Abstract
CD4+ T cell dysfunction in HIV-1 infection is associated with increased CTLA-4 and TGF-β expression. In this study we described a population of TGF-β-positive CD4+ T cells with multiple HIV specificities. These HIV-specific TGF-β-positive CD4+ T cells did not display the immunophenotypic patterns traditionally attributed to regulatory CD4+ T cells. TGF-β-positive CD4+ T cells were FOXP3 negative, CD25 negative, and displayed a heterogeneous surface expression of CD127. We also examined one potential mechanism for regulating TGF-β expression by HIV-specific CD4+ T cells. Blocking of the TGF-β receptor II led to increased HIV-specific IFN-γ-positive CD4+ and CD8+ T cell responses. Interestingly, HIV-specific TGF-β-positive CD4+ T cells did not substantially express CTLA-4. Nevertheless, CTLA-4 blockade resulted in a significant decrease in HIV-specific TGF-β-positive CD4+ T cell responses, and a concomitant increase in HIV-specific IFN-γ-positive CD4+ T cell responses. Our study proposes a mechanism by which HIV-specific TGF-β production may be regulated by CTLA-4 engagement.
Introduction
Several subsets of regulatory CD4+ T cells (CD4+ Treg) have been described.1,2 These CD4+ Treg express high levels of FOXP3 and CD253–6 and low surface expression of CD127.7,8 CD4+ Treg cells exert their inhibitory effects on T cell proliferation and cytokine production through a cell–cell contact-dependent mechanism4,9–11 and the secretion of immunosuppressive cytokines such as interleukin (IL)-10 and transforming growth factor β (TGF)-β.12,13 Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is constitutively expressed on CD4+ Treg cells and is believed to be critical in mediating T cell suppression.14 CTLA-4 inhibits IL-2 production and cell cycle progression by binding to its ligands B7-1 (CD80) and B7-2 (CD86).14,15 The frequency of CTLA-4-positive CD4+ Treg is increased in patients with chronic HIV-1 infection and is suspected to play a critical immunomodulatory role leading HIV-associated immune dysfunction.16 Increased CTLA-4 expression correlates with markers of HIV disease progression.17–19 Upregulation of CTLA-4 also increases CCR5 expression and enhances susceptibility of CD4+ T cells to HIV infection,20 and in vitro blockade of CTLA-4 augments HIV-specific CD4+ T cell functions.17
TGF-β is an antiinflammatory cytokine and its increased production leads to suppression of T cell function.21–25 TGF-β upregulates CTLA-4 expression26–28 and inhibits T cell responses either through a direct or an indirect mechanism.21–25,29 TGF-β expression is upregulated in HIV-infected cells.30,31 The increased plasma TGF-β observed in advanced HIV-1 disease is believed to be associated with ineffective antiviral immune responses.32 In this study, we describe the production of TGF-β by HIV-specific CD4+ T cells that is regulated by a CTLA-4-mediated mechanism and assessed the immunophenotype profile of these TGF-β-positive CD4+ T cells.
Materials and Methods
Study subjects and samples
HIV-positive volunteers (n = 20) were recruited from the “The Research in Access to Care in the Homeless” (REACH) cohort in San Francisco as previously described.10,33 Demographic information and CD4+ T cell count were obtained at the time of enrollment and blood draw. Institutional Review Board approvals were obtained from the California Department of Public Health and UCSF Committee on Human Research, and all study participants gave written informed consent. None of the study participants has received antiretroviral therapy (ART) for at least 6 months prior to enrollment in the study. The HIV RNA level was determined from plasma using the Roche Amplicor 1.5 (Roche, Branchburg, NJ) as per the manufacturer's recommendations. Peripheral blood mononuclear cells (PBMCs) were separated and cryopreserved in liquid nitrogen until assay time.
