Abstract
Recombinant Silk-Elastinlike Protein polymers (SELPs) are well-known for their highly tunable properties on both the molecular and macroscopic hydrogel level. One specific structure of these polymers, SELP-815K, has been investigated as an injectable controlled delivery system for the treatment of head and neck cancer via a gene-directed enzyme prodrug therapy (GDEPT) approach. Due to its pore size and gelation properties in vivo, SELP restricts the distribution and controls the release of therapeutic viruses for up to one month. It has been shown that SELP-mediated delivery significantly improves therapeutic outcome of the herpes simplex virus thymidine kinase (HSVtk)/ganciclovir (GCV) system in xenograft models of human head and neck cancer. However little is known about potential benefits of this approach with regard to toxicity in the presence of a fully intact immune system. The studies presented here were designed to assess the change in toxicity of the SELP mediated viral delivery compared to free viral injection in a non-tumor bearing immune competent mouse model. Toxicity was assessed at 1, 2, 4, and 12 weeks via body weight monitoring, complete blood count (CBC), and blood chemistry. It was found that in the acute and subacute phases (weeks 1-4) there is significant toxicity in groups combining the virus and the prodrug, and matrix-mediated gene delivery with SELP demonstrates a reduction in toxicity from the 2 week time point through the 4 week time point. At the end of the subchronic phase (12 weeks), signs of toxicity had subsided in both groups. Based on these results, recombinant SELPs offer a significant reduction in toxicity of virus-mediated GDEPT treatment compared to free virus injection in the acute and subacute phases.
Keywords: Viral gene delivery, Recombinant polymers, Silk elastinlike protein polymers, Hydrogels
Introduction
In spite of its superior transfection efficiency, the application of adenoviruses as gene delivery vectors has been plagued with problems of toxicity which have delayed their advancement to clinical use. It is very likely that if the safety of viral vectors can be improved then they will become the preferred gene delivery method. Non-viral vectors have been unable to compete with viral gene carriers in terms of their ability to deliver large quantities of genetic material in an efficient and reliable manner. Improving the safety of viral vectors is a difficult proposition however, as the main toxicity concern is the ability of adenoviruses to trigger a strong systemic immune response, and high levels of transfection in the liver whether this is desirable to treatment or not1. Due to the immune system’s essential role in protecting the body, co-administration of viruses and immunosuppressants would leave the patient vulnerable to infection for the duration of treatment. Hence, evasion of the immune system is the best option, e.g. the viruses must be disguised or carefully administered in order to be a clinically viable and useful treatment.
One way of achieving this goal would be to surface-modify or package the viruses such that they do not activate the immune system, which would require surface antigen molecules to be modified or covered to avoid detection. This approach has previously been attempted by conjugation of poly [N-(2-hydroxypropyl) methacrylamide] (HPMA)2 or polyethylene glycol (PEG)3, 4 for disguising of adenovirus from detection and neutralization by host immune system. These approaches do accomplish the goal of reducing neutralization of the viral particles by various mechanisms, and in the case of HPMA actually affect an increase in uptake by cells2. However reduced immunogenicity comes at a cost of reduced efficacy of gene transduction. PEG showed similar results in vitro, almost completely eliminating transfection of cells in a coxsackie and adenovirus receptor (CAR)-dependent manner 4. It is important to note that some organ-specific gene transduction is unaffected or even aided by surface functionalization of the viruses3, 4. However this will not be appropriate for most applications of gene therapy as it is a result of interactions with the surface hexon proteins with specific blood components (primarily clotting factors) leading to increased uptake by the liver5, 6. In terms of CAR-dependent transfection, surface functionalization thus far appears to completely ablate transfection.
An approach which may also have significant potential for increasing the safety of adenoviral gene delivery while not sacrificing efficacy is spatial and temporal delivery of viruses following injection into a localized area. Applications for this type of treatment are primarily solid tumor growths, as these present a well-defined target and can easily be modeled in vivo. Direct intratumoral injection of virus without a controlled delivery system has been shown to still result in significant systemic dissemination of viral particles7, which clearly is undesirable both in terms of viral toxicity and off-target transgene expression. In addition to these toxicological concerns, the rapid dissemination of viruses from the injection site does not afford the virus the opportunity to transfect a significant number of tumor cells, reducing the efficacy of therapy. Administration of the viruses in a matrix which will aid in controlling their release results in a more favorable outcome both in terms of efficacy and biodistribution 7-9. This approach has been shown to localize, prolong, and increase overall gene expression levels at the site of interest 8, 9.
