Abstract
The changes in tissue architecture that accompany the development of breast cancer have been the focus of investigations aimed at developing new cancer therapeutics. As we learn more about the normal mammary gland, we have begun to understand the complex signaling pathways underlying the dramatic shifts in the structure and function of breast tissue. Integrin-, growth factor-, and steroid hormone-signaling pathways all play an important part in maintaining tissue architecture; disruption of the delicate balance of signaling results in dramatic changes in the way cells interact with each other and with the extracellular matrix, leading to breast cancer. The extracellular matrix itself plays a central role in coordinating these signaling processes. In this review, we consider the interrelationships between the extracellular matrix, integrins, growth factors, and steroid hormones in mammary gland development and function.
Introduction
Tissue architecture depends on proper cell-cell and cell-extracellular matrix interactions involving a reciprocal exchange of mechanical and biochemical information to maintain homeostasis (Grobstein 1967, Bissell et al. 1982, Sakakura 1983). Disruption of these delicately balanced interactions can result in loss of tissue structure, with severe consequences for the tissue as well as the organism. The mammary gland is a versatile model to investigate how functional and structural homeostasis is achieved and maintained because it undergoes cycles of development, differentiation, and apoptosis during the adult life of the organism. These cyclic changes involve dramatic shifts in the structure and function of the tissue, and are regulated through complex multi-hormonal and growth factor signaling pathways. Furthermore, the tumors of the breast provide examples of changes in signaling pathways and gene expression, and the corresponding loss in tissue structure and function.
In both the normal mammary gland and breast cancer, the steroid hormones, estrogen and progesterone, have important roles in tissue function (reviewed in Lapidus et al. 1998, Russo & Russo 1998). Estrogen and progesterone regulate mammary gland development at puberty and pregnancy, and are associated with the initiation, development, and progression of breast cancer. Numerous studies have established that the reproductive history of a woman is associated with the risk of developing breast cancer, and factors that inhibit or decrease exposure to steroid hormones reduce this risk. Early pregnancy, late menarche, and early menopause all provide a protective effect against breast cancer (Staszewski 1971, Trichopoulos et al. 1972, Lambe et al. 1996). In spite of this evidence and decades of examining the mechanisms of steroid hormone action, however, we have yet much to learn about how estrogen and progesterone integrate with other signals that contribute to homeostasis, and how they may be involved in processes that lead to breast cancer.
The identification of the extracellular and intracellular components that are responsible for the maintenance of mammary gland structure and function has been the focus of the work of many investigators, including our own. We now know that the tissue microenvironment plays a dominant role in this process, and that the extracellular matrix (ECM), an essential component of the microenvironment, is intimately involved in processes that determine tissue specificity (Fig. 1; reviewed in Bissell et al. 1999). We know that the tissue microenvironment has an impact also on the initiation and maintenance of estrogen and/or progesterone responsiveness (reviewed in Woodward et al. 1998). In this brief review, we discuss the importance of cell–cell and cell–ECM interactions in maintaining tissue structure and function, and hormone responsiveness.
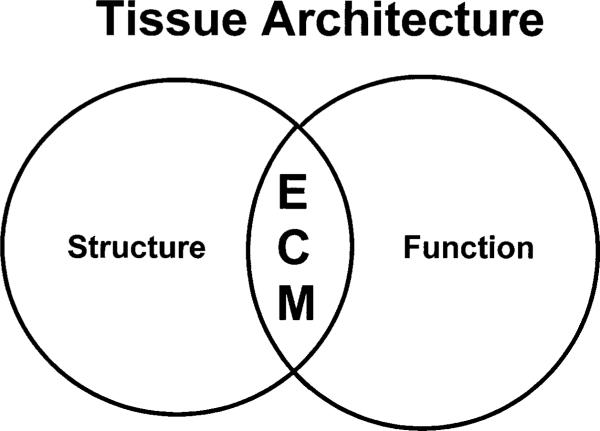
Figure 1.
Integration of structure and function by the ECM. The ECM, an essential component of the mammary gland, is intimately involved in processes that determine tissue architecture (reviewed in Bissell et al. 1999).
The organization of the mammary gland
Normal mammary gland development
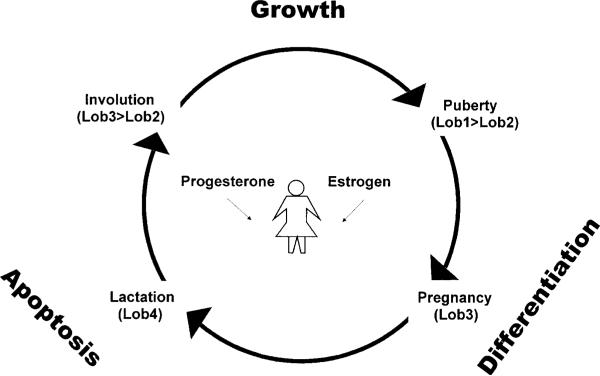
Mammary gland development occurs throughout the female life and can be divided into several stages that differ in morphology, function, and hormonal responsiveness (Fig. 2; reviewed in Ronnov-Jessen et al. 1996, Russo & Russo 1998). From birth to the onset of puberty, few changes occur in the mammary gland, and it is essentially the same in both males and females. During puberty, however, the female mammary gland responds to the production of the ovarian steroid hormone, estrogen, and the epithelium branches into numerous ducts with terminal endbuds or alveoli, collectively referred to as the terminal ductal lobular unit (TDLU). For humans, this fairly undifferentiated structure is composed of clusters of 6 to 11 ductules per lobule, and has been called lobule type 1 (or Lob 1) (Russo & Russo 1998). Lob 1 progresses to lobule type 2 (Lob 2) in the postpubertal virgin gland, with only modest alveolar proliferation producing a higher number of ductular structures per lobule during the menstrual cycle. Once pregnancy occurs, elevated levels of estrogen and progesterone, another ovarian steroid hormone, as well as pituitary hormones stimulate Lob 1 and Lob 2 to progress to lobule type 3 (Lob 3). Lob 3 is formed by epithelial expansion of existing pubertal alveoli to 80 small lobules per alveoli. These changes prime the mammary gland for milk secretion from the alveoli, now called secretory lobules type 4 (Lob 4). After parturition, the lactating mammary gland becomes insensitive to estrogen-dependent regulation of growth; during the post-weaning involution phase, responsiveness to estrogen is restored. Finally, with the cessation of lactation, the alveoli collapse and the mammary gland regresses apoptotically to its resting, pre-pregnancy state, reverting to Lob 3 and Lob 2, retaining a more extensive framework of branching than Lob 1. Thus the adult female mammary gland experiences recurrent cycles of regulated growth, differentiation, and apoptosis, and estrogen and progesterone play important roles in this process (Fig. 2; reviewed in Ronnov-Jessen et al. 1996, Russo & Russo 1998).
Figure 2.
The structure of the mammary gland is dependent on the developmental stage. The adult female mammary gland experiences recurrent cycles of regulated growth, differentiation, and apoptosis, and estrogen and progesterone play a central role in this process. The cycles that occur in the mammary gland can be divided into several stages: puberty, pregnancy, lactation, and involution. Each stage can be further described by the structure of the gland, called lobules or Lobs (reviewed in Russo & Russo 1998).
Mammary gland composition
Irrespective of the developmental status of the gland, the mammary epithelium is a bilayered structure consisting of an inner continuous layer of luminal epithelial cells and an outer layer of myoepithelial cells. The epithelial bilayer is polarized; the apical layer (luminal epithelial cells) faces the lumen of the ducts and the alveoli, and the basal layer (myoepithelial cells) is in close contact with a laminin-rich basement membrane (BM). The epithelium is embedded in the mammary stroma, which makes up >80% of the breast volume (Ronnov-Jessen et al. 1996). Types of breast stroma include fat tissue, interstitial/interlobular dense connective tissue, intralobular loose connective tissue, and blood vessels. In addition to contacting the BM, stromal cells are also surrounded by ECM that is sometimes referred to as `stromal ECM'. For the purpose of this review, we will use the term ECM to describe all the acellular, insoluble proteinaceous components that exist in the mammary tissue, including BM and stromal ECM.
In the normal human breast, approximately 20% of the luminal epithelial cells are in direct contact with the BM; the remaining cells are adjacent to the myoepithelial cells (Gusterson et al. 1982, Petersen & van Deurs 1988). The precise relationship between the luminal epithelial cells and myoepithelial cells, and the origin of these cells is largely unknown, making this an important problem for developmental biology of the mammary gland. We have recently shown that a portion of luminal epithelial cells, cultivated in culture to maintain correct functional characteristics, give rise to myoepithelial cells in an appropriate medium, but myoepithelial cells do not produce luminal epithelial cells (Pechoux et al. 1999). This finding suggests a linear relationship between these two epithelial cell types, and may be important to tumor biology because most breast cancers are luminal rather than myoepithelial in origin (Wellings et al. 1975, Rudland 1993). Myoepithelial cells have been hypothesized to play a `tumor suppressive' role by maintaining the differentiated state of luminal epithelial cells (Bani et al. 1994, Liu et al. 1996). While we have also arrived at this hypothesis, we believe that luminal epithelial cell transformation may prevent conversion to myoepithelial cells. This may explain why in premalignant lesions there are fewer myoepithelial cells, and in invasive breast cancer, myoepithelial cells are either missing or less differentiated (Gusterson et al. 1982, Guelstein et al. 1988, Rudland et al. 1995). In >90% of cases, tumor cells are restricted to a luminal-like phenotype (Altmannsberger et al. 1986, Nagle et al. 1986, Dairkee et al. 1988, Guelstein et al. 1988, Bocker et al. 1992), and only a small proportion of these cells are in contact with myoepithelial cells (Gusterson et al. 1982, Petersen & van Deurs 1988).
Although breast cancer cells originate mainly in the epithelium, evidence suggests that the stroma is an active participant in cancer progression (and possibly even induction), and constitutes the majority of the tumor mass (Dvorak 1986, Thomasset et al. 1998). Compared with normal mammary gland stroma, which mainly consists of fibroblasts and adipocytes, tumor stroma contains changes in the cellular composition and in the amounts of certain protein constituents, often referred to as `reactive stroma' or `desmoplasia'. For example, the most prominent cellular change in tumor stroma is the appearance of myofibroblasts, which are found in close proximity to tumor cell nests (reviewed in Ronnov-Jessen et al. 1996). Myofibroblasts produce proteases such as urokinase plasminogen activator and stromelysin-3, which degrade ECM and contribute to tumor cell invasion (Wolf et al. 1993, Unden et al. 1996).