Antigens
Peptides corresponding to the clade B consensus sequences of HIV-1 for Gag and Nef were synthesized as 15 amino acids (aa) overlapping by 11 aa (Mitochor Mimotopes, Victoria, Australia). Synthetic peptides for Gag (total = 123) and Nef (total = 49) used for all T cell assays were pooled into one single pool of peptides with a final concentration of 1 μg/ml per peptide.34
Flow-based intracellular cytokine staining
Detection of HIV-specific TGF-β, IL-10, and interferon (IFN)-γ production was performed as previously described.35 PBMCs (1 × 106) were incubated with HIV peptide pools for 2 h at 37°C in 5% CO2 in the presence of one of these following antibodies: anti-TGF-β R II (2 μg/ml, R&D systems, Minneapolis MN), anti-CTLA-4 (5 μg/ml, BD Pharmingen), or the respective isotype control for 12–14 h at 37°C in 5% CO2 in the presence of costimulatory anti-CD49d (1 μg/ml, Becton-Dickinson) and Golgi stop (BD Pharmingen). Phorbol myristate acetate (PMA, 50 ng/ml) and ionomycin (1 μg/ml; Sigma-Aldrich, St. Louis, MO), lipopolysacccharide (LPS, 1 ng/ml; Sigma-Aldrich), and phytohemagglutinin (PHA, 10 μg/ml; Sigma-Aldrich) were used as positive controls for TGF-β, IL-10, and IFN-γ production, respectively. Media alone without antigen stimulation were used as negative control. All samples were stained with an amine reactive viability dye as a dead cell exclusion marker (Molecular probes, Eugene, OR).36 Cells were then stained with specific combinations of the following antibodies: CD4 PerCP CY5.5, CD127 Alexa Fluor 647, CD8 PE CY7, and CD3 AmCyan (BD Pharmingen). PBMCs were permeabilized and stained with different combinations of the following antibodies: CTLA-4 APC, IL-10 PE, IL-10 APC, IFN-γ FITC (BD Pharmingen), TGF-β PE (Biotest Diagnostics, Denville, NJ) and analyzed by flow cytometry. A minimum of 30,000 CD3+ T cells per sample was acquired using a 6-color flow cytometer (LSRII, BD Biosciences) and analysis was performed by FLOWJO software (TreeStar, San Carlos, CA). Results were expressed as Percent TGF-β, IL-10, or IFN-γ positive CD3+/CD4+, T cells (Percent positive = % antigen-specific − % negative control). Responses greater than or equal to 0.1% and two times the background were considered positive. The extent of CTLA-4 and CD127 expression was also assessed. Gating was performed using the fluorescence-minus-one (FMO) control for each marker. All volunteers demonstrated significant TGF-β, IL-10, and IFN-γ production following PMA/ionomycin, LPS, and PHA stimulation, respectively. Background expression was <0.1%.
Flow-based FOXP3 staining assay
PBMCs were stained for FOXP3 expression following the manufacturer's protocol (eBioscience, San Diego, CA) with some modifications.10 Briefly, PBMCs (1 × 106) were incubated with HIV peptide pools for 12–14 h as described above for intracellular cytokine staining. Cells were then stained with an amine reactive viability dye as a dead cell exclusion marker (Molecular probes)36 and the following antibodies: FOXP3 FITC (eBioscience), TGF-β PE, CD4 PerCP Cy5.5, CD8 PE CY7, CD25 allophycocyanin, and CD3 AmCyan (BD Pharmingen). Analysis was performed by flow cytometry as described above. The percentage of TGF-β positive CD4+ T cells was determined and the extent of FOXP3 and CD25 expression was also assessed. Gating was performed using the FMO control for each marker. Results were expressed as the fraction of TGF-β-positive cells that expressed FOXP3 or CD25 over the total number of TGF-β-positive cells (equivalent to 100%).
Statistical analysis
Groups were compared using the paired t test and analysis was performed with PRISM software version 4.02 (Graph-Pad). Statistical significance was defined as p < 0.05.
