Abstract
Aim
First, to compare the characterization of neurocognitive deficits in milder stages of HIV-associated neurocognitive disorder (HAND) derived from existing dementia rating scales of the American Academy of Neurology (AAN) and Memorial Sloan Kettering (MSK) with the 2007 consensus (‘Frascati’) classification. Second, to identify potential sociodemographic and clinical predictors of HAND progression during 1-year follow-up.
Methods
104 HIV-infected subjects in an existing cohort system were evaluated with a medical history, exam, neuropsychological test battery and functional assessments. The degree of HAND was rated using the AAN, MSK and Frascati scales. The degree of concordance among these scales was determined. In addition, 45 subjects were reassessed for changes in their neurocognitive status at 1-year follow-up. Associations between age, education, sex, depression ratings, substance abuse, race, hepatitis C serostatus, CD4 count and progression of HAND were examined.
Results
There was excellent concordance (γ > 0.8) among the Frascati, MSK and AAN ratings. Subjects rated as having minor cognitive motor disorder on the AAN scale (n = 45) were evenly split between Frascati rating of asymptomatic neurocognitive impairment (n = 24) and mild neurocognitive disorder (n = 21). At 1-year follow-up of 45 subjects, 31% had worsened, 13% had improved and 56% were stable. Predictors of progression included age older than 50 years (odds ratio: 5.57; p = 0.013) and female gender (odds ratio: 3.13; p = 0.036).
Conclusion
The Frascati HAND rating scale has excellent concordance with previous neurocognitive rating scales and can be used to better characterize milder stages of cognitive impairment. Older individuals and women appeared to be more likely to show neurocognitive progression.
Keywords: dementia rating scales, Frascati, HIV, HIV-associated neurocognitive disorders, progression
One of the earlier scales used to diagnose HIV-associated neurocognitive disorders (HAND) was the Memorial Sloan Kettering (MSK) scale, which contains gradations that range from minor cognitive disturbance to profound and incapacitating disorders [1]. However, the scales integrate neurological deficits related to myelopathy, focusing on ambulatory function. While this scale has been useful in many contexts it does not adequately separate the cognitive and behavioral impairments originating from brain disease specifically from myelopathic impairments.
In 1991 the American Academy of Neurology (AAN) detailed criteria for defining different levels of HAND in research studies and clinical practice [2]. The 1991 criteria described two levels of cognitive impairment: minor cognitive motor disorder (MCMD) and the more severe HIV-associated dementia (HAD). MCMD was defined to encompass less profound forms of cognitive motor and other behavioral dysfunction.
In 2007, a revised classification was proposed by a working group assembled in Frascati, Italy, which aimed to categorize the presentation of neurocognitive disturbances in the post-HAART era, which is characterized by persistence of primarily milder forms of neurocognitive disturbance. The ‘Frascati’ criteria emphasizes that the essential feature of HAND is cognitive disturbance; this revision eliminated the possibility of HIV neurocognitive disorders being diagnosed on the basis of neuromotor and noncognitive psychiatric changes such as changes in personality or mood. More precise criteria for three syndromes within the framework of HAND were established: asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and HAD. Specifically, the introduction of the terms ANI and MND were proposed to better characterize the neurocognitive deficits seen within milder stages of HAND [3].
Many individuals with HAND remain neuro logically stable after starting HAART, or may show partial reversal of neurological deficits. However, there is a subset of individuals whose neurocognitive function continues to decline despite antiretroviral treatment [4–6]. Both demographic and medical factors may play a role in this progression, which, if recognized, might trigger different strategies of antiretroviral or adjunctive therapies.
Previous studies have mostly looked for predictive factors associated with a risk of developing HAD rather than progression through various hierarchical levels of neurocognitive dysfunction [7–9].
The current study was designed to examine the concordance between the Frascati scale and two other scales; the MSK scale and the AAN scale. The Frascati scale has more precise deline- delineation of neurocognitive impairment in milder stages of this disease and may thus be more sensitive to neurological progression in HAART-experienced HIV-infected individuals. We thus evaluated the predictive influence of baseline sociodemographic and clinical characteristics on progression of HAND using this newer scale.