The ECM of the normal mammary gland is a dense, sheetlike meshwork of collagens, fibrillar glycoproteins, and proteoglycans that are secreted by the cells, the composition of which changes during preadult development, estrous cycles, and pregnancy, lactation, and involution (reviewed in Lochter & Bissell 1995). In addition, there are both quantitative and qualitative modifications in ECM components in breast cancer (reviewed in Kosmehl et al. 1996). Since myofibroblasts produce proteases that degrade ECM, tumor stroma may alter the ECM surrounding a tumor and thus enhance the malignant potential of epithelial cells (Ronnov-Jessen et al. 1996, Thomasset et al. 1998). There are several lines of evidence that these changes in the epithelial cell microenvironment can lead to changes in cell behavior. First, cells grown in two-dimensional (2D) monolayers exhibit morphological differences compared with cells in vivo and fail appropriately to express tissue-specific genes (Emerman et al. 1977, reviewed in Stoker et al. 1990, Roskelley et al. 1995). Secondly, coculture of MCF-7 breast cancer cells with various types of fibroblasts (normal or tumor) alters the expression of genes such as estrogen receptor (ER) and progesterone receptor (PR) (Adam et al. 1994). And thirdly, three-dimensional (3D) cultured cells grown in reconstituted basement membrane (rBM) recapture some of the normal features lost in 2D cultures, such as expression of milk proteins, and the correct alveolar structure of the human breast (Barcellos-Hoff et al. 1989, Petersen et al. 1992). Thus cell–cell and cell–ECM interactions within the mammary gland are important to maintain normal epithelial structure and function.
Mammary gland morphology and breast cancer origin
The development of breast cancer is characterized by the acquisition or loss of discrete cellular functions, resulting in altered tissue organization, which has long been recognized by pathologists and used to classify breast tumors as specific morphological types (reviewed in Beckmann et al. 1997). Russo and Russo (1998) have observed that specific morphological types of breast cancer are associated with specific breast structures or developmental stages of the mammary gland. For example, the common breast malignancy, ductal carcinoma, which is thought to originate within the fairly undifferentiated epithelial cells of the TDLU, corresponds to Lob 1 (described in the first section; Fig. 2). Similarly, lobular carcinomas in situ are found in Lob 2, benign breast lesions originate in Lob 3, and lactating adenomas arise in Lob 4. Russo and Russo (1998) concluded from these observations that less functionally differentiated breast cells (Lob 1) are more susceptible to giving rise to the most undifferentiated, aggressive neoplasms. Thus the developmental stage of the breast appears to affect neoplastic transformation. Supporting this hypothesis are studies demonstrating the higher risk of malignancy in nulliparous and late parous women (Lambe et al. 1996). In spite of this evidence, we do not yet understand how the morphological and developmental stages of the mammary gland are associated with breast cancer.
It is generally accepted that development of invasive breast cancer occurs through the multistep transformation of epithelial cells via steps of hyperplasia, premalignant change, in situ carcinoma, and invasive carcinoma (reviewed in Wellings et al. 1975, Gould 1993, Beckmann et al. 1997). However, there is no evidence that each step is a necessary precursor of the next stage because it has been difficult to develop model systems with cells representing various types of breast lesions, from benign tumors to invasive carcinoma. Markers of malignant cells have been partially defined, but the characteristics of the precursor cells are less well-known, making identification difficult. Evidence does suggest, however, that certain regions of the mammary gland may be predisposed to tumor formation. Studies by Tsai et al. (1996) indicate that whole regions of the breast may originate from the same cells, i.e. that they are clonal. If these cells are `primed' for tumor formation by harboring genetic mutations, one might expect to find normal-appearing cells with genetic abnormalities in the region surrounding tumors. We now know that morphologically normal epithelia do contain many genetic mutations and that these mutations may give rise to cancer (Deng et al. 1996).
The role of steroid hormones
Mechanisms of steroid hormone action
Estrogen and progesterone promote proliferation and differentiation in the normal breast epithelium. They function via binding to their corresponding intracellular receptors, ER and PR, which are members of the nuclear hormone receptor superfamily (reviewed in Evans 1988). The process by which estrogen and progesterone interact with their receptors is similar for all members of the nuclear hormone receptor family (reviewed in White & Parker 1998). In the absence of hormones, the receptors are in an inactive complex with a number of other proteins, including heat shock proteins. When hormones pass through the cell membrane and bind the receptors, the inactive oligomeric complex dissociates and the receptors are transformed into an active state that regulates gene expression either directly as a transcription factor by binding DNA at a specific response-element (Beato & Sanchez-Pacheco 1996, Glass et al. 1996, Horwitz et al. 1996), or indirectly by cooperative interactions with other transcription factors (e.g. AP-1) (Gaub et al. 1990, Philips et al. 1993, Umayahara et al. 1994). As DNA-binding transcription factors, steroid hormone receptors do not function alone, but interact with general transcription factors and receptor interacting proteins.
In addition to this complexity, members of the nuclear hormone receptor superfamily are expressed in multiple forms. Two forms of ER are known to exist, ERα and ERβ (Green et al. 1986, Greene et al. 1986, Kuiper et al. 1996), and although most of the work described in this review concerns ERα, it is important to mention ERβ (reviewed in Enmark & Gustafsson 1998, Hansen & Fuqua 1999). ERβ is expressed in the mammary gland and in some breast cancers (Dotzlaw et al. 1997, Saji et al. 2000), but since it was very recently identified, its role in mammary gland function is largely unknown. In the rat mammary gland, the presence and cellular distribution of ERα and ERβ differs depending on the developmental stage, but during most stages there are more cells expressing ERβ than ERα (Saji et al. 2000). ERβ has a similar affinity for estradiol, and binds DNA in a manner similar to ERα, as a homodimer interacting with estrogen response elements (EREs) (Cowley et al. 1997, Kuiper et al. 1997, Pace et al. 1997, Pettersson et al. 1997). In addition, ERβ and ERα form functional heterodimers on EREs, suggesting the existence of a previously unrecognized pathway of estrogen signaling (Cowley et al. 1997, Pettersson et al. 1997). Estrogen signaling may occur through ERβ in cells expressing this subtype, through ERα in cells expressing this subtype, and through ERβ/ERα in cells expressing both subtypes. Furthermore, varying ratios of ERβ and ERα in cells expressing both subtypes may be important for estrogen responsiveness (Enmark & Gustafsson 1998).
At least two forms of PR are also known to exist, PR-A and PR-B, but unlike the ER subtypes, which are separate genes, these isoforms are transcribed from two distinct transcription start sites within the same gene (Rousseau-Merck et al. 1987, reviewed in Giangrande & McDonnell 1999). PR-A and PR-B have different transcriptional activation properties; their activity varies depending on the cell type (Tora et al. 1988, Tung et al. 1993, Vegeto et al. 1993). PR-A can either inhibit or enhance PR-B activity, depending on the cell type and promoter context (Vegeto et al. 1993). The ratio between PR forms also varies depending on cell type, and is believed to be an important factor for appropriate progesterone responsiveness (Tsai & O'Malley 1994, McDonnell 1995). For example, an imbalance in the expression and/or activities of PR-A and PR-B can result in inappropriate progesterone signaling and an aberration in mammary development (Shyamala et al. 1998, reviewed in Shyamala et al. 1999). Progesterone signaling may also be affected by the presence of a third PR, PR-C (Wei et al. 1990, 1996). PR-C is missing the first 594 amino acids at the N-terminus common to PR-A and PR-B, but still binds hormone and is capable of forming heterodimers with PR-B. Both PR-A and PR-B also modulate ER and estrogen-dependent gene expression (Chalbos & Galtier 1994, McDonnell & Goldman 1994, Kraus et al. 1995).
Finally, there are variant forms of ER and PR, which may have unique properties that influence hormone responsiveness (Fuqua et al. 1991, Leygue et al. 1999, reviewed in Lapidus et al. 1998). For ERα, variants with point mutations at a single amino acid have been identified, as well as numerous messenger RNA splice variants. ERα splice variants are usually coexpressed along with the wild-type ER message (Zhang et al. 1996), and may affect the activity of the wild-type receptor. For example, an ERα splice variant lacking exon 5 has constitutive and hormone-independent transcriptional activity (Fuqua et al. 1991). Other variants have also been identified with altered functional activities (reviewed in Hopp & Fuqua 1998).
Expression of steroid hormone receptors
The cellular distribution of ER and PR in the mammary gland is important in the complex relationships between the epithelial cells and stroma. Normal human mammary epithelial cells express very low levels of ER and PR, with only approximately 7–30% of these cells staining positive by immunohistochemistry, depending on the status of the mammary gland (Petersen et al. 1987, Ricketts et al. 1991, reviewed in Ronnov-Jessen et al. 1996). These levels fluctuate in conjunction with the cyclic changes in estrogen and progesterone during the menstrual cycle (Markopoulos et al. 1988). ER has not been detected in human mammary stroma by immunohistochemistry, although primary cultures of human breast fibroblasts have been reported to express this receptor (McCarty et al. 1986, Malet et al. 1991). Similar to ER, PR has not been detected in human mammary stroma (Press & Greene 1988). In the mouse mammary gland, ER has been detected in both epithelial and stromal cells, with only approximately 4–20% of these cells staining positive by immunohistochemistry, depending on the status of the mammary gland (Haslam & Nummy 1992). Initial PR localization studies suggested that this receptor was also expressed by both epithelial and stromal cells (Haslam & Shyamala 1981). Subsequent studies, however, revealed that PR is expressed only by epithelial cells, as is the case in human tissues (Silberstein et al. 1996, Shyamala et al. 1997). The distribution of ER and PR within the mammary gland, and the function of the receptors expressed by epithelial versus stromal cells are critical for determining how cells in the mammary gland communicate.
Steroid hormones have long been implicated in the transformation of the normal mammary gland to invasive carcinoma. Although ER and PR are expressed by a small number of cells in the normal human mammary gland, approximately two-thirds of breast cancers express ER, and half of these express both ER and PR; only one-third of breast cancers lack both ER and PR (McGuire 1978). ER- and PR-positive tumors tend to be more differentiated and are often responsive to hormonal therapies, and ER- and PR-negative tumors are generally associated with poor differentiation, and rarely respond to hormonal therapies. Even though the majority of steroid receptor-positive tumors initially respond to hormonal therapies, invariably they progress from hormone dependence to independence. The presence and concentration of these receptors, therefore, are useful markers of prognosis and survival, and can be used as clinical indices of the effectiveness of potential therapeutic intervention (McGuire & Clark 1992, Mansour et al. 1994).