Results
HIV-specific CD4+ T cells produce TGF-β
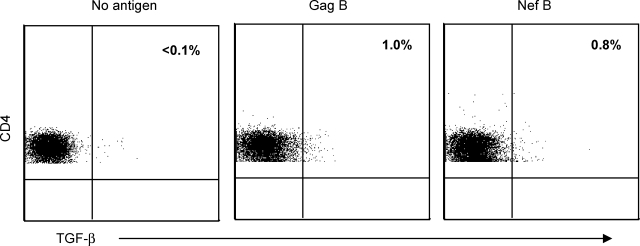
We first measured the frequency of HIV-specific TGF-β-positive CD4+ T cells in 20 HIV-1-infected volunteers. IL-10 and IFN-γ production by HIV-specific CD4+ T cells was determined concurrently.37 Volunteers had a median age of 42 years (range, 31–61), CD4+ T cell count of 209 cells/mm3 (range, 200–243), and median HIV plasma RNA of 100,000 copies/ml (range, 20,000–460,000). Representative plots of the frequency of the HIV-specific TGF-β-positive CD4+ T cells are shown in Fig. 1. Fifteen and seven volunteers demonstrated significant Gag- and Nef-specific IFN-γ-positive CD4+ T cell responses (median = 0.3%; range = 0.1–1.0%, and median = 0.11%; range = 0.1–0.22% for Gag and Nef, respectively). Six and five volunteers demonstrated significant Gag-specific TGF-β and IL-10 CD4+ T cell responses (median = 0.65%; range = 0.1–1.5%, and median = 0.16%; range = 0.13–0.3% for TGF-β and IL-10, respectively). Similarly, nine and eight volunteers demonstrated significant Nef-specific TGF-β and IL-10 CD4+ T cell responses (median = 0.5%; range = 0.1–1.4%, and median = 0.14%; range = 0.1–0.3% for TGF-β and IL-10, respectively). No significant differences in age, CD4+ count, or plasma HIV RNA were observed between volunteers with or without HIV-specific TGF-β-positive CD4+ T cell responses (p = 0.3, p = 0.1, and p = 0.7, for age, CD4+ count, and plasma HIV RNA, respectively; data not shown). No overlap between HIV-specific CD4+ T cells producing TGF-β, IL-10, or IFN-γ was observed (Fig. 2 and data not shown for Gag and Nef, respectively).
FIG. 1.
HIV-specific CD4+ T cells produce TGF-β. PBMCs were stimulated with HIV peptides then stained with anti-TGF-β PE, anti-CD3 AmCyan, anti-CD4 PerCP CY5.5, and anti-CD8 PE CY7, and analyzed by flow cytometry. Samples were first gated on the CD3+/CD4+ lymphocyte population and then the percent of TGF-β-positive CD4+ T cells was determined. Representative plots of HIV-specific CD4+ T cells expressing TGF-β after subtraction of the background values.
FIG. 2.
HIV-specific TGF-β-positive CD4+ T cells do not produce IL-10 or IFN-γ. PBMCs were stimulated with HIV peptides and then stained with anti-IFN-γ FITC, anti-TGF-β PE, anti-IL-10 APC, anti-CD3 AmCyan, anti-CD4 PerCP CY5.5, and anti-CD8 PE CY7, and analyzed by flow cytometry. Samples were first gated on the CD3+/CD4+ lymphocyte population and then the percent of TGF-β, IL-10, and IFN-γ-positive CD4+ T cells was determined. Representative plots of the number of TGF-β, IL-10, and IFN-γ-positive CD4+ T cells after subtraction of the background values.
TGF-β receptor II blockade increases IFN-γ expression by HIV-specific T cells
HIV-specific TGF-β-positive CD4+ T cells may have an immunosuppressive function that is mediated by TGF-β.21,22,30,31 To determine the effect of TGF-β on the HIV-specific T cell responses (as measured by IFN-γ production), PBMCs from three HIV-positive volunteers with evidence of HIV-specific TGF-β-positive CD4+ T cells were cocultured in the presence of anti-TGF-β receptor II Ab (or isotype control). Blocking of the TGF-β receptor II led to increased Gag-specific IFN-γ-positive CD4+ (range without blocking = 0.06–0.4% vs. range with blocking = 0.3–0.9%; Fig. 3) and CD8+ (data not shown) T cell responses.38 Blocking of the TGF-β receptor II also led to increased Nef-specific IFN-γ-positive CD4+ T cell responses (range without blocking = 0.04–0.2% vs. range with blocking = 0.2–0.5%; data not shown). These results support the inhibitory effect mediated by TGF-β production from HIV-specific CD4+ T cells. We also examined the effect of TGF-β receptor II blockade on IL-2 production. IL-2 expression by the HIV-specific CD4+ T cells was not detected with or without TGF-β RII blockade. These results may reflect the low CD4 T cell count and advanced disease of the volunteers in our study.
FIG. 3.