Methods
Participants
The study included 104 HIV-infected individuals at the General Clinical Research Clinic at Johns Hopkins Hospital in Baltimore, MD, USA, who were evaluated from 2007 to 2009. The study was approved by the Johns Hopkins Institutional Review Board. HIV-infected patients were recruited from two cohorts; the Northeast AIDS Dementia (NEAD) cohort [10] and the Oxidative Stress Cohort [11], and were chosen using the following inclusion criteria: adults older than 18 years of age, HIV-1-seropositive status, ability to provide written informed consent, and ability to ambulate at first clinic visit. Exclusion criteria were the following: history or current opportunistic CNS infection, history of or current schizophrenia, current severe affective disorder believed to explain subject’s cognitive impairment, and history of chronic neurological disorders such as epilepsy or multiple sclerosis. Substance abusers and heavy alcohol users, remote or active, were not excluded but were not examined if judged by the examiner to be intoxicated during the visit
Written informed consent was obtained from all patients participating in the study.
Questionnaires
Every 6 to 12 months subjects underwent the following: standardized questionnaires were used to capture medical information and demographics, along with medical, psychiatric and neurological history. A detailed substance abuse questionnaire was also self-administered. Subjects were asked to report their current amount of alcohol intake and whether they used cocaine and/or heroin in the past 6 months.
Clinical exam
Subjects underwent a clinical examination consisting of a macroneurologic examination created for the AIDS Clinical Trials Group (ACTG) and the motor subscale (part III) of the Unified Parkinson’s Dementia Rating Scale (UPDRS) [12], to assess extrapyramidal signs associated with HAD.
Depression assessment
Mood was assessed with the self-administered Beck Depression Inventory (BDI). A score greater than or equal to 16 was defined as the presence of depression symptomatology.
Laboratory assessment
CD4+ lymphocyte count and levels of HIV RNA in cerebrospinal fluid (CSF) and plasma were obtained. HIV RNA levels were determined using the Roche PCR assay (Amplicor HIV-1 Monitor assay, version 1.5; Roche Diagnostics, Basel, Switzerland). Hepatitis C viral status was obtained via history and evaluation of laboratory findings for HCV antibodies.
Diagnosis of HAND
Dementia assessment was performed according to the original AAN classification and MSK staging classification using previously published methodology for neurocognitive testing and definition of functional impairment [13]. Neuropsychological domains tested were: attention and working memory (using Computerized Reaction Time Test), verbal memory (using Rey Auditory Verbal Learning Test), visual memory and visuo-construction (using Rey Complex Figure Test), psychomotor and motor speed (using Symbol Digit Modalities Test, Trailmaking Test A & B, Grooved Pegboard Test and Timed Gait), frontal/executive functioning (using Verbal Fluency Test and Odd Man Out) and literacy (using National Adult Reading Test). Functional assessment was performed using the Karnofsky Performance Scale, Instrumental Activities of Daily Living (IADL) assessment and subjective functional complaints. In addition, new outcomes developed in the 2007 Frascati consensus classification were also assigned by the same study team [3].
Data analyses
The primary outcomes for this study were neurocognitive status using the previously mentioned three classifications at baseline visit. Concordance between MSK and both AAN rating and Frascati rating scales were tested using the Goodman–Kruskal γ statistic [14]. The Goodman–Kruskal γ statistic is defined as the difference between the probability of getting a concordant pair and that of getting a nonconcordant pair, conditional on all such concordant and nonconcordant pairs. The Goodman–Kruskal γ statistic ranges between +1 and −1. If the two rating scales are independent of one another, γ would equal 0. A positive γ statistic means that we are more likely to obtain concordant pairs than discordant ones, Similarly, a negative γ statistic implies that subjects with larger scores on one scale (e.g., Frascati) are more likely to be associated with smaller scores on the other scale (e.g., MSK).
A subset of 45 HIV-infected individuals was assessed at 1 year following baseline. Change in the Frascati rating was used to document progression of HAND during this time period. To analyze variables as predictors of change in cognitive status, associations between the independent sociodemographic characteristics measured at baseline (age ≥ 50 years, race, college education, gender, clinical depression per BDI, substance abuse history, hepatitis C status and CD4 count less than 200 cells/mm3) and change in cognitive status (improved, stable or worse) were examined using a polytomous logistic regression model [15]. This model was fit using SAS PROC CATMOD.
