Abstract
Background
Cognitive deficits that persist up to a month have been detected among adult marijuana users, but decrements and their pattern of recovery are less known in adolescent users. Previously, we reported cognitive deficits among adolescent marijuana users after one month of abstinence (Medina, Hanson, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007). In this longitudinal study, we characterized neurocognitive changes among marijuana-using adolescents across the first three weeks of abstinence.
Method
Participants were adolescent marijuana users with limited alcohol and other drug use (n = 19) and demographically similar non-using controls (n = 21) ages 15–19. Participants completed a brief neuropsychological battery on three occasions, after 3 days, 2 weeks, and 3 weeks of stopping substance use. Abstinence was ascertained by decreasing tetrahydrocannabinol metabolite values on serial urine drug screens. Verbal learning, verbal working memory, attention and vigilance, and time estimation were evaluated.
Results
Marijuana users demonstrated poorer verbal learning (p<.01), verbal working memory (p<.05), and attention accuracy (p<.01) compared to controls. Improvements in users were seen on word list learning after 2 weeks of abstinence and on verbal working memory after 3 weeks. While attention processing speed was similar between groups, attention accuracy remained deficient in users throughout the 3-week abstinence period.
Conclusions
This preliminary study detected poorer verbal learning and verbal working memory among adolescent marijuana users that improved during three weeks of abstinence, while attention deficits persisted. These results implicate possible hippocampal, subcortical, and prefrontal cortex abnormalities.
Keywords: Adolescence, Neuropsychology, Cognition, Cannabis, Drug effects, Recovery
1. Introduction
Marijuana is the most frequently used illicit drug among adolescents in the United States (Johnston, O’Malley, Bachman, & Schulenberg, 2009). In 2008, 43% of high school seniors reported having tried marijuana, 19% used in the past month, and 5% endorsed daily use (Johnston et al., 2009). Of individuals who initiated marijuana use before age 15, 14% went on to meet criteria for drug abuse or dependence during adulthood (Substance Abuse and Mental Health Services Administration, 2009). Because adolescence is a period of significant neurodevelopment (Giedd et al., 1996; Sowell et al., 2002), the neurocognitive effects of marijuana use are a concern (Ehrenreich et al., 1999; Pope et al., 2003; Wilson et al., 2000).
Marijuana use during adulthood has known effects on cognition. For example, acute marijuana intoxication may interfere with time estimation, suggesting a “speeding up of an internal clock” (Chait & Pierri, 1992; Jones & Stone, 1970; Lieving et al., 2006; Perez-Reyes et al., 1991). Within several hours to days since last use, heavy marijuana users have shown decrements in attention, memory, executive function, time estimation, and psychomotor speed (Pope & Yurgelun-Todd, 1996; Solowij et al., 2002; Varma et al., 1988). A meta-analysis found selective deficits in learning and memory among chronic users with a range of abstinence while other cognitive domains appeared unaffected (Grant et al., 2003). Others suggested that executive function, motor speed, and manual dexterity deficits may persist after a month of abstinence among heavy users (Bolla et al., 2002). In general, most deficits related to marijuana use seem to be temporary, and cognition may improve with sustained abstinence of at least a week (Pope et al., 2002; Pope et al., 2001). When deficits are found, they tend to be dose-related (Bolla et al., 2002) and may relate to the age of onset of cannabis use (Ehrenreich et al., 1999).
However, adult research may not generalize to adolescents, as significant neuromaturation occurs until early adulthood (Giedd et al., 1996; Sowell et al., 2002). Specifically, gray matter volume peaks in early childhood and decreases thereafter (Pfefferbaum et al., 1994), largely due to synaptic pruning (Huttenlocher, 1990). Gray matter in more basic sensorimotor areas matures earlier than in areas requiring more complex cognitive functions, such as the prefrontal cortex and lateral temporal lobes, which appear to reach maturity during late adolescence or young adulthood (Gogtay et al., 2004). White matter development continues through the late 20s and possibly into middle adulthood as myelination increases (Benes et al., 1994; Jernigan & Gamst, 2005; Pfefferbaum et al., 1994). Importantly, brain maturation during adolescence appears to mirror developments in cognition (Fryer et al., 2008; Nagel et al., 2005; Sowell et al., 2001). Given the confluence of neuromaturational activity and drug use initiation, the potential impact of cannabis use on neuroanatomical and neurocognitive maturation is important to understand.