In addition to being useful markers for the treatment and outcome of breast cancer, steroid hormone receptors also appear to play a role in tumor initiation and/or progression to malignancy. The role of ER in breast cancer development is based on the ability of estrogens to stimulate proliferation of mammary epithelial cells (Osborne et al. 1985, Katzenellenbogen et al. 1987). Since premalignant breast lesions are often characterized by intense epithelial cell proliferation, the presence of ER (and estrogen) could confer a selective growth advantage to these cells. The role of PR in breast cancer has not been as clear because it is an estrogen-induced product, and because progesterone modulates ER and estrogen-dependent gene expression (Chalbos & Galtier 1994, McDonnell & Goldman 1994, Kraus et al. 1995). Evidence now suggests, however, that PR may also play an important role in the development of breast cancer (Groshong et al. 1997). The proliferative index for mammary epithelial cells parallels that of progesterone levels, and progesterone also stimulates epithelial cell proliferation (Masters et al. 1977, Meyer 1977, Hissom & Moore 1987, Robinson & Jordan 1987).
Although estrogen and progesterone are key hormones in the regulation of normal and malignant mammary cell growth, there are many additional contributing factors that also regulate mammary cell behavior. For example, the peptide hormone, prolactin, is intimately involved in mammary gland development and differentiation (reviewed in Vonderhaar 1999); we will not discuss prolactin further in this review. Growth factors and their receptors also regulate mammary cell behavior. The expression of epidermal growth factor receptor (EGFR) varies throughout the menstrual cycle, and is inversely related to ER expression (van Agthoven et al. 1994, Gompel et al. 1996). From these and many other studies, it has become clear that steroid hormones and their receptors do not act independently, but function with multiple other regulatory factors through a maze of interrelated intracellular signaling pathways. The combined effect of these events may have an impact on the tissue architecture, as will be discussed in the following sections.
Models to study the mammary gland
Mammary cells require normal cell–cell and cell–ECM interactions for the maintenance of structure and function, and these interactions change during the conversion to malignancy (Lochter et al. 1997, Sternlicht et al. 1999). Steroid hormone receptor expression levels also change during the conversion to malignancy. Therefore, studies of the effect of these changes on the transformation process should accommodate the need for cells to interact with other cells and with the ECM. The ability to study how normal and malignant cells interact with each other and with the ECM, and the effect of steroid hormones on these interactions have been facilitated by the availability of ECM-like biomatrices, human breast cell lines, and animal models.
Culture models to study normal breast and malignant tumors
As discussed in the previous section, certain features of normal mammary cell cytoarchitecture are lost in 2D cultures. To simulate the 3D condition of the normal mammary gland for culture experiments, several ECM-like biomatrices have been used, including collagen I matrices (rat tail collagen) (Emerman & Pitelka 1977, Hallowes et al. 1980, Yang et al. 1980), and reconstituted basement membrane (rBM; Englebreth-Holm-Swarm tumor or EHS; Matrigel) (Barcellos-Hoff et al. 1989, Petersen et al. 1992). Using these biomatrices, we asked whether normal human breast epithelial cells could respond and recapitulate certain aspects of their normal growth and differentiation program. Normal breast epithelial cells proliferate inside collagen matrices attached to a rigid surface, and form duct-like structures but do not differentiate (Hallowes et al. 1980, Taylor-Papadimitriou et al. 1980, Foster et al. 1983). In contrast, if grown in rBM (EHS; Matrigel), these cells form polarized ducts surrounded by an endogenous BM, with the size of these structures reaching the physiological size of TDLUs, thus simulating the structure of the normal mammary gland (Petersen et al. 1992). Armed with this culture system, we tested breast carcinoma cells to determine whether they react differently from normal cells to the 3D rBM microenvironment (Petersen et al. 1992). We found that these cells did not form duct-like structures and did not growth arrest, but instead formed large clusters of cells that were not polarized or surrounded by an organized BM. Since the phenotypes of normal and tumorigenic breast cells are apparent only in 3D cultures, we hypothesized that cell–ECM interactions are important for growth and differentiation, and that changes in these interactions might influence breast cancer initiation and/or progression. Therefore, we have recently combined the 3D rBM culture system with a breast cancer progression series (reviewed in Weaver et al. 1996) to create tools to study cell–ECM interactions during breast cancer progression.
When rodent or human breast epithelial cells are cultured, they rapidly lose their functional and morphological characteristics including all steroid hormone receptor expression, in spite of the development of advanced strategies for epithelial cell isolation and culture (Emerman & Pitelka 1977, reviewed in Ronnov-Jessen et al. 1996). Most culture studies of mammary function have depended on the availability of breast cancer cell lines and immortalized nonmalignant mammary epithelial cells.
There are at least 90 malignant human breast cancer cell lines originating from primary, recurrent, or metastatic carcinomas (e.g. MCF-7, MDA-231, MDA-435, T47D, BT-20), and at least 20 nonmalignant `normal' breast epithelial cell lines immortalized spontaneously, chemically, or virally which are available for study (reviewed in Ronnov-Jessen et al. 1996). Some of these cell lines have been extensively characterized, and have been valuable tools for breast cancer studies. However, there are some limitations to the information obtained from cell lines. First, the nonmalignant cells are not exactly normal because they are immortal, and often have a number of genetic lesions. Secondly, functional comparisons between these unpaired nonmalignant and malignant cell types are limited by the possibility that the observed differences may be due to variability in cell type and not restricted to the conversion process from a normal to malignant phenotype. Thirdly, there is an enormous gap in our knowledge of the steps between normal breast epithelial cells and breast carcinoma, as discussed in the previous section. To address the second and third of these problems, several breast cancer progression models derived from single cell lineages have been developed (Stampfer & Bartley 1985, Clark et al. 1988, Band et al. 1990, Bartek et al. 1991, Shearer et al. 1992, Miller et al. 1993, Stampfer & Yaswen 1993, Wazer et al. 1994). We have chosen to use the human mammary epithelial cell (MEC) model, HMT-3522, for the reasons discussed below.
The HMT-3522 series was derived from a reduction mammoplasty of a woman with benign fibrocystic disease (Briand et al. 1987, 1996). This cell line (known as HMT-3522 S1) was established from a purified luminal epithelial cell population, and has been in culture for over 10 years (i.e. 500+ passages) without becoming tumorigenic. HMT-3522 S1 cell culture medium is completely defined and contains insulin, transferrin, epidermal growth factor (EGF), hydrocortisone, estradiol, prolactin, and sodium selenite (Briand et al. 1987). At passage 118, EGF was removed from HMT-3522 medium to test whether S1 cells could become EGF-independent and progress to malignancy. After 120 passages in the absence of EGF, the cells became tumorigenic; the cells explanted from one of these tumors were named HMT-3522 T4-2 (Briand et al. 1996). Nontumorigenic EGF-independent cells from an intermediate stage between HMT-3522 S1 and T4-2 were called S2.
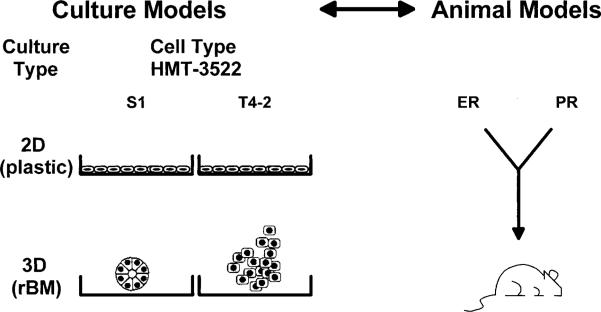
During the past five years, we have been characterizing the cells of the HMT-3522 series in 2D and 3D cultures. In 2D monolayers on plastic, S1 and T4-2 cells display similar morphology, but in 3D rBM, they look very different, as predicted from our initial studies in 3D (Fig. 3; Petersen et al. 1992, Weaver et al. 1997). S1 cells form phenotypically normal structures reminiscent of TDLUs, with regular boundaries. In contrast, T4-2 cells form disorganized clusters, with irregular boundaries and dissemination into the EHS. The first objection raised above, i.e. that established cell lines are not genetically normal, applies also to HMT-3522. However, since the HMT-3522 series appears to be a useful model system, and early passages are phenotypically normal in 3D, we have used these cells to address the role of cell adhesion, growth factor signaling, and tissue architecture in normal and malignant breast (discussed in the following sections) (Weaver et al. 1996, Wang et al. 1998, reviewed in Bissell et al. 1999).
Figure 3.
Models to study the role of ECM and steroid hormones. Culture and animal models provide complementary systems for the study of breast structure and function. Cells from the HMT-3522 human breast cancer series exhibit similar phenotypes in 2D cultures on plastic, but exhibit very different phenotypes in 3D cultures in rBM. S1 cells form phenotypically normal structures reminiscent of terminal ductal lobular units, with regular boundaries, and T4-2 cells form disorganized clusters with irregular boundaries and dissemination into the EHS (reviewed in Bissell et al. 1999). Although mouse mammary tissue varies somewhat with respect to the overall mammary gland organization, it is now clear that there are many similarities. As such, the mouse has been a valuable model for studying the effects of ER and PR function.
Animal and culture models to study steroid hormone receptors
Animal models are yet another powerful tool for studying the mammary gland. Although the system of implanting intact human breast tissue into athymic nude mice has been used to investigate the effect of steroid hormones on tumor growth for many years, the recent development of transgenic and knockout mouse models has allowed us more specifically to address molecular mechanisms of steroid hormone action (Fig. 3). For example, ERα (ERKO) and PR (PRKO) knockout mice provide definitive evidence that these hormones play an important role in mammary gland development (Lydon et al. 1995, Bocchinfuso & Korach 1997). ERKO mice develop mammary glands with only vestigial ducts present at the nipples, resembling the mammary glands found in wild-type (WT) mice before puberty (Bocchinfuso & Korach 1997). Unlike WT mice, however, there is no further development of the ERKO gland during adulthood, suggesting that estrogen signaling through ERα is critical for ductal morphogenesis. ERα is apparently not required for lobuloalveolar development, since it can be initiated in mammary glands of ERKO mice. These results contrast with those from the recently generated ERβ (BERKO) knockout mice, in which mammary development was unaffected (Krege et al. 1998).
ERα is present in both the epithelial and stromal cell components of normal mice. Therefore, it was not clear whether the lack of mammary development in the ERKO mice was due to a lack of epithelial or stromal ER. This question was addressed by performing surgical recombinations of WT ER-positive epithelium and ERKO-negative stroma or ERKO-negative epithelium and WT ER-positive stroma (Cunha et al. 1997). Ductal morphogenesis was only observed when ER-positive stroma was combined with either ER-positive or ER-negative epithelium, supporting yet another role for stroma in mammary ductal proliferation. Stromal ER is necessary for estrogen-induced epithelial proliferation, but epithelial ER is neither necessary nor sufficient for this process. Thus paracrine mechanisms play a role in ductal morphogenesis in the mammary gland.
To exclude the possibility that the lack of mammary development in ERKO mice was due to downregulation of estrogen-inducible PR, ovariectomized WT and ERKO females were treated with estradiol and the mammary glands were analyzed for PR mRNA (Bocchinfuso & Korach 1997). PR mRNA was induced 3-fold by estradiol treatment in WT mice, but was not induced in ERKO mice, suggesting that loss of ER may result in loss of PR in the mammary gland. Further insights into the role of PR in the mammary gland have been gained from analysis of PRKO mice.