Results
Study demographics
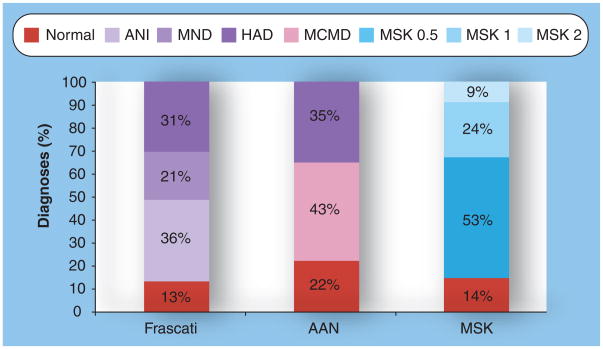
In Table 1, there is a summary of the demographics of the total 104 HIV-infected patients evaluated in this study and then subdivided by Frascati rating of cognitive status. The mean age was 46.9 years (standard deviation [SD] = 6.4). A majority of the patients (72%) were men. Mean CD4+ T-cell count was 354 cells/mm3 (SD = 206.5). Depression based on a BDI score greater than or equal to 16 was found in 22.1% of HIV-infected patients in this study. A majority of patients (58.7%) were seropositive for HCV. No significant differences between the dementia rating subgroups were seen in these demographics. Figure 1 illustrates the distribution of neurocognitive diagnoses according to the Frascati, AAN and MSK rating scales.
Table 1.
Demographics for the total clinical outcomes cohort and Frascati ratings.
| Total | Frascati ratings | ||||
|---|---|---|---|---|---|
| N | ANI | MND | HAD | ||
| n (%) | 104 (100) | 13 (12.5) | 37 (35.6) | 22 (21.2) | 32 (30.8) |
| Mean age (years) | 46.9 | 46.2 | 45.9 | 46.8 | 48.4 |
| Mean education (years) | 12.8 | 12.5 | 13.0 | 12.7 | 12.8 |
| Male sex (n; %) | 75 (72.1) | 11 (10.6) | 27 (26.0) | 12 (11.5) | 25 (24.0) |
| Depression (BDI ≥ 16) (n; %) | 23 (22.1) | 4 (3.9) | 8 (7.7) | 4 (3.9) | 7 (6.7) |
| HCV infected (n; %) | 61 (58.7) | 7 (6.7) | 21 (20.2) | 13 (12.5) | 20 (19.2) |
| Mean CD4 count/mm3 (mean) | 354 | 321 | 353 | 418 | 325 |
| Mean HIV RNA in plasma (copies/ml) | 2.45 | 2.34 | 2.50 | 2.22 | 2.59 |
| Mean HIV RNA in CSF (copies/ml) | 2.07 | 1.94 | 2.06 | 1.91 | 2.27 |
ANI: Asymptomatic neurocognitive impairment; BDI: Beck Depression Index; CSF: Cerebrospinal fluid; HAD: HIV-associated dementia; MND: Mild neurocognitive disorder; N: Normal.
Figure 1. Distribution of neurocognitive diagnoses according to Frascati, asymptomatic neurocognitive impairment and Memorial Sloan Kettering ratings.
MSK ratings are defined as follows: MSK 0.5: equivocal/subclinical cognitive impairment; MSK 1: mild dementia; MSK 2: moderate dementia. AAN: American Academy of Neurology; ANI: Asymptomatic neurocognitive impairment; HAD: HIV-associated dementia; MCMD: Minor cognitive motor disorder; MND: Mild neurocognitive disorder; MSK: Memorial Sloan Kettering.
Concordance between rating scales
The concordance between the Frascati and MSK scales was excellent (Goodman–Kruskal γ = 0.969) (Table 2). However, there were some cases where there was differential rating. For instance, 32 out of the 55 cases of MSK 0.5 defined as subclinical/equivocal dementia were rated as Frascati ANI.
Table 2.
Concordance between Frascati and Memorial Sloan Kettering rating scale†.
| MSK rating | Total | |||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | |||
| Frascati rating | Normal | 12 | 1 | 0 | 0 | 13 |
| ANI | 3 | 32 | 2 | 0 | 37 | |
| MND | 0 | 22 | 0 | 0 | 22 | |
| HAD | 0 | 0 | 23 | 9 | 32 | |
| Total | 15 | 55 | 25 | 9 | 104 | |
Goodman–Kruskal γ = 0.969.
