Abstract
This study demonstrated the concept of using speed modulation in a continuous-flow total artificial heart (CFTAH) to shape arterial pressure waveforms and to adjust pressure pulsatility. A programmable function generator was used to determine the optimum pulsatile speed profile. Three speed profiles (sinusoidal, rectangular, and optimized [a profile optimized for generation of a physiologic arterial pressure waveform]) were evaluated using the CFTAH mock circulatory loop. Hemodynamic parameters were recorded at average pump speeds of 2,700 rpm and a modulation cycle of 60 beats per minute. The effects of varying physiologically relevant vascular resistance and lumped compliance on the hemodynamics were assessed. The feasibility of using speed modulation to manipulate systemic arterial pressure waveforms, including a physiologic pressure waveform, was demonstrated in vitro. The additional pump power consumption needed to generate a physiologic pulsatile pressure was 16.2% of the power consumption in nonpulsatile continuous-flow mode. The induced pressure waveforms and pulse pressure were shown to be very responsive to changes in both systemic vascular resistance and arterial compliance. This system also allowed pulsatile pulmonary arterial waveform. Speed modulation in the continuous-flow total artificial heart could enable physicians to obtain desired pressure waveforms by simple manual adjustment of speed control input waveforms.
Keywords: Pulsatile, Pulse, Artificial, Mechanical Support
Introduction
The two total artificial hearts (TAHs) approved for clinical use in the United States are the CardioWest (SynCardia, Tucson, AZ) and the AbioCor (Abiomed, Danvers, MA).1,2 Both are pulsatile devices requiring valved conduits and moving blood chambers. CardioWest’s percutaneous pneumatic drive lines and console and AbioCor’s large size and high incidence of thromboembolic events are major unsolved factors limiting the use of TAHs to date.
We are developing an innovative, sensorless, pulsatile, continuous-flow TAH (CFTAH).3,4 The CFTAH has one motor and one rotating assembly supported on a hydrodynamic bearing. The alpha prototype of the device was used in this experiment. The design is evolving into a smaller envelope. Centrifugal impellers supporting the left and right circulations are mounted on opposing ends of the rotor. The CFTAH is uniquely configured to act as an inlet (atrial) pressure balancing regulator, thereby passively balancing right and left flows without the need for sensors. A programmable function generator was used with the speed controller to change the shape of the speed modulation waveform, allowing for iteration to a desired arterial pressure waveform. This report demonstrates the concept of CFTAH speed modulation to simulate a physiologic arterial pressure waveform.
MATERIALS AND METHODS
CFTAH Speed Modulation Design
The CFTAH design and our preliminary in vitro and in vivo testing have been described previously.3, 4 In those experiments, the CFTAH was run in a pulsatile mode by cyclic modulation of the pump speed to simulate a systolic/diastolic cardiac cycle at physiologic beat rates. Using a sinusoidal cyclic speed modulation waveform at a frequency of 80 beats per minute (bpm), previous CFTAH in vivo studies demonstrated significant induced systemic and pulmonary arterial pulse pressures of 18 and 11 mm Hg, respectively, at 2,600 rpm ± 650 rpm without compromising pump output or atrial balance. The effects of induced pulsatility on CFTAH hemodynamics were minimal, with mean pump output and the passive atrial balance remaining essentially unchanged from nonpulsatile operation when the amplitude and frequency of sinusoidal speed modulation were varied up to ± 25% of the mean speed and from 60 to 80 bpm.