Unlike ERKO mice, PRKO mice did not initially exhibit changes in the mammary gland; PRKO mice have normal ductal morphogenesis (Lydon et al. 1995). However, when ovariectomized WT and PRKO mice were treated with exogenous estrogen and progesterone, a dramatic difference was observed. WT mice developed a fully differentiated mammary gland, the typical pregnancy-associated phenotype. PRKO mice, however, exhibited limited ductal branching and lobuloalveolar maturation, suggesting that PR has a role in both ductal branching and lobuloalveolar development. As mentioned above, PR is now known to be in the epithelial and not stromal mammary components of mice. Surgical recombination of WT PR-positive epithelium and PRKO-negative stroma indicated that lobuloalveolar morphogenesis was normal in these mice, confirming that epithelial PR is required for lobuloalveolar maturation (Humphreys et al. 1997).
In addition to PRKO mice, PR-A transgenic mice have been made to simulate an imbalanced ratio of PR-A to PR-B (Shyamala et al. 1998, reviewed in Shyamala et al. 1999). These mice exhibit aberrant mammary development, as characterized by extensive lateral branching and very thick ducts. The lateral branches resembled those found in an early pregnant female except that they terminated in bulbous structures, and the ducts were composed of multilayered luminal epithelial cells, in contrast to the single layer found in normal ducts. The morphology of these mice resembles that of mice transgenic for stromelysin-1, a matrix metalloproteinase (MMP) that destroys basement membrane and is usually expressed in the glands of involuting animals (Sympson et al. 1994, Witty et al. 1995, Thomasset et al. 1998, Sternlicht et al. 1999). In PR-A transgenic mice, disorganized masses of epithelial cells accompanied by an indistinct epithelial–stromal boundary were also seen at the tip of some ducts, suggesting there was a disruption of the basement membrane, and an alteration of MMP expression and/or activity. This observation is especially relevant to this review because it suggests that steroid hormone signaling also plays a role in regulating cell–cell and cell–ECM interactions (Simian et al. 2000).
In addition to the animal models discussed above, culture systems have also been utilized in studies of steroid hormone receptors, although these studies have been challenging because receptor expression is rapidly lost in normal mammary cells that are placed in culture. There are only a few reports of the isolation of steroid hormone receptor expressing nonmalignant mammary epithelial cells. One group reported the isolation of epithelial cells expressing both ER and PR that were stimulated to proliferate by estradiol, although there was some question as to whether the cells were derived from normal epithelium or a fibrocystic lesion (Malet et al. 1991). Recently, another group has reported the successful culture of ER- and PR-positive human luminal epithelial cells (Yang et al. 2000). Cells obtained from reduction mammoplasty specimens were cultured inside 3D collagen matrices and immunostained for ER and PR, or cultured inside matrices then implanted in nude mice before immunostaining; both methods produced ER- and PR-positive cells.
Other attempts to produce receptor-positive cells have focused on transfection of immortalized human breast epithelial cells (Zajchowski et al. 1993, Lundholt et al. 1996, Pilat et al. 1996). For example, HMT-3522 S1 cells, which are ER-negative, were stably transfected with ER (ERα) to study the effect of estrogen on nonmalignant human breast epithelial cells (Lundholt et al. 1996). These ER-transfected cells were growth inhibited by both estrogen and antiestrogens, in agreement with results from another group (Zajchowski et al. 1993), suggesting that the introduction of ER into cells is not sufficient for restoring estrogen responsiveness. ER activity may depend on the availability of receptor interacting proteins and other regulatory factors, and on cellular context (reviewed in White & Parker 1998). For example, isolated mammary epithelial cells in culture do not grow in response to estrogen, but when epithelial and stromal cells are combined in co-cultures, they do respond (McGrath 1983, Haslam 1986). Our preliminary data indicate that, in addition to these cell–cell interactions, cell–ECM interactions may also be important for ER activity; we have found that the ERE is activated by ECM in a mouse epithelial cell line (C D Roskelley, V Novaro & M J Bissell, unpublished observations).
Integrating mammary gland communication
Connections between ECM and steroid hormones
Collectively, the studies utilizing ECM-like biomatrices, mammary cell lines, co-cultures, and animal models indicate that the relationships between epithelial cells, stroma, and ECM are important for tissue structure and steroid hormone responsiveness. We have demonstrated that the ECM surrounding epithelial and stromal cells is more than an acellular, insoluble proteinaceous mix of components; it coordinates diverse cellular behaviors via a combination of cell adhesion and growth factor connections between the different cells that make up the mammary gland (reviewed in Howlett & Bissell 1993). Therefore, the hormone responsiveness of mammary epithelial cells may be influenced by stromal cell production of soluble factors and/or specific ECM molecules.
Cell adhesion connections
Cell–cell and cell–ECM connections involve adhesion molecules, which when engaged, initiate cell adhesion-mediated signals that are transduced from the cell membrane to the nucleus. The bulk of studies of cell adhesion proteins have concentrated on integrins (reviewed in Hynes 1992) and cadherins (reviewed in Knudsen et al. 1998), although other molecules such as dystroglycan (reviewed in Durbeej et al. 1998, Henry & Campbell 1999) and CD44 (reviewed in Herrera-Gayol & Jothy 1999) are also important. Here we will briefly summarize integrin-related studies in the mammary gland.
The integrins are a major family of transmembrane receptors that connect the cell to the ECM on the outside, and anchor the cytoskeleton to the plasma membrane on the inside of cells (reviewed in Clark & Brugge 1995, Giancotti & Ruoslahti 1999). There are more than 20 integrin receptors, formed by heterodimerization between different α and β subunits. Normal human breast epithelial cells express at least two β-integrins (β1 and β4) and four α-integrins (α1, α2, α3, α6), which dimerize to form α1β1, α2β1, α3β1, and α6β4 receptors (Glukhova et al. 1995, Alford & Taylor-Papadimitriou 1996). Mouse epithelial cells also express at least two β-integrins (β1 and β4), and possibly as many as 7 α-integrins (α1, α2, α3, α5, α6, α7, αv) (Delcommenne & Streuli 1995). These transmembrane receptors bind the ECM glycoproteins laminin and/or collagens, and associate with components of the cytoskeleton, forming an `extracellular–intracellular bridge' involved in the transmission of signals (Clark & Brugge 1995). The integrin bridge is a bi-directional conduit for the transfer of information, as signals can be transmitted from the outside to the inside of the cell and vice versa (O'Toole et al. 1991, Du et al. 1993, Dedhar & Hannigan 1996). Much of what we know about integrin-mediated signaling has been determined through analysis of biochemical events triggered by integrin engagement. These investigations indicate that the signaling pathways stimulated by integrins overlap with those identified for growth factors, suggesting a role for integrins in tumor progression.
Both quantitative and qualitative changes in integrin expression have been associated with breast cancer, and changes in β1-, β4-, α2-, α3-, and α6-integrins have all been reported in breast cancer cell lines and mammary tumor tissue sections (Natali et al. 1992, Berdichevsky et al. 1994, Gui et al. 1995, Zutter et al. 1995). These changes in expression may result in altered cell surface ratios of individual integrins, which could affect tissue organization and tumor progression via altered intracellular signaling (Schoenenberger et al. 1994, Sastry et al. 1996). For example, in normal breast epithelial cells, signaling via α6β4 plays a role in cell morphology and growth arrest, but in malignant cells, signaling through α6β4 is impaired (Spinardi et al. 1995). Instead, tumor cells express high levels of β1 integrin, which promotes growth and may contribute to the tumorigenic phenotype of these cells (Howlett et al. 1995, Weaver et al. 1997).
The significance of β1 integrin overexpression in the phenotype of breast cancer cells was examined in our laboratory using the HMT-3522 breast cancer progression model described in the previous section (Weaver et al. 1997). β1 integrin expression was higher in tumorigenic T4-2 cells than in nontumorigenic S1 cells, and its cell surface distribution was altered. In S1 cells, integrin distribution was restricted to the basal or basolateral surface, but in T4-2 cells, it was random and disorganized. To address the significance of increased β1 integrin signaling in the phenotype of these cells, inhibitory antibodies were used to attenuate β1 integrin function in T4-2 cells. When β1 signaling was reduced, the tumorigenic T4-2 cells reverted to morphologically normal structures (resembling S1 cells), stopped growing, produced a basement membrane, and reorganized their cytoskeleton, supporting the concept that the relative distribution and proportion of integrins helps maintain normal breast structure and function. We have also shown that the integration of downstream signaling events is important in this process (Weaver et al. 1997, Wang et al. 1998). These results have led us to hypothesize that not all tumor cells necessarily lose their ability to respond to ECM-generated signals, but instead may have alterations in the level of signaling that, if attenuated, could lead to normal behavior.
Although integrins themselves lack intrinsic tyrosine kinase activity, integrin–ECM interactions recruit a number of proteins involved in signal transduction, including focal adhesion kinase, mitogen-activated protein kinase, and Rho GTPases (Miyamoto et al. 1995, reviewed in Giancotti & Ruoslahti 1999). In addition, integrins appear to be linked to activation of growth factor receptors, such as EGFR, as discussed below.
Growth factor connections
Stromal cells secrete a variety of growth factors, many of which have been shown to interact with ECM in at least three ways (reviewed in Adams & Watt 1993, Streuli 1999). First, growth factors bind the glycosaminoglycan side chains or the protein cores of specific ECM molecules. For example, platelet-derived growth factor-B binds heparin/heparan sulfate chains (LaRochelle et al. 1991), and transforming growth factor-β (TGFβ) binds the proteoglycan core proteins betaglycan and decorin (Andres et al. 1989, Yamaguchi et al. 1990). These binding interactions result in a local source of growth factors within the ECM that persists after growth factor production has ceased. Secondly, growth factors can induce production of ECM proteins, and ECM in return can affect growth factor production. TGFβ upregulates the expression of a variety of ECM components (Ignotz & Massague 1986), and ECM downregulates TGFβ expression (Streuli et al. 1993). Thirdly, cell–ECM and growth factor signaling pathways can converge or overlap. One growth factor family that is believed to play an important role in mammary gland proliferation and differentiation as well as breast cancer pathogenesis, and shares signaling components with integrins is EGF.