Thus far, the current literature suggests that adolescents have a heightened vulnerability to the effects of drug and alcohol use (Monti et al., 2005; Spear, 2000). For example, chronic cannabis exposure in adolescent rats has long-lasting effects on learning and memory compared to similar exposure during adulthood (Cha et al., 2006; Schneider & Koch, 2003; Schneider et al., 2008; Stiglick & Kalant, 1982), possibly due to fewer or less efficient synaptic connections in the hippocampus (Rubino et al., 2009). Both cognitive and brain imaging studies in humans suggest critical periods of development that may be impacted by marijuana use (Ehrenreich et al., 1999; Pope et al., 2003; Wilson et al., 2000). Within several hours of intake, regular marijuana use in human adolescents or young adults (≥21 years old) appears to negatively affect learning, memory, attention, and spatial working memory (Fried et al., 2005; Harvey et al., 2007). After at least three weeks of abstinence, memory, complex attention, psychomotor speed, and planning and sequencing decrements are evident among adolescent marijuana users (Medina, Hanson et al., 2007; Millsaps et al., 1994; Schwartz et al., 1989). The effects of abstinence among youth are mixed, but poorer cognition is generally associated with heavier and more recent use (Fried et al., 2005).
Marijuana use during adolescence may have neuroanatomical and functional consequences. Youth recently abstinent from marijuana and alcohol use have demonstrated abnormal associations between verbal learning and hippocampal asymmetry (Medina, Schweinsburg et al., 2007). Irregular activation patterns have been observed in multiple brain regions, including the frontal and temporal lobes, during spatial working memory despite similar task performance (Padula et al., 2007; Schweinsburg et al., 2008; Schweinsburg et al., 2005). Marijuana users have also demonstrated aberrant brain activation patterns in posterior and frontoparietal regions while completing a verbal working memory task (Jacobsen et al., 2007). Finally, increased parietal and dorsolateral prefrontal cortex activity has been observed during a response inhibition task (Tapert et al., 2007). Collectively, these findings suggest that adolescent marijuana use may have detrimental anatomical and functional consequences in the brain, which may result in altered neural networks or compensatory mechanisms during cognitive tasks (Padula et al., 2007; Schweinsburg et al., 2008).
Given that previous studies of adolescent marijuana users have not yet examined the neurocognitive recovery process following regular use, the current study monitored cognition throughout the first three weeks of abstinence from marijuana use. A brief neuropsychological battery, including measures of verbal learning, verbal working memory, visual attention, and time estimation, was administered an average of 3 days, 2 weeks, and 3 weeks after cessation of marijuana use. Based on previous findings, we hypothesized that marijuana users would perform worse than control teens on all measures (Medina, Hanson et al., 2007), but that improvement would be seen over the follow-up period (Pope et al., 2001). While the adult literature suggests that marijuana users may improve to the same level as controls with sustained abstinence (Pope et al., 2002; Pope et al., 2001), the adolescent research to date suggests continued impairment after a month of non-use (Medina, Hanson et al., 2007; Millsaps et al., 1994; Schwartz et al., 1989).
2. Methods
2.1. Participants
Adolescents were recruited from local high schools and colleges via distribution of flyers. Teens responded to the ad as an opportunity to earn money and participate in developmental research, without knowledge that the study examined marijuana use. No information regarding eligibility criteria was described in the flyer or discussed before screening. To assess eligibility, comprehensive screening measures were administered to adolescents and their parents/guardians. Written informed assent (adolescent participant) and consent (adult participant and parent/legal guardian) were obtained prior to participation, in accordance with University of California, San Diego Human Research Protections Program procedures. Teens and their guardians were administered separate, detailed, structured clinical interviews assessing demographic and psychosocial functioning, Axis I psychiatric disorders, and substance use history. To facilitate open disclosure, parents and youths were interviewed by different research associates, and confidentiality was guaranteed within ethical and legal limits.
Inclusion criteria required that youth were fluent in English, and had a parent or legal guardian available to consent and provide medical and psychiatric history. Exclusionary criteria included history of DSM-IV (Diagnostic and Statistical Manual for Mental Disorders, 4th Ed.) (APA, 2000) Axis I disorder (other than substance use disorder) or use of psychoactive medications; history of chronic medical illness, neurological condition (e.g., meningitis, human immunodeficiency virus [HIV]), or head trauma with loss of consciousness <2 min; significant prenatal alcohol (≥4 drinks in a day or ≥7 drinks in a week) or drug exposure; complicated delivery or premature birth (<33 weeks gestation); learning disability or mental retardation; first-degree relative with history of bipolar I or psychotic disorders; left-handedness; non-correctable vision, colorblindness or hearing impairments; and substance use during the abstinence protocol.
