–coinfected subjects under HAART. We selected this particular genomic region because it is the most highly conserved part of the viral genome, its amplification is efficient, and any differences between PBMC- and plasma-derived sequences are likely to be important.
Second, comparison of sequences in more-variable regions is less reliable because, in the presence of large numbers of variants, artefactual polymorphism related to sampling error becomes a major problem.
Ten patients with HIV/HCV coinfection followed up at the Infectious Diseases Unit of the Fernandez Hospital were included (nine men aged 33–48 years; mean age, 40.3 years). These patients are part of a larger cohort of HIV-HCV coinfected patients who were selected based on a history of 4–8 years of HAART and the absence of interferon-based anti-HCV therapy. We longitudinally analyzed the variability of HCV 5′UTR genomic sequences in plasma samples yearly taken (10 ± 2 months). All had chronic HIV/HCV coinfection with a mean ± standard deviation (SD) of disease duration of 11.44 ± 4.4 and 11.0 ± 6.3 years, respectively. The duration of the disease was considered as starting at the time of diagnosis. In three of them (patients II, V, and, IX), the first available plasma sample was taken before HAART initiation.
At the last-studied point (immediately previous to the start of anti-HCV therapy), peripheral blood mononuclear cells (PBMCs) were also separated to characterize HCV 5′UTR sequences. Plasma and cells were separated directly after collection from whole blood by centrifugation on a Ficoll-Hypaque density gradient. PBMC pellets (1 × 107 cells) and plasma (200 μl) were aliquoted in Trizol LS (GIBCO, Life Technologies, NY) and stored at −80°C until use. HCV RNA was isolated from plasma samples and PMBCs by following strictly the manufacturer's instructions. The 5′UTR was amplified by using an RT-nested PCR, producing a 250-bp amplicon, which was further directly sequenced to establish phylogenetic relatedness for genotype assignment based on distance methods, as previously described.13 Each amplicon was also cloned (pGEM-T Easy vector, Promega, WI), and 10 sequences were used to define the extent or presence of any 5′UTR sequence heterogeneity.14 Positive and negative controls were included in all extractions and amplifications reactions, and samples from the same individual were handled separately to avoid contamination.
HCV (Roche Amplicor Monitor HCV 2.0, lower limit of detection, 600 IU/ml) and HIV (VERSANT HIV-1 RNA 3.0 Assay, Siemens; lower limit of detection, 50 copies/ml) plasma viral load dynamics were measured as well as the CD4+ T-cell count (flow cytometry) and alanine aminotransferase (ALT; upper limit normal value, 41 IU/ml; BioSystems S.A. Costa Brava 30, Barcelona, Spain) levels during HAART. HCV-RNA and HIV-RNA data were transformed to the log10 scale to obtain better distributional properties for statistical analysis. The lower limit of HIV quantification was used for calculation purposes in cases in which levels were undetectable (Fig. 1).