TGF-β receptor II blockade increases IFN-γ expression by HIV-specific T cells. PBMCs were stimulated with Gag peptides in the presence of anti-TGF-β R II (or isotype control). PBMCs were then stained with anti-IFN-γ FITC, anti-CD3 AmCyan, anti-CD4 PerCP Cy5.5, and anti-CD8 PE Cy7 and analyzed by flow cytometry. Samples were first gated on the CD3+/CD4+ and CD3+/CD8+ lymphocyte populations and then the percent of IFN-γ-positive cells was determined after subtraction of the background values. Plots are from three independent experiments yielding similar results.
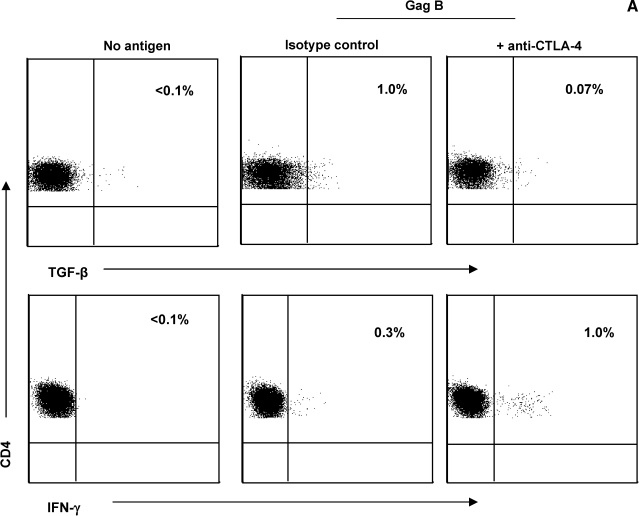
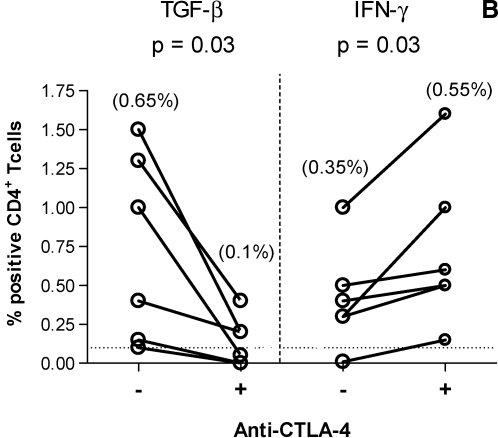
CTLA-4 blockade decreases TGF-β expression by HIV-specific CD4+ T cells
In vitro blockade of CTLA-4 engagement augments HIV-specific CD4+ T cell proliferation, IL-2, and IFN-γ production.17 We explored whether a similar inhibitory mechanism is involved in HIV-specific TGF-β CD4+ T cells. PBMCs from six HIV-positive volunteers with demonstrated HIV-specific TGF-β-positive CD4+ T cell responses were incubated with anti-CTLA-4 Ab (or isotype control). Representative plots are shown in Fig. 4A. CTLA-4 blockade resulted in a significant decrease in the frequency of Gag-specific TGF-β-positive CD4+ T cell responses (Fig. 4B). In contrast, blockade of CTLA-4 led to a significant increase in the frequency of Gag-specific IFN-γ-positive CD4+ T cell responses. Blocking of CTLA-4 engagement also led to a significant decrease in the frequency of Nef-specific TGF-β-positive CD4+ T cell responses and a concurrent significant increase in the frequency of Nef-specific IFN-γ-positive CD4+ T cell responses (p = 0.03 and p = 0.04 for TGF-β and IFN-γ, respectively; data not shown). These results suggest that engagement of CTLA-4 regulates TGF-β production by HIV-specific CD4+ T cells. The effect of CTLA-4 blockade on CD4+ T cells with antigenic specificity other than HIV was also analyzed in the same study population. CMV-specific TGF-β-positive CD4+ T cell responses were not detected in most volunteers irrespective of the presence of anti-CTLA-4 Ab. Blocking of CTLA-4 engagement led to a significant increase in the frequency of CMV-specific IFN-γ-positive CD4+ T cell responses (p = 0.01; data not shown).
FIG. 4.