In Vitro System Performance Test Methods
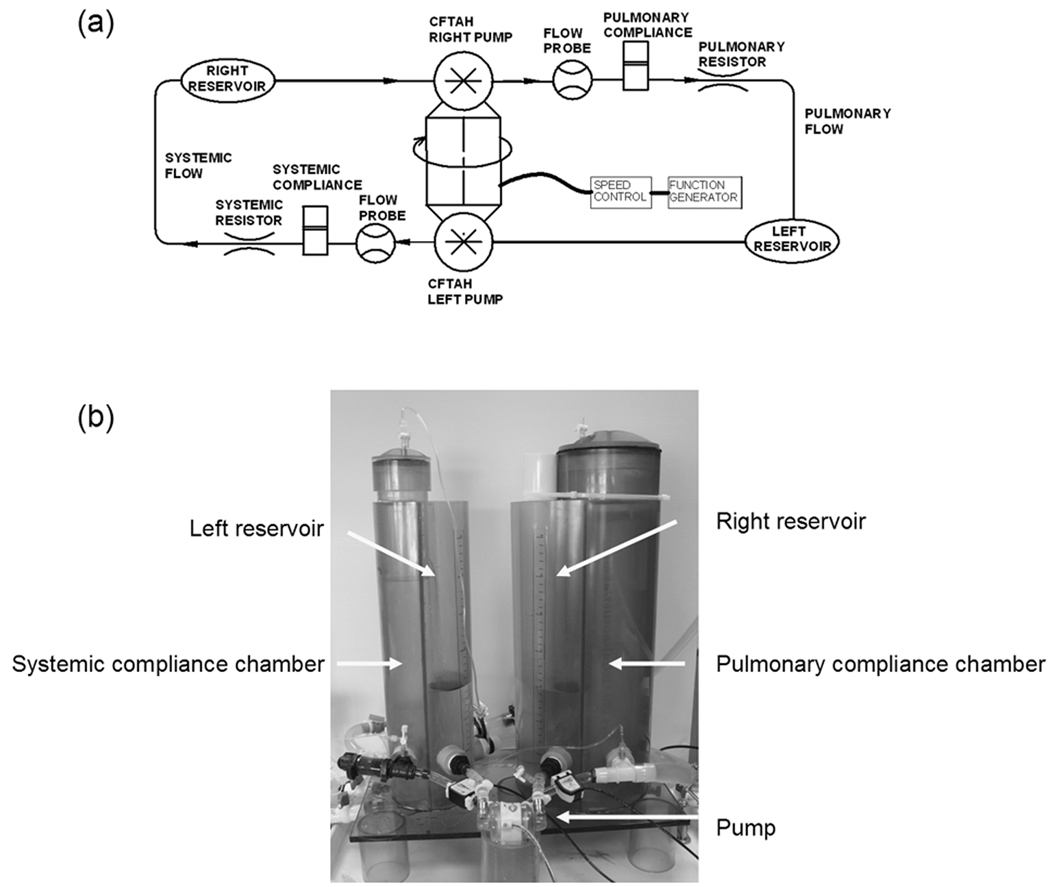
The CFTAH system performance was evaluated using the CFTAH mock circulatory loop. A schema and picture of the in vitro test setup used in this study are shown in Figure 1. To test both pump performances as a CFTAH, the left pump output from the systemic arterial compliance chamber returned to the right venous reservoir and the right pump output from the pulmonary arterial compliance chamber returned to the left venous reservoir. A mixture of water and glycerin (specific gravity 1.060) was used as the working fluid. The systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) were adjusted using valves on the outflow conduit to provide a constant SVR and PVR vs. flow characteristic. Arterial and pulmonary compliance values were adjusted by increasing or decreasing the air volumes in the systemic and pulmonary arterial compliance reservoirs. As we have reported in our initial in vitro and acute animal experiments, CFTAH pump flows range from 4 to 12 L/min and the pump speed can be varied between 1,500 and 4,500 rpm.3, 4
Figure 1.
Schematic drawing (a) and picture (b) of total artificial heart mock circulatory loop used in the in vitro tests.
Speed Modulation Test
A motor controller capable of varying the pump speed modulation profile was used to determine the effects of speed modulation parameters on the arterial pressure waveform. Three speed profiles (sinusoidal, rectangular, and optimized [a profile optimized for generation of a physiologic arterial pressure waveform]) were evaluated. The optimized waveform was generated by varying the speed modulation profiles and observation of resultant pressure waveforms to achieve a “classical” physiologic arterial pressure waveform. For each speed profile assessed, hemodynamic parameters (pump flow, systemic and pulmonary arterial pressures, systemic and pulmonary arterial pulse pressures, systemic and pulmonary arterial waveforms, and power consumption) were recorded at an average pump speed of 2,700 rpm and a modulation cycle of 60 bpm. Furthermore, the effects of varying SVR (600, 1,200, and 1,800 dyne·sec·cm−5) and arterial compliance (1.0, 1.5, and 2.0 ml/mm Hg) on the hemodynamics were assessed for the speed modulation profile that produced physiologic arterial pressure waveforms. The value of PVR was defined as an eighth part of the value of SVR (75, 150, and 225 dyne·sec·cm−5). The pulmonary compliance was defined as four times of the systemic arterial compliance (4.0, 6.0, and 7.5 ml/mm Hg). The pulmonary compliance for the arterial compliance of 2.0 ml/mm Hg was changed from .8.0 to 7.5 ml/mm Hg because the maximum value of the pulmonary compliance in our mock loop system was 7.5 ml/mm Hg. The degree of speed modulation (speed amplitude) was quantified by the maximum pump speed minus the minimum pump speed. The generated arterial pulse pressure was quantified by the resulting maximum minus minimum pressures.