One class of materials used for such applications is the family of silk-elastinlike protein polymers (SELPs)9-11. SELPs are recombinant polymers comprised of tandem repeats of a six amino acid sequence commonly found in silkworm silk fibroin (GAGAGS)12 and a five amino acid sequence commonly found in mammalian elastin (GVGVP)13, 14. This combination of silk and elastin molecular properties results in a polymer which is responsive to temperature increases and irreversibly forms hydrogels at 37°C, via the spontaneous β-sheet formation by the silk units, effectively producing a crosslinked polymer network15. These hydrogels display swelling and in vitro release properties for bioactive agents in a structure-dependent manner15-25. SELP hydrogels have been investigated in vivo as controlled release materials for viral gene delivery, and have distinct advantages compared with direct intratumoral injection in terms of localization of gene expression, efficacy of anticancer treatment (using virus-mediated gene directed enzyme prodrug therapy, or GDEPT), and prolonging intratumoral gene expression9, 11. SELP hydrogels have also been shown to provoke minimal inflammatory response following intradermal and subcutaneous injection up to one month, suggesting that the material itself is unlikely to be harmful25.
The specific enzyme-prodrug system under investigation is the herpes simplex virus thymidine kinase (HSVtk)-ganciclovir (GCV) system, in which expression of HSVtk allows cells to phosphorylate GCV into a highly toxic triphosphate which competitively inhibits incorporation of dGTP into the host cell’s DNA, causing DNA damage, incomplete transcription, and resulting apoptotic cell death not only in cells expressing the HSVtk gene, but also in surrounding cells via the “bystander effect”26. GCV has a very low affinity for non-viral thymidine kinases, and therefore causes little toxicity in cells not expressing HSVtk26. In systematic studies evaluating SELPs, we have shown that SELP-815K increases gene expression to a greater extent than the other analogs and increases efficacy of virus mediated GDEPT in xenograft tumor models of human head and neck cancer9, 11. SELP-815K, with the single-letter amino acid sequence of (head sequence[GAGS(GAGAGS)2(GVGVP)4GKGVP(GVGVP)11(GAGAGS)5GA]6(tail sequence with 6-histidine tag), (Silk units in bold, Elastin italic, and Lys-substitution underlined) is so named due to its monomer repeat of eight silk units followed by 15 elastin units, with the K representing one additional elastin unit in which a lysine substitution is made (GKGVP).
While this copolymer has shown increased localization, duration of gene expression, and efficacy in an immunocompromised mouse model, it must also show an improvement in safety compared to a non-controlled delivery treatment in an immunocompetent model to be a viable treatment option. Here we present evidence that the safety of subcutaneously injected adenovirus-mediated GDEPT is significantly improved by delivery in SELP-815K compared to injection of virus in saline.
Experimental Section
Adenoviruses
Human adenovirus serotype 5 encoding for herpes simplex virus thymidine kinase (Ad.HSVtk) was purchased from Vector Biolabs (Philadelphia, PA) and used at a viral titer of 1.5×1011 PFU/ml. Viruses were stored at −80°C and used immediately following defrosting to avoid unnecessary freeze-thaw cycles.
Animals
All animal studies were carried out with the permission and under the rules and guidelines of the University of Utah Institutional Animal Care and Use Committee. All mice used in this study were 4-6 week old female standard CD-1 mice, purchased from Charles River Laboratories (Boston, MA). CD-1 mice have an intact immune system, which was anticipated to show adverse reactions to the viral vectors comparable to those observed in human trials. Mouse cages and bedding were changed weekly and food and water was checked and replenished daily as needed.
