Abstract
Current therapeutic approaches to cancer are designed to target molecules that contribute to malignant behavior but leave normal tissues intact. β1 integrin is a candidate target well known for mediating cell-extracellular matrix (ECM) interactions that influence diverse cellular functions; its aberrant expression has been implicated in breast cancer progression and resistance to cytotoxic therapy. The addition of β1 integrin inhibitory agents to breast cancer cells at a single-cell stage in a laminin-rich ECM (three-dimensional lrECM) culture was shown to down-modulate β1 integrin signaling, resulting in malignant reversion. To investigate β1 integrin as a therapeutic target, we modified the three-dimensional lrECM protocol to approximate the clinical situation: before treatment, we allowed nonmalignant cells to form organized acinar structures and malignant cells to form tumor-like colonies. We then tested the ability of β1 integrin inhibitory antibody, AIIB2, to inhibit tumor cell growth in several breast cancer cell lines (T4-2, MDA-MB-231, BT474, SKBR3, and MCF-7) and one nonmalignant cell line (S-1). We show that β1 integrin inhibition resulted in a significant loss of cancer cells, associated with a decrease in proliferation and increase in apoptosis, and a global change in the composition of residual colonies. In contrast, nonmalignant cells that formed tissue-like structures remained resistant. Moreover, these cancer cell–specific antiproliferative and proapoptotic effects were confirmed in vivo with no discernible toxicity to animals. Our findings indicate that β1 integrin is a promising therapeutic target, and that the three-dimensional lrECM culture assay can be used to effectively distinguish malignant and normal tissue response to therapy
Introduction
Development of monoclonal antibody therapies designed to target aberrant cell surface signaling receptors, such as HER-2 and epidermal growth factor receptor (EGFR), have shown great promise in cancer therapy (1, 2). One other class of cell surface receptors that is critical in mediating cell-extracellular matrix (ECM) interactions is β1 integrin, a major contributor for growth factor receptor signaling. β1 integrins belong to a family of heterodimeric transmembrane receptors that transmit biomechanical cues that critically mediate cell-ECM interactions (reviewed in ref. 3). β1 integrin is aberrantly expressed in human breast carcinomas and has been shown to play a central role in growth, apoptosis, invasion, and metastasis (4–8). In addition to its role in cancer progression, an emerging body of evidence indicates that β1 integrin signaling plays a significant role in mediating resistance to cytotoxic chemotherapies by enhancing cell survival in hematologic malignancies, lung, and breast cancers (9–12). Inhibition of β1 integrin has also been shown to abrogate the formation of metastasis in gastric and breast cancer models (13–15). Thus, several aspects of β1 integrin signaling point to it as a multifaceted target for breast cancer therapy.
Using a three-dimensional lrECM cell culture model, which emulates a more physiologically relevant microenvironment (16), we showed previously that down-modulation of β1 integrin and growth factor signaling pathways resulted in reversion of the malignant phenotype (17), leading to growth arrest and reformation of tissue polarity (18). In addition, β1 integrin and growth factor signaling were found to be integrated in the context of the three-dimensional lrECM but not on tissue culture plastic (18, 19).
We reasoned that a modified version of this culture model could provide an accurate surrogate for testing therapies for human breast cancer cells and tumors. We developed the modified three-dimensional lrECM assay and show that inhibition of β1 integrin results not only in antiproliferative and proapoptotic effects in malignant cell lines in three-dimensional cultures, but that these results were recapitulated also in vivo. β1 integrin inhibition preferentially affected malignant cells both in culture and in vivo; the nonmalignant acini and normal tissues were not affected, and remarkably, there was little or no toxicity to the animals.
Materials and Methods
Cell culture
HMT-3522-S1 (S-1) mammary epithelial cells were originally derived from a woman with nonmalignant fibrocystic breast disease (20) and cultured in H14 medium as previously described (17). S-1 cells were propagated on plastic in medium containing 10 ng/mL EGF, and T4-2 cells were grown on collagen type I–coated flasks in the absence of EGF (17). Human breast cancer cell lines MCF-7 and MDA-MB-231 were obtained from the American Type Culture Collection (Manassas, VA), and SKBR-3 and BT474 were a gift from Dr. Joe Gray (University of California in San Francisco, UCSF). Three-dimensional cultures were plated with cells trypsinized from monolayer cultures and plated on top of commercially available matrix produced from Englebreth-Holm-Swarm tumors (Matrigel, Collaborative Research, Waltham, MA). Cell lines were maintained in media described above, conditioned with 5% Matrigel. This assay is distinct from previously published reversion assays that were done with cells completely embedded within Matrigel (17). Cells were plated on day 0. For S-1 cultures, AIIB2 was added on day 6 of culture, after acinar formation had occurred. For malignant cell lines, AIIB2 was added on day 4 of culture, after cells had undergone several population doublings. All cultures were analyzed after 3 days of AIIB2 treatment.
β1 integrin and HER-2 inhibitory antibodies
AIIB2, a β1 integrin function-blocking antibody (originally a gift from C. Damsky, UCSF) was isolated and prepared from a hybridoma cell line (Sierra Biosources, Milipitas, CA). AIIB2 is a rat monoclonal IgG1 that was originally isolated from a human choriocarcinoma hybridoma that specifically binds β1 integrin extracellular domain (21–23). Experiments using F(ab′)2 fragments of enzyme-digested AIIB2 indicated that the epitope-binding portion of the antibody was active and resulted in down-modulation of β1 integrin–mediated signaling (17, 19). AIIB2 was added to culture medium on alternate days. Herceptin is a humanized monoclonal antibody against the erbB2 or HER-2 receptor (24) that was used (20 µg/mL) to treat SKBR3 cells on day 6. Control cultures for all experiments were treated with the same concentration of nonspecific IgG.
Immunofluorescence
Cells from three-dimensional cultures were fixed onto a glass slide using 4% paraformaldehyde or methanol/acetone. Nonspecific sites were blocked with 0.5% casein/PBS solution for 1 hour at room temperature. Primary β1 integrin monoclonal rat anti-mouse antibody (PharMingen, San Diego, CA; 1:100) was diluted in blocking buffer and was applied for 1 hour at room temperature in a humidified chamber. Slides were washed in PBS containing 0.1% bovine serum albumin, before incubating in secondary antibody conjugated to FITC (Molecular Probes, Eugene, OR) for 1 hour in a dark humidified chamber at room temperature. The slides were then washed and counterstained with 4′,6-diamidino-2-phenylindole before mounting with Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
Confocal microscopy
Confocal images were acquired by using a Zeiss LSM 410 inverted laser scanning confocal microscope equipped with an external argon/krypton laser. Using a Zeiss Fluor ×40 (1.3 numerical aperture) objective, images were captured at the colony midsection. Relative immunofluorescence intensity of images was standardized by comparing only cultures that were processed identically and stained in the same experiment.
Western immunoblot
Cells propagated in three-dimensional lrECM were first treated with ice-cold PBS/EDTA [0.01 mol/L sodium phosphate (pH 7.2) containing 138 mmol/L sodium chloride and 5 mmol/L EDTA] to isolate the cells and then lysed in radioimmunoprecipitation assay buffer as previously described (17). Equal amounts of protein were loaded onto reducing SDS gels. After transfer onto nitrocellulose membrane (Invitrogen, Carlsbad, CA), blots were blocked with 5% nonfat milk and probed. Primary antibodies used include β1 integrin, clone 18 (1:1,000), phospho-FAK, clone 14 (1:1,000; BD Transduction Laboratories, Lexintgon, KY); phospho-β1 integrin (1:1,000; Biosource, Camarillo, CA); β-actin, clone AC-15 (1:5,000; Sigma, St. Louis, MO). Blots were washed, incubated with secondary antibody, and exposed to X-ray film.