CTLA-4 blockade decreases TGF-β expression by HIV-specific CD4+ T Cells. PBMCs (n = 6) were stimulated with Gag peptides in the presence of anti-CTLA4 (or isotype control), then stained with anti-IFN-γ FITC, anti-TGF-β PE, anti-CD3 AmCyan, anti-CD4 PerCP CY5.5, and anti-CD8 PE CY7, and analyzed by flow cytometry. Samples were first gated on the CD3+/CD4+ lymphocyte population and then the percent of TGF-β- and IFN-γ-positive cells was determined. Results were expressed as percent of Gag-specific CD4+ T cells expressing TGF-β or IFN-γ after subtraction of the background values. (A) Representative plots of Gag-specific CD4+ T cells expressing TGF-β or IFN-γ in the presence or absence of anti-CTLA-4. (B) Dashed line represents the cutoff for significant cytokine expression. Percentages in parentheses are median values. The two dots joined by a line represent the values obtained from the same individual and analysis was performed by paired t test.
HIV-specific TGF-β-positive CD4+ T cells are CTLA-4 negative
Upregulation of CTLA-4 in HIV-specific CD4+ T cells has been observed in patients with chronic HIV-1 infection.16,17 We next determined whether CTLA-4 is also expressed on TGF-β-positive CD4+ T cells. Representative plots are shown in Fig. 5. High CTLA-4 expression was detected on CD4+ T cells (median = 2.8%; range = 1.3–7.0%; Fig. 5A). In contrast, most of the CTLA-4-positive CD4+ T cells did not express TGF-β (median = 3%: range = 0–4.8%, and median = 2%: range = 0–3.5% for Gag and Nef, respectively; Fig. 5B). The lack of CTLA-4 expression on the TGF-β-positive CD4+ T cells suggests that the effect of CTLA-4 blockade on the TGF-β-positive CD4+ T cells is mediated by an indirect mechanism.
FIG. 5.
HIV-specific TGF-β-positive CD4+ T cells are CTLA-4 negative. PBMCs (n = 6) were stimulated with HIV peptides and then stained with anti-TGF-β PE, anti-CD3 AmCyan, anti-CD4 PerCP Cy5.5, anti-CD8 PE Cy7, and anti-CTLA-4 APC, and analyzed by flow cytometry. Gating on the CTLA-4-positive cells was performed using the fluorescence-minus-one (FMO) control for CTLA-4. (A) Representative plots of samples that were first gated on the CD3+/CD4+ and CD3+/CD8+ lymphocyte population and then the percentages of CTLA-4-positive cells were determined. (B) Representative plots of samples that were first gated on the CD3+/CD4+ lymphocyte population and then the percent of TGF-β-positive cells that expressed CTLA-4 was determined after subtraction of the background values. The values marked with an asterisk represent the fraction of TGF-β-positive cells that expresses CTLA-4 over the total number of TGF-β-positive cells (equivalent to 100%).
Analysis of the immunophenotypic profile of HIV-specific TGF-β-positive CD4+ T cells
CD4+ Treg classically express FOXP3 and CD25 and display low surface expression of CD127.7,8 In this study, we examined the immunophenotypic profile of the HIV-specific TGF-β-positive CD4+ T cells. PBMCs were stimulated with HIV peptides and the extent of FOXP3, CD25, and CD127 expression by the HIV-specific TGF-β-positive CD4+ T cells was assessed. Representative plots are shown in Fig. 6. Gag-specific TGF-β-positive CD4+ T cells were mainly FOXP3 negative, CD25 negative, and displayed heterogeneous expression of CD127 (median for FOXP3 = 3.5; range = 0.2–5, median for CD25 = 1; range = 0.2–4, median for CD127 = 35.5; range = 20–55; data not shown). A similar immunophenotypic profile of the Nef-specific CD4+ T cells was also observed (median = 3; range = 0.1–4, median = 1; range = 0.4–3, median = 38; range = 23–58 for FOXP3, CD25, and CD127, respectively; data not shown). These results indicate that the HIV-specific TGF-β-positive CD4+ T cells are distinct from the previously defined CD4+ Treg population.7,8
FIG. 6.