ANI: Asymptomatic neurocognitive impairment; HAD: HIV-associated dementia; MND: Mild neurocognitive disorder; MSK: Memorial Sloan Kettering.
Comparing the Frascati rating and AAN rating scales, we again found that there was excellent concordance (Goodman–Kruskal γ = 0.960) (Table 3). Those cases that were rated as MCMD on the AAN scale (n = 45) were split approximately evenly between ANI and MND on the Frascati scale. Ten individuals who were considered normal under the AAN rating scale fell within the ANI category in the new Frascati rating.
Table 3.
Concordance between Frascati and American Academy of Neurology rating scale†.
| AAN rating | Total | ||||
|---|---|---|---|---|---|
| Normal | MCMD | HAD | |||
| Frascati rating | Normal | 13 | 0 | 0 | 13 |
| ANI | 10 | 24 | 3 | 37 | |
| MND | 0 | 21 | 1 | 22 | |
| HAD | 0 | 0 | 32 | 32 | |
| Total | 23 | 45 | 36 | 104 | |
Goodman–Kruskal γ = 0.960.
AAN: American Academy of Neurology; ANI: Asymptomatic neurocognitive impairment; HAD: HIV-associated dementia; MND: Mild neurocognitive disorder; MCMD: Minor cognitive motor disorder.
Predictors of HAND progression
Of the original 104 enrolled, 45 patients had a follow-up examination within 1 year. Others were seen at different time intervals so we excluded them from this ana lysis to ensure we had consistency with regard to the time elapsed from baseline to determine progression of HAND. Of the 45 patients, 32 (71%) were men, which was not significantly different from the total cohort. Of the 45 patients, 14 (31%) showed declines in neurocognitive function, 25 (56%) were stable and six (13%) showed improved function at 1-year follow-up relative to their baseline neuro cognitive status. Based on polytomous logistic regression, individuals with an age greater than or equal to 50 years were more likely to demonstrate neurocognitive decline compared with those with an age younger than 50 years (odds ratio [OR]: 5.57; 95% CI: 1.44–21.53; p = 0.013), after adjusting for college education, gender, race, depression symptoms, substance abuse, hepatitis C status and CD4+ cell count less than 200 cells/mm3. In the same model, women were more likely to demonstrate worsening of neurocognitive status compared with men (OR: 3.13; 95% CI: 1.08–9.09; p = 0.036) (Table 4). Owing to this finding, we stratified the demographic and clinical data by gender. In this subset of 45 follow-up visits, of the 15 people aged 50 years or greater, four (27%) were women. Of the 30 people aged under 50 years, nine (30%) were women. There was no difference in distribution of gender between these two age groups (p = 0.816). Women, however, had significantly fewer years of education (11.83 vs 13.15 years; p = 0.006) and higher CD4+ cell counts (461.17 vs 312.27 cells/mm3; p < 001) compared with men.
Table 4.
Decline or improvement of neurocognitive status as a function of sociodemographic and clinical characteristics.
| Progression | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Decline | |||
| Age ≥ 50 years | 5.57 | 1.44–21.53 | 0.013 |
| College education | 2.04 | 0.57–7.27 | 0.274 |
| Women | 3.13 | 1.08–9.09 | 0.036 |
| Depression | 0.45 | 0.12–1.66 | 0.228 |
| Hepatitis C coinfection | 0.37 | 0.11–1.22 | 0.104 |
| CD4 < 200 cells/mm3 | 0.95 | 0.33–2.71 | 0.922 |
| Improve | |||
| Age ≥ 50 years | 0.61 | 0.16–2.22 | 0.449 |
| College education | 1.37 | 0.38–4.93 | 0.633 |
| Women | 0.61 | 0.18–2.11 | 0.437 |
| Hepatitis C coinfection | 1.64 | 0.55–4.87 | 0.374 |
| Depression | NA | NA | NA |
| CD4 < 200 cells/mm3 | 0.88 | 0.22–3.45 | 0.855 |
Discussion
The AIDS Task Force of the AAN published nomenclature and research case definitions to guide the diagnosis of neurologic manifestations of HIV-1 infection in 1991 [2]. The different levels of HAND they described subsequently became known as the ‘AAN rating scale’.