Injection of therapeutic Ad.HSVtk
For the toxicity studies, Ad.HSVtk was prepared by dilution in either sterile 0.9% physiological saline for injection (Baxter, Deerfield, IL) or SELP-815K which was previously synthesized15 to a final concentration of 1.5×1011 PFU of virus per ml of solution. SELP-815K was used at a final concentration of 4 wt%, determined to be the leading candidate for improvement of virus-mediated GDEPT in previous studies9, 11. Mice were anesthetized and maintained in anesthesia with 3% isofluorane in oxygen. After shaving and cleaning the injection site with 60% isopropanol, mice were subcutaneously injected with 50μl of solution in the right flank via a 26G needle on a 250μl Hamilton syringe. This resulted in an actual viral titer of ~7.6×109 PFU administered per mouse. The injection site was gently clamped for 10 seconds to prevent leakage from the injection site, after which mice were returned to their cages. For consistency, the day of viral injection was considered day 0. The exact conditions administered are shown in Table 1.
Table 1.
Animal numbers and end points of toxicity studies. See list of abbreviations for acronyms
| Group | 1 Week | 2 Weeks | 4 Weeks | 12 Weeks |
|---|---|---|---|---|
| Control | 3 | 9 | 9 | 3 |
| Ad.HSVtk+GCV | 4 | 9 | 9 | 4 |
| SELP-815K 4%+Ad.HSVtk+GCV | 4 | 9 | 9 | 4 |
Injection of ganciclovir (GCV)
For treatment groups, a suspension of 1mg GCV (Sigma Aldrich, St. Louis, MO) per milliliter of 0.9% physiological saline was prepared and sterilized through a 0.22 μm filter. Each day, animal weights were recorded, injection sites cleaned with 60% isopropanol, and mice injected i.p. with 1mg/ml GCV suspension through a 26G needle sufficient for a total dose of 25 mg/kg. Average dosing volume for these studies was approximately 0.6 ml. Mice were injected daily for the first four weeks of the 12 week recovery study, and for the entirety of the 1, 2, and 4 week studies.
Necropsy and data collection
Upon completion of each study, mice were individually euthanized using 70% CO2 in oxygen, with euthanasia confirmed by lack of breathing for 10s. Blood was taken via inferior vena cava stick, and drawn into a heparinized syringe through a 25G needle and deposited into a heparinized plastic blood tube. Minimal evidence of hemolysis was observed in blood samples. Organs (heart, lungs, liver, spleen, kidney, and for 2 and 4 week studies, injection site) were removed, sealed in aluminum foil, and snap-frozen by immersion in liquid nitrogen. Complete blood counts (CBCs) were performed within 2 hours of blood sample collection using a CBC-DIFF (Heska, Loveland, CO) blood count analyzer. Following CBC, samples were centrifuged at 10,000 rpm for 2.5 minutes and rapidly frozen at −80°C for future analysis. A blood chemistry panel consisting of blood urea nitrogen, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, total protein, and albumin was used as indicators of both kidney and liver toxicity. Additionally, globulin was calculated by subtracting albumin from total protein, and albumin/globulin ratio was calculated. Blood chemistry assessments were made using a DRI-CHEM (Heska, Loveland, CO) veterinary blood chemistry analyzer.
Data interpretation
Mouse weight data was used to compare with values on day 0. The percentage weight gain was calculated for each mouse and used as a general indicator of animal health. Animal weight, blood count, and chemistry data were compared using two-tailed Student’s t-tests, with results considered significant if p-values of <0.05 were obtained. Only data reflecting significant changes compared to control is included here. Specifically, animal weight, differential white blood cell count, and ALT/AST values are reported.
Results and Discussion
Virus mediated GDEPT administered in SELP hydrogels for the treatment of cancer has been shown to be an effective treatment in a xenograft model of human head and neck cancer 11. Due to legitimate concerns in the presence of a fully intact immune system, it becomes necessary to carefully assess the ability of the delivery system to improve the safety of this treatment in an immunocompetent model. The toxicity of SELP-controlled, virus-mediated GDEPT was examined in the acute (one week) phase, and a 12 week recovery study was performed to investigate the ability of the mice to return to normal weight and blood count after treatment had been stopped. Two additional time points were selected, two weeks and four weeks, corresponding to the apparent peak and resolution of toxicity based on mouse weight data gathered in the recovery study. It was considered that examination of the specific indicators of toxicity and organ inflammation/damage at these time points would allow diagnosis of the mechanism of toxicity, or at the very least the specific organs affected. This information could potentially be useful in the design of future systems and strategies for controlled delivery of viral gene therapy to localized diseases.