Fluorescence-activated cell sorting analysis
Cells were propagated on tissue culture plastic and harvested using 0.25% trypsin. After resuspending in 1 mL DMEM/F-12 media with trypsin inhibitor, cells were spun down and washed in 1× PBS, 5% fetal bovine serum, and 0.1% sodium azide on ice. Cells were incubated with primary antibody (AIIB2, 1:10) at 4°C for 30 minutes to 1 hour, washed, and incubated with a fluorescein-conjugated IgG secondary antibody (1:100) for 30 minutes. After washing, 1 mL of 1% paraformaldehyde solution was added to the pellet and suspended immediately. Cells were analyzed using a Beckman-Coulter EPICS XL-MCL Analyzer. System II Data Acquisition and Display software, version 2.0 was used for data analysis.
Apoptosis and proliferation assays
Apoptosis was assayed in cell culture using a commercially available kit (In Situ Cell Death Detection kit, fluorescein; Roche, Nutley, NJ) designed to detect terminal deoxynucleotidyl transferase (TdT)–mediated nick end labeling (TUNEL). Cells were fixed in 4% paraformaldehyde and permeabilized in cold 0.1% Triton X-100 in 0.1% sodium citrate. After washing in PBS, cells were incubated in TUNEL reaction mixture at 37°C for 60 minutes, washed, and mounted. Proliferation was detected by indirect immunofluorescence of Ki-67 nuclear antigen. Cells were fixed in methanol/acetone and blocked using 10% goat serum, then incubated in primary rabbit antibody against Ki-67, clone MIB-1 (1:200; Novocastra Laboratories, Norwell, MA) for 1 hour and washed before FITC-conjugated anti-rabbit secondary antibody (The Jackson Laboratory, Bar Harbor, ME) was applied. Nuclei were counterstained with DAPI.
For assay of apoptosis in paraffin-embedded tissues, Apoptag In Situ Apoptosis Detection kit (Intergen, Burlington, MA) was used to detect TUNEL reaction. Paraffin-embedded xenograft tumors were sectioned at 5- to 10-µm-thick sections. Sections were deparaffinized and rehydrated using xylene and ethanol washes. Tissues were then treated with proteinase K at room temperature, washed, and quenched using 3% hydrogen peroxide. Buffer solution was applied, and sections were incubated in TdT enzyme at 37°C for 1 hour. Stop/wash buffer was used before anti-digoxigenin peroxidase conjugate was applied. Proliferation was assayed in paraffin-embedded tissues using indirect immunohistochemistry. Sections were deparaffinized as above and blocked using 10% normal horse serum, then incubated with mouse monoclonal antibody against Ki-67 (Oncogene, San Diego, CA) overnight at 4°C, and washed in PBS. They were then serially incubated with biotinylated anti-mouse antibody, and steptavidin-horseradish peroxidase and 3,3′-diaminobenzidine (DAPI) medium. After counterstaining with hematoxylin, sections were dehydrated in serial concentrated ethanol and xylene and mounted. Cells were scored by counting the total number of nuclei in five high-power microscopic fields (×40) using a ×10 objective, or a minimum of 200 nuclei per tumor section.
Tumor growth and toxicity assessment in vivo
Female nu−/− mice were obtained from Charles River (Wilmington, MA) or Taconic (Germantown, NY) and housed five per cage with chow and water ad libitum in a controlled animal barrier. Animals were injected s.c. with 5 to 10 × 106 T4-2 cells or 107 MCF-7 cells into the upper back posterior to the right front limb. Estradiol pellets were inserted s.c. above the tail for animals bearing MCF-7 xenografts. AIIB2 antibody or nonspecific rat IgG was injected into the i.p. cavity biweekly beginning on day 4 or day 28 after cell implantation. Tumor dimensions (width, height, and depth) were measured biweekly. At the time of sacrifice, animals were euthanized, and tumors were harvested and either immediately frozen in ornithine carbamyl transferase or fixed in formalin. Serum was collected using cardiac puncture techniques.
Animals were monitored for evidence of toxicity by measuring weight, assessing overall activity, and necropsy. Additional toxicity studies were done using β1 integrin inhibitory antibody, clone Ha 2/5 (PharMingen), which specifically recognizes murine β1 integrin. Antibody was administered at doses of 1 to 20 mg/kg biweekly over 4 weeks. All experimental procedures were followed according to the UCSF, and LBNL Animal Welfare Committees approved policies and guidelines.
Statistical analysis
For each dose of AIIB2 or control IgG in culture, pairwise differences in Ki-67 or TUNEL were tested among the six cell lines using Student’s t test (25). Multivariate ANOVA was used for analysis of tumor volume at each time point. For each dose of AIIB2 or control IgG in vivo, pairwise Student’s t test or χ2 comparison was used to analyze differences between TUNEL and Ki-67 expression. MINITAB (Minitab, Inc., State College, PA) statistical software was used for all calculations.
Results
β1 integrin inhibition results in cytostasis and apoptosis in breast cancer cell colonies treated in three-dimensional cultures
We showed previously that down-modulation of β1 integrin downstream signaling pathways in single cancer cells embedded within three-dimensional lrECM was associated with phenotypic reversion, exemplified by growth arrest and acinar differentiation (17, 26), whereas single nonmalignant mammary epithelial cells underwent apoptosis (27). We sought to explore whether there is a role for β1 integrin as a molecular target in breast cancer, which relies on the differential response between normal and malignant tissues. In patients, tumors are commonly discovered after a multicellular three-dimensional tumor has already been formed, and normal cells are found in an organized three-dimensional context. We reasoned that this scenario could be emulated also in the three-dimensional lrECM assay. In addition, we wanted to know whether we could then distinguish between the response of normal and malignant structures. Accordingly, we modified the three-dimensional lrECM assay to test these concerns.