After the introduction of HAART in 1996, the incidence of HAD declined along with most other HIV-associated comorbidities [16]. In a study by one of the authors, the incidence of moderate or severe dementia fell from approximately 7% in 1989 to only 1% in 2000 [16]. HAART was found to improve cognitive performance in some patients [8], and patients could survive for years with HAND. In 2000, approximately 5–10% of HIV-infected individuals with advanced infection had HAD and approximately 37% had less severe forms of HAND [16]. The prevalence of HAD in other cohorts from more recent years is even lower, for example in the large National Institute of Mental Health (NIMH)-funded cohort CNS HIV Antiretroviral Therapy Effects Research (CHARTER) it is 2% [Heaton R, Manuscript Under Review]. However, despite the remarkable effect of HAART on HAND incidence rates, the prevalence of HAND continues to be very high at between 15 and 50%, depending on the cohort [Heaton R, Pers. Comm.], and in several cohorts appears almost ‘resistant’ to the impact of HAART. For example, in CHARTER, 53% of the total sample had neurocognitive impairment, with increasing rates in those groups with greater comorbid illnesses. Prevalence estimates were 33% for ANI, 12% for MND and 2% for HAD [Heaton R, Pers. Comm.]. In those with incidental comorbidities, a history of advanced HIV disease (AIDS status and low nadir CD4) was a stronger predictor of impairment than current disease status indicators (current CD4, plasma and CSF viral loads).
Thus, neurocognitive impairment still presents a significant problem for many HIV-infected patients, and yet clinical guidelines appear to ignore this issue. For example, the latest Department of Health and Human Services guidelines recommend initiation of HAART at any level of CD4 count for individuals with HIV-associated nephropathy, but essentially ignore HAND [101].
After the advent of HAART, the NIMH and the National Institute of Neurological Diseases and Stroke identified aspects that required updating. A limitation of the AAN criteria was that they did not recognize a subgroup of HIV-infected patients (<15%) who actually have mild neuro cognitive impairment despite the absence of overt functional decline. They therefore suggested inclusion of the term ANI to categorize individuals with subclinical impairment. This is characterized by measureable neurocognitive impairment that is not recognized by the infected individual (or clinician), or fails to impact upon function. In some sense, this can be considered a ‘presymptomatic’ form of HAND, analogous to the presymptomatic phases of Alzheimer’s disease or Huntington’s disease. Despite the absence of symptoms, individuals with ANI may be more likely to go on to develop the more severe forms of HAND. These new criteria came to be known as the Frascati rating scale and an algorithm was proposed to assist in standardized diagnostic classification of HAND by this scale.
According to the AAN criteria, MCMD required a history of impaired cognitive/behavioral function in two areas (e.g., impaired attention and concentration, mental slowing, abnormal memory or other cognitive dysfunctions, slowed movements, incoordination, personality change, irritability and emotional lability), and these abnormalities should be associated with mild impairment in work or activities of daily living (ADLs). Those patients who had impairments in cognitive/behavioral function but no impairments in work or ADLs were classified as normal. In Frascati, the categories ANI and MND were developed specifi- cally to address this differentiation, namely the presence or absence of impairment in work or ADLs among patients with mild abnormalities on neuropsychological testing.
In this study, we compared a cohort of patients assigned HAND ratings using the older AAN and MSK rating scale with those of the newer Frascati rating scale. We found that concordance is excellent between the older and new rating scales and that 45 MCMD patients were split almost evenly between ANI (n = 24) and MND (n = 21). In addition, ten patients that were considered normal under the older AAN rating scale ‘shifted’ into ANI in the new Frascati rating, highlighting how this older scale may fail to detect early signs of neurocognitive impairment and thereby under-represent the true prevalence of dysfunction.
When comparing MSK and Frascati rating scales, we again noted excellent overall concordance. Of note, MND cases were all classified as MSK 0.5. A majority of MSK 0.5 cases fell into Frascati ANI rating, probably due to lack of any functional impairment or insight regarding their impairments.