Acute toxicity
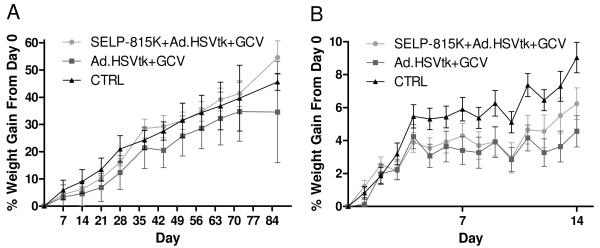
Figure 1 shows animal weight data for the toxicity studies. Acute toxicity was assessed with an endpoint of seven days, at which a lower weight gain was observed for the free viral delivery group compared to control. SELP-mediated delivery did not show statistically significant weight loss at any point during the first seven days compared to both free viral delivery and control, although the mean values were less than control. It is noteworthy that virus injected without the hydrogels stopped gaining weight for 24 hours after injection, while this effect appeared at day 3 in SELP-mediated group.
Figure 1.
Animal weight data for toxicity assessment of viral delivery with and without SELP-815K 4wt% as a controlled delivery vehicle. Data represents the mean percentage of animal weight gained compared to day of injection (Day 0) for each group. Figure 1a shows weight data for the entire study, while Figure 1b highlights the first two weeks of the study in greater detail.
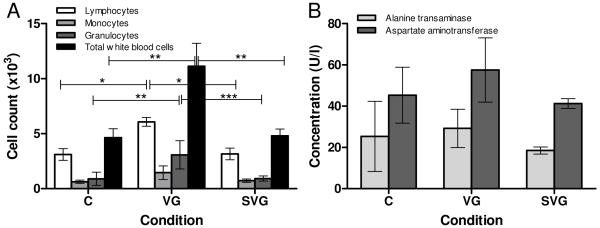
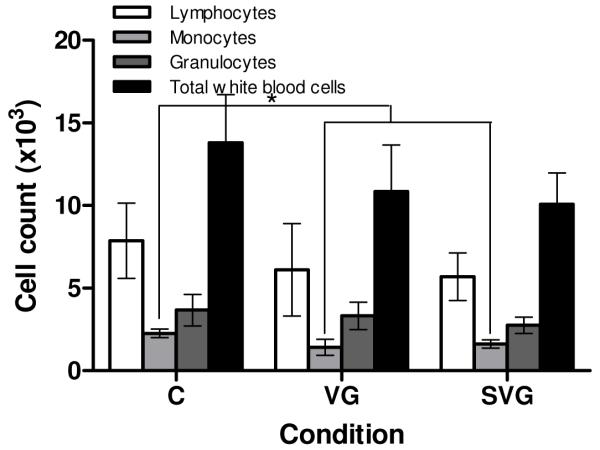
White blood cell counts, shown in Figure 2A, indicated a significant increase in total white blood cell count in the free virus group compared to both the SELP-mediated condition and the control. This overall increase is primarily due to elevated granulocyte and lymphocyte counts in the virus group, suggesting an acute immune response to the administered virus. The absence of this increase in total white blood cell count and differentials in the SELP-mediated group could potentially indicate that locally controlling viral gene delivery using SELP-815K hydrogel reduces or eliminates the systemic immune response to the adenoviral vectors.
Figure 2.
White blood cell counts (A) and liver enzyme levels (B) at one week post-treatment. C: Control, VG: Ad.HSVtk + GCV, SVG: SELP-815K + Ad.HSVtk + GCV. *p<0.05, **p<0.01, ***p<0.001
Blood chemistry results for the acute toxicity study indicated the beginnings of hepatotoxicity (Figure 2B), with elevated AST levels appearing in some mice in the free virus group. It is possible that this increase in AST is a result of damage to liver cells, which would likely be attributed to the therapeutic activity as opposed to immune mechanisms. This is because adenoviruses are known to be removed from circulation by the liver, increasing the probability of gene transfection in this organ. This phenomenon has been observed in previous studies performed in our lab9. Statistical analysis did not yield any significant differences between groups in either ALT or AST, however. At this early stage substantial liver toxicity is unexpected. It has been shown that adenoviral gene expression in vivo peaks at 6 days following exposure to the virus27, however as the therapeutic gene itself does not cause cytotoxicity in our system26, it requires additional time for sufficient quantities of converted prodrug to accumulate and cause toxicity.