Results
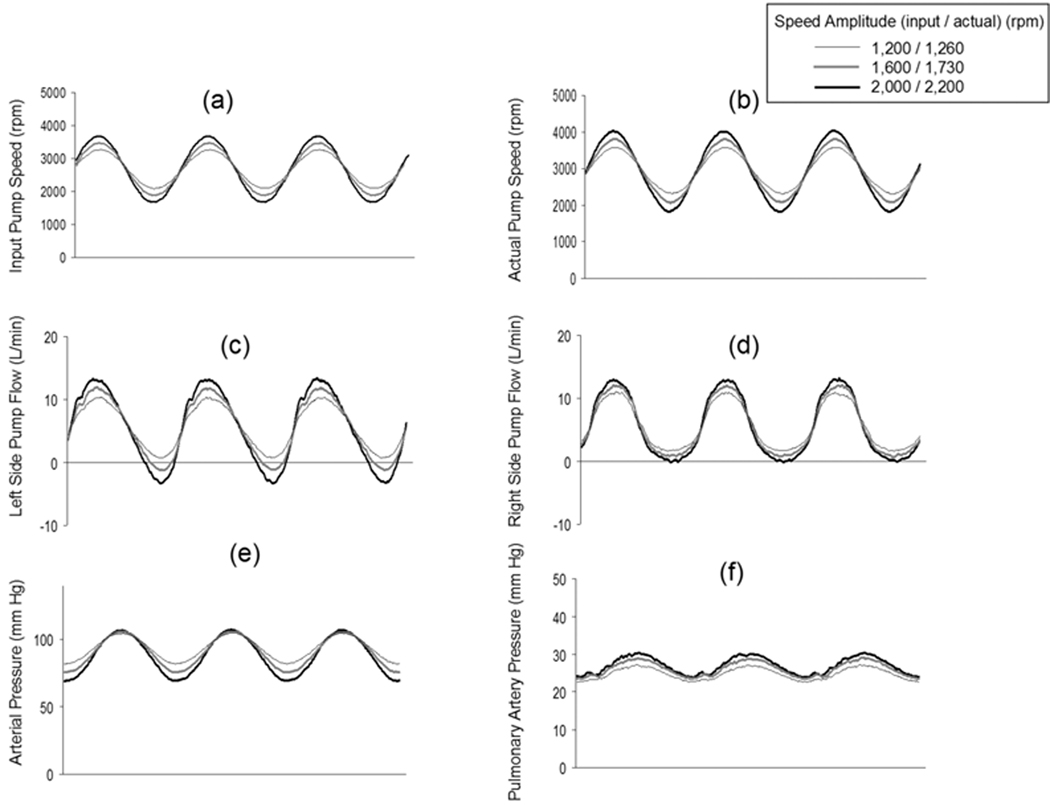
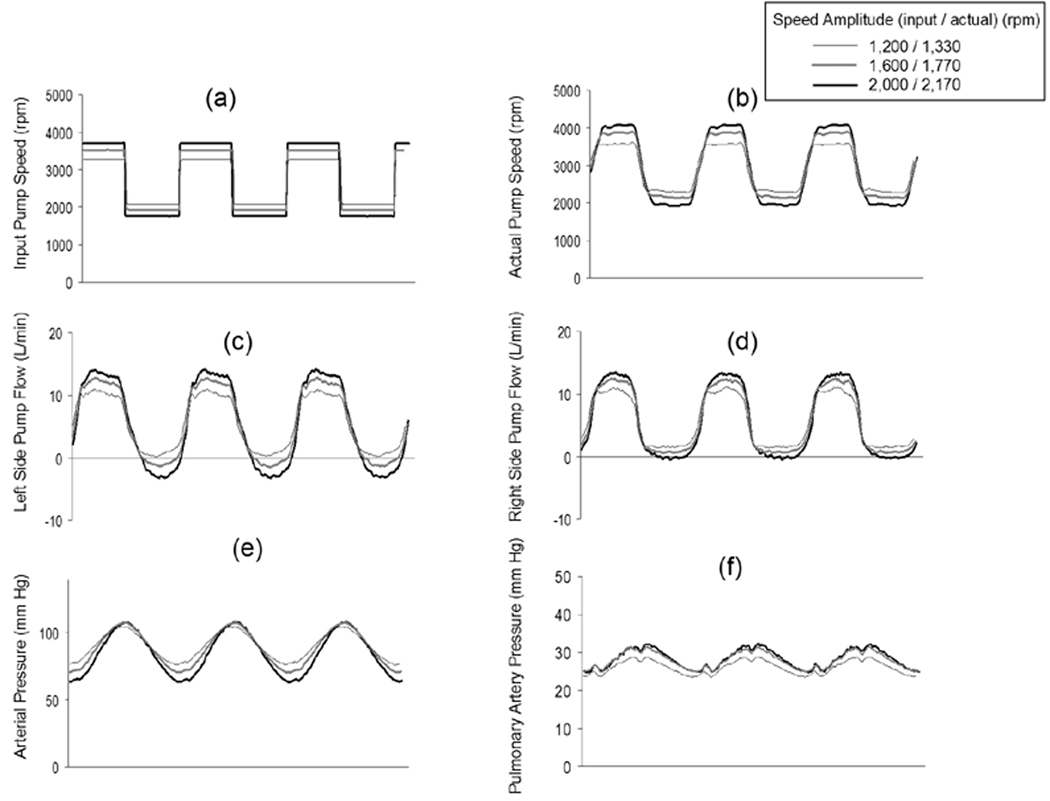
The pump speed response and the resulting systemic arterial pressure waveforms for the sinusoidal and rectangular speed modulation profiles are shown in Figure 2. All data collected for Figure 2 were at a fixed average pump speed of 2,700 rpm and a flow of 5.6 L/min, and at the fixed SVR of 1,200 dyne·sec·cm−5, the fixed PVR of 150 dyne·sec·cm−5, and compliance 1.5 ml/mm Hg. The actual pump speed waveforms achieved by the controller closely matched the sinusoidal control inputs (Figures 2a and 2b). The left and right sides pump flow were shown in Figures 2c and 2d, respectively. The systemic arterial pressure waveform for the sinusoidal input closely matched the sinusoidal speed waveforms (Figure 2e). The pulmonary arterial waveform was also pulsatile (Figure 2f). In response to increasing input speed amplitudes of 1,200, 1,600, and 2,000 rpm (actual speed amplitudes: 1,260, 1,730, and 2,200 rpm, respectively), all cases showed increasing arterial pulse pressures of 23, 31, and 38 mm Hg. The same characteristics were shown for the rectangular profile (Figure 3). The effectively instantaneous change in speed seen with the rectangular speed waveforms also produced a near sinusoidal flow and pressure waveform due to the filtering effects of the systemic arterial compliance.
Figure 2.
Pump speed modulation control input (Figure 2a), the actual pump speed achieved for this control input (Figure 2b), the left side pump flow (Figure 2c), the right side pump flow (Figure 2d), the resulting induced systemic arterial pressure waveforms (Figure 2e), and the resulting induced pulmonary artery pressure waveforms (Figure 2f) for the sinusoidal profile at three speed modulation amplitudes. All data displayed were collected at the same fixed pump and hemodynamic conditions designated as “Baseline” and defined as an average pump speed of 2,700 rpm, pump flow of 5.6 L/min, systemic vascular resistance of 1,200 dyne·sec·cm−5, and systemic compliance of 1.5 ml/mm Hg.
Figure 3.
Pump speed modulation control input (Figure 3a), the actual pump speed achieved for this control input (Figure 3b), the left side pump flow (Figure 3c), the right side pump flow (Figure 3d), the resulting induced systemic arterial pressure waveforms (Figure 3e), and the resulting induced pulmonary artery pressure waveforms (Figure 3f) for the rectangular profile at three speed modulation amplitudes. All data displayed were collected at Baseline pump and hemodynamic conditions.