The EGF family consists of a subfamily of at least three neuregulins and a subfamily of at least six different growth factors, including EGF (reviewed in Normanno & Ciardiello 1997, Pinkas-Kramarski et al. 1997, Rosfjord & Dickson 1999). EGF family expression changes throughout mammary gland development, pregnancy, and lactation (Schroeder & Lee 1998, Sebastian et al. 1998). For example, expression of EGF is low in the virgin and pregnant mammary gland, but increases dramatically towards the end of pregnancy, peaking during lactation with high levels of EGF in milk, and decreases in the involuting mammary gland (Carpenter 1980, Beardmore & Richards 1983, Kenney & Dickson 1996). EGF mRNA has been detected in 83% of human breast cancer biopsy samples (Dotzlaw et al. 1990), and EGF protein has been detected by immunohistochemistry in 15–30% of human primary breast carcinomas (Mizukami et al. 1991, Umekita et al. 1992). EGF, as well as a number of other ligands, binds the EGFR, and EGFR forms homodimers or heterodimers with the ErbB2, ErbB3, and ErbB4 receptors (reviewed in Earp et al. 1995, Pinkas-Kramarski et al. 1997). Studies using EGFR knockout mice have shown that EGFR is essential for ductal growth and branching morphogenesis, but not lobuloalveolar development, in the mammary gland (Wiesen et al. 1999). The EGFR is overexpressed in approximately 14–93% of breast carcinomas (Koenders et al. 1992), and is associated with poor patient prognosis (reviewed in Fox & Harris 1997). Since the majority of breast cancers that overexpress growth factors also overexpress EGFR, signaling through this receptor may be important in breast tumor development and acquisition of hormone independence (Lundy et al. 1991, Umekita et al. 1992, reviewed in Normanno & Ciardiello 1997, Fox & Harris 1997). For example, overexpression of EGFR and the ErbB2 receptor are associated with progression to hormone independence in human breast cancer (Fitzpatrick et al. 1984, Sainsbury et al. 1985, Klijn et al. 1992), and both receptors are being explored as potential targets for cancer therapy.
Crosstalk between integrins and growth factor receptors
Integrins and growth factor receptors (i.e. EGFR) are interconnected in at least two ways (reviewed in Giancotti & Ruoslahti 1999). First, integrins are necessary for optimal activation of growth factor receptors. Although a systematic analysis has not been performed, certain integrins appear preferentially to associate with specific growth factor receptors. For example, β1 integrin appears to associate with EGFR in fibroblasts and they form a complex on the cell membrane (Miyamoto et al. 1996, Moro et al. 1998). The formation of integrin–growth factor receptor complexes enhances growth factor-dependent autophosphorylation and activation of downstream substrates. Secondly, there are reciprocal interactions between integrins and EGFR, and coupling of their signal transduction pathways. Using the HMT-3522 breast cancer progression model we examined crosstalk between β1 integrin and EGFR (Wang et al. 1998). Similar to β1 integrin, EGFR is expressed at a higher level in T4-2 cells. When either β1 or EGFR inhibitory antibodies were used to treat the cells, both β1 integrin and EGFR protein expression decreased, and the cells formed morphologically normal structures. Additional experiments determined that these changes were mediated by bidirectional integrin–growth factor receptor crosstalk via the mitogen-activated protein kinase pathway.
Crosstalk between growth factors, integrins, steroid hormones, and ECM
In addition to crosstalk between growth factor receptors and integrins, there is evidence for crosstalk between growth factors and steroid hormones (reviewed in Nicholson et al. 1999). Estrogen and progesterone can regulate the synthesis of EGFR (Berthois et al. 1989, Haslam et al. 1992, Chrysogelos et al. 1994). Numerous studies have also shown that steroid hormone receptors can be activated by growth factors. For example, ER is phosphorylated and activated by EGF, and is a target for several growth factor-induced kinases, including mitogen-activated protein kinase, casein kinase II, and p60c-src (Ignar-Trowbridge et al. 1992, Ali et al. 1993, Arnold et al. 1994, Le Goff et al. 1994, Bunone et al. 1996, Arnold et al. 1997). Growth factors appear to function cooperatively with steroid hormones to regulate mammary gland structure and function. For example, EGF, estrogen, and progesterone regulate ductal sidebranching in the mature mammary gland (Haslam et al. 1993). Mammary epithelial cells may require cooperative interactions between growth factors and estrogen for estrogen-induced responsiveness, but may not require these interactions for progesterone-induced responsiveness (Imagawa et al. 1990, Xie & Haslam 1997). Thus steroid hormone receptors may function not only as direct transducers of hormonal effects, but also as key points for the convergence of multiple signal transduction pathways (Nicholson et al. 1999). Since integrins share some of the same signal transduction pathways with growth factors, we can hypothesize that there may also be crosstalk between integrins and steroid hormones.
As discussed in the previous section, changes in integrin expression may result in altered cell surface ratios of individual integrins, which could affect tissue organization and tumor progression via altered intracellular signaling (Schoenenberger et al. 1994, Sastry et al. 1996). Since α6 integrin can dimerize with β1 and β4 integrins, and α2 and α3 can also dimerize with β1, there is a competition for β1 binding by the α integrins. Generally in this competition, α2 is the preferred β1 partner over α3, which is preferred over α6 (Schoenenberger et al. 1994); however alterations in α2, α3, or α6 expression can shift this balance. In this respect, it is very interesting that the α2 gene promoter contains ER binding sites (EREs) (Zutter et al. 1994), and the α6 gene promoter contains PR binding sites (progesterone response elements (PREs)) (Nishida et al. 1997), suggesting that ER and PR may play a role in regulation of integrin expression. ER has been correlated with α2β1 expression in breast carcinomas; in 82 ductal carcinomas of no special type, lack of ER corresponded to low or absent α2β1 expression (Lanzafame et al. 1996). α2β1 does not appear to be a major ECM receptor in ER-negative breast cancer cell lines, but it does function in ER-positive cell lines (Maemura et al. 1995).
Finally, there is a connection between growth factors, integrins, steroid hormones/steroid hormone receptors and ECM. ECM composition can be altered by a large number of proteolytic enzymes, including tissue serine proteases and the large family of matrix metalloproteinases (MMPs), in a process called ECM remodeling (reviewed in Schroen & Brinckerhoff 1996, Chambers & Matrisian 1997, Benaud et al. 1998, Lochter et al. 1998). In the normal mammary gland, MMPs cooperate with hormonal stimuli to induce some of the morphological and functional changes required at various stages, and in breast cancer, MMPs have been associated with tumor growth, invasion, metastasis, and angiogenesis (reviewed in Chambers & Matrisian 1997, Benaud et al. 1998, Lochter et al. 1998). In general, the number of different MMP family members expressed and the relative expression level of individual MMP family members tends to increase with tumor progression (Benaud et al. 1998). MMP regulation provides another link between growth factors, integrins, and steroid hormones/steroid hormone receptors. MMP expression can be regulated by growth factors, such as EGF. In response to EGF, the transcription factors Ets and AP-1 cooperate to stimulate transcription of matrilysin and stromelysins (Wasylyk et al. 1991, Gaire et al. 1994). MMP expression can also be regulated by integrins. For example, α2β1 integrin has been implicated in MMP-1 production, and α3β1 integrin is associated with MMP-2 production (Langholz et al. 1995, Kubota et al. 1997, Sugiura & Berditchevski 1999). Finally, MMP expression may be regulated by steroid hormones. Progesterone inhibits MMP-1, -2, and -9 expression (Marbaix et al. 1992), although, in a similar manner to growth factors, the effect of steroid hormones on MMP expression may be indirect, through regulation of AP-1 binding (Schroen & Brinckerhoff 1996). Finally, as discussed above, PR overexpression affects mammary gland organization by disrupting basement membrane organization, possibly through altering MMP expression and/or activity (Shyamala et al. 1998, Simian et al. 2000).
Conclusions
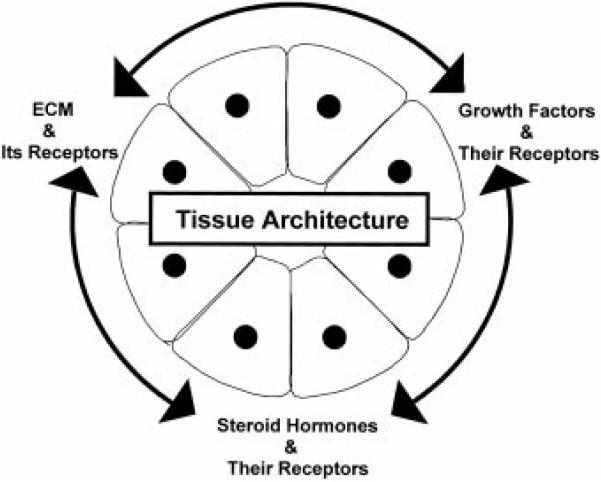
Years of investigations have established that the normal structure and function of the mammary gland depends on the reciprocal exchange of information between the different types of mammary cells and between these cells and the ECM. The studies reviewed in this article suggest that integrin-, growth factor-, and steroid hormone-signaling pathways play an important part in maintaining the structure and function of the mammary gland (Fig. 4); disruption of the delicate balance of signaling results in dramatic changes in the way cells interact with each other and with the ECM. Therefore, the integrity of the mammary gland depends on the correct integration of signaling pathways. While we do not yet know how these signals are integrated, it is clear that the ECM, its receptors, and ECM-degrading enzymes play essential roles in this process.
Figure 4.
Tissue architecture integrates, and in turn depends on, the integration of signals from ECM and its receptors, growth factors and their receptors, and steroid hormones and their receptors.
We have previously hypothesized that the unit of function in higher organisms is not the individual cell, but the tissue itself (Bissell & Hall 1987). Tissue architecture appears to be intimately involved in controlling the signaling processes within cells, and although we are just beginning to investigate how tissue morphology and signaling are connected, our current information supports this hypothesis. Future studies will provide a better understanding of tissue architecture, and they may reveal new concepts of tissue function. Experimental approaches aimed at determining the mechanisms underlying structure and function in the normal mammary gland and the changes that occur in the malignant mammary gland may provide the basis for a revolutionary approach to the prevention and treatment of breast cancer.
Acknowledgements
The authors would like to thank Ali C Ravanpay for assistance with the figures, and Albert R Davalos for constructive criticism. This work was supported by the U S Department of Energy, Office of Biological and Environmental Research (contract DE-AC03-76SF00098) to M J B, by National Institute of Health Grant CA-64786 to M J B, and by National Institute of Health Grant CA-57621 to M J B. R K H is partially supported by Cooperative Research and Development Agreement No. BG98-053(00).