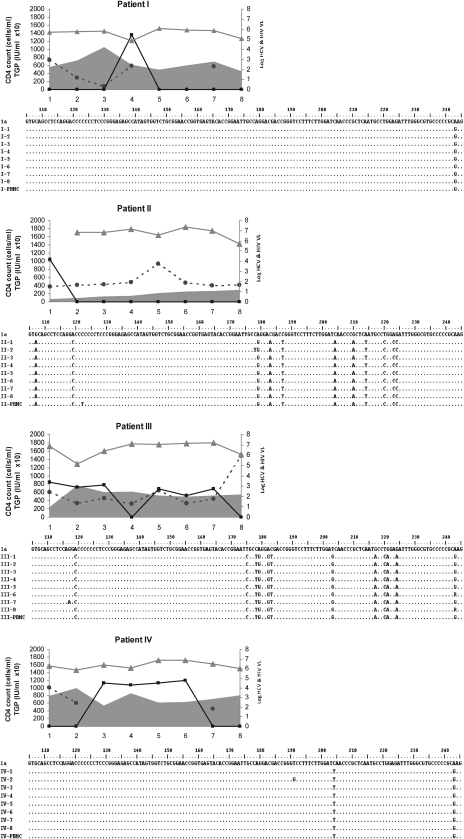
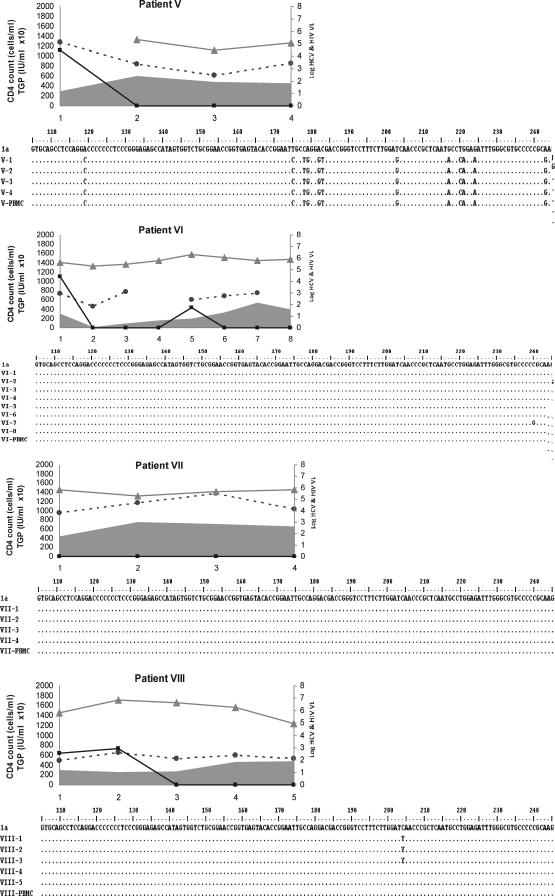
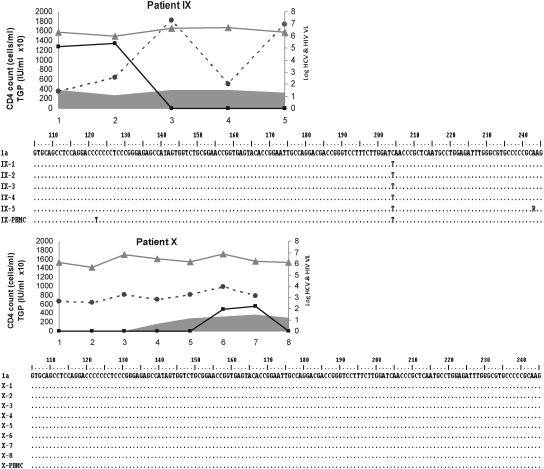
FIG. 1.
Patient-by-patient dynamics of viral, immune, and biochemical parameters during the follow-up are plotted as a function of time (expressed in years in the x-axis). Individual alignment of partial (nucleotide 106 to 244) 5′UTR consensus nucleotide sequences from yearly sequential HCV strains characterized from consecutive plasma samples and PBMCs samples. HCV isolates belonging to genotype 1 are those from patients I, IV, VI, VII, VIII, IX, and X; genotype 2 are those from patient II, and genotype 3 are those from patients III and V.  , Hepatitis C virus-RNA: (log10 scale IU/ml);
, Hepatitis C virus-RNA: (log10 scale IU/ml);  , HIV (log10 scale copies/ml); gray curve, CD4 T cell count (cells/ml); and
, HIV (log10 scale copies/ml); gray curve, CD4 T cell count (cells/ml); and  , ALT (IU × 10/ml).
, ALT (IU × 10/ml).
Descriptive statistics are presented to describe the study sample and to estimate population parameters. In general, categoric variables are summarized with counts and percentages. Descriptive statistics (N, mean, standard deviation) are used to summarize continuous variables. Fisher's Exact Test was used to analyze qualitative variables and the Mann-Whitney test, to analyze quantitative variables. All reported p values are two-sided; p < 0.05 was considered statistically significant.