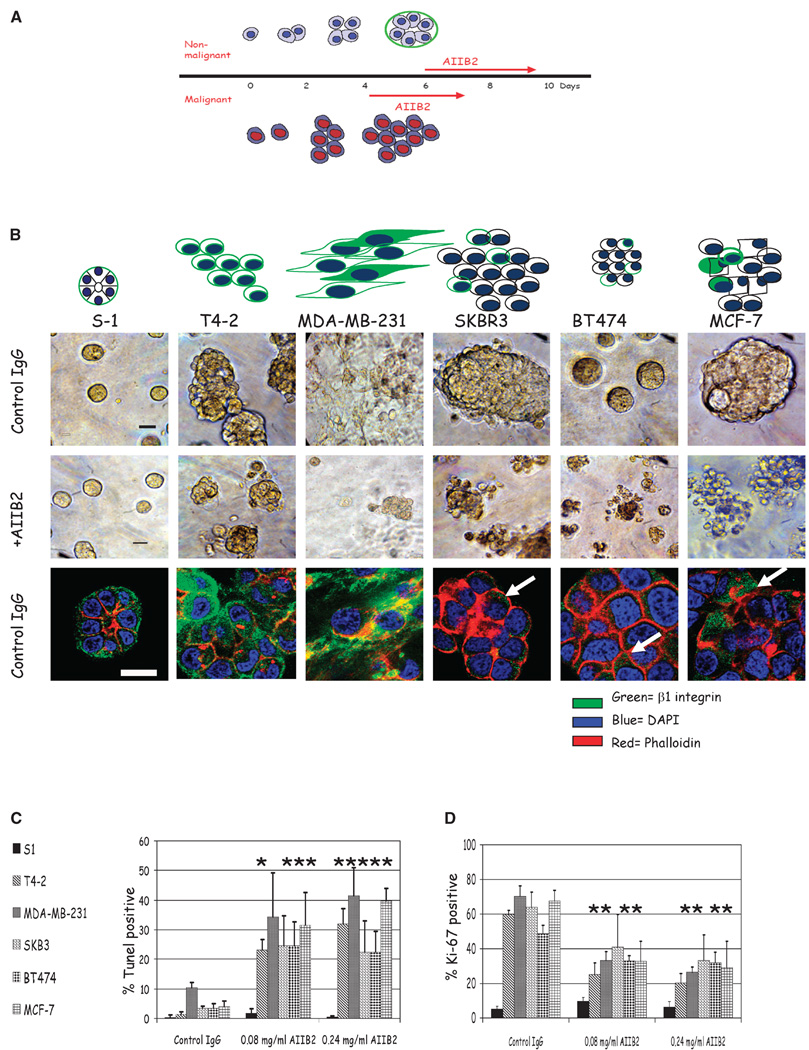
When cultured on top of three-dimensional lrECM gels with 5% Matrigel conditioned media, nonmalignant breast cells undergo morphogenesis and, after 6 days, form acini with polarized cells oriented around a central lumen with a well organized basement membrane, recapitulating normal acinar structures found in vivo (Fig. 1A; for review, see ref. 28). In contrast, all malignant breast cell lines tested (T4-2, MDA-MB-231, SKBR3, BT474, and MCF-7) continued to proliferate and formed disorganized tumor colonies (Fig. 1A). Our previous studies have shown that β1 integrin inhibitory monoclonal antibody, AIIB2, or its F(ab′)2 fragments applied to single cells were capable of down-modulating β1 integrin signaling pathways (17, 19). In the present studies, breast cancer cell lines were propagated in three-dimensional lrECM until colonies were formed (4 days) and were then treated with AIIB2 at doses ranging from 0.08 to 0.24 mg/mL, or with isotype-matched nonspecific rat IgG1 as control (Fig. 1B). Using confocal microscopy, we show that β1 integrin was appropriately localized to the basolateral surfaces of the S-1 cells within the acini, as is the case in vivo. In contrast, it was diffusely distributed around the surfaces of each cell within T4-2 and MDA-MB-231 colonies in three-dimensional lrECM, and little expression was seen on three of the other cell lines (Fig. 1B).
Figure 1.
Morphology and response of breast cell lines to β1 integrin inhibition in three-dimensional lrECM. Five malignant breast cell lines (T4-2, MDA-MB-231, SKBR3, BT474, and MCF-7) and one nonmalignant breast cell line (S-1) were propagated in three-dimensional lrECM and treated with β1 integrin inhibitory antibody, AIIB2, after colonies were formed. A, schema of the three-dimensional lrECM assay used in this study. B, top and middle, phase-contrast micrographs of cultured colonies before and after antibody treatment, respectively. Bar, 20 µm. Bottom, confocal midsections of β1 integrin immunofluorescence ( ) of colonies cultured in three-dimensional lrECM. Phalloidin was used to stain actin filaments (
) of colonies cultured in three-dimensional lrECM. Phalloidin was used to stain actin filaments ( ), and DAPI was used to stain individual nuclei (
), and DAPI was used to stain individual nuclei ( ). Bar, 13 µm for all cell lines shown. C, whereas there were no significant changes in Ki-67 or TUNEL expressing cells among nonmalignant S-1 cells after β1 integrin inhibition, malignant cell lines, except SKBR3, had a significant decrease in Ki-67 expressing cells and a significant increase in TUNEL-positive cells after antibody treatment. Columns, mean (n = 3); bars, SE. P < 0.05, t test.
). Bar, 13 µm for all cell lines shown. C, whereas there were no significant changes in Ki-67 or TUNEL expressing cells among nonmalignant S-1 cells after β1 integrin inhibition, malignant cell lines, except SKBR3, had a significant decrease in Ki-67 expressing cells and a significant increase in TUNEL-positive cells after antibody treatment. Columns, mean (n = 3); bars, SE. P < 0.05, t test.
In assays starting from single cells, AIIB2 concentrations of 0.10 to 0.16 mg/mL were sufficient to induce reversion (17, 19). In the current procedure, colonies were analyzed for percentage of proliferating cells using Ki-67 nuclear antigen and for apoptosis by TUNEL assay. After 3 days of treatment, all but one of the malignant cell lines showed a significant proportional decrease in the percentage of proliferating cells (46–54% of Ki-67 expressing cells at 0.08 mg/mL AIIB2 and 0.24 mg/mL AIIB2, respectively; P < 0.02, Student’s t test for any AIIB2 dose compared with controls for T4-2, MDA-MB-231, BT-474, and MCF-7; Fig. 1C). The only exception among the five malignant cell lines was SKBR3, which did not show a significant decrease in the percentage of Ki-67–positive cells with AIIB2 treatment. Apoptosis was assayed simultaneously: there was a dramatic increase in TUNEL-positive nuclei for all malignant cell lines (85–88% at 0.08 mg/mL AIIB2 and 0.24 mg/mL AIIB2, respectively; Fig. 1D; n = 3). For the MDA-MB-231 cell line, the higher dose of AIIB2 was associated with a statistically significant increase in TUNEL-positive nuclei, whereas the P approached significance for the lower dose (hence the absence of the *).
In contrast, the nonmalignant cell line S-1 formed acinar structures when cultured on top of three-dimensional lrECM for 6 days and, unlike colonies made of malignant cells, did not undergo increased apoptosis or cytostasis upon addition of AIIB2 regardless of the dose used (Fig. 1C and D). Similar results were obtained for S-1 cells treated at day 4 (data not shown). In addition, there was no significant change in the distribution of the size or number of total colonies (data not shown). Previous studies have shown that AIIB2 applied to single S-1 cells induce apoptosis (27); however, in the present study, we show that when S-1 cells are in the context of organized structures, they are resistant to apoptosis. This indicated that the signaling context of β1 integrin is critical to response to AIIB2 treatment: nonmalignant mammary epithelial cells with intact cell-cell and cell-ECM interactions were resistant to the inhibitor. These results confirm and extend studies of conventional apoptotic and chemotherapeutic agents tested previously in the single-cell assay in three-dimensional lrECM (29).