These discrepancies point to a possible limitation of the new Frascati rating scale, in that it relies on the individual’s subjective assessment of their functional status. Under-reporting of functional deficits can thereby occur when there is poor insight, and over-reporting can occur if the patient is depressed. Overall, however, our comparisons show that the Frascati ranking scale performs well and specifically addresses the limitations of the existing HAND rating scales by identifying and more precisely classifying individuals with the milder stages of HAND. A limitation of our study was that our raters were not blinded to the ratings of AAN and MSK when determining the Frascati rank.
Although many individuals with HAND remain neurologically stable for years after starting HAART, there are certain individuals whose cognitive decline continues. In a study that used MRI assessment, HIV-infected patients on ART still demonstrated greater rates of white matter volume loss than HIV-negative control individuals regardless of viral load [4,5]. Understanding the etiology for this continued decline is complicated as several factors may be at play. Both demographic and medical factors such as age, educational status, coinfection with hepatitis C, as well as severity of HIV infection, may all play a role in the progression of the disorder.
In the second part of the ana lysis, we explored variables associated with progression of HAND. Most prior studies have focused on predictors of HAD rather than progression through the full spectrum of HAND, especially those performed prior to the widespread use of HAART. Older studies prior to HAART have found HIV-related medical symptoms [8], lower hemoglobin levels [8], higher plasma viral load [9], lower CD4+ cell counts [17] and intravenous drug use [18] to be associated with a greater risk of developing dementia. Other pre-HAART studies have found CSF HIV RNA levels associated with HIV encephalitis [19], cognitive impairment [20] and severity of dementia [21–23]. More recent post-HAART studies, which also focused on predictors of HAD, have found conflicting results when looking at blood and CSF markers of immune activation, HIV RNA levels and CD4 counts [24,25].
In the second part of the analysis, when exploring variables associated with progression of HAND, we found that individuals who were older than 50 years of age and women were more likely to have neurocognitive decline. No other predictors for the progression of HAND were found, including self-reported substance abuse. In fact, in this population, men were more likely to abuse alcohol compared with women (p = 0.047) and there was no significant difference between genders and cocaine and heroin use. Older age has been previously found to increase the risk of developing HAD [26–29]. In the pre-HAART era, two studies reported that women have more rapid progression of neurological signs and symptoms [8,30]. In the post-HAART era, female gender as a predictor for progression of HAND has not been previously reported. This may be in part owing to a finding that women with HAND have decreased function and activity of antioxidant enzymes in CSF and monocytes, thus possibly resulting in more oxidative stress in the CNS and thereby promoting peroxynitrite formation and neuronal damage [31,32]. On further analysis of this subset, we did find that women had significantly fewer years of education compared with men, which may explain their worsening HAND status. Lower education is associated with more variability on repeat neurocognitive assessments, and it is possible that the change in HAND status reflects random test–retest variability rather than a true biological change. Less education may also result in less cognitive reserve, which is the efficient utilization of brain networks or of enhanced ability to recruit alternate brain networks to cope with or compensate for pathology [33]. Therefore, although this finding is potentially interesting, it needs to be validated by larger studies. Of note, most of our subjects were taking HAART and their clinical disease was well controlled; as judged by plasma HIV RNA control, approximately 57% were undetectable. On HAART, HAND is known to be usually stable and the majority of individuals with HAND do not progress on HAART but instead show frequent bidirectional transitions. We had 14 subjects (31%) who progressed in their HAND ranking using the Frascati scale in the 12-month period. However, if we had used the older AAN scale for our assessment, three of these 14 would have been considered stable as progression was from ANI to MND. Thus, the Frascati scale may be more sensitive to neurological progression due to the more precise delineation in this milder stage of this disease.
This study shows that the Frascati dementia rating scale has good concordance with the MSK and AAN rating scale and can be used to more precisely describe the disorder, particularly in milder stages of cognitive impairment. Progression of HAND in this study was more likely in subjects older than 50 years and women, and the proportion showing progression was surprisingly high, even though the overall study numbers were small. In the post-HAART era of HIV, progression of HAND is thought to be less likely but larger studies are needed to evaluate those factors that might allow for the more accurate prediction of change in neurocognitive status ratings and thus validate our findings.
Executive summary.
Introduction
Original scales used to diagnose HIV-associated neurocognitive disorders (HAND) were the Memorial Sloan Kettering (MSK) scale and the American Academy of Neurology (AAN) scale.