References
- Adam L, Crepin M, Lelong JC, Spanakis E, Israel L. Selective interactions between mammary epithelial cells and fibroblasts in co-culture. International Journal of Cancer. 1994;59:262–268. doi: 10.1002/ijc.2910590219. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- van Agthoven T, Timmermans M, Foekens JA, Dorssers LC, Henzen-Logmans SC. Differential expression of estrogen, progesterone, and epidermal growth factor receptors in normal, benign, and malignant human breast tissues using dual staining immunohistochemistry. American Journal of Pathology. 1994;144:1238–1246. [PMC free article] [PubMed] [Google Scholar]
- Alford D, Taylor-Papadimitriou J. Cell adhesion molecules in the normal and cancerous mammary gland. Journal of Mammary Gland Biology and Neoplasia. 1996;1:207–218. doi: 10.1007/BF02013644. [DOI] [PubMed] [Google Scholar]
- Ali S, Metzger D, Bornert JM, Chambon P. Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO Journal. 1993;12:1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmannsberger M, Dirk T, Droese M, Weber K, Osborn M. Keratin polypeptide distribution in benign and malignant breast tumors: subdivision of ductal carcinomas using monoclonal antibodies. Virchows Archiv B. Cell Pathology Including Molecular Pathology. 1986;51:265–275. doi: 10.1007/BF02899036. [DOI] [PubMed] [Google Scholar]
- Andres JL, Stanley K, Cheifetz S, Massague J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. Journal of Cell Biology. 1989;109:3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SF, Obourn JD, Jaffe H, Notides AC. Serine 167 is the major estradiol-induced phosphorylation site on the human estrogen receptor. Molecular Endocrinology. 1994;8:1208–1214. doi: 10.1210/mend.8.9.7838153. [DOI] [PubMed] [Google Scholar]
- Arnold SF, Melamed M, Vorojeikina DP, Notides AC, Sasson S. Estradiol-binding mechanism and binding capacity of the human estrogen receptor is regulated by tyrosine phosphorylation. Molecular Endocrinology. 1997;11:48–53. doi: 10.1210/mend.11.1.9876. [DOI] [PubMed] [Google Scholar]
- Band V, Zajchowski D, Swisshelm K, Trask D, Kulesa V, Cohen C, Connolly J, Sager R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Research. 1990;50:7351–7357. [PubMed] [Google Scholar]
- Bani D, Riva A, Bigazzi M, Bani Sacchi T. Differentiation of breast cancer cells in vitro is promoted by the concurrent influence of myoepithelial cells and relaxin. British Journal of Cancer. 1994;70:900–904. doi: 10.1038/bjc.1994.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Kyprianou N, Lalani EN, Staskova Z, Shearer M, Chang S, Taylor-Papadimitriou J. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. PNAS. 1991;88:3520–3524. doi: 10.1073/pnas.88.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardmore JM, Richards RC. Concentrations of epidermal growth factor in mouse milk throughout lactation. Journal of Endocrinology. 1983;96:287–292. doi: 10.1677/joe.0.0960287. [DOI] [PubMed] [Google Scholar]
- Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocrine Reviews. 1996;17:587–609. doi: 10.1210/edrv-17-6-587. [DOI] [PubMed] [Google Scholar]
- Beckmann MW, Niederacher D, Schnurch HG, Gusterson BA, Bender HG. Multistep carcinogenesis of breast cancer and tumour heterogeneity. Journal of Molecular Medicine. 1997;75:429–439. doi: 10.1007/s001090050128. [DOI] [PubMed] [Google Scholar]
- Benaud C, Dickson RB, Thompson EW. Roles of the matrix metalloproteinases in mammary gland development and cancer. Breast Cancer Research and Treatment. 1998;50:97–116. doi: 10.1023/a:1006061115909. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Alford D, D'Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. Journal of Cell Science. 1994;107:3557–3568. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- Berthois Y, Dong XF, Martin PM. Regulation of epidermal growth factor-receptor by estrogen and antiestrogen in the human breast cancer cell line MCF-7. Biochemical and Biophysical Research Communications. 1989;159:126–131. doi: 10.1016/0006-291x(89)92413-3. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG. Form and function in the mammary gland: the role of extracellular matrix. In: Neville MC, Daniel CW, editors. The Mammary Gland. Plenum Publishing Corporation; New York: 1987. pp. 97–146. [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? Journal of Theoretical Biology. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Research. 1999;59:1757–1763s. discussion 1763s–1764s. [PubMed] [Google Scholar]
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. Journal of Mammary Gland Biology and Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- Bocker W, Bier B, Freytag G, Brommelkamp B, Jarasch ED, Edel G, Dockhorn-Dworniczak B, Schmid KW. An immunohistochemical study of the breast using antibodies to basal and luminal keratins, alpha-smooth muscle actin, vimentin, collagen IV and laminin. Part II: epitheliosis and ductal carcinoma in situ. Virchows Archiv A. Pathological Anatomy and Histopathology. 1992;421:323–330. doi: 10.1007/BF01660979. [DOI] [PubMed] [Google Scholar]
- Briand P, Petersen OW, Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cellular and Developmental Biology. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Research. 1996;56:2039–2044. [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO Journal. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Epidermal growth factor is a major growth-promoting agent in human milk. Science. 1980;210:198–199. doi: 10.1126/science.6968093. [DOI] [PubMed] [Google Scholar]
- Chalbos D, Galtier F. Differential effect of forms A and B of human progesterone receptor on estradiol-dependent transcription. Journal of Biological Chemistry. 1994;269:23007–23012. [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. Journal of the National Cancer Institute. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- Chrysogelos SA, Yarden RI, Lauber AH, Murphy JM. Mechanisms of EGF receptor regulation in breast cancer cells. Breast Cancer Research and Treatment. 1994;31:227–236. doi: 10.1007/BF00666156. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Clark R, Stampfer MR, Milley R, O'Rourke E, Walen KH, Kriegler M, Kopplin J, McCormick F. Transformation of human mammary epithelial cells by oncogenic retroviruses. Cancer Research. 1988;48:4689–4694. [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. Journal of Biological Chemistry. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Hom YK, Coole PS, Taylor JA, Lubahn DR. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombination experiments. Journal of Mammary Gland Biology and Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- Dairkee SH, Puett L, Hackett AJ. Expression of basal and luminal epithelium-specific keratins in normal, benign, and malignant breast tissue. Journal of the National Cancer Institute. 1988;80:691–695. doi: 10.1093/jnci/80.9.691. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Current Opinion in Cell Biology. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- Delcommenne M, Streuli CH. Control of integrin expression by extracellular matrix. Journal of Biological Chemistry. 1995;270:26794–26801. doi: 10.1074/jbc.270.45.26794. [DOI] [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Dotzlaw H, Miller T, Karvelas J, Murphy LC. Epidermal growth factor gene expression in human breast cancer biopsy samples: relationship to estrogen and progesterone receptor gene expression. Cancer Research. 1990;50:4204–4208. [PubMed] [Google Scholar]
- Dotzlaw H, Leygue E, Watson PH, Murphy LC. Expression of estrogen receptor-beta in human breast tumors. Journal of Clinical Endocrinology and Metabolism. 1997;82:2371–2374. doi: 10.1210/jcem.82.7.4212. [DOI] [PubMed] [Google Scholar]
- Du X, Gu M, Weisel JW, Nagaswami C, Bennett JS, Bowditch R, Ginsberg MH. Long range propagation of conformational changes in integrin alpha IIb beta 3. Journal of Biological Chemistry. 1993;268:23087–23092. [PubMed] [Google Scholar]
- Durbeej M, Henry MD, Campbell KP. Dystroglycan in development and disease. Current Opinion in Cell Biology. 1998;10:594–601. doi: 10.1016/s0955-0674(98)80034-3. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. New England Journal of Medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Research and Treatment. 1995;35:115–132. doi: 10.1007/BF00694752. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Enami J, Pitelka DR, Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. PNAS. 1977;74:4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmark E, Gustafsson JA. Estrogen receptor beta – a novel receptor opens up new possibilities for cancer diagnosis and treatment. Endocrine-Related Cancer. 1998;5:213–222. [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick SL, Brightwell J, Wittliff JL, Barrows GH, Schultz GS. Epidermal growth factor binding by breast tumor biopsies and relationship to estrogen receptor and progestin receptor levels. Cancer Research. 1984;44:3448–3453. [PubMed] [Google Scholar]
- Foster CS, Smith CA, Dinsdale EA, Monaghan P, Neville AM. Human mammary gland morphogenesis in vitro: the growth and differentiation of normal breast epithelium in collagen gel cultures defined by electron microscopy, monoclonal antibodies, and autoradiography. Developmental Biology. 1983;96:197–216. doi: 10.1016/0012-1606(83)90323-8. [DOI] [PubMed] [Google Scholar]
- Fox SB, Harris AL. The epidermal growth factor receptor in breast cancer. Journal of Mammary Gland Biology and Neoplasia. 1997;2:131–141. doi: 10.1023/a:1026399613946. [DOI] [PubMed] [Google Scholar]
- Fuqua SA, Fitzgerald SD, Chamness GC, Tandon AK, McDonnell DP, Nawaz Z, O'Malley BW, McGuire WL. Variant human breast tumor estrogen receptor with constitutive transcriptional activity. Cancer Research. 1991;51:105–109. [PubMed] [Google Scholar]
- Gaire M, Magbanua Z, McDonnell S, McNeil L, Lovett DH, Matrisian LM. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. Journal of Biological Chemistry. 1994;269:2032–2040. [PubMed] [Google Scholar]
- Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos–jun complex. Cell. 1990;63:126–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Progress in Hormone Research. 1999;54:291–313. [PubMed] [Google Scholar]
- Glass CK, Rose DW, Rosenfeld MG. Nuclear receptor coactivators. Current Opinion in Cell Biology. 1996;9:222–232. doi: 10.1016/s0955-0674(97)80066-x. [DOI] [PubMed] [Google Scholar]
- Glukhova M, Koteliansky V, Sastre X, Thiery JP. Adhesion systems in normal breast and in invasive breast carcinoma. American Journal of Pathology. 1995;146:706–716. [PMC free article] [PubMed] [Google Scholar]
- Gompel A, Martin A, Simon P, Schoevaert D, Plu-Bureau G, Hugol D, Audouin J, Leygue E, Truc JB, Poitout P. Epidermal growth factor receptor and c-erbB-2 expression in normal breast tissue during the menstrual cycle. Breast Cancer Research and Treatment. 1996;38:227–235. doi: 10.1007/BF01806677. [DOI] [PubMed] [Google Scholar]
- Gould MN. Cellular and molecular aspects of the multistage progression of mammary carcinogenesis in humans and rats. Seminars in Cancer Biology. 1993;4:161–169. [PubMed] [Google Scholar]
- Green S, Walter P, Greene G, Krust A, Goffin C, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human oestrogen receptor cDNA. Journal of Steroid Biochemistry. 1986;24:77–83. doi: 10.1016/0022-4731(86)90035-x. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. National Cancer Institute Monographs. 1967;26:279–299. [PubMed] [Google Scholar]
- Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p 21 and p 27(Kip1) Molecular Endocrinology. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- Guelstein VI, Tchypysheva TA, Ermilova VD, Litvinova LV, Troyanovsky SM, Bannikov GA. Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. International Journal of Cancer. 1988;42:147–153. doi: 10.1002/ijc.2910420202. [DOI] [PubMed] [Google Scholar]
- Gui GP, Wells CA, Browne PD, Yeomans P, Jordan S, Puddefoot JR, Vinson GP, Carpenter R. Integrin expression in primary breast cancer and its relation to axillary nodal status. Surgery. 1995;117:102–108. doi: 10.1016/s0039-6060(05)80236-3. [DOI] [PubMed] [Google Scholar]
- Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Research. 1982;42:4763–4770. [PubMed] [Google Scholar]
- Hallowes RC, Bone EJ, Jones W. A new dimension in the culture of human breast. In: Richards J, Rajan KT, editors. Tissue Culture in Medical Research (II); Second International Symposium; Cardiff, Wales, UK. London: Pergamon; 1980. pp. 213–220. [Google Scholar]
- Hansen RK, Fuqua SAW. The estrogen receptor and breast cancer. In: Bowcock AM, editor. Breast Cancer: Molecular Genetics, Pathogenesis, and Therapeutics. Humana Press; Totowa, New Jersey: 1999. pp. 1–30. [Google Scholar]
- Haslam SZ. Mammary fibroblast influence on normal mouse mammary epithelial cell responses to estrogen in vitro. Cancer Research. 1986;46:310–316. [PubMed] [Google Scholar]
- Haslam SZ, Shyamala G. Relative distribution of estrogen and progesterone receptors among the epithelial, adipose, and connective tissue components of the normal mammary gland. Endocrinology. 1981;108:825–830. doi: 10.1210/endo-108-3-825. [DOI] [PubMed] [Google Scholar]
- Haslam SZ, Nummy KA. The ontogeny and cellular distribution of estrogen receptors in normal mouse mammary gland. Journal of Steroid Biochemistry and Molecular Biology. 1992;42:589–595. doi: 10.1016/0960-0760(92)90449-s. [DOI] [PubMed] [Google Scholar]
- Haslam SZ, Counterman LJ, Nummy KA. EGF receptor regulation in normal mouse mammary gland. Journal of Cellular Physiology. 1992;152:553–557. doi: 10.1002/jcp.1041520315. [DOI] [PubMed] [Google Scholar]
- Haslam SZ, Counterman LJ, Nummy KA. Effects of epidermal growth factor, estrogen, and progestin on DNA synthesis in mammary cells in vivo are determined by the developmental state of the gland. Journal of Cellular Physiology. 1993;155:72–78. doi: 10.1002/jcp.1041550110. [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP. Dystroglycan inside and out. Current Opinion in Cell Biology. 1999;11:602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- Herrera-Gayol A, Jothy S. Adhesion proteins in the biology of breast cancer: contribution of CD44. Experimental and Molecular Pathology. 1999;66:149–156. doi: 10.1006/exmp.1999.2251. [DOI] [PubMed] [Google Scholar]
- Hissom JR, Moore MR. Progestin effects on growth in the human breast cancer cell line T-47D – possible therapeutic implications. Biochemical and Biophysical Research Communications. 1987;145:706–711. doi: 10.1016/0006-291x(87)91022-9. [DOI] [PubMed] [Google Scholar]
- Hopp TA, Fuqua SAW. Estrogen receptor variants. Journal of Mammary Gland Biology and Neoplasia. 1998;3:73–83. doi: 10.1023/a:1018726418931. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Molecular Endocrinology. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biology. 1993;2:79–89. [PubMed] [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. Journal of Cell Science. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Humphreys RC, Lydon JP, O'Malley BW, Rosen JM. Use of PRKO mice to study the role of progesterone in mammary gland development. Journal of Mammary Gland Biology and Neoplasia. 1997;2:343–354. doi: 10.1023/a:1026343212187. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. PNAS. 1992;89:4658–4662. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. Journal of Biological Chemistry. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Imagawa W, Bandyopadhyay GK, Nandi S. Regulation of mammary epithelial cell growth in mice and rats. Endocrine Reviews. 1990;11:494–523. doi: 10.1210/edrv-11-4-494. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Kendra KL, Norman MJ, Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Research. 1987;47:4355–4360. [PubMed] [Google Scholar]
- Kenney NJ, Dickson RB. Growth factor and sex steroid interactions in breast cancer. Journal of Mammary Gland Biology and Neoplasia. 1996;1:189–198. doi: 10.1007/BF02013642. [DOI] [PubMed] [Google Scholar]
- Klijn JG, Berns PM, Schmitz PI, Foekens JA. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocrine Reviews. 1992;13:3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Frankowski C, Johnson KR, Wheelock MJ. A role for cadherins in cellular signaling and differentiation. Journal of Cellular Biochemistry (Suppl) 1998;31:168–176. doi: 10.1002/(SICI)1097-4644(1998)72:30/31+<168::AID-JCB21>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Koenders PG, Faverly D, Beex LV, Bruggink ED, Kienhuis CB, Benraad TJ. Epidermal growth factor receptors in human breast cancer: a plea for standardisation of assay methodology. European Journal of Cancer. 1992;28:693–697. doi: 10.1016/s0959-8049(05)80128-5. [DOI] [PubMed] [Google Scholar]
- Kosmehl H, Berndt A, Katenkamp D. Molecular variants of fibronectin and laminin: structure, physiological occurrence and histopathological aspects. Virchows Archiv. 1996;429:311–322. doi: 10.1007/BF00198435. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Weis KE, Katzenellenbogen BS. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Molecular and Cellular Biology. 1995;15:1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. PNAS. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota S, Ito H, Ishibashi Y, Seyama Y. Anti-alpha3 integrin antibody induces the activated form of matrix metalloprotease-2 (MMP-2) with concomitant stimulation of invasion through matrigel by human rhabdomyosarcoma cells. International Journal of Cancer. 1997;70:106–111. doi: 10.1002/(sici)1097-0215(19970106)70:1<106::aid-ijc16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. PNAS. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Lambe M, Hsieh CC, Chan HW, Ekbom A, Trichopoulos D, Adami HO. Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Research and Treatment. 1996;38:305–311. doi: 10.1007/BF01806150. [DOI] [PubMed] [Google Scholar]
- Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. Journal of Cell Biology. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame S, Emmanuele C, Torrisi A. Correlation of alpha 2 beta 1 integrin expression with histological type and hormonal receptor status in breast carcinomas. Pathology, Research and Practice. 1996;192:1031–1038. doi: 10.1016/s0344-0338(96)80045-8. [DOI] [PubMed] [Google Scholar]
- Lapidus RG, Nass SJ, Davidson NE. The loss of estrogen and progesterone receptor gene expression in human breast cancer. Journal of Mammary Gland Biology and Neoplasia. 1998;3:85–94. doi: 10.1023/a:1018778403001. [DOI] [PubMed] [Google Scholar]
- LaRochelle WJ, May-Siroff M, Robbins KC, Aaronson SA. A novel mechanism regulating growth factor association with the cell surface: identification of a PDGF retention domain. Genes and Development. 1991;5:1191–1199. doi: 10.1101/gad.5.7.1191. [DOI] [PubMed] [Google Scholar]
- Le Goff P, Montano MM, Schodin DJ, Katzenellenbogen BS. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. Journal of Biological Chemistry. 1994;269:4458–4466. [PubMed] [Google Scholar]
- Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered expression of exon 6 deleted progesterone receptor variant mRNA between normal human breast and breast tumour tissues. British Journal of Cancer. 1999;80:379–382. doi: 10.1038/sj.bjc.6690366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Niranjan B, Gomes P, Gomm JJ, Davies D, Coombes RC, Buluwela L. Inhibitory effects of activin on the growth and morphogenesis of primary and transformed mammary epithelial cells. Cancer Research. 1996;56:1155–1163. [PubMed] [Google Scholar]
- Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in breast cancer. Seminars in Cancer Biology. 1995;6:165–173. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. Journal of Cell Biology. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Sternlicht MD, Werb Z, Bissell MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Annals of the New York Academy of Sciences. 1998;857:180–193. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- Lundholt BK, Madsen MW, Lykkesfeldt AE, Petersen OW, Briand P. Characterization of a nontumorigenic human breast epithelial cell line stably transfected with the human estrogen receptor (ER) cDNA. Molecular and Cellular Endocrinology. 1996;119:47–59. doi: 10.1016/0303-7207(96)03793-8. [DOI] [PubMed] [Google Scholar]
- Lundy J, Schuss A, Stanick D, McCormack ES, Kramer S, Sorvillo JM. Expression of neu protein, epidermal growth factor receptor, and transforming growth factor alpha in breast cancer. Correlation with clinicopathologic parameters. American Journal of Pathology. 1991;138:1527–1534. [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes and Development. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- McCarty KS, Jr, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, Konrath J, Soper JT, Budwit DA, Creasman WT, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Research. 1986;46:4244s–4248s. [PubMed] [Google Scholar]
- McDonnell DP. Unraveling the human progesterone receptor signal transduction pathway: insights into anti-progestin action. Trends in Endocrinology and Metabolism. 1995;6:133–138. doi: 10.1016/1043-2760(95)00065-p. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Goldman ME. RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. Journal of Biological Chemistry. 1994;269:11945–11949. [PubMed] [Google Scholar]
- McGrath CM. Augmentation of the response of normal mammary epithelial cells to estradiol by mammary stroma. Cancer Research. 1983;43:1355–1360. [PubMed] [Google Scholar]
- McGuire WL. Hormone receptors: their role in predicting prognosis and response to endocrine therapy. Seminars in Oncology. 1978;5:428–433. [PubMed] [Google Scholar]
- McGuire WL, Clark GM. Prognostic factors and treatment decisions in axillary-node-negative breast cancer. New England Journal of Medicine. 1992;326:1756–1761. doi: 10.1056/NEJM199206253262607. [DOI] [PubMed] [Google Scholar]
- Maemura M, Akiyama SK, Woods VL, Jr, Dickson RB. Expression and ligand binding of alpha 2 beta 1 integrin on breast carcinoma cells. Clinical and Experimental Metastasis. 1995;13:223–235. doi: 10.1007/BF00133478. [DOI] [PubMed] [Google Scholar]
- Malet C, Gompel A, Yaneva H, Cren H, Fidji N, Mowszowicz I, Kuttenn F, Mauvais-Jarvis P. Estradiol and progesterone receptors in cultured normal human breast epithelial cells and fibroblasts: immunocytochemical studies. Journal of Clinical Endocrinology and Metabolism. 1991;73:8–17. doi: 10.1210/jcem-73-1-8. [DOI] [PubMed] [Google Scholar]
- Mansour EG, Ravdin PM, Dressler L. Prognostic factors in early breast carcinoma. Cancer. 1994;74:381–400. doi: 10.1002/cncr.2820741326. [DOI] [PubMed] [Google Scholar]
- Marbaix E, Donnez J, Courtoy PJ, Eeckhout Y. Progesterone regulates the activity of collagenase and related gelatinases A and B in human endometrial explants. PNAS. 1992;89:11789–11793. doi: 10.1073/pnas.89.24.11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markopoulos C, Berger U, Wilson P, Gazet JC, Coombes RC. Oestrogen receptor content of normal breast cells and breast carcinomas throughout the menstrual cycle. British Medical Journal. 1988;296:1349–1351. doi: 10.1136/bmj.296.6633.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters JR, Drife JO, Scarisbrick JJ. Cyclic variation of DNA synthesis in human breast epithelium. Journal of the National Cancer Institute. 1977;58:1263–1265. doi: 10.1093/jnci/58.5.1263. [DOI] [PubMed] [Google Scholar]