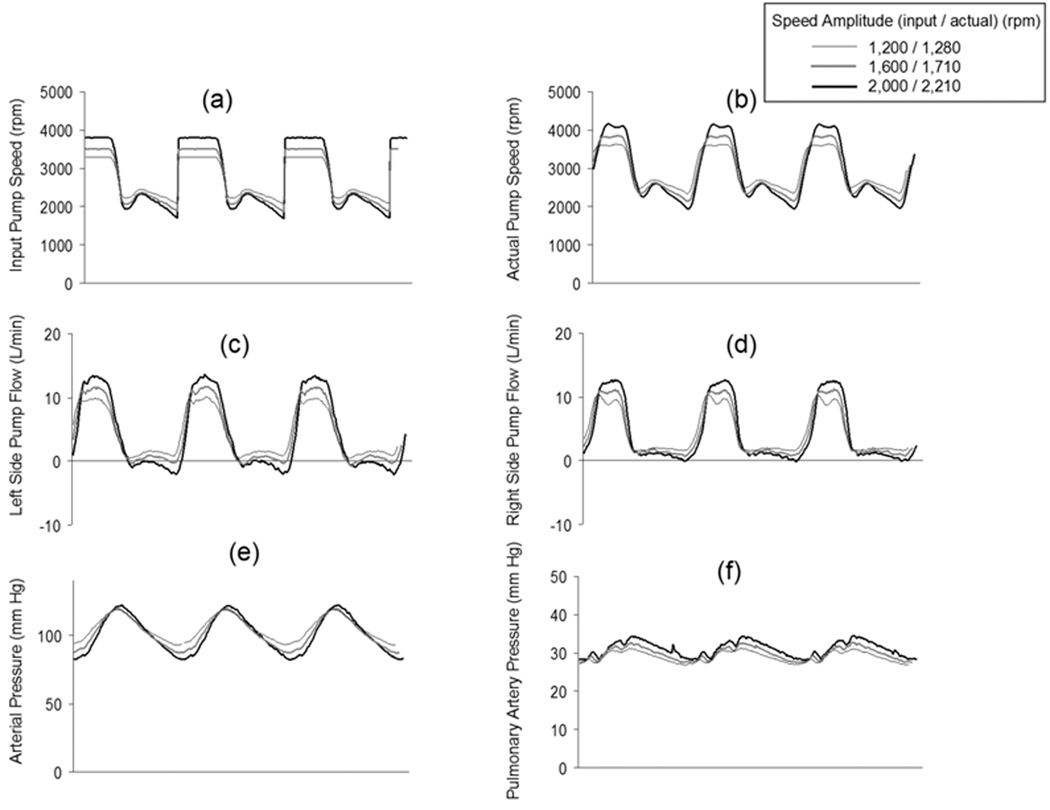
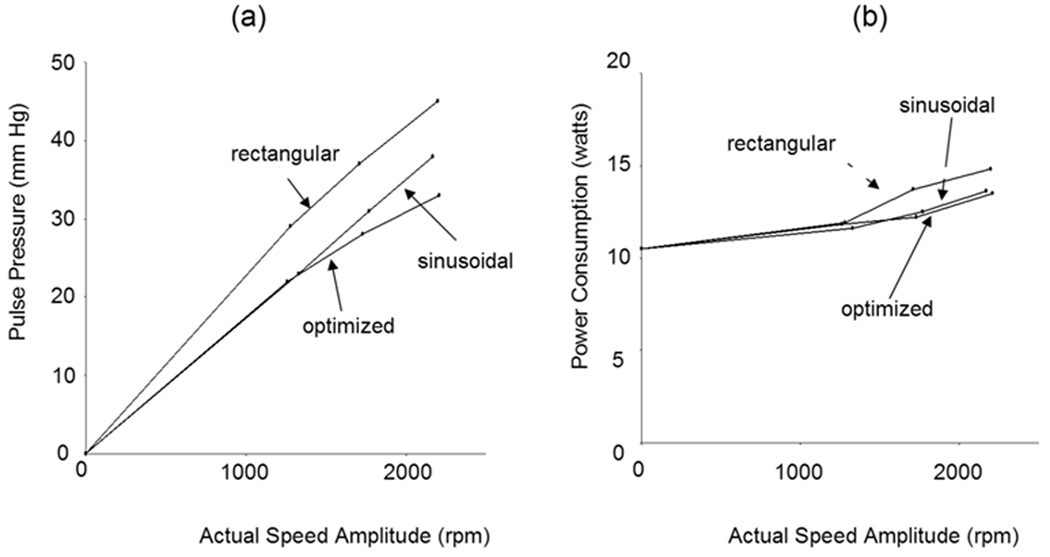
The speed control input and resulting speed waveforms required to simulate a physiologic arterial pressure waveform are shown in Figure 4. These data were also collected at an average pump speed and flow of 2,700 rpm and 5.6 L/min and at the fixed SVR of 1,200 dyne·sec·cm−5, the fixed PVR of 150 dyne·sec·cm−5, and SVR and compliance of 1.5 ml/mm Hg. Likewise, the arterial pulse pressure increased when the input speed amplitude was increased from 1,200, 1,600, and 2,000 rpm (actual speed amplitudes: 1,280, 1,710, and 2,210 rpm, respectively) (Figure 4c). The pulmonary pressure waveform was also pulsatile (Figure 4f). The maximum systemic arterial pulse pressures of 33, 28, and 22 mm Hg was achieved at actual speed amplitude of 2,210, 1,710, and 1,280 rpm for the optimized profile, respectively (Figure 5). The pump power consumption increased by 16.2% from a nonpulsatile (0% speed amplitude) to input speed amplitude of 1,600 rpm (actual speed amplitude: 1,710 rpm) at the same average pump speed and flow. These data demonstrated the modest lower relative efficiency of pulsatile flow vs. nonpulsatile flow in this CFTAH. The rectangular profile was most suitable for making a large pulse pressure, but needed more power (Figure 5).