The current study examined 40 adolescents (ages 15–19) who were classified into two groups: marijuana users (n = 19) and non-using controls (n = 21). Similar sample sizes in other studies of adolescent substance users yielded small effect sizes or p-values ranging from p<.05 to p = .001 in group comparisons (Hanson et al., in press; Jacobsen et al., 2007; Medina, Schweinsburg et al., 2007). Therefore, we regard the current sample size as sufficient to detect group differences on behavioral tasks. Groups were similar in gender and racial composition, general intellectual ability (average to high average range), grades completed in school, grade point averages, and self-reported mood and anxiety scores (see Table 1). Classification criteria for the marijuana-using (MJ user) group included >200 lifetime marijuana use episodes, >4 past month marijuana episodes, <30 lifetime experiences with other drugs, and not meeting DSM-IV criteria for alcohol abuse or dependence. Controls had <5 lifetime experiences with marijuana, no previous use of any other drug except nicotine or alcohol, and did not meet DSM-IV criteria for abuse or dependence on alcohol or any drug (see Table 1). As expected, MJ users reported higher levels of marijuana, alcohol, and other drug use than controls. Several MJ users used marijuana the same day as the first testing session, and all had used within the two weeks prior to study initiation. All but one MJ user met DSM-IV criteria for marijuana abuse or dependence. Controls had very limited or no previous use of marijuana, cigarettes, or other recreational drugs and were lighter drinkers than MJ users.
Table 1.
Demographic and substance use information for participants.
| Controls (n=21) M (SD) or % |
MJ users (n=19) M (SD) or % |
|
|---|---|---|
| Age ** | 17.4 (1.0) | 18.1 (0.8) |
| Male: Female | 16:5 | 17:2 |
| % Caucasian | 48% | 54% |
| Hollingshead SES scorea | 31.3 (17.4) | 24.1 (12.3) |
| WASI Vocabulary T-score | 57.4 (8.4) | 59.5 (8.3) |
| Grades completed | 11.0 (1.4) | 11.5 (0.8) |
| Grade point average | 3.4 (0.6) | 3.1 (0.6) |
| Beck Depression Inventory total | 2.3 (2.6) | 1.9 (1.9) |
| Spielberger State Anxiety T-score | 26.5 (8.9) | 29.0 (6.5) |
| Age regular (weekly) marijuana use began | – | 15.6 (1.6) |
| Days since last marijuana use at first testing session |
277.9 (345.0)b | 3.3 (3.2) |
| Marijuana use days, past month *** | 0.1 (0.2) | 16.0 (9.2) |
| Lifetime marijuana use episodes *** | 1.2 (1.8) | 465.0 (294.5) |
| Met lifetime MJ abuse or dependence criteria *** |
0% | 95% |
| Drinks per month, past 3 months *** | 3.2 (6.3) | 54.4 (48.9) |
| Lifetime alcohol use episodes *** | 13.5 (26.7) | 241.4 (178.5) |
| Lifetime occasions “drunk” *** | 3.7 (7.7) | 93.3 (104.0) |
| Other drug use days, past month ** | 0.0 (0.0) | 0.7 (1.0) |
| Lifetime other drug use episodes *** | 0.1 (0.4) | 12.4 (23.6) |
| Average cigarettes smoked per day, past week ** |
0.0 (0.0) | 0.5 (0.8) |
Notes. WASI = Wechsler Abbreviated Scale of Intelligence. MJ = marijuana.
p<.01
p<.001.
Socioeconomic status (SES) determined by Hollingshead scores (higher scores reflect lower SES).
Includes only controls who have used marijuana in their lifetime (n=15).
2.2. Measures
2.2.1. Screening interview
The detailed screening interview included a structured clinical interview measuring psychosocial functioning, activities, estimated pubertal stage, last menstruation (for females), health history, and handedness. The computerized National Institutes of Mental Health Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001; Shaffer et al., 2000) determined probable DSM-IV mood, anxiety, attention deficit hyperactivity disorder, and conduct disorders. Parallel modules of the computerized Diagnostic Interview Schedule (C-DIS-IV) (Robins et al., 1996) were used for 18-year-olds who lived independently. Family history of psychiatric and substance use disorders was also assessed (Rice et al., 1995).
2.2.2. Substance use
Youth were administered the Customary Drinking and Drug Use Record to assess lifetime and past 3-month use, withdrawal symptoms, DSM-IV abuse and dependence criteria, and substance-related life problems (Brown et al., 1998; Stewart & Brown, 1995). The Timeline Followback (Sobell & Sobell, 1992) provided a detailed substance use pattern using a calendar format with temporal cues to aid recall. Teens were asked about their use of each of the following drugs: marijuana, alcohol, nicotine, stimulants (cocaine, amphetamine, methamphetamine, and methylenedioxymethamphetamine [MDMA]/ecstasy), opiates (heroin, narcotic pain relievers other than as prescribed), dissociatives/hallucinogens (phencyclidine [PCP], mushrooms, lysergic acid diethylamide [LSD], and ketamine), sedatives (GHB, barbiturates, and benzodiazepines), and misuse of other prescription or over-the-counter medications.