Once HAART was initiated, the HIV viral load was reduced to an undetectable level (patients II, V, and IX), and all studied subjects reached an undetectable HIV viral load (<50 copies/ml) at the last study (Fig. 1). No statistically significant differences (p = 0.1) were found between mean CD4+ T-cell count (±SD) at first-time analysis (333 ± 205) and during the follow-up (447 ± 204). The HCV genomic characterization based on phylogenetic relatedness among viral isolates was as follows: seven of 10 genotype (Gt) 1; one of 10 Gt2; two of 10 Gt3. The hepatitis C viral load, assumed as the replication rate, exhibited a highly sustained level through the study period, independent of HAART, HIV replication, and CD4+ T-cell dynamics (Fig. 1). No correlation was observed between the dynamics of both HCV and HIV RNA levels (data not shown) and considering the small sample size, we analyzed too few subjects with the HCV genotype other than 1 to evaluate the correlation genotype and HCV-RNA levels on HAART.
ALT-level fluctuations were frequent, but grade 3 or higher elevations were absent (Fig. 1), and consequently, no HAART discontinuation was decided because of hepatotoxicity. A transient increase was observed to parallel the increase in HCV-RNA level and CD4+ T-cell count, but the analysis based on data during the follow-up revealed no significant differences (p = 0.4) between first-time values (72 ± 29.3 IU/ml) and last-time ones (86.1 ± 44.1 IU/ml).
At the intrapatient level, the analysis showed a high degree of genomic conservation among HCV 5′UTR sequences during the follow-up, regardless of the HAART regimen, CD4+ T-cell count, and HCV genotype (Fig. 1). The phylogenetic analysis of HCV strains revealed that all samples from the same host (plasma and PBMCs) were closely related to each other, with (mean ± SD) 98.6 ± 0.5 nucleotide identity.
In agreement with a previous report,15 when a successful limitation of HIV-infected lymphocytes by means of HAART was reached, high HCV-RNA levels were sustained. This may reflect the replication of a diverse repertoire of quasispecies, the diversity of which was enhanced during a period of immune compromise. With successful enhancement of the specific anti-HCV immune response, the newly applied selection pressure could lead to replication toward minority quasispecies, maintaining HCV-RNA levels. This hypothesis could not be supported, because it has not been examined at a quasispecies level in an appropriate genomic region, such as the hypervariable region coding for envelope glycoproteins, raising the possibility of missing changes in minor viral variants; and second, considering that 5′UTR appears to be subjected to stronger conservative constraints than other regions of the HCV genome, probably because of the need for structural (and functional) conservation of the IRES.16
The role of HAART in this setting has also been examined, finding increased HCV quasispecies diversity after immune restoration in some groups5,9 and others with no differences.6,10 It is difficult to reconcile the differences in these studies because they used different study designs (cross-sectional vs. longitudinal), a variable duration of HCV infection was noted among study subjects, and the HCV sequence-analysis methods also differed.
Alternatively, the HCV viral load level could be explained as more susceptible target cells become available, considering that HCV appears to replicate within PBMCs, CSF, brain, dendritic cells, female genital tract, and other extrahepatic tissues. This particular cell tropism is known to be influenced by the 5′UTR nucleotide sequence,17–19 which could differ in plasma compared with other compartments or according the route of infection.20 All of these studies represent single time-point comparisons between compartments, and so are limited in demonstrating the evolution or stability over time.