Coexpression of total β1 integrin, phosphorylated β1 integrin, and phosphorylated 397FAK among breast cell lines cultured in three-dimensional lrECM
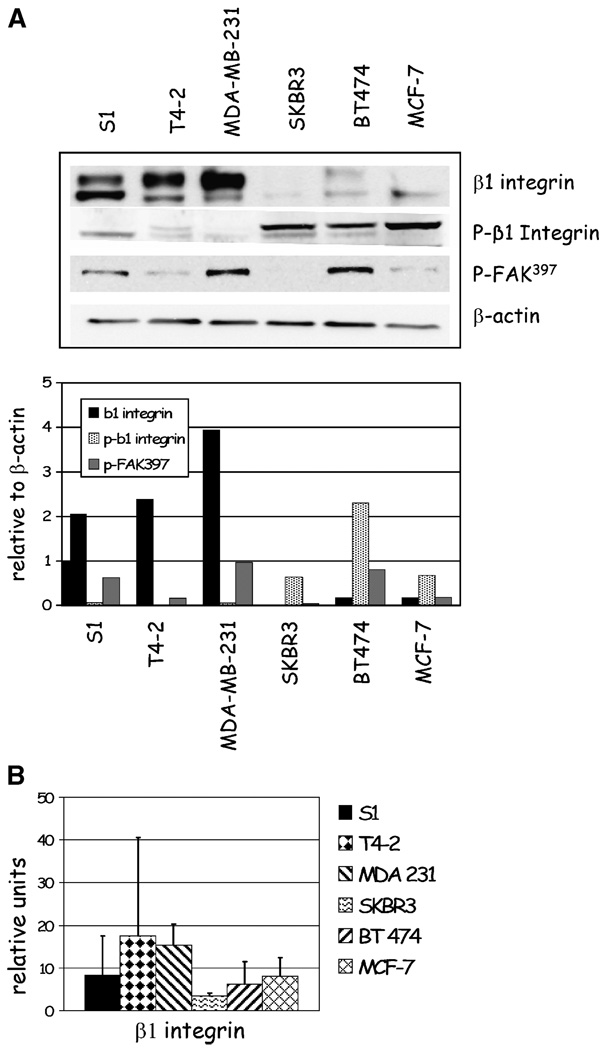
β1 Integrin expression detected by immunofluorescence was characterized by basolateral localization in nonmalignant S-1 acinar structures and disorganized and aberrant expression in the malignant cell lines. To further characterize β1 integrin expression, we analyzed cell lysates for total β1 integrin levels using Western immunoblotting. Total β1 integrin expression corresponded to that detected using immunofluorescence; three cell lines (S-1, T4-2, and MDA-MB-231) showed relatively higher levels of β1 integrin compared with SKBR3, BT474, and MCF-7 (Fig. 2A). In addition, fluorescence-activated cell sorting (FACS) analysis confirmed the surface expression of β1 integrin reflected that detected using immunofluorescence and Western blot (Fig. 2B). We concluded that β1 integrin expression was variable, and response to β1 integrin inhibitory antibody did not seem to correlate with total levels of β1 integrin expression in individual cell lines.
Figure 2.
β1 Integrin, p-β1 integrin, and p-397FAK are expressed at different levels in breast cell lines in three dimensions. Lysates from five breast cancer cell lines (T4-2, MDA-MB-231, SKBR3, BT474, and MCF-7) and one nonmalignant cell line (S-1) in three-dimensional lrECM were probed by Western immunoblotting for total and p-β1 integrin and p-397FAK and for surface β1 integrin expression by FACS analysis. A, total β1 integrin levels are relatively high in S-1, T4-2, and MDA-MB-231 cells compared with SKBR3, BT474, and MCF7 cells. Conversely, p-β1 integrin levels are relatively low in S-1, T4-2, and MDA-MB-231 cells compared with SKBR3, BT474, and MCF7 cells. Expression of p-397FAK is expressed among all cell lines except SKBR3, where it is undetectable by Western blots. B, surface expression of β1 integrin by FACS analysis corresponds to that seen by immunofluorescence (Fig. 1B) and Western blots (Fig. 1A).
We reasoned that signaling proteins that are critical in β1 integrin signaling, such as phosphorylation of β1 integrin cytoplasmic tail (30), or focal adhesion kinase (FAK; refs. 31, 32), may correlate with response to AIIB2 treatment. To test this, protein lysates from the six cell lines propagated in three-dimensional lrECM were used to detect relative coexpression of β1 integrin, phosphorylated β1 integrin (p-β1 integrin), and phosphorylated 397FAK (p-397FAK). We found that p-β1 integrin levels were relatively lower in S-1, T4-2, and MDA-MB-231 cells compared with SKBR3, BT474, and MCF-7 cell lines, which inversely correlated with total β1 integrin levels (Fig. 2A). p-397FAK levels did not seem to correlate with total β1 integrin or p-β1 integrin levels. Interestingly, p-397FAK levels were lowest in SKBR3 cells, which were refractory to AIIB2-induced cytostasis.
SKBR3 colonies respond to a combination of AIIB2 and Herceptin
The SKBR3 cell line overexpresses erbB2 (HER-2), a member of the EGF family of growth factor receptors. β1 integrin has been shown to cooperate with other members of the EGF family, such as erbB1 (19); however, the relationship between HER-2 and β1 integrin signaling is not well understood. We reasoned that HER-2 signaling was one factor that could contribute to the decreased cytostatic response of SKBR3 cells treated with AIIB2. Herceptin is a monoclonal antibody directed against HER-2 and has a significant role in treatment of patients with HER-2 overexpressing breast cancer (33). Therefore, we tested the effect of Herceptin and AIIB2 in combination in SKBR3 cells. Compared with colonies treated with nonspecific control IgG, SKBR3 colonies treated with AIIB2 or Herceptin alone showed a proportional decrease in Ki-67–positive cells (44.8% for AIIB2 and 39.1% for Herceptin). However, colonies that were treated with both AIIB2 and Herceptin had an augmented proportional decrease in Ki-67–positive cells (68.8%; P < 0.05, χ2; Supplementary Fig. S1).
β1 integrin inhibition preferentially affects larger tumor masses with a global redistribution in colony size and morphology
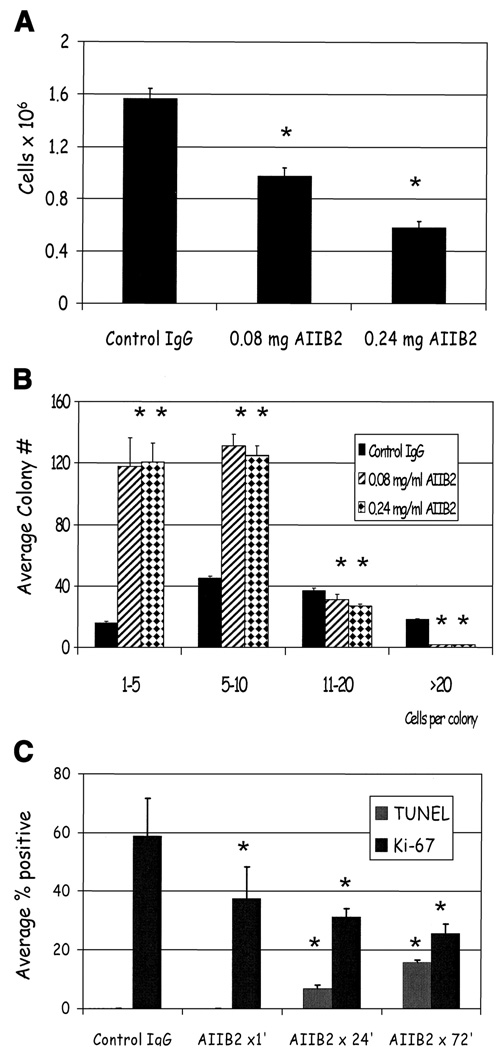
To determine the effect of treatment on the colony population as a whole, we counted the total number of cells and then scored for individual colonies by size. Using T4-2 cells as a prototype, we found that β1 integrin inhibition resulted in a significant decrease in total cell number (Fig. 3A, mean ± SE; P < 0.05, χ2). To further examine how the treatment influenced the global composition of colonies in the population, we counted the number of cells within each colony after 3 days of treatment. The mean colony size decreased from 12 to 6 cells with treatment reflected by the distribution of the size of colonies (Fig. 3B, mean ± SE; P < 0.05, t test). Similar results were seen for all other cancer cell lines (data not shown). To investigate the time course and mechanism of these changes, we counted the average number of Ki-67– and TUNEL-positive nuclei in the T4-2 cultures as a function of time after addition of AIIB2 (Fig. 3C, mean ± SE; P < 0.05, t test). The number of proliferating cells decreased dramatically even within 1 hour after addition of AIIB2, indicating an immediate growth arrest. The percentage of TUNEL-positive nuclei increased from 24 to 72 hours.