2.2.3. Parent interview
A parent or guardian underwent a detailed screening interview, covering information on prenatal/infant development, childhood behavior, age of developmental milestones, parental socioeconomic status (SES) (Hollingshead, 1965), family history of psychiatric and substance use disorders (Rice et al., 1995), and youth and family medical and psychiatric history. Parents/guardians were also administered the parent version of the Diagnostic Interview Schedule for Children Predictive Scales to improve the reliability of the youth diagnostic reports.
2.2.4. Mood
Youth were administered the Beck Depression Inventory (BDI) (Beck, 1978) and the Spielberger State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1970) to assess mood and anxiety.
2.2.5. General intelligence
The Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) Vocabulary subtest was used as a brief measure of general intellectual ability.
2.2.6. Neuropsychological battery
A brief battery of neuropsychological (NP) tests was administered to participants at three time points spaced throughout the first three weeks of a monitored abstinence period. Verbal learning was assessed with the Hopkins Verbal Learning Test-Revised (HVLT-R) (Brandt & Benedict, 2001). A list of 12 words was read aloud and the participant was instructed to recite as many words as possible. The list was presented three times. To reduce practice effects, alternate word lists were presented at each NP session. The number of words correctly recalled at each learning trial and the total words recalled across all three trials were tabulated. Visual attention was assessed with Ruff 2 & 7 (Ruff & Allen, 1996), a visual search and cancellation task that measures both sustained attention (consistency of performance across time) and selective attention (accurately detecting stimuli among distractors). The participant was instructed to place a slash through each ‘2’ and ‘7’ embedded among either letters (10 automatic detection trials) or numbers (10 controlled search trials). For each trial, the correct hits and errors were tabulated and used to determine speed scores (total targets correctly identified [hits]; speed of processing T-score) and accuracy scores (number of hits in relation to number of possible hits; total accuracy T-score). Verbal working memory was estimated with the Letter–Number Sequencing subtest from the Wechsler Adult Intelligence Scale-3rd Edition (WAIS-III) (Wechsler, 1997). This subtest measured verbal attention and working memory. Participants were verbally presented with a series of randomly ordered numbers and letters (e.g., R–3–J–8–T–5), and they were asked to first say the numbers in numerical order and then the letters in alphabetical order (e.g., 3–5–8–J–R–T). The first trials contained only two digits (one number and one letter) and the number of digits presented increased throughout the test until two trials at the same level were failed. Age-corrected scaled scores were used for data analysis. Time estimation (Benton et al., 1964) was assessed by asking participants to estimate when one minute had elapsed. The examiner recorded the number of seconds elapsed when the participant indicated that 60 s was up. All 19 MJ users completed the four NP tests. All 21 non-using controls completed the HVLT-R and Ruff 2 & 7, but only 13 controls completed the Letter–Number Sequencing and Time Estimation tasks.
2.3. Procedures
Adolescents who remained eligible after screening interviews began a monitored abstinence protocol. Youth were asked to abstain from all alcohol and drug use, monitored with serial supervised urine (Quest Diagnostics) and breath samples (Intoximeter, St. Louis, MO) every 3–4 days for the duration of the protocol. Serial tetrahydrocannabinol (THC) metabolite data were normed to creatinine and evaluated based on Smith et al. (2009). Of MJ-using youth who initiated monitored abstinence, 71% had data suggesting compliance with the abstinence protocol (non-compliers not described here).
The NP tests were administered by trained research associates on three occasions over three weeks of abstinence, which reflected an average of 2.7, 13.3, and 21.0 days of abstinence from marijuana. As part of the larger study, all participants received an evaluation between the 23rd and 27th day of abstinence including mood questionnaires and interviews. Upon completion of the study, youth and parents/guardians received financial compensation for participation.
3. Data analysis
Fisher’s Exact Tests compared categorical variables between groups, and analysis of variance (ANOVA) examined group differences on continuous measures. Some alcohol and drug use variables did not meet the requirements for parametric analysis; therefore the Mann–Whitney procedure compared these characteristics between groups. T-scores for the HVLT-R were calculated based on the control group (i.e., the mean of the control group was T = 50 across the three time points). Because MJ users were approximately 8 months older than controls, on average (p = .013), age was controlled in analyses. Marijuana users had slightly, though non-significantly, higher parental SES than controls. Because SES may influence neuropsychological performance (Farah et al., 2007), Hollingshead SES scores were entered as a second covariate.
Repeated measures analysis of covariance (ANCOVA) compared groups on each NP measure across the three testing time points, controlling for age and SES. When significant differences were detected, post-hoc univariate ANCOVAs examined group differences at each time point. Main effects of time were followed up with t-tests. Effect sizes are presented as partial eta-squared (, range = 0 to 1), and interpretations of statistical significance were made if p <.05.