SELP matrix appears to reduce the overall acute systemic toxicity of the treatment. Its apparent ability to reduce the magnitude of the acute systemic immune response to the adenoviral vector, as evidenced by reduced leukocytes (Figure 2) highlights the utility of this polymer for localized viral delivery.
Subacute toxicity – 2 week time point
A two week time point was selected in order to monitor the progression of toxicity of this system, as only initial phases appeared to occur in the one week study. Animal weight data over the first two weeks of study (Figure 1B) showed the difference in the progression of toxicity between the SELP-mediated release and free virus conditions. Weight gains were significantly reduced for the free virus group compared to the control group for days 10-14. SELP-mediated delivery reduced this effect to only a single day (day 11) on which weight gain was significantly reduced compared to control.
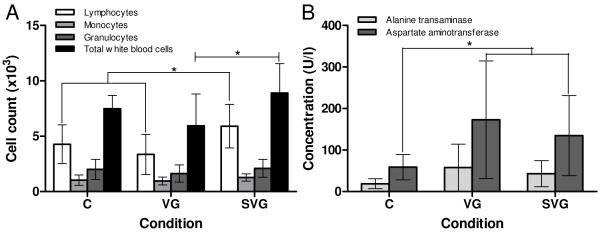
Figure 3A represents the white blood cell count data gathered for animals at the two week time point. While the primary mechanism of toxicity in the free virus delivery case at one week appeared to be immune response-related based on significantly elevated lymphocytes, monocytes, and granulocytes, at two weeks this response appears to have resolved as levels returned comparable to control. In contrast, at two weeks there was a significant elevation in total white cell and lymphocyte counts in the SELP-mediated delivery case compared to both control and free viral delivery. However, in both cases the levels were less than those at one week in the free virus group. These results suggest that delivering the virus in SELP delays the onset of an immune response to the virus, and possibly reduces its magnitude.
Figure 3.
White blood cell counts (A) and liver enzyme levels (B) at two weeks. C: Control, VG: Ad.HSVtk + GCV, SVG: SELP-815K + Ad.HSVtk + GCV. *p<0.05
The results of blood chemistry analysis at the two week time point are displayed in Figure 3B. The elevated ALT and AST levels in the two treatment groups as compared to control suggest that liver damage is occurring. Hepatotoxicity is indicated most readily by AST, with statistically significant elevations in both treatment groups compared to control. This evidence is supported by ALT levels, which were also elevated for several animals in the virus treatment groups. While not statistically significant, the AST and ALT levels were reduced in the SELP-mediated delivery group versus free virus. This damage is likely caused by transfection of the cells of the liver and subsequent cell death due to sensitivity to the prodrug and the bystander effect26, as opposed to an immune reaction to the viral constructs themselves.
Subacute toxicity – four week time point
The next time point considered was four weeks after the initial injection of virus, chosen based on previous results 9 indicating that viral load is essentially completely released by the end of four weeks, making this a likely “turning point” after which toxicity should resolve. Between two weeks and four weeks all groups displayed weight gain, however the order of weight gain between the groups established early in the study remained. Based on the animal weight data (Figure 1) at four weeks (28 days), the free virus group appeared to be delayed in growth by 7-14 days as compared to the control group, while the SELP-mediated group appears to be delayed by less than 7 days. This demonstrates an advantage of SELP-mediated delivery over free viral injection in terms of general health as measured by body weight increase.
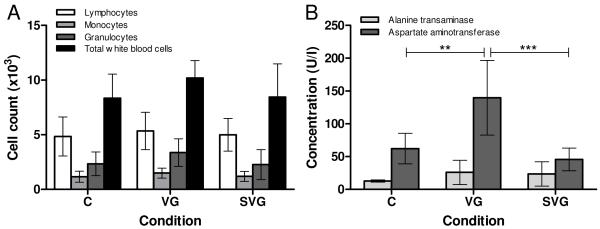
White blood cell counts, shown in Figure 4A, did not indicate any significant differences in immune state of the mice at four weeks post-injection, possibly due to complete or near-complete clearance of the virus. There was a minor elevation, although not statistically significant, of the mean values for all three differential counts and in the total white cell count in the free virus group suggesting a possible low-level immune activation. Further evidence of SELP’s utility in improving the safety of this treatment is shown in Figure 4B. It is apparent from this data that at four weeks the uncontrolled delivery of virus in combination with prodrug was still causing hepatotoxicity as evidenced by AST elevation in the free virus group compared to the control and the SELP-mediated conditions. The significance of this effect suggests that there was some lingering toxicity of the initial free viral delivery.