Figure 4.
The pump speed modulation control input designated as “Physiologic” (Figure 4a), the resulting actual pump speed (Figure 4b), the left side pump flow (Figure 4c), and the right side pump flow (Figure 4d) achieved to simulate a Physiologic systemic arterial pressure waveform (Figure 4e) and pulsatile pulmonary artery pressure waveform (Figure 4f) at three speed modulation amplitudes. All data displayed were collected at Baseline pump and hemodynamic conditions.
Figure 5.
The loop arterial pulse pressure (a) and CFTAH motor power consumption (b) as a function of speed modulation amplitude for sinusoidal, rectangular, and optimized speed modulation control inputs at the Baseline pump and hemodynamic conditions.
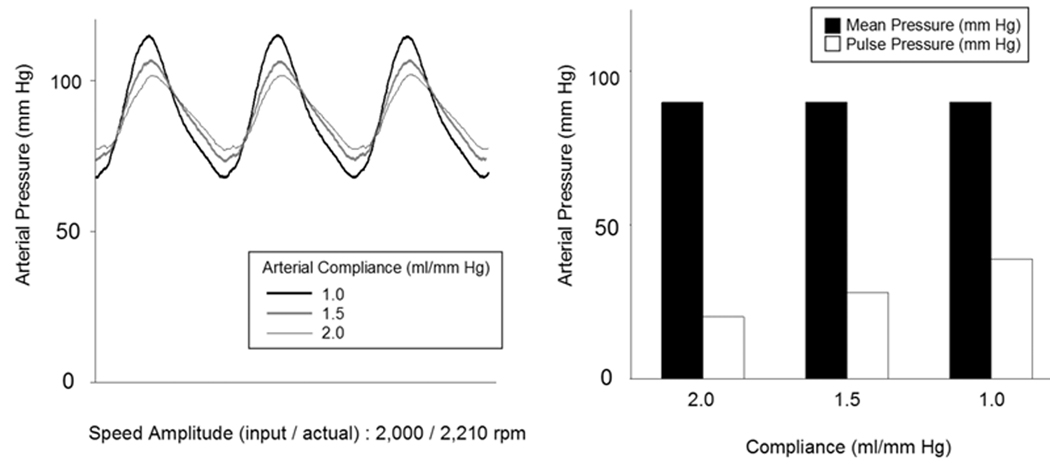
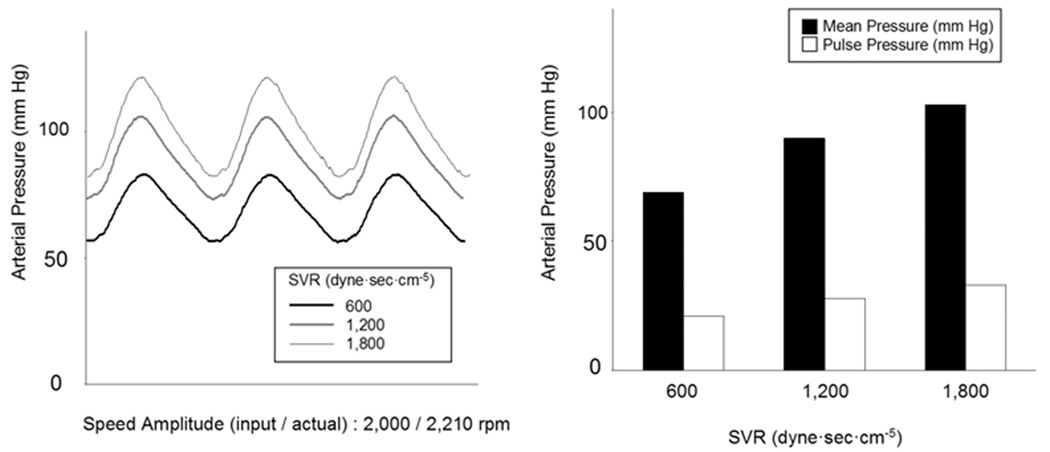
Figure 6a shows the effects of varying compliance on arterial waveforms and systemic pressure and pulse pressures while fixing the input speed amplitude and pump flow at 2,000 rpm and 5.6 L/min and fixing the speed modulation profile at that optimized for a physiologic arterial pressure waveform at the fixed SVR of 1,200 dyne·sec·cm−5 and the fixed PVR of 150 dyne·sec·cm−5. Mean arterial pressure did not show a significant change as the compliance (1.0, 1.5, and 2.0 ml/mm Hg) increased; however, the pulse pressure did show a decreasing trend (Figure 6). Figures 7 shows the effects of varying SVR on arterial waveforms and systemic pressure and pulse pressures while fixing the input speed amplitude at 2,000 rpm and fixing the speed modulation profile at that optimized for a physiologic arterial pressure waveform at compliance 1.5 ml/mm Hg. Mean arterial pressure and pulse pressures increased as the SVR (600, 1,200, and 1,800 dyne·sec·cm−5) increased (Figure 7).