Analysis of the immunophenotypic profile of HIV-specific TGF-β-positive CD4+ T cells. PBMCs (n = 6) were stimulated with HIV peptides, then stained for regulatory markers and the percentage of TGF-β-positive CD4+ T cells was determined by flow cytometry. Samples were first gated on the CD3+/CD4+ lymphocyte population and then the percentages of TGF-β-positive cells were determined and the extent of FOX P3, CD25, and CD127 expression was also examined. Gating was performed using the fluorescence-minus-one (FMO) control for each marker. Representative plots of the phenotype of the HIV-specific TGF-β-positive CD4+ T cells expressing FOX P3, CD25, and CD127. The values marked with an asterisk represent the fraction of TGF-β-positive cells that expresses FOX P3, CD25, or CD127 over the total number of TGF-β-positive cells (equivalent to 100%).
Discussion
The mechanisms leading to the progressive loss of immune function and eventual failure of T cell responses in chronic HIV-1 infection remain unresolved. One hypothesis is that effector T cells eventually succumb to an immunosuppressive environment. Chemokine- and cytokine-mediated regulations are believed to play a key role in the loss of immune functions. The role of TGF-β in the inhibitory pathways involving HIV is complex and not fully elucidated.39. We evaluated HIV-specific TGF-β-positive CD4+ T cells in chronic HIV infection and hypothesized that the induction and function of cells are regulated by multiple factors including antigen-specific T cell receptors and inhibitory coreceptors such as CTLA-4.
Blocking the binding of TGF-β to its receptor resulted in increased IFN-γ expression by HIV-specific CD4+ and CD8+ T cells. These data suggest that TGF-β-positive CD4+ T cells exert an inhibitory effect on HIV-specific effector function in vitro. Signaling through the TGF-β receptor may promote a differential and temporal effect on T cells in different stages of HIV disease. The induction and detection of these TGF-β-positive CD4+ T cells in the course of HIV disease are still unknown. In addition, the intriguing role by which distinct antigen specificities of these TGF-β-positive CD4+ T cells contribute to the level of suppression may be highly significant.24 Nevertheless, the suppressive impact of these TGF-β CD4+ T cells in vivo remains to be confirmed. Direct cell–cell contact may also be required for these TGF-β-positive CD4+ T cells to exert their maximum inhibitory effect.
Inhibitory functions of regulatory T cells are believed to be mediated by direct binding of CTLA-4 and by cytokine production.39–41 CTLA-4 is upregulated on HIV-specific CD4+ T cells in advanced disease,17–19 and CTLA-4 is also constitutively expressed on CD4+ Treg.14 The generation of antigen-specific regulatory T cells requires weaker TCR stimulation42 and has been shown to be highly dependent on CTLA-4 signaling.43 Surprisingly, blockade of CTLA-4 also prevented the production of TGF-β, a suppressive cytokine, by the CTLA-4-negative HIV-specific CD4+ T cells. We postulate that CTLA-4-positive CD4+ T cells indirectly influence the inhibitory function of the TGF-β-positive CD4+ T cells by modulating CTLA-4 engagement. Clearly, the presence of CD4+ Treg in HIV infection adds to the complexity in understanding the mechanisms of immune regulation and suppression. Treg are believed to regulate the production of soluble factors including IL-10 and TGF-β. Because CTLA-4 is needed for the optimal function of regulatory Treg,50 one key question is whether CD4+ Treg that express CTLA-4 can also manipulate the expression of TGF-β by HIV-specific CD4+ T cells that do not express CTLA-4.
We describe the presence of TGF-β-positive CD4+ T cells that do not display the immunophenotypic patterns traditionally attributed to CD4+ Treg. FOXP3 and CD25 surface expressions are considered the traditional hallmark for identifying CD4+ Treg in diseases other than HIV infection.3–6 However, FOXP3-negative and CD25-negative CD4+ Treg have also been described.44,45 The lack of CD127 expression on FOXP3-positive CD4+ T cells is used to distinguish between regulatory and effector T cells, although this is not an exclusive phenotypic marker.7,8 We hypothesize that the mechanisms leading to the induction of these TGF-β-positive CD4+ T cells share similar pathways with other regulatory CD4+ T cells. IL-10-producing HIV-specific CD4+ Treg suppress HIV-specific T cell function in vitro,46,47 and their detection is associated with progressive HIV disease.10,37,48,49 We found no significant IL-10 production by the HIV-specific TGF-β-positive CD4+ T cells in our study. We proposed that the concomitant presence of suppressor IL-10-positive CD8+ T cells and HIV-specific TGF-β-positive CD4+ T cells may promote additive suppression of HIV-specific T cell responses.