Figure 4.
White blood cell counts (A) and liver enzyme levels (B) at four weeks. C: Control, VG: Ad.HSVtk + GCV, SVG: SELP-815K + Ad.HSVtk + GCV. **p<0.01, ***p<0.001
Recovery study
A twelve week timepoint served as an indication of how well animals are able to recover from the effects of the treatment previously observed. Animal weight is the primary indicator of recovery at this point, as CBC and blood chemistry evidenced toxicity is very likely to be resolved, particularly considering the rapid metabolism and healing capability of mice. As shown in Figure 1A, between 4 and 12 weeks following viral injection, signs of toxicity had generally resolved and except for two instances, animals in all groups gained weight at a steady rate. However, between days 36 and 44 and between days 72 and 86, the mean weight gains for the free virus group decreased; the only weight losses recorded throughout the entire study. In contrast, the weight gains of the SELP-mediated group actually surpassed the control group from day 36 through the end of the study. CBC analysis of the animals at 12 weeks, shown in Figure 5, reveals no overt evidence of toxicity, although mononuclear leukocytes appear to be depressed slightly in the two treatment groups compared to control. These low values are not particularly of concern as their quantity can fluctuate significantly for any number of reasons, and there was no other independent evidence of an issue related to this analyte. These data indicate that animals were able to completely recover from the toxic effects previously observed in the treatment timepoints.
Figure 5.
White blood cell counts at twelve weeks post-treatment. C: Control, VG: Ad.HSVtk + GCV, SVG: SELP-815K + Ad.HSVtk + GCV. *p<0.05
Conclusion
Administration of Ad.HSVtk in SELP-815K 4wt% hydrogel was shown to reduce the systemic toxicity of gene-directed enzyme-prodrug therapy compared to free viral injection. This reduction in toxicity in conjunction with previously collected data showing improved efficacy makes SELP-mediated delivery a promising approach for viral cancer gene therapy.
Acknowledgements
Support was provided by the National Institutes of Health (R01CA107621) and the Utah Science Technology and Research (USTAR) Initiative.
Abbreviations
- A
Alanine
- Ad.HSVtk
Adenovirus carrying herpes simplex virus thymidine kinase gene
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CAR
Coxsackie and adenovirus receptor
- CBC
Complete blood count
- G
Glycine
- GCV
Ganciclovir
- GDEPT
Gene-directed enzyme-prodrug therapy
- GRA
Granulocyte
- HNSCC
Head and neck squamous cell carcinoma
- HSVtk
Herpes simplex virus thymidine kinase
- LYM
Lymphocyte
- MON
Mononuclear leukocyte
- P
Proline
- S
Serine
- SELP
Silk-elastinlike protein polymer
- V
Valine
- WBC
White blood cell
References
- 1.Descamps D, Benihoud K. Two key challenges for effective adenovirus-mediated liver gene therapy: innate immune responses and hepatocyte-specific transduction. Curr Gene Ther. 2009;9(2):115–27. doi: 10.2174/156652309787909544. [DOI] [PubMed] [Google Scholar]
- 2.Sims K, Ahmed Z, Read ML, Cooper-Charles L, Gonzalez AM, Fisher KD, Berry M, Seymour LW, Logan A. In vitro evaluation of a ‘stealth’ adenoviral vector for targeted gene delivery to adult mammalian neurones. J Gene Med. 2009;11(4):335–44. doi: 10.1002/jgm.1306. [DOI] [PubMed] [Google Scholar]
- 3.Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11(1):66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Hofherr SE, Shashkova EV, Weaver EA, Khare R, Barry MA. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther. 2008;16(7):1276–82. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- 5.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132(3):397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A. 2008;105(14):5483–8. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Hu JK, Krol A, Li YP, Li CY, Yuan F. Systemic dissemination of viral vectors during intratumoral injection. Mol Cancer Ther. 2003;2(11):1233–42. [PubMed] [Google Scholar]
- 8.Wang Y, Liu S, Li CY, Yuan F. A novel method for viral gene delivery in solid tumors. Cancer Res. 2005;65(17):7541–5. doi: 10.1158/0008-5472.CAN-05-1112. [DOI] [PubMed] [Google Scholar]