4. Results
4.1. Verbal learning: HVLT-R
Repeated measures ANCOVAs showed significant main effects of group on HVLT-R Trial 1 performance [F(1,36) = 10.81, p = .002, ] (see Fig. 1), HVLT-R Trial 2 [F(1,36) = 5.73, p =.022, ], and the HVLT-R Total Score over the three testing sessions [F(1,36) = 8.20, p = .007, ], above and beyond the effects of age and SES. Follow-up analyses showed that at the first test session, MJ users recalled one less word on average than controls on HVLT-R Trial 1 (controls: 7.4±1.8 words; MJ users: 6.1±1.4 words) [F(1,36)= 12.33, p=.001, ] and Trial 2 (controls: 9.5±1.8 words; MJ users: 8.3±1.2 words) [F(1,36) = 6.62, p = .014, ], and had lower HVLT-R Total Scores than controls [F(1,36)=11.24, p=.002, ]. No main effects of time or group by time interactions were seen.
Fig. 1.
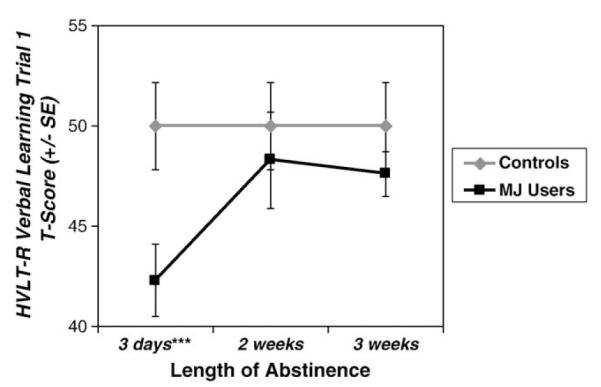
MJ users performed worse than controls in first trial verbal list learning, but improved with abstinence (group main effect p<.01). Error bars depict standard errors. ***p=.001.
4.2. Attention: Ruff 2 & 7
Repeated measures ANCOVAs revealed that MJ users were less accurate than controls on the Ruff 2 & 7 [Total Accuracy T-score: F(1,36) = 7.68, p = .009, ] (see Fig. 2) above and beyond the effects attributable to age and SES. No time or group by time interaction effects were seen. MJ users were less accurate than controls at the first testing session [F(1,36) = 8.52, p = .006, ] and the third testing session [F(1,36) = 6.22, p = .017, ], and marginally worse at the second session [F(1,36) = 3.99, p = .053, ]. No group or time effects, or group by time interactions were seen for Speed of Processing T-score.
Fig. 2.
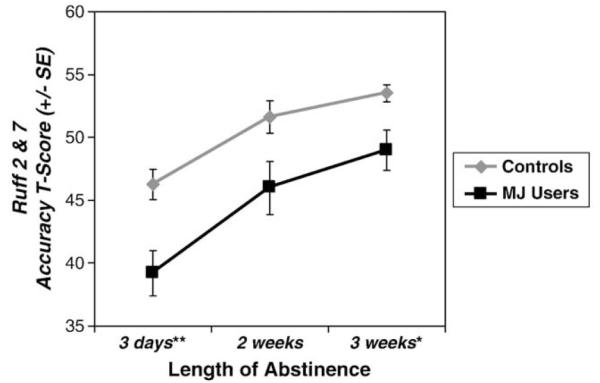
MJ users showed poorer attention accuracy relative to non-using controls across the first three weeks of abstinence (group main effect p<.01). Error bars depict standard errors. *p<.05; **p<.01.
4.3. Verbal working memory: WAIS-III Letter–Number Sequencing
A main effect of group was found for Letter–Number Sequencing scaled score, above and beyond age and SES [F(1,28) = 4.71, p = .039, ] (see Fig. 3). MJ users performed worse than controls at the first and second testing sessions, and the post-hoc univariate ANCOVA revealed that this difference was significant at the second session [F(1,28)=8.18, p=.008, ]. No time or group by time interaction effects were seen.
Fig. 3.
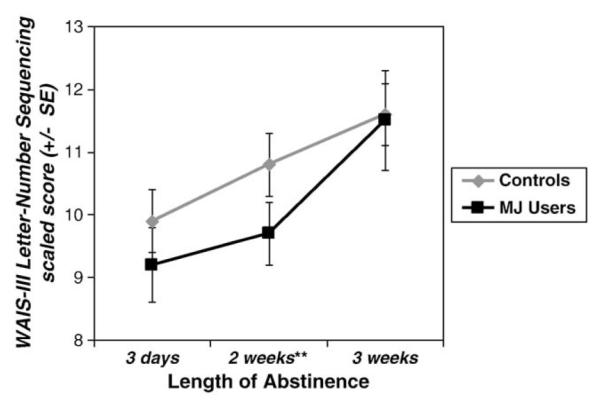
MJ users performed worse than controls in verbal working memory, but improved with abstinence (group main effect p<.05). Error bars depict standard errors. **p<.01.