Figure 6.
The effects of the varying circulatory loop systemic arterial compliance on loop systemic arterial pressure waveforms at the Physiologic speed modulation control input, Baseline pump speed and flow conditions and a fixed input speed amplitude of 2,000 rpm (actual speed amplitude: 2,210 rpm).
Figure 7.
The effects of the varying circulatory loop systemic vascular resistance on loop systemic arterial pressure waveforms at the Physiologic speed modulation control input, Baseline pump speed and flow conditions and a fixed input speed amplitude of 2,000 rpm (actual speed amplitude: 2,210 rpm).
Discussion
This study demonstrated the concept of using speed modulation in a CFTAH to shape arterial pressure waveforms and to adjust pressure pulsatility. A physiologic arterial pressure waveform similar to that produced by the native heart was achieved by controlling the speed profile input to the CFTAH controller. This is the first report of an induced physiologic arterial pressure waveform by a mechanical device.
The clinical success of current continuous-flow left ventricular assist devices (LVADs) and of early animal research conducted by our laboratories on nonpulsatile perfusion indicate that there are no gross end-organ adverse effects induced by partial or even complete arterial depulsation.5 Sandner et al.6 demonstrated that patients with continuous flow devices and patients with pulsatile flow devices have comparable renal function after LVAD implantation. Still, there is a longstanding debate over whether or not pulsatile flow provides better perfusion than nonpulsatile flow and what are or will be the long-term chronic effects of depulsation.7 We reported recently that reduced pulsatility resulting from a continuous-flow LVAD implantation induced periarteritis in the calf kidney.8 The local renin-angiotensin system was shown to be upregulated in the renal inflammatory cells in this continuous-flow LVAD group.
Additional factors complicating the analysis of the effects of pulsatility is the fact that the arterial pressure waveform with an LVAD is affected by the relative contribution to cardiac output by the pump vs. the native heart. Even when the native heart does not produce any cardiac output, left ventricular contraction affects LVAD differential pressure, thus creating some pulsatility in the LVAD flow. In contrast, a TAH is the sole source to produce cardiac output and is directly responsible along with the SVR and compliance for arterial pressure pulsatility. Manipulation of the speed modulation control input to a CFTAH can allow varying degrees of physiologic pressure pulsatility, from completely depulsed to that typical of normal cardiac physiology generated by the native heart.
This approach will provide a valuable tool for researchers in the study of end-organ histopathology and other local and systemically mediated effects of reduced pulsatile circulation. It will provide a unique ability to biopsy end organs and evaluate the reversibility of any pathological findings by varying the degree of induced arterial pressure pulsatility within the same animal during the course of the same chronic pump run. This approach will provide valuable answers to how these changes are mediated and possibly how to prevent them in patients on chronic continuous-flow cardiac support. In addition, specific pathological flow and pressure waveforms (such as those seen in valvular diseases) will be reproducible with this system, which may help us to understand their effects on the peripheral organs.