Figure 3.
AIIB2 reduces both total cell number and average colony size in malignant cell lines. A, average number of T4-2 cells after 3 days of treatment with AIIB2. Columns, mean; bars, SE. P < 0.05, χ2. B, histogram showing the average colony size decreased with AIIB2 compared with controls. Columns, mean; bars, SE. P < 0.05, t test. C, percentage of Ki-67 and TUNEL expressing nuclei among T4-2 cells 1, 24, and 72 hours after addition of AIIB2 to cultures. Columns, mean; bars, SE. P < 0.05, t test.
Treatment with AIIB2 results in decreased tumor formation, increased apoptosis, and cytostasis in vivo
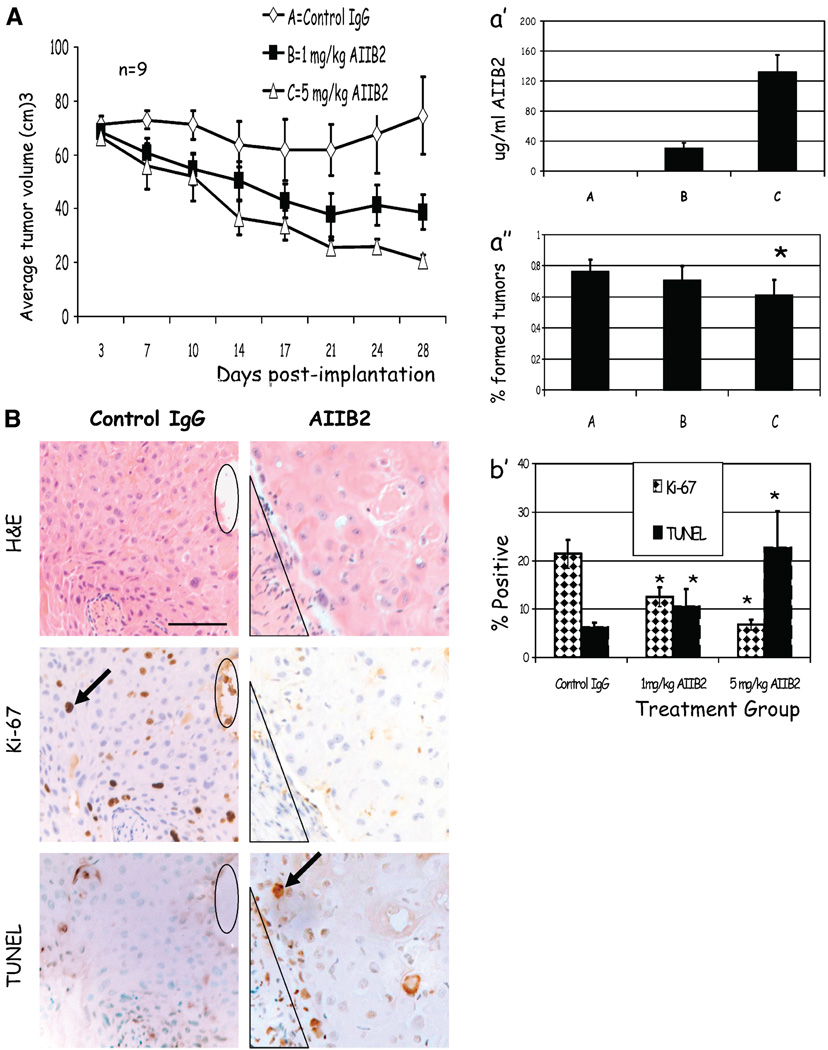
We have shown previously that breast cancer cells that have been pretreated with β1 integrin inhibitors before injection into nude mice have decreased ability to form tumors in vivo (17, 26). To determine the efficacy and optimal dose of AIIB2 that effectively inhibits tumor formation in vivo, we tested the ability of AIIB2 to inhibit untreated T4-2 cells to form tumors in adult female nu−/− mice. Animals were implanted with 5 to 10 × 106 T4-2 cells or 107 MCF-7 cells either s.c. or into the mammary fat pad on day 0. Three groups of mice (n = 9) received biweekly i.p. injections of (a) isotype-matched nonspecific rat IgG1, (b) 1 mg/kg AIIB2, or (c) 5 mg/kg AIIB2 in a blinded fashion beginning on day 4. Tumors were measured biweekly, and volume was estimated by multiplying width × length × depth. Compared with tumors propagated in animals that received control IgG, there was a significant dose-dependent decrease in the volume of treated tumors (Fig. 4A) and in the number of animals harboring tumors (Fig. 4A′; P < 0.03, χ2). After 4 weeks, animals were sacrificed, serum was analyzed for AIIB2 levels, and tumors were analyzed for histology. Compared with controls, treated animals had a dose-dependent level of AIIB2 detectable in serum samples (Fig. 4A″, mean ± SE; P < 0.05, χ2). Representative micrographs of the same coregistered region of a tumor stained with H&E, Ki-67, and TUNEL are shown (Fig. 4B)
Figure 4.
AIIB2 suppresses tumor growth in vivo by enhancing apoptosis and decreasing proliferation. A, mean tumor volumes among animals that received 1 mg/kg AIIB2 (■) or 5 mg/kg AIIB2 (△) were significantly smaller than control animals (♦). Points, mean (n = 9); bars, SE. A′, average serum concentration (µg/mL) of AIIB2 at the time of sacrifice was significantly higher in animals receiving AIIB2 compared with control vehicle. Columns, mean; bars, SD. A″, average number of animals in each treatment group that had histologic evidence of tumor at the time of sacrifice was significantly lower among treated animals compared to controls. Columns, mean for three separate experiments; bars, SE. *, P < 0.03, χ2. B, micrographs of serial sections of tumors that were scored for apoptosis by the presence of TUNEL-positive nuclei and for proliferation by the presence of Ki-67 nuclear antigen. Black ovals and triangles, coregistered regions in serial sections; arrows, examples of positive staining. B′, with AIIB2 treatment, the average number of TUNEL nuclei increased, and the number of Ki-67–positive nuclei decreased. Columns, mean; bars, SE. *, P < 0.05, χ2. Bar, 247 µm.