4.4. Time estimation
Repeated measures ANCOVAs controlling for age and SES detected no group, time, or group by time interaction effects for time estimation of one minute.
5. Discussion
This preliminary study examined neuropsychological function among adolescent marijuana users without comorbid psychiatric disorders compared to non-using community control teens at three time points over three weeks of abstinence. Marijuana users performed worse than controls on measures of verbal learning and verbal working memory, above and beyond the effects attributable to age or SES. Specifically, adolescent marijuana users learned fewer words after approximately 3 days of abstinence, but they performed similarly to controls after 2 and 3 weeks without substance use. Marijuana users had poorer verbal working memory at 2 weeks of abstinence but were similar to controls after 3 weeks. Finally, although they had similar attention processing speeds, marijuana users were less accurate than controls on an attention/vigilance task throughout the 3-week abstinence period. No group differences were found on time estimation. Overall, these results suggest decrements in verbal learning and verbal working memory among marijuana-using youth that may resolve with abstinence, while attention accuracy deficits appear to persist.
To our knowledge, this is the first study of adolescent marijuana users to examine neurocognitive recovery throughout the first three weeks of abstinence. The presence of memory, attention, and working memory deficits among adolescent marijuana users is in concordance with several previous studies of youth (Fried et al., 2005; Harvey et al., 2007; Medina, Hanson et al., 2007; Millsaps et al., 1994; Schwartz et al., 1989; Tapert et al., 2002). The current findings also concur with animal studies reporting learning and memory deficits in adolescents after protracted cannabis exposure (Cha et al., 2006; Schneider & Koch, 2003; Schneider et al., 2008; Stiglick & Kalant, 1982), and with human adult reports of attention, memory, and executive deficits (Bolla et al., 2002; Grant et al., 2003; Pope & Yurgelun-Todd, 1996; Solowij et al., 2002).
Previously, our group reported memory, attention, psychomotor, and sequencing deficits among marijuana-using youth after approximately one month of abstinence (Medina, Hanson et al., 2007). The previous report included a large comprehensive neuropsychological battery that was summarized with composite scores, while the current study used an abbreviated battery that tested a few key areas of functioning. The complex attention composite score used in Medina, Hanson et al. (2007) mirrors the current measures of first trial verbal word list learning, verbal working memory, and attention accuracy; deficits in complex attention after a month of abstinence were primarily driven by poorer performance among marijuana users on several working memory tasks, including first trial word list learning. The current study found that deficits in verbal learning and working memory improved by 2 or 3 weeks of abstinence. The relatively intact performances after 3 weeks of abstinence here may be due to differences in measure sensitivity and sample size (N =65 in the previous study).
We did not find reduced accuracy of time estimation in this sample of adolescent marijuana users. Further, a recent study of adolescent marijuana users did not find an association between time estimation and cerebellar volumes (Medina et al., 2010). Although several adult studies have reported deficits in the accurate estimation of time (Chait & Pierri, 1992; Lieving et al., 2006; Perez-Reyes et al., 1991), these studies were conducted during acute marijuana intoxication, whereas the current study was conducted after several days to weeks of abstinence. It is possible that marijuana’s purported effects on time estimation may only occur during intoxication with the drug. Further, previous reports used alternate approaches to measure time estimation. Overall, deficits in time estimation do not appear to persist after acute intoxication, but further research is warranted.
Deficits in learning and working memory are consistent with adult research that marijuana use is associated with hippocampal and prefrontal cortex abnormalities (Block et al., 2002; Eldreth et al., 2004; Gruber & Yurgelun Todd, 2005; Loeber & Yurgelun-Todd, 1999; Lundqvist et al., 2001). Attention accuracy deficits also implicate dysfunction within attention networks (Eldreth et al., 2004; Gruber & Yurgelun Todd, 2005). Marijuana use during adolescence may disrupt the normal maturational processes that take place during this period, including pruning of gray matter and myelination of white matter (Benes et al., 1994; Gogtay et al., 2004; Jernigan & Gamst, 2005; Pfefferbaum et al., 1994), resulting in altered structure and function of the brain (Jacobsen et al., 2007; Medina, Nagel et al., 2007; Medina et al., 2010; Medina, Schweinsburg et al., 2007; Padula et al., 2007; Rubino et al., 2009; Schweinsburg et al., 2008; Schweinsburg et al., 2005; Tapert et al., 2007). It is also important to emphasize that in some functional imaging studies, marijuana-using youth demonstrated aberrant patterns of activation despite similar performance to non-users (Padula et al., 2007; Schweinsburg et al., 2008; Schweinsburg et al., 2005), suggesting that even once cognitive deficits are no longer detectable, brain function may remain affected. That is, marijuana users may use alternate strategies or may need to “work harder” to obtain the same result as teens who do not use drugs. More research is needed to determine the nature, extent, and duration of any cognitive or neurological abnormalities related to marijuana use.