In agreement with the present data, one study showed no difference in 5′UTR sequences after interferon therapy in HCV-monoinfected subjects.16 In this regard, the nucleotide variations observed among HCV isolates in the studied population were genotype dependent (Fig. 1). Most of the substitutions were in unpaired regions of the IRES or clustered, so that base-pairing was maintained. Among nucleotide changes, three well-characterized point mutations residing within the IRES were found; these could favor lymphotropism, possibly because of more-efficient cap-independent translation.21–23 These were consistently exhibited in plasma (and PBMCs in the last time sample collected) in six HCV isolates ascribed to genotype 1 (G243A) and in one belonging to genotype 2 (G107A, C204A, and G243A), but did not emerge de novo in other HCV isolates during the follow-up, providing evidence that the 5′UTR-related lymphotropism was not affected. Other HCV coding genomic regions are more prone to vary under immune-response dynamics during HIV infection.9 However, their relation with the lymphotropism of HCV is still obscure. Because viral particles in serum are thought to be released from the liver but also from other compartments of the organism, such as PBMCs, the observed minimal diversity within the 5′UTR might reflect the existence of various HCV IRES sequences targeting RNA translation specifically to the liver or extrahepatic compartments. In this framework, initiation of protein translation may appear as one rate-limiting factor for viral replication. It will be of interest to assess HCV 5′UTR polymorphism due to viral tropism in different parts of the organism and its impact on the selection of replicative variants.1 The strong constrains for variability in the 5′UTR of HCV may explain that, even in HIV-coinfected patients, any improvement in the immune pressure against HCV as result of HAART, may be too limited to be recognized.
Acknowledgments
This work was partly supported by grants from Universidad de Buenos Aires (UBA; M431), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP6116), and Fogarty International Center/NIH grant, AIDS International, Training and Research Program, Mount Sinai School of Medicine (Grant D43TW 001037).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roe B. Hall WW. Cellular and molecular interactions in coinfection with hepatitis C virus and human immunodeficiency virus. Expert Rev Mol Med. 2008;10:30. doi: 10.1017/S1462399408000847. [DOI] [PubMed] [Google Scholar]
- 2.Laskus T. Radkowski M. Piasek A, et al. Hepatitis C virus in lymphoid cells of patients coinfected with human immunodeficiency virus type 1: evidence of active replication in monocytes/macrophages and lymphocytes. J Infect Dis. 2000;181(2):442–448. doi: 10.1086/315283. [DOI] [PubMed] [Google Scholar]
- 3.Barría MI. González A. Vera-Otarola J, et al. Analysis of natural variants of the hepatitis C virus internal ribosome entry site reveals that primary sequence plays a key role in cap-independent translation. Nucleic Acids Res. 2009;37(3):957–971. doi: 10.1093/nar/gkn1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Labrador FX. Dove L. Hui CK, et al. Trends for genetic variation of hepatitis C virus quasispecies in human immunodeficiency virus-1 coinfected patients. Virus Res. 2007;130:285–291. doi: 10.1016/j.virusres.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuhart MC. Sullivan DG. Bekele K, et al. HIV infection and antiretroviral therapy: effect on hepatitis C virus quasispecies variability. J Infect Dis. 2006;193(9):1211–1218. doi: 10.1086/502974. [DOI] [PubMed] [Google Scholar]
- 6.Netski DM. Mao Q. Ray SC, et al. Genetic divergence of hepatitis C virus: the role of HIV-related immunosuppression. J Acquir Immune Defic Syndr. 2008;49(2):136–141. doi: 10.1097/QAI.0b013e3181869a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman KE. Andreatta C. O'Brien J, et al. Hepatitis C in human immunodeficiency virus-coinfected patients:increased variability in the hypervariable envelope coding domain. Hepatology. 1996;23:688–694. doi: 10.1002/hep.510230405. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y. Hanada K. Hanabusa H, et al. Increasing genetic diversity of hepatitis C virus in haemophiliacs with human immunodeficiency virus coinfection. J Gen Virol. 2007;88:2513–2519. doi: 10.1099/vir.0.82974-0. [DOI] [PubMed] [Google Scholar]