Sections from each tumor were evaluated for apoptosis by TUNEL assay and proliferation by Ki-67 (Fig. 4B′). Compared with controls, treated tumors had significantly decreased percentage of Ki-67–positive cells and a significantly increased level of TUNEL-positive nuclei (mean ± SE; P < 0.05, χ2). In addition, tumors treated with 5 mg/kg AIIB2 had significantly higher percentage of caspase-3–positive cells (11 ± 2%) compared with controls (3.13 ± 0.7%; P < 0.05, χ2). Similar results were obtained for MCF-7 xenografts treated with AIIB2 in vivo (data not shown).
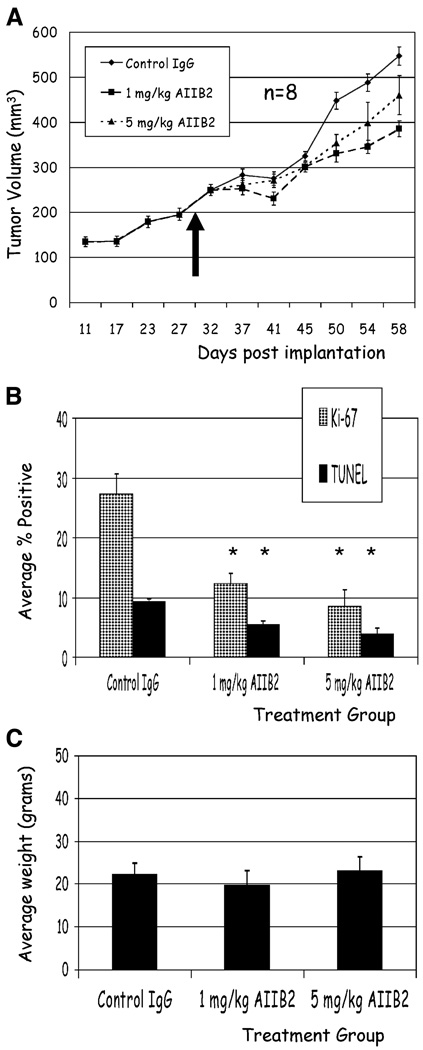
AIIB2 is effective against established tumors in vivo
To further evaluate the efficacy of AIIB2 in vivo, we allowed MCF-7 cells to continue to grow for ~4 weeks and then randomized animals to receive nonspecific rat IgG1, 1 mg/kg AIIB2, or 5 mg/kg AIIB2 for four additional weeks. Compared with controls, treated animals had significantly less tumor growth (Fig. 5A). In addition, histologic analysis showed that treated tumors had significantly fewer Ki-67–positive cells compared with controls (Fig. 5B, mean ± SE; P < 0.001, χ2) and significantly decreased TUNEL-positive nuclei (Fig. 5B, mean ± SE; P < 0.01, χ2). Similar results were found for T4-2 xenografts treated in vivo (data not shown).
Figure 5.
AIIB2 effectively induces cytostasis in established MCF-7 tumors in vivo with no toxicity. A, animals were randomized to receive treatment or control IgG 4 weeks after MCF-7 cell implantation (arrow, time of randomization at day 30). Mean tumor volumes for animals receiving 1 mg/kg AIIB2 (■) and 5 mg/kg AIIB2 (△) and nonspecific rat IgG1 (♦). Points, mean (n = 8); bars, SE. B, compared with controls, tumors from animals that received AIIB2 treatment had significantly fewer Ki-67–positive nuclei and fewer TUNEL-positive nuclei. Columns, mean; bars, SE. *, P < 0.001, χ2. C, average weight of control animals, and animals that received AIIB2 treatment were not significantly different at any biweekly time point measured throughout the experiment (data not shown) and at the end of the experiment. Columns, mean; bars, SE.
There is no discernible toxicity with β1 integrin inhibition in vivo
Animals were monitored for any signs of toxicity by measuring weekly weight and assessing activity and general appearance. There was no difference in animal weight between the treated or control groups (Fig. 5C), and no discernible toxicity among any groups, up to AIIB2 doses of 20 mg/kg administered biweekly over 4 weeks (data not shown).
Although AIIB2 seems to cross react with murine β1 integrin,6 we sought to further evaluate the potential toxicity of broad β1 integrin inhibition in vivo. Therefore, we used clone Ha2/5, a β1 integrin function-blocking antibody that recognizes murine β1 integrin. Adult female nu−/− mice were treated with serially increasing doses of antibody from 1 to 20 mg/kg over 4 weeks via biweekly i.p. injection. There were no differences in body weight, activity, overall appearance, or examination at necropsy among animals receiving antibody compared with controls, and no evidence of toxicity among any groups (data not shown).
Discussion
Recent advances in cancer therapy have taken advantage of the aberrant receptors in tumor cells to inhibit growth and enhance the efficacy of conventional cytotoxic treatments (2). β1 Integrin belongs to a class of cell surface receptors that not only facilitates growth factor receptor signaling but also plays diverse roles in mediating multiple aspects of malignant cell behavior. Indeed, expression of β1 integrin was shown recently to be necessary for formation of mammary tumors in engineered murine models (4). In addition, β1 integrin has been shown to enhance survival by mediating resistance to cytotoxic treatment in several cancers (9, 34). The success of any therapy depends on its ability to distinguish between malignant and normal tissues or the therapeutic index. Taking advantage of the modified three-dimensional lrECM culture assay, we show that β1 integrin inhibitory monoclonal antibody effectively distinguishes between normal and malignant tissue structures. Treatment of malignant colonies with AIIB2 resulted in a dramatic loss in total cell number with a concomitant decrease in proliferation and increase in apoptosis. In addition, there was a global redistribution in the malignant colony size and morphology, reflected by a decrease in mean colony size. In contrast, nonmalignant epithelial cells that were capable of forming organized and polar structures with appropriate cell-ECM interactions remained intact and were resistant to β1 integrin inhibition. In vivo, AIIB2 treatment inhibited tumor growth with an associated decrease in proliferation and increase in cell death in early treated tumors and a decrease in proliferation in treatment of established tumors, with no measurable toxicity to the host. Overall, these results indicate that β1 integrin inhibition is a potentially viable therapeutic approach in the treatment of breast cancer.
The use of three-dimensional cultures provides a physiologically relevant context in which to emulate cells in vivo (35, 36) and has been used previously to investigate novel mechanisms of drug resistance in cancer cells that are demonstrable specifically only in a three-dimensional setting when the appropriate basement membrane molecules are present (37, 38). To model the differences between normal and malignant tissues, we took advantage of the ability of a nonmalignant cell line, HMT-3522-S-1, to undergo normal morphogenesis in three-dimensional lrECM, in contrast to malignant cells that continue to form disorganized invasive colonies. This allowed us to examine the effects of β1 integrin inhibition on the morphology of cancer cell colonies as a population and to distinguish the potential effects on nonmalignant acini. We had shown previously that nonmalignant cells that were treated with β1 integrin inhibition as single cells were susceptible to apoptosis (27, 39). However, the response of cells within acinar-like tissue structures where β1 integrin function is relatively intact has not been investigated. We found that in response to 3 days of AIIB2 treatment, all but one malignant cell line in three-dimensional lrECM showed a dramatic loss in total number of cells, coupled with a significant increase in the percentage of apoptotic cells and a significant decrease in the percentage of proliferating cells. In contrast, S-1 cells that formed polar acinar-like structures were entirely resistant to AIIB2. These results indicate that most malignant cells that form colonies in three-dimensional lrECM rely on β1 integrin signaling for proliferation and survival, whereas in the context of an organized structure, cells were either no longer dependent on β1 integrin signaling for survival, or that β1 integrin was not accessible to the antibody.