The clinical implications of the current findings should also be discussed. The current deficits detected in verbal learning, working memory, and attention were less than one standard deviation worse than controls (small to medium effect sizes) and do not indicate a clinical impairment. However, these marijuana-using high school and college students may have more difficulty learning and processing new information and accurately completing their schoolwork or other tasks, and as a consequence, may not perform to expected levels (Lynskey & Hall, 2000). Although the grades achieved in school were not statistically different between groups in the current study, our previous larger study indicated that marijuana users had lower grades than non-users (Medina, Hanson et al., 2007), and they may need to work harder to achieve this grade level (Tapert et al., 2007). Furthermore, participants may be considered high functioning among the general population of adolescent cannabis users, given their high average estimated intelligence, middle to upper class socioeconomic status, and lack of comorbid psychiatric or medical disorders. Thus, if relatively advantaged young marijuana users manifest some deficits, it is possible that those with fewer protective factors may be more vulnerable to cognitive, academic, and occupational consequences. Finally, as significant deficits were detected after several days to 3 weeks of abstinence, youth with frequent use patterns are likely to manifest similar deficits on an ongoing basis.
Some limitations of this study should be considered. This preliminary study used a brief cognitive assessment at three time points, but additional deficits may have been detected with a more comprehensive battery. Although the groups were generally well-matched, marijuana users were slightly older and had higher parental SES than controls; however, this difference was counter to the direction of our hypotheses. Further, the sample consisted mostly of males, so confirmation of observed effects among female users is needed. As with most studies of substance users, it is difficult to determine whether the deficits reported here were the consequences of marijuana use. For example, attention accuracy deficits may predate substance use, may take longer to recover, or may be due to other substance use. However, the relatively small sample size restricted our ability to examine relationships between cognition and the extent of marijuana or other substance use. Because alcohol was used at relatively high levels among users, we cannot attribute any deficits solely to the effects of marijuana use. Nevertheless, regular marijuana use in the context of moderate to heavy alcohol use may be detrimental to cognitive function in teens.
In summary, this preliminary study found poorer verbal learning and verbal working memory among recent marijuana-using adolescents that improved with continued abstinence, and deficits in attention accuracy that persisted at least through 3 weeks of abstinence. These results implicate possible hippocampal and prefrontal cortex abnormalities, as well as dysfunction within attention networks. Adolescent marijuana users could be unaware of any cognitive difficulties, yet academic, behavioral, and occupational functioning may be negatively affected. Additional research is needed to determine whether marijuana use during adolescence results in alterations to neurological or cognitive functions that persist into adulthood.
Acknowledgements
We would like to thank the research associates in the Adolescent Brain Imaging Project at the University of California San Diego, as well as the adolescent participants and their families.
Portions of this paper were presented at the 2008 annual meeting of the International Neuropsychological Society.
Role of funding sources Funding was provided by grants from the National Institute on Drug Abuse (R01 DA021182, PI: Tapert; P20 DA024194, PI: Mason) and the National Institute on Alcohol Abuse and Alcoholism (5 T32 AA1352505, PI: Riley). NIDA and NIAAA had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Contributors Susan F. Tapert designed the study and wrote the protocol. Karen L. Hanson, Alecia Schweinsburg, and Krista Lisdahl Medina conducted literature searches and provided summaries of previous research studies. Karen L. Hanson, Jennifer L. Winward, and Susan F. Tapert conducted the statistical analysis. Karen L. Hanson wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
Conflict of interest All authors declare that they have no conflicts of interest.