In summary, our study described an HIV-specific CD4+ T cell population that mediated suppression of HIV-specific T cell responses. These TGF-β-positive CD4+ T cells most likely confer regulatory properties, possibly with comparable suppressive activity to the classic Treg. The relative contribution of these TGF-β-positive CD4+ T cells to the general immune dysfunction observed in HIV infection remains to be determined. The pathways by which immune regulatory mechanisms modulate the adaptive antiviral immune response are complex. The ability to manipulate and regulate an effective antiviral immunity is clearly needed to decrease the number of infected cells and improve disease outcome.
Appendix
See Appendix Fig. 7.
FIG. 7.
CTLA-4 blockade increases IFN-γ expression by CMV-specific CD4+ T cells. PBMCs (n = 6) were stimulated with CMV PP65 peptides in the presence of anti-CTLA4 (or isotype control), then stained with anti-IFN-γ FITC, anti-TGF-β PE, anti-CD3 AmCyan, anti-CD4 PerCP CY5.5, and anti-CD8 PE CY7, and analyzed by flow cytometry. Samples were first gated on the CD3+/CD4+ lymphocyte population and the percent of IFN-γ-positive cells was determined. Results were expressed as percent of CMV-specific CD4+ T cells expressing IFN-γ after subtraction of the background values. The dashed line represents the cutoff for significant cytokine expression. Percentages in parentheses are median values. The two dots joined by a line represent the values obtained from the same individual and analysis was performed by paired t test.
Acknowledgments
This work was supported by NIH Grants AI43885, MM54907, and AI71772, and California Research Center for the Biology of HIV in Minorities grant, UC Davis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Groux H. Bigler M. de Vries JE, et al. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanchot C. Guillaume S. Delon J, et al. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity. 1998;8:581–590. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- 3.Asseman C. von Herrath M. About CD4pos CD25pos regulatory cells. Autoimmun Rev. 2002;1:190–197. doi: 10.1016/s1568-9972(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 4.Baecher-Allan C. Brown JA. Freeman GJ, et al. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 5.Fontenot JD. Gavin MA. Rudensky AY. Foxp3 programs the development and function of CD4+CD25+regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 6.Khattri R. Cox T. Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 7.Liu W. Putnam AL. Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddiki N. Santner-Nanan B. Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scotto L. Naiyer AJ. Galluzzo S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28− T suppressor cells. Hum Immunol. 2004;65:1297–1306. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Elrefaei M. Ventura FL. Baker CA, et al. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007;178:3265–3271. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 11.Cosmi L. Liotta F. Lazzeri E, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 12.Levings MK. Sangregorio R. Sartirana C, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 14.Greenwald RJ. Freeman GJ. Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 15.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 16.Andersson J. Boasso A. Nilsson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann DE. Kavanagh DG. Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4(+) T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 18.Leng Q. Bentwich Z. Magen E, et al. CTLA-4 upregulation during HIV infection: Association with anergy and possible target for therapeutic intervention. AIDS. 2002;16:519–529. doi: 10.1097/00002030-200203080-00002. [DOI] [PubMed] [Google Scholar]
- 19.Steiner K. Waase I. Rau T, et al. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin Exp Immunol. 1999;115:451–457. doi: 10.1046/j.1365-2249.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley JL. Schlienger K. Blair PJ, et al. Modulation of susceptibility to HIV-1 infection by the cytotoxic T lymphocyte antigen 4 costimulatory molecule. J Exp Med. 2000;191:1987–1997. doi: 10.1084/jem.191.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letterio JJ. Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 22.Valdez H. Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997:187–228. [PubMed] [Google Scholar]