Further analysis of cell cultures during and after AIIB2 treatment revealed that the largest cancer cell colonies were being affected, resulting in a global change in the morphology and distribution of proliferating cells, reflected in a decrease in mean colony size. This pattern of multiple residual “tumor foci” was seen also in vivo (data not shown). The morphologic characteristics of these clones were distinctly different from the untreated tumors, as were features of cell-cell and cell-ECM interactions. These results have implications for clinical treatment. β1 Integrin has been implicated in mediating resistance to cytotoxic chemotherapies (9, 10), and inhibition of different tumor types may enhance response by abrogating resistance. In addition, ionizing radiation was shown to up-regulate β1 integrin in cancer cells (40, 41), and our preliminary studies of β1 integrin inhibition combined with ionizing radiation are promising and may lead to novel strategies for combinatorial therapies to eradicate or further reduce tumor viability in vivo.
Several promising biological therapies aimed at signaling pathways have entered clinical trials; however, despite evidence of response to treatment, useful biomarkers have frequently been difficult to validate (1, 42, 43). For example, the current treatment of cancers with EGFR inhibition illustrates the complexity of some molecular targets and the lack of robust predictive markers that would aid in the selection of individuals for treatment (42). The mechanisms that are involved in cytostasis and apoptosis associated with β1 integrin inhibition in malignant cells are likely to involve interactions between multiple signaling pathways. For example, our previous studies have shown that β1 integrin signaling pathway integrates and cooperates with the EGFR signaling pathway via mitogen-activated protein kinase and phosphatidylinositol 3-kinase (18, 19). In the present study, we found that β1 integrin expression on the six breast cell lines used was variable. We probed the cell lines for p-β1 integrin and p-397FAK to investigate potential markers for β1 integrin signaling activity. Interestingly, p-β1 integrin expression was inversely correlated with total β1 integrin, suggesting that either species is required for β1 integrin signaling to occur. Although p-397FAK is a requisite protein for focal adhesion formation, its role in β1 integrin signaling in the context of the cell lines we investigated remains unclear. We recognize that β1 integrin signaling involves several steps, including activation, heterodimerization, ligand binding, and clustering (44, 45); these functional aspects of β1 integrin signaling activity may not be reflected by the level of receptor expression and/or status of any single signaling protein alone. The major factor that distinguished the nonmalignant S-1 cells and the malignant cell lines is the organization and polarity of β1 integrin localization, indicating that the context of signaling may be the most important feature that enhances the therapeutic window. Studies are ongoing to investigate which pathways may be the most robust predictors of response in to β1 integrin inhibition in the clinical setting.
SKBR3 cells were less responsive to β1 integrin inhibition compared with other cancer cell lines. This cell line is characteristically devoid of E-cadherin and overexpresses growth factor receptor HER-2 features that could contribute to uncoupling of β1 integrin signaling and survival (46, 47). Interestingly, BT474 cells, which overexpress HER-2 and estrogen receptor (ER), remain sensitive to AIIB2. In contrast, SKBR3 cells overexpress HER-2 but are ER negative, a phenotype that has implicated growth factor signaling pathways with resistance to tamoxifen (48). Herceptin, a monoclonal antibody against HER-2, has been shown to down-modulate the HER-2 receptor, resulting in cytostasis (24). We found that the addition of Herceptin to AIIB2 in SKBR3 cells in three-dimensional lrECM resulted in a significantly decreased percentage of Ki-67–positive cells compared with cultures treated with AIIB2 alone. These data indicate that an additive cytostatic effect is achieved by using the combination of inhibitory antibodies. Further investigations of the features of SKBR3 that may confer resistance to AIIB2 are warranted and may help identify subsets of tumors that may respond to a combination of β1 integrin inhibition and Herceptin or hormonal therapy.
We found that β1 integrin inhibition was effective in both T4-2 and MCF-7 xenografts in nude mice in vivo, confirming our results in three-dimensional lrECM. Similar to the response observed in culture, tumor xenografts treated in vivo showed decreased proliferation and increased apoptosis compared with controls in the animals that received treatment beginning 4 days after tumor implantation. In animals where the tumors were treated after 4 weeks of implantation, there was a significant decrease in tumor size and proliferation and a decrease in apoptosis in treated animals compared with controls. The decrease in observed TUNEL-positive cells in the larger tumors could be due to the increased amounts of necrosis, an alternate mechanism of cell death, seen in larger tumors (data not shown).
Toxicity studies using AIIB2 and clone Ha2/5 revealed no discernible toxicity in animals, even with 20 mg/kg doses. These results indicate that β1 integrin signaling confers growth and survival advantages in cancer cells in vivo that can be discriminated from normal β1 integrin signaling by AIIB2. Other mechanisms also should be considered. For example, immune-mediated secondary effects of the antibody have been shown to play a significant role in antibody-mediated therapies (47). We have previously shown that the F(ab′)2 fragments of AIIB2 are active in three-dimensional lrECM assays (17), and others have shown that AIIB2 binds to a region of β1 integrin extracellular domain between two putative ligand binding sites that are thought to induce a conformational change (48), resulting in down-modulation of signaling. The activity or presence of Fc-directed immune response in vivo has not been isolated from the activity of the F(ab′)2 region per se. These studies, in addition to humanization of the AIIB2 clone, are necessary next steps towards clinical drug development.
In summary, β1 integrin inhibition using monoclonal antibody AIIB2 results in cytostasis and apoptosis in malignant breast cancer colonies but not normal tissue structures propagated on top of three-dimensional lrECM gels. The three-dimensional lrECM assay appropriately distinguishes the difference in response between normal structures and malignant colonies and reveals global changes in morphology associated with treatment. In addition, AIIB2 inhibits breast cancer growth in vivo by eliciting increased apoptosis and decreased proliferation, with no discernible toxicity to animals. We conclude that β1 integrin inhibition using monoclonal antibodies is a promising approach to breast cancer treatment, and that the modified three-dimensional lrECM assay and protocol is an appropriate assay for testing differences in malignant and normal cell response to targeted therapeutic agents.
Acknowledgments
Grant support: UCSF-REAC; Cooperative Institutional Research Program; NIH P50 Specialized Programs of Research Excellence grant CA CA58207-08 (C. Park); U.S. Department of Energy, Office of Biological and Environmental Research grant DE-AC03-76SF00098 (M.J. Bissell); NIH/National Cancer Institute grants CA64786-09 (M.J. Bissell) and P50 CA112970-01 (J.W. Gray and M.J. Bissell); and U.S. Department of Defense Breast Cancer Research Program’s Innovator Award DAMD17-02-1-0438 (M.J. Bissell).
We thank Donghui Wang and Evelyn Yao for expert technical assistance.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Unpublished data.