- 9.Blackard JT. Yang Y. Bordoni P, et al. AIDS Clinical Trials Group 383 Study Team. Hepatitis C virus (HCV) diversity in HIV-HCV-coinfected subjects initiating highly active antiretroviral therapy. J Infect Dis. 2004;189(8):1472–1481. doi: 10.1086/382959. [DOI] [PubMed] [Google Scholar]
- 10.Babik JM. Holodniy M. Impact of highly active antiretroviral therapy and immunologic status on hepatitis C virus quasispecies diversity in human immunodeficiency virus/hepatitis C virus-coinfected patients. J Virol. 2003;77(3):1940–1950. doi: 10.1128/JVI.77.3.1940-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solmone M. Girardi E. Lalle E, et al. Evolution of HVR-1 quasispecies after 1-year treatment in HIV/HCV-coinfected patients according to the pattern of response to highly active antiretroviral therapy. Antivir Ther. 2006;11:87–94. [PubMed] [Google Scholar]
- 12.Wang XP. Goodwin L. Kahn P, et al. Influence of increased CD4 cell counts on the genetic variability of hepatitis C virus in patients co-infected with human immunodeficiency virus. Int J Biomol Tech. 2006;17:228–239. [PMC free article] [PubMed] [Google Scholar]
- 13.Bolcic F. Bull L. Martinez L, et al. Analysis of sequence configurations of the PKR-interacting HCV proteins from plasma and PBMC as predictors of response to interferon-alpha and ribavirin therapy in HIV-coinfected patients. Intervirology. 2008;51(4):261–264. doi: 10.1159/000158523. [DOI] [PubMed] [Google Scholar]
- 14.Cabot B. Esteban JI. Martell M, et al. Structure of replicating hepatitis C virus (HCV) quasispecies in the liver may not be reflected by analysis of circulating HCV virions. J Virol. 1997;71(2):1732–1734. doi: 10.1128/jvi.71.2.1732-1734.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung RT. Evans SR. Yang Y, et al. Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS. 2002;16(14):1915–1923. doi: 10.1097/00002030-200209270-00008. and the AIDS Clinical Trials Group 383 Study Team. [DOI] [PubMed] [Google Scholar]
- 16.Soler M. Pellerin M. Malnou CE, et al. Quasispecies heterogeneity and constraints on the evolution of the 5′ noncoding region of hepatitis C virus (HCV): relationship with HCV resistance to interferon-alpha therapy. Virology. 2002;298(1):160–173. doi: 10.1006/viro.2002.1494. [DOI] [PubMed] [Google Scholar]
- 17.Zignego AL. Giannini C. Monti M, et al. Hepatitis C virus lymphotropism: lessons from a decade of studies. Dig Liver Dis. 2007;39(Suppl 1):S38–S45. doi: 10.1016/s1590-8658(07)80009-0. [DOI] [PubMed] [Google Scholar]
- 18.Fishman SL. Murray JM. Eng FJ, et al. Molecular and bioinformatic evidence of hepatitis C virus evolution in brain. J Infect Dis. 2008;197(4):597–607. doi: 10.1086/526519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minosse C. Calcaterra S. Abbate I, et al. Possible compartmentalization of hepatitis C viral replication in the genital tract of HIV-1-coinfected women. J Infect Dis. 2006;194(11):1529–1536. doi: 10.1086/508889. [DOI] [PubMed] [Google Scholar]
- 20.Roque-Afonso AM. Ducoulombier D. Di Liberto G, et al. Compartmentalization of hepatitis C virus genotypes between plasma and peripheral blood mononuclear cells. J Virol. 2005;79(10):6349–6357. doi: 10.1128/JVI.79.10.6349-6357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laporte J. Malet I. Andrieu T, et al. Comparative analysis of translation efficiencies of hepatitis C virus 5′ untranslated regions among intraindividual quasispecies present in chronic infection: opposite behaviors depending on cell type. J Virol. 2000;74(22):10827–10833. doi: 10.1128/jvi.74.22.10827-10833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima N. Hijikata M. Yoshikura H, et al. Characterization of long-term cultures of hepatitis C virus. J Virol. 1996;70(5):3325–3329. doi: 10.1128/jvi.70.5.3325-3329.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araújo FM. Machado-Lima A. Durham AM, et al. Sequence and structural analysis of the 5′ noncoding region of hepatitis C virus in patients with chronic infection. J Med Virol. 2009;81(7):1212–1219. doi: 10.1002/jmv.21507. [DOI] [PubMed] [Google Scholar]