References
- American Psychiatric Association . Diagnostic and Statistical Manual for Mental Disorders. 4th Ed. Author; Washington, DC: 2000. text revision. [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) Psychological Corp.; San Antonio, TX: 1978. [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Benton AL, Van Allen MW, Fogel ML. Temporal orientation in cerebral disease. The Journal of Nervous and Mental Disease. 1964;139:110–119. doi: 10.1097/00005053-196408000-00003. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, et al. Effects of frequent marijuana use on memory related regional cerebral blood flow. Pharmacology, Biochemistry and Behavior. 2002;72(1–2):237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised. Professional manual. Psychological Assessment Resources, Inc.; Lutz, FL: 2001. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacology, Biochemistry and Behavior. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chait LD, Pierri J. Effects of smoked marijuana on human performance: A critical review. In: Murphy LL, Bartke A, editors. Marijuana/cannabinoids: Neurobiology and neurophysiology. CRC Press; Boca Raton, FL: 1992. pp. 387–423. [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142(3):295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23(3):914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Noble KG, Hurt H. The developing adolescent brain in socioeconomic context. In: Romer D, Walker EF, editors. Adolescent psychopathology and the developing brain: Integrating brain and prevention science. Oxford University Press; New York, NY, US: 2007. pp. 373–387. [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana – A comparison with pre drug performance. Neurotoxicology and Teratology. 2005;27(2):231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, et al. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain and Cognition. 2008;67:225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non acute (residual) neurocognitive effects of cannabis use: A meta analytic study. Journal of the International Neuropsychological Society. 2003;9(5):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Todd D. A. Yurgelun. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Cognitive Brain Research. 2005;23(1):107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. How does adolescent alcohol and drug use affect neuropsychological functioning in young adulthood?: 10-year outcomes. Journal of Child & Adolescent Substance Abuse. doi: 10.1080/1067828X.2011.555272. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26(3):309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age: Consistency and interpretation of observed effects. Neurobiology of Aging. 2005;26(9):1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students (NIH Publication No. 09-7402), Volume I. National Institute on Drug Abuse; Bethesda, MD: 2009. Monitoring the future national survey results on drug use, 1975–2008. [Google Scholar]
- Jones RT, Stone GC. Psychological studies of marijuana and alcohol in man. Psychopharmacologia. 1970;18:108–117. doi: 10.1007/BF00402390. [DOI] [PubMed] [Google Scholar]
- Lieving LM, Lane SD, Cherek DR, Tcheremissine OV. Effects of marijuana on temporal discriminations in humans. Behavioural Pharmacology. 2006;17:173–183. doi: 10.1097/01.fbp.0000197458.08892.fc. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Yurgelun-Todd DA. Human neuroimaging of acute and chronic marijuana use: Implications for frontocerebellar dysfunction. Human Psychopharmacology: Clinical and Experimental. 1999;14:291–304. [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicology and Teratology. 2001;23(5):437–443. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: A review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry. 2007;48(6):592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Cerebellar vermis abnormality in adolescent marijuana users. Psychiatry Research: Neuroimaging. 2010;182(2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millsaps CL, Azrin RL, Mittenberg W. Neuropsychological effects of chronic cannabis use on the memory and intelligence of adolescence. Journal of Child & Adolescent Substance Abuse. 1994;3:47–54. [Google Scholar]
- Monti PM, Miranda R, Jr., Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, et al. Adolescence: Booze, brains, and behavior. Alcoholism, Clinical and Experimental Research. 2005;29(2):207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Barlett VC, Schweinsburg AD, Tapert SF. Neuropsychological predictors of BOLD response during a spatial working memory task in adolescents: What can performance tell us about fMRI response patterns? Journal of Clinical and Experimental Neuropsychology. 2005;27(7):823–839. doi: 10.1080/13803390490919038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interactions in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21(4):478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes M, Burstein SH, White WR, McDonal SA, Hicks RE. Antagonism of marijuana effects by indomethacin in humans. Life Sciences. 1991;48:507–515. doi: 10.1016/0024-3205(91)90465-n. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Todd D. Yurgelun. Early onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. Journal of Clinical Pharmacology. 2002;42:41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Todd D. Yurgelun. Neuropsychological performance in long term cannabis users. Archives of General Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA: Journal of the American Medical Association. 1996;275(7):521–527. [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism, Clinical and Experimental Research. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. The Diagnostic Interview Schedule, Version 4.0 (DIS 4.0) Washington University; St. Louis, MO: 1996. [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Allen CC. Ruff 2 and 7 Selective Attention Test professional manual. Psychological Assessment Resources, Inc.; Odessa, FL: 1996. [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addiction Biology. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases of Children. 1989;143:1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. Journal of Analytical Toxicology. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. Journal of the International Neuropsychological Society. 2001;7(3):312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Developmental Medicine & Child Neurology. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. 1995;90:627–635. doi: 10.1046/j.1360-0443.1995.9056274.x. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Learning impairment in the radial arm maze following prolonged cannabis treatment in rats. Psychopharmacology. 1982;77(2):117–123. doi: 10.1007/BF00431932. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2008 National Survey on Drug Use and Health: National findings (Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434) Rockville, MD: 2009. [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VK, Anil KM, Ravinder D, Karobi D, Nehra R. Cannabis and cognitive functions: A prospective study. Drug and Alcohol Dependence. 1988;21:147–152. doi: 10.1016/0376-8716(88)90061-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-III. Psychological Corp.; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corp.; San Antonio, TX: 1999. [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: A magnetic resonance and positron emission tomography study. Journal of Addictive Diseases. 2000;19(1):1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]