In 2007, revised classification was proposed in Frascati, Italy, to represent the presentation of neurocognitive disturbances in the post-HAART era, characterized by milder forms of neurocognitive disturbance.
More precise criteria for three syndromes within the framework of HAND was established: asymptomatic neurocognitive impairment (ANI); mild neurocognitive disorder (MND); and HIV-associated dementia (HAD).
Although most individuals with HAND remain neurologically stable after starting HAART, there is a subset of individuals whose neurocognitive function continues to decline.
The current study was designed to examine the concordance between the Frascati scale and the two older scales and to evaluate the predictive influence of baseline sociodemographic and clinical characteristics on progression of HAND using this newer scale.
Methods
A total of104 HIV-infected individuals every 6–12 months completed the following: standardized questionnaires to capture demographics and medical, psychiatric and neurological history; clinical examination; depression assessment; and laboratory assessment.
Diagnosis of HAND made using the AAN, MSK and Frascati classification.
Neuropsychological testing and functional assessment performed at each visit.
Concordance between MSK rating scales carried out using the Goodman-Kruskal γ statistic.
A subset of 45 HIV-infected individuals assessed at 1 year following baseline for change in Frascati rating.
Associations between sociodemographic characteristics measured at baseline (age ≥ 50 years, race, college education, gender, clinical depression per Beck Depression Inventory, substance abuse history, hepatitis C status and CD4+ count less than 200 cells/mm3) and change in cognitive status (improved, stable or worse) examined using logistic regression.
Results
Concordance between the Frascati and the MSK and AAN scales was excellent (Goodman-Kruskal γ > 0.8)
Cases that were rated as minor cognitive motor disorder on the AAN scale were split between ANI and MND on the Frascati scale.
Ten individuals considered normal under the AAN rating scale fell within the ANI category in the Frascati scale.
Of the 45 patients followed-up after 1 year, 31% showed declines in neurocognitive function, 56% were stable and 13% showed improvement.
Individuals older than 50 years and women were more likely to demonstrate neurocognitive decline.
Women, however, had significantly fewer years of education compared with men.
Discussion
After the introduction of HAART in 1996, the incidence of HAD has declined.
The prevalence of HAD is estimated at 2%; the prevalence of less severe forms of HAND is high, between 15 and 50%.
The inclusion of the term asymptomatic neurocognitive impairment to categorize individuals with subclinical impairment and mild neurocognitive disorder was added to new criteria known as the Frascati rating scale.
The categories ANI and MND were developed specifically to address the presence or absence of impairment in work or activities of daily living among patients with mild abnormalities on neuropsychological testing.
Prevalence estimates are 33% for ANI and 12% for MND.
Concordance is excellent between the older and new rating scales.
The category ANI helps detect early signs of neurocognitive impairment that were missed with the older rating scales.
The Frascati scale addresses limitations of the older scales by identifying and more precisely classifying individuals with milder stages of HAND.
Although many individuals with HAND remain neurologically stable for years after starting HAART, in certain individuals cognitive decline continues.
HIV-infected patients on ART still demonstrated greater rates of white matter volume loss.
In this study, variables associated with progression of HAND were age greater than 50 years and female gender.
No other predictors for the progression of HAND were found, including self-reported substance abuse.
In the post-HAART era, female gender as a predictor for progression of HAND has not been previously reported.
However, women had significantly fewer years of education, which may explain their worsening HAND status.
We had 31% who progressed in their HAND ranking using the Frascati scale. Using the older AAN scale, three out of 14 would have been considered stable as progression was from ANI to MND, resulting in only 24% progression.