- 23.Sousa AE. Chaves AF. Doroana M, et al. Kinetics of the changes of lymphocyte subsets defined by cytokine production at single cell level during highly active antiretroviral therapy for HIV-1 infection. J Immunol. 1999;162:3718–3726. [PubMed] [Google Scholar]
- 24.Garba ML. Pilcher CD. Bingham AL, et al. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J Immunol. 2002;168:2247–2254. doi: 10.4049/jimmunol.168.5.2247. [DOI] [PubMed] [Google Scholar]
- 25.Gorelik L. Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 26.Chen W. Jin W. Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HB. Paik DJ. Jang E, et al. Acquisition of anergic and suppressive activities in transforming growth factor-beta-costimulated CD4+CD25− T cells. Int Immunol. 2004;16:1203–1213. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- 28.Zheng SG. Gray JD. Ohtsuka K, et al. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 29.Sung JL. Lin JT. Gorham JD. CD28 co-stimulation regulates the effect of transforming growth factor-beta1 on the proliferation of naive CD4+ T cells. Int Immunopharmacol. 2003;3:233–245. doi: 10.1016/S1567-5769(02)00276-X. [DOI] [PubMed] [Google Scholar]
- 30.Sharma V. Knobloch TJ. Benjamin D. Differential expression of cytokine genes in HIV-1 tat transfected T and B cell lines. Biochem Biophys Res Commun. 1995;208:704–713. doi: 10.1006/bbrc.1995.1395. [DOI] [PubMed] [Google Scholar]
- 31.Reinhold D. Wrenger S. Kahne T, et al. HIV-1 Tat: Immunosuppression via TGF-beta1 induction. Immunol Today. 1999;20:384–385. doi: 10.1016/s0167-5699(99)01497-8. [DOI] [PubMed] [Google Scholar]
- 32.Wiercinska-Drapalo A. Flisiak R. Jaroszewicz J, et al. Increased plasma transforming growth factor-beta1 is associated with disease progression in HIV-1-infected patients. Viral Immunol. 2004;17:109–1013. doi: 10.1089/088282404322875502. [DOI] [PubMed] [Google Scholar]
- 33.Moss AR. Hahn JA. Perry S, et al. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: A prospective study. Clin Infect Dis. 2004;39:1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 34.McEvers K. Elrefaei M. Norris P, et al. Modified anthrax fusion proteins deliver HIV antigens through MHC Class I and II pathways. Vaccine. 2005;23:4128–4135. doi: 10.1016/j.vaccine.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Elrefaei M. Baker CA. Jones NG, et al. Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. J Immunol. 2008;180:7757–7763. doi: 10.4049/jimmunol.180.11.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perfetto SP. Chattopadhyay PK. Lamoreaux L, et al. Amine reactive dyes: An effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Elrefaei M. Barugahare B. Ssali F, et al. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J Immunol. 2006;176:1274–1280. doi: 10.4049/jimmunol.176.2.1274. [DOI] [PubMed] [Google Scholar]
- 38.Zanussi S. Simonelli C. D'Andrea M, et al. CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin Exp Immunol. 1996;105:220–224. doi: 10.1046/j.1365-2249.1996.d01-746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor A. Verhagen J. Blaser K, et al. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sansom DM. Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 41.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 42.Read S. Greenwald R. Izcue A, et al. Blockade of CTLA-4 on CD4 + CD25 + regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng SG. Wang JH. Stohl W, et al. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4 + CD25 + regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 44.Han Y. Guo Q. Zhang M, et al. CD69 + CD4 + CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 45.Li R. Perez N. Karumuthil-Melethil S, et al. Enhanced engagement of CTLA-4 induces antigen-specific CD4 + CD25 + Foxp3+ and CD4 + CD25− TGF-beta 1+ adaptive regulatory T cells. J Immunol. 2007;179:5191–5203. doi: 10.4049/jimmunol.179.8.5191. [DOI] [PubMed] [Google Scholar]
- 46.Kinter AL. Hennessey M. Bell A, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss L. Donkova-Petrini V. Caccavelli L, et al. Human immunodeficiency virus-driven expansion of CD4 + CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 48.Graziosi C. Pantaleo G. Gantt KR, et al. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 49.Ostrowski MA. Gu JX. Kovacs C, et al. Quantitative and qualitative assessment of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cell immunity to gag in HIV-1-infected individuals with differential disease progression: Reciprocal interferon-gamma and interleukin-10 responses. J Infect Dis. 2001;184:1268–1278. doi: 10.1086/324005. [DOI] [PubMed] [Google Scholar]
- 50.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]