References
- 1.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 4.White DE, Kurpios NA, Zuo D, et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Berry MG, Gui GP, Wells CA, Carpenter R. Integrin expression and survival in human breast cancer. Eur J Surg Oncol. 2004;30:484–489. doi: 10.1016/j.ejso.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Gui GP, Wells CA, Yeomans P, et al. Integrin expression in breast cancer cytology: a novel predictor of axillary metastasis. Eur J Surg Oncol. 1996;22:254–258. doi: 10.1016/s0748-7983(96)80013-8. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LM. Integrin function in breast carcinoma progression. J Mammary Gland Biol Neoplasia. 1999;4:367–376. doi: 10.1023/a:1018766317055. [DOI] [PubMed] [Google Scholar]
- 8.Zutter MM, Krigman HR, Santoro SA. Altered integrin expression in adenocarcinoma of the breast Analysis by in situ hybridization. Am J Pathol. 1993;142:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- 9.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 10.Damiano JS. Integrins as novel drug targets for overcoming innate drug resistance. Curr Cancer Drug Targets. 2002;2:37–43. doi: 10.2174/1568009023334033. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JM, Truong TN, Schwartz MA. Integrins regulate the apoptotic response to DNA damage through modulation of p53. Proc Natl Acad Sci U S A. 2002;99:3627–3632. doi: 10.1073/pnas.062698499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura T, Endo Y, Yonemura Y, et al. Significance of integrin α2/β1 in peritoneal dissemination of a human gastric cancer xenograft model. Int J Oncol. 2001;18:809–815. [PubMed] [Google Scholar]
- 14.Fujita S, Watanabe M, Kubota T, et al. Alteration of expression in integrin β1-subunit correlates with invasion and metastasis in colorectal cancer. Cancer Lett. 1995;91:145–149. doi: 10.1016/0304-3835(95)03735-f. [DOI] [PubMed] [Google Scholar]
- 15.Elliott BE, Ekblom P, Pross H, et al. Anti-β1 integrin IgG inhibits pulmonary macrometastasis and the size of micrometastases from a murine mammary carcinoma. Cell Adhes Commun. 1994;1:319–332. doi: 10.3109/15419069409097263. [DOI] [PubMed] [Google Scholar]
- 16.Bissell MJ, Weaver VM, Lelievre SA, et al. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757s–1763s. discussion 1763–4s. [PubMed] [Google Scholar]
- 17.Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol. 2004;164:603–612. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Weaver VM, Petersen OW, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briand P, Petersen OW, Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 21.Hall DE, Reichardt LF, Crowley E, et al. The α1/β1 and α6/β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomaselli KJ, Damsky CH, Reichardt LF. Purification and characterization of mammalian integrins expressed by a rat neuronal cell line (PC12): evidence that they function as α/β heterodimeric receptors for laminin and type IV collagen. J Cell Biol. 1988;107:1241–1252. doi: 10.1083/jcb.107.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werb Z, Tremble PM, Behrendtsen O, et al. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28:4–11. doi: 10.1016/s0093-7754(01)90276-3. [DOI] [PubMed] [Google Scholar]
- 25.Ott RL. Introduction to statistical methods and data analysis. 5th ed. Belmont (CA): Duxbury Press; 2001. [Google Scholar]
- 26.Wang F, Hansen RK, Radisky D, et al. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howlett AR, Bailey N, Damsky C, et al. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- 28.Lelievre SA, Bissell MJ. Three dimensional cell culture: the importance of microenvironment in regulation of function. In: Meyers RA, editor. Encyclopedia of molecular cell biology and molecular medicine. 2nd ed. New York: Wiley; 2005. [Google Scholar]
- 29.Weaver VM, Lelievre S, Lakins JN, et al. Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wennerberg K, Fassler R, Warmegard B, Johansson S. Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin β1A. Requirement for threonines 788–789 in receptor activation. J Cell Sci. 1998;111:1117–1126. doi: 10.1242/jcs.111.8.1117. [DOI] [PubMed] [Google Scholar]
- 31.Yamada KM, Pankov R, Cukierman E. Dimensions and dynamics in integrin function. Braz J Med Biol Res. 2003;36:959–966. doi: 10.1590/s0100-879x2003000800001. [DOI] [PubMed] [Google Scholar]
- 32.Gu J, Tamura M, Pankov R, et al. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol. 1999;146:389–403. doi: 10.1083/jcb.146.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 34.Truong T, Sun G, Doorly M, et al. Modulation of DNA damage-induced apoptosis by cell adhesion is independently mediated by p53 and c-Abl. Proc Natl Acad Sci U S A. 2003;100:10281–10286. doi: 10.1073/pnas.1635435100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 36.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green SK, Francia G, Isidoro C, Kerbel RS. Anti-adhesive antibodies targeting E-cadherin sensitize multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer Ther. 2004;3:149–159. [PubMed] [Google Scholar]
- 38.St Croix B, Florenes VA, Rak JW, et al. Impact of the cyclin-dependent kinase inhibitor p27Kip1 on resistance of tumor cells to anticancer agents. Nat Med. 1996;2:1204–1210. doi: 10.1038/nm1196-1204. [DOI] [PubMed] [Google Scholar]
- 39.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci U S A. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordes N, Blaese MA, Meineke V, Van Beuningen D. Ionizing radiation induces up-regulation of functional β1-integrin in human lung tumour cell lines in vitro. Int J Radiat Biol. 2002;78:347–357. doi: 10.1080/09553000110117340. [DOI] [PubMed] [Google Scholar]
- 41.Meineke V, Gilbertz KP, Schilperoort K, et al. Ionizing radiation modulates cell surface integrin expression and adhesion of COLO-320 cells to collagen and fibronectin in vitro. Strahlenther Onkol. 2002;178:709–714. doi: 10.1007/s00066-002-0993-9. [DOI] [PubMed] [Google Scholar]
- 42.Rosell R, Fossella F, Milas L. Molecular markers and targeted therapy with novel agents: prospects in the treatment of non-small cell lung cancer. Lung Cancer. 2002;38 Suppl 4:43–49. doi: 10.1016/s0169-5002(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 43.Sledge GW., Jr HERe-2 stay: the continuing importance of translational research in breast cancer. J Natl Cancer Inst. 2004;96:725–727. doi: 10.1093/jnci/djh156. [DOI] [PubMed] [Google Scholar]
- 44.Ruoslahti E. Integrins as signaling molecules and targets for tumor therapy. Kidney Int. 1997;51:1413–1417. doi: 10.1038/ki.1997.193. [DOI] [PubMed] [Google Scholar]
- 45.Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol. 2000;2:E13–E14. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- 46.Ojakian GK, Ratcliffe DR, Schwimmer R. Integrin regulation of cell-cell adhesion during epithelial tubule formation. J Cell Sci. 2001;114:941–952. doi: 10.1242/jcs.114.5.941. [DOI] [PubMed] [Google Scholar]
- 47.Celetti A, Garbi C, Consales C, et al. Analysis of cadherin/catenin complexes in transformed thyroid epithelial cells: modulation by β1 integrin subunit. Eur J Cell Biol. 2000;79:583–593. doi: 10.1078/0171-9335-00083. [DOI] [PubMed] [Google Scholar]
- 48.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 49.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 50.Takada Y, Puzon W. Identification of a regulatory region of integrin β1 subunit using activating and inhibiting antibodies. J Biol Chem. 1993;268:17597–17601. [PubMed] [Google Scholar]