- 9.Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: from molecular definition to controlled gene expression. J Control Release. 2009;140(3):256–61. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greish K, Araki K, Li D, O’Malley BW, Jr., Dandu R, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels for localized adenoviral gene therapy of head and neck tumors. Biomacromolecules. 2009;10(8):2183–8. doi: 10.1021/bm900356j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greish K, Frandsen J, Scharff S, Gustafson J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers improve the efficacy of adenovirus thymidine kinase enzyme prodrug therapy of head and neck tumors. J Gene Med. 2010 doi: 10.1002/jgm.1469. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandberg LB, Soskel NT, Leslie JG. Elastin structure, biosynthesis, and relation to disease states. N Engl J Med. 1981;304(10):566–79. doi: 10.1056/NEJM198103053041004. [DOI] [PubMed] [Google Scholar]
- 13.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnol Prog. 1990;6(3):198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 14.Lucas F, Shaw JT, Smith SG. The amino acid sequence in a fraction of the fibroin of Bombyx mori. Biochem J. 1957;66(3):468–79. doi: 10.1042/bj0660468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandu R, Cresce AV, Briber R, Dowell P, Cappello J, Ghandehari H. Silk–elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties. Polymer. 2009;50(2):366–374. [Google Scholar]
- 16.Hwang D, Moolchandani V, Dandu R, Haider M, Cappello J, Ghandehari H. Influence of polymer structure and biodegradation on DNA release from silk-elastinlike protein polymer hydrogels. Int J Pharm. 2009;368(1-2):215–9. doi: 10.1016/j.ijpharm.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatefi A, Cappello J, Ghandehari H. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm Res. 2007;24(4):773–9. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 18.Dandu R, Ghandehari H, Cappello J. Characterization of structurally related adenovirus-laden silk-elastinlike hydrogels. Journal of Bioactive and Compatible Polymers. 2008;23(1):5–19. [Google Scholar]
- 19.Dandu R, Ghandehari H. Delivery of bioactive agents from recombinant polymers. Progress in Polymer Science. 2007;32(8-9):1008–1030. [Google Scholar]
- 20.Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H. Molecular engineering of silk-elastinlike polymers for matrix-mediated gene delivery: biosynthesis and characterization. Mol Pharm. 2005;2(2):139–50. doi: 10.1021/mp049906s. [DOI] [PubMed] [Google Scholar]
- 21.Megeed Z, Haider M, Li D, O’Malley BW, Jr., Cappello J, Ghandehari H. In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy. J Control Release. 2004;94(2-3):433–45. doi: 10.1016/j.jconrel.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Megeed Z, Cappello J, Ghandehari H. Controlled release of plasmid DNA from a genetically engineered silk-elastinlike hydrogel. Pharm Res. 2002;19(7):954–9. doi: 10.1023/a:1016406120288. [DOI] [PubMed] [Google Scholar]
- 23.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv Drug Deliv Rev. 2002;54(8):1075–91. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 24.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Swelling behavior of a genetically engineered silk-elastinlike protein polymer hydrogel. Biomaterials. 2002;23(21):4203–10. doi: 10.1016/s0142-9612(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 25.Cappello J, Crissman JW, Crissman M, Ferrari FA, Textor G, Wallis O, Whitledge JR, Zhou X, Burman D, Aukerman L. In-situ self-assembling protein polymer gel systems for administration, delivery, and release of drugs. Journal of Controlled Release. 1998;53(1-3):105–117. doi: 10.1016/s0168-3659(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 26.Mesnil M, Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res. 2000;60(15):3989–99. [PubMed] [Google Scholar]
- 27.Sen L, Hong YS, Luo H, Cui G, Laks H. Efficiency, efficacy, and adverse effects of adenovirus vs. liposome-mediated gene therapy in cardiac allografts. Am J Physiol Heart Circ Physiol. 2001;281(3):H1433–41. doi: 10.1152/ajpheart.2001.281.3.H1433. [DOI] [PubMed] [Google Scholar]