Acute in vivo CFTAH experiments are planned to validate the feasibility of both systemic and pulmonary physiologic pressure waveform shaping using variable speed modulation waveform inputs to the current CFTAH controller. The CFTAH will additionally be subjected to long-term in vivo pump performance, biocompatibility and reliability preclinical studies in which the chronic effects of a physiologic pressure waveform shaping can be further assessed. The influence on hemolysis and thrombosis due to speed modulation will be tested with human blood. The retrograde flow observed in some conditions (Figures 2–4) may potentially increase hemolysis, increase ventricular loading, and augment pulse amplitude; however, our preliminary evaluations of our LVAD with retrograde flow showed no evidence of hemolysis.9
A limitation of this study is that the in vivo pulsatility reported previously with the CFTAH4 was not at the same conditions of pump speed, speed modulation and pump flow as were controlled in this in vitro study. Also the in vivo systemic pulse pressures achieved are believed to be low, given that they were obtained in an anesthetized animal. Another limitation is that the systemic compliance values used in the Cleveland Clinic CFTAH mock loop is a simple lumped compliance value simulated by the volume of air in a single fluid reservoir, which was lower than aortic compliance. Use of this figure makes it difficult to fully evaluate the distributed physiologic compliance of the large vessels seen in the body. This is why we tested over such a wide range of vascular compliance. We hope the results of our acute and chronic animal studies will also provide a comparative probe of the actual vascular resistance and compliance seen by the CFTAH and allow us to better optimize our CFTAH mock circulatory system to better simulate the native circulation. There is great potential for CFTAH speed modulation to be used clinically to eliminate or decrease the severity of any potential complications to chronic depulsed circulation that may be found during clinical trials or that may be identified in preliminary animal studies on non-pulsatile circulation. Speed modulation may also prove to be advantageous in terms of biocompatibility by eliminating fixed low velocity and or high residence time areas of the device and its conduits by inducing time varying pulsatile pressure and blood velocity profiles. The influence of speed modulation on hemolysis and thrombosis will be evaluated with human blood hemolysis tests and chronic in vivo animal studies and analyzed by a coupled electromagnetic and CFD multiphysics analysis of the pump.
Conclusion
CFTAH speed modulation was demonstrated in vitro with the potential for physicians to use this technique to shape the systemic arterial pressure waveforms to minimize the learned pathophysiologic end-organ and possibly systemic effects of depulsed circulation by simple manual adjustment of speed control input waveforms. The mean pressure induced by speed modulation was also shown to be affected primarily by SVR changes and not by changes in systemic arterial compliance. The increase in power consumption required to induce this pulsatility was 16.2%.
Acknowledgments
Disclosures: This work was supported by the Trawick Fund and a grant from the National Heart, Lung, and Blood Institute/National Institutes of Health (Grant 1R21HL089052 to L.A.R.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copeland JG, Smith RG, Arabia FA, et al. CardioWest Total Artificial Heart Investigators: Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2000;351:859–867. doi: 10.1056/NEJMoa040186. [DOI] [PubMed] [Google Scholar]
- 2.Dowling RD, Gray LA, Jr, Etoch SW, et al. Initial experience with the AbioCor implantable replacement heart system. J Thorac Cardiovasc Surg. 2004;127:131–141. doi: 10.1016/j.jtcvs.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Fukamachi K, Horvath DJ, Massiello AL, et al. An innovative, sensorless, pulsatile, continuous-flow total artificial heart: Device design and initial in vitro study. J Heart Lung Transplant. 2010;29:13–20. doi: 10.1016/j.healun.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fumoto H, Horvath DJ, Rao S, et al. In vivo acute performance of the Cleveland Clinic self-regulating, continuous-flow total artificial heart. J Heart Lung Transplant. 2010;29:21–26. doi: 10.1016/j.healun.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tominaga R, Smith WA, Massiello A, Harasaki H, Golding LA. Chronic nonpulsatile blood flow. I. Cerebral autoregulation in chronic nonpulsatile biventricular bypass: carotid blood flow response to hypercapnia. J Thorac Cardiovasc Surg. 1994;108:907–912. [PubMed] [Google Scholar]
- 6.Sandner SE, Zimpfer D, Zrunek P, et al. Renal function after implantation of continuous versus pulsatile flow left ventricular assist devices. J Heart Lung Transplant. 2008;27:469–473. doi: 10.1016/j.healun.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Haines N, Wang S, Myers JL, Undar A. Comparison of two types of neonatal extracorporeal life support systems with pulsatile and nonpulsatile flow. Artif Organs. 2009;33:993–1001. doi: 10.1111/j.1525-1594.2009.00934.x. [DOI] [PubMed] [Google Scholar]
- 8.Ootaki C, Yamashita M, Ootaki Y, et al. Reduced pulsatility induces periarteritis in kidney: role of the local renin-angiotensin system. J Thorac Cardiovasc Surg. 2008;136:150–158. doi: 10.1016/j.jtcvs.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi K, Golding LA, Massiello AL, et al. Preclinical readiness testing of the Arrow International CorAide left ventricular assist system. Ann Thorac Surg. 2004;77:2103–2110. doi: 10.1016/j.athoracsur.2003.07.048. [DOI] [PubMed] [Google Scholar]