Thus, the Frascati scale is more sensitive to neurological progression due to the more precise delineation in this milder stage of disease.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investi gations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors were supported by the Johns Hopkins NIMH Center for Novel Therapeutics in HIV Associated Cognitive Disorder (MH 075673), (MH71150) and (NS 049465). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Price RW, Brew B. The AIDS dementia complex. J Infect Dis. 1988;158:1079–1083. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- 2▪.Janssen RS, Cornblath DR, Epstein LG. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. Describes in detail the American Academy of Neurology (AAN) rating scale. [DOI] [PubMed] [Google Scholar]
- 3▪▪.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. Describes in detail the Frascati rating scale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas V, Meyerhoff D, Studholme C, et al. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009;15(4):324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gongvatana A, Schweinsburg BC, Taylor MJ, et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 7.Childs EA, Lyles RH, Selnes OA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 8.Stern Y, McDermott MP, Albert S, et al. Factors associated with incident human immunodeficiency virus-dementia. Arch Neurol. 2001;58(3):473–479. doi: 10.1001/archneur.58.3.473. [DOI] [PubMed] [Google Scholar]
- 9.McArthur JC, Hoover DR, Bacellar H, et al. for the Multicenter AIDS Cohort Study. Dementia in AIDS patients: incidence and risk factors. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 10.McArthur JC, McDermott MP, McClernon D, et al. Attenuated CNS infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61(11):1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- 11.Sacktor N, Skolasky R, Moxley M, et al. Magnetic resonance spectroscopy abnormalities and markers of oxidative stress for individuals with HIV-associated neurocognitive disorders. Neurology. 2008;70:A4. (Abstract) [Google Scholar]
- 12.Fahn S, Marsden C, Caine D. Recent developments in Parkinson’s disease. Macmillan Healthcare Information; Florham Park, NJ, USA: 1987. [Google Scholar]
- 13.Butters N, Grant I, Haxby J, et al. Assessment of AIDS-related cognitive changes: recommendations of the NIMH Workshop on Neuropsychological Assessment Approaches. J Clin Exp Neuropsychol. 1990;12:963–978. doi: 10.1080/01688639008401035. [DOI] [PubMed] [Google Scholar]
- 14.Goodman LA, Kruskal WH. Measures of association for cross classifications. J American Statistical Association. 1954;49:732–764. [Google Scholar]
- 15.Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley; NY, USA: 1989. [Google Scholar]
- 16.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 17.Bouwman FH, Skolasky RL, Hes D, et al. Variable progression of HIV-associated dementia. Neurology. 1998;50:1814–1820. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- 18.Chiesi A, Vella S, Dally LG, et al. for AIDS in Europe Study Group. Epidemiology of AIDS dementia complex in Europe. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Cinque P, Vago L, Ceresa D, et al. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 21.Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175:963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 22.McArthur JC, McClernon DR, Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 23.Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- 24.De Luca A, Ciancio BC, Larussa D, et al. Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology. 2002;59:342–347. doi: 10.1212/wnl.59.3.342. [DOI] [PubMed] [Google Scholar]
- 25▪▪.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–11. doi: 10.1016/j.jneuroim.2004.08.042. Review of HIV dementia in the pre-HAART and post-HAART eras. [DOI] [PubMed] [Google Scholar]
- 26.Valcour V, Shiramizu B, Shikuma C, et al. Neurocognitive function among older compared with younger HIV-1 seropositive individuals. J Neurovirol. 2002;8:69. [Google Scholar]
- 27.Jevtovic DJ, Vanovac V, Veselinovic M, Salemovic D, Ranin J, Stefanova E. The incidence of and risk factors for HIV-associated cognitive-motor complex among patients on HAART. Biomed Pharmacother. 2009;63(8):561–565. doi: 10.1016/j.biopha.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004;18(Suppl 1):S79–S86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol. 2008;14:362–367. doi: 10.1080/13550280802216494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Marder K, Stern Y, et al. Gender differences in HIV-related neurological progression in a cohort of injecting drug users followed for 3.5 years. J NeuroAIDS. 1996;1:17–29. doi: 10.1300/j128v01n04_03. [DOI] [PubMed] [Google Scholar]
- 31▪.Velázquez I, Plaud M, Wojna V, Skolasky R, Laspiur JP, Meléndez LM. Antioxidant enzyme dysfunction in monocytes and CSF of Hispanic women with HIV-associated cognitive impairment. Neuroimmunology. 2009;206(1–2):106–111. doi: 10.1016/j.jneuroim.2008.10.013. Theories to explain female gender as a predictor of HIV-associated neurocognitive disorders (HAND) progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Mollace V, Nottet HS, Clayette P, et al. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. Theories to explain female gender as a predictor of HAND progression. [DOI] [PubMed] [Google Scholar]
- 33.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
Website
- 101.Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, December 1, 1–161 (2009). www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.