- Meyer JS. Cell proliferation in normal human breast ducts, fibroadenomas, and other ductal hyperplasias measured by nuclear labeling with tritiated thymidine. Effects of menstrual phase, age, and oral contraceptive hormones. Human Pathology. 1977;8:67–81. doi: 10.1016/s0046-8177(77)80066-x. [DOI] [PubMed] [Google Scholar]
- Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ, Heppner GH. Xenograft model of progressive human proliferative breast disease. Journal of the National Cancer Institute. 1993;85:1725–1732. doi: 10.1093/jnci/85.21.1725. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. Journal of Cell Biology. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. Journal of Cell Biology. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Nonomura A, Noguchi M, Taniya T, Koyasaki N, Saito Y, Hashimoto T, Matsubara F, Yanaihara N. Immunohistochemical study of oncogene product ras p21, c-myc and growth factor EGF in breast carcinomas. Anticancer Research. 1991;11:1485–1494. [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO Journal. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle RB, Bocker W, Davis JR, Heid HW, Kaufmann M, Lucas DO, Jarasch ED. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. Journal of Histochemistry and Cytochemistry. 1986;34:869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Botti C, Mottolese M, Bigotti A, Segatto O. Changes in expression of alpha 6/beta 4 integrin heterodimer in primary and metastatic breast cancer. British Journal of Cancer. 1992;66:318–322. doi: 10.1038/bjc.1992.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RI, McClelland RA, Robertson JFR, Gee JMW. Involvement of steroid hormone and growth factor cross-talk in endocrine response in breast cancer. Endocrine-Related Cancer. 1999;6:373–387. doi: 10.1677/erc.0.0060373. [DOI] [PubMed] [Google Scholar]
- Nishida K, Kitazawa R, Mizuno K, Maeda S, Kitazawa S. Identification of regulatory elements of human alpha 6 integrin subunit gene. Biochemical and Biophysical Research Communications. 1997;241:258–263. doi: 10.1006/bbrc.1997.7808. [DOI] [PubMed] [Google Scholar]
- Normanno N, Ciardiello F. EGF-related peptides in the pathophysiology of the mammary gland. Journal of Mammary Gland Biology and Neoplasia. 1997;2:143–151. doi: 10.1023/a:1026351730785. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Hobbs K, Clark GM. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Research. 1985;45:584–590. [PubMed] [Google Scholar]
- O'Toole TE, Mandelman D, Forsyth J, Shattil SJ, Plow EF, Ginsberg MH. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991;254:845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. Journal of Biological Chemistry. 1997;272:25832–25838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Developmental Biology. 1999;206:88–99. doi: 10.1006/dbio.1998.9133. [DOI] [PubMed] [Google Scholar]
- Petersen OW, van Deurs B. Growth factor control of myoepithelial cell differentiation in cultures of human mammary gland. Differentiation. 1988;39:197–215. doi: 10.1111/j.1432-0436.1988.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Hoyer PE, van Deurs B. Frequency and distribution of estrogen receptor-positive cells in normal, nonlactating human breast tissue. Cancer Research. 1987;47:5748–5751. [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. PNAS. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Molecular Endocrinology. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- Philips A, Chalbos D, Rochefort H. Estradiol increases and anti-estrogens antagonize the growth factor-induced activator protein-1 activity in MCF7 breast cancer cells without affecting c-fos and c-jun synthesis. Journal of Biological Chemistry. 1993;268:14103–14108. [PubMed] [Google Scholar]
- Pilat MJ, Christman JK, Brooks SC. Characterization of the estrogen receptor transfected MCF10A breast cell line 139B6. Breast Cancer Research and Treatment. 1996;37:253–266. doi: 10.1007/BF01806507. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Alroy I, Yarden Y. ErbB receptors and EGF-like ligands: cell lineage determination and oncogenesis through combinatorial signaling. Journal of Mammary Gland Biology and Neoplasia. 1997;2:97–107. doi: 10.1023/a:1026343528967. [DOI] [PubMed] [Google Scholar]
- Press MF, Greene GL. Localization of progesterone receptor with monoclonal antibodies to the human progestin receptor. Endocrinology. 1988;122:1165–1175. doi: 10.1210/endo-122-3-1165. [DOI] [PubMed] [Google Scholar]
- Ricketts D, Turnbull L, Ryall G, Bakhshi R, Rawson NS, Gazet JC, Nolan C, Coombes RC. Estrogen and progesterone receptors in the normal female breast. Cancer Research. 1991;51:1817–1822. [PubMed] [Google Scholar]
- Robinson SP, Jordan VC. Reversal of the antitumor effects of tamoxifen by progesterone in the 7,12-dimethylbenzanthracene-induced rat mammary carcinoma model. Cancer Research. 1987;47:5386–5390. [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiological Reviews. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- Rosfjord EC, Dickson RB. Growth factors, apoptosis, and survival of mammary epithelial cells. Journal of Mammary Gland Biology and Neoplasia. 1999;4:229–237. doi: 10.1023/a:1018789527533. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Current Opinion in Cell Biology. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau-Merck MF, Misrahi M, Loosfelt H, Milgrom E, Berger R. Localization of the human progesterone receptor gene to chromosome 11q22–q23. Human Genetics. 1987;77:280–282. doi: 10.1007/BF00284486. [DOI] [PubMed] [Google Scholar]
- Rudland PS. Epithelial stem cells and their possible role in the development of the normal and diseased human breast. Histology and Histopathology. 1993;8:385–404. [PubMed] [Google Scholar]
- Rudland PS, Fernig DG, Smith JA. Growth factors and their receptors in neoplastic mammary glands. Biomedicine and Pharmacotherapy. 1995;49:389–399. doi: 10.1016/0753-3322(96)82676-x. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. Journal of Mammary Gland Biology and Neoplasia. 1998;3:49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- Sainsbury JR, Farndon JR, Sherbet GV, Harris AL. Epidermal-growth-factor receptors and oestrogen receptors in human breast cancer. Lancet. 1985;1:364–366. doi: 10.1016/s0140-6736(85)91385-6. [DOI] [PubMed] [Google Scholar]
- Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. PNAS. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura T. Epithelial-mesenchymal interactions in mammary gland development and its perturbation in relation to tumorigenesis. In: Rich MA, Hager JC, Furmanski P, editors. Understanding Breast Cancer: Clinical and Laboratory Concepts. Marcel Dekker, Inc.; New York: 1983. pp. 261–284. [Google Scholar]
- Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. Journal of Cell Biology. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger CA, Zuk A, Zinkl GM, Kendall D, Matlin KS. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. Journal of Cell Science. 1994;107:527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Lee DC. Dynamic expression and activation of ERBB receptors in the developing mouse mammary gland. Cell Growth and Differentiation. 1998;9:451–464. [PubMed] [Google Scholar]
- Schroen DJ, Brinckerhoff CE. Nuclear hormone receptors inhibit matrix metalloproteinase (MMP) gene expression through diverse mechanisms. Gene Expression. 1996;6:197–207. [PMC free article] [PubMed] [Google Scholar]
- Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, Hom YK, Cunha GR, DiAugustine RP. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth and Differentiation. 1998;9:777–785. [PubMed] [Google Scholar]
- Shearer M, Bartkova J, Bartek J, Berdichevsky F, Barnes D, Millis R, Taylor-Papadimitriou J. Studies of clonal cell lines developed from primary breast cancers indicate that the ability to undergo morphogenesis in vitro is lost early in malignancy. International Journal of Cancer. 1992;51:602–612. doi: 10.1002/ijc.2910510417. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Barcellos-Hoff MH, Toft D, Yang X. In situ localization of progesterone receptors in normal mouse mammary glands: absence of receptors in the connective and adipose stroma and a heterogeneous distribution in the epithelium. Journal of Steroid Biochemistry and Molecular Biology. 1997;63:251–259. doi: 10.1016/s0960-0760(97)00128-3. [DOI] [PubMed] [Google Scholar]
- Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. PNAS. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala G, Louie SG, Camarillo IG, Talamantes F. The progesterone receptor and its isoforms in mammary development. Molecular Genetics and Metabolism. 1999;68:182–190. doi: 10.1006/mgme.1999.2897. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Van Horn K, Shyamala G, Daniel CW. Progesterone receptors in the mouse mammary duct: distribution and developmental regulation. Cell Growth and Differentiation. 1996;7:945–952. [PubMed] [Google Scholar]
- Simian M, Vigil A, Bissell MJ, Shyamala G. Involvement of progesterone receptor (PR) in the organization of basement membrane integrity in mammary glands. Proceedings of the American Association for Cancer Research. 2000;91:139. [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. A recombinant tail-less integrin beta 4 subunit disrupts hemidesmosomes, but does not suppress alpha 6 beta 4-mediated cell adhesion to laminins. Journal of Cell Biology. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MR, Bartley JC. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. PNAS. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MR, Yaswen P. Culture systems for study of human mammary epithelial cell proliferation, differentiation and transformation. Cancer Surveys. 1993;18:7–34. [PubMed] [Google Scholar]
- Staszewski J. Age at menarche and breast cancer. Journal of the National Cancer Institute. 1971;47:935–940. [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AW, Streuli CH, Martins-Green M, Bissell MJ. Designer microenvironments for the analysis of cell and tissue function. Current Opinion in Cell Biology. 1990;2:864–874. doi: 10.1016/0955-0674(90)90085-s. [DOI] [PubMed] [Google Scholar]
- Streuli C. Extracellular matrix remodelling and cellular differentiation. Current Opinion in Cell Biology. 1999;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. Journal of Cell Biology. 1993;120:253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Berditchevski F. Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2) Journal of Cell Biology. 1999;146:1375–1389. doi: 10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. Journal of Cell Biology. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Fentiman IS, Burchell J. Markers for malignancy in human mammary epithelial cells in vitro: problems and directions. In: McGrath C, Brennan MJ, Rich MA, editors. Cell Biology of Breast Cancer. Academic Press; New York: 1980. pp. 347–362. [Google Scholar]
- Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, Werb Z, Bissell MJ. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. American Journal of Pathology. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L, Gronemeyer H, Turcotte B, Gaub MP, Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988;333:185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. Journal of the National Cancer Institute. 1972;48:605–613. [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual Review of Biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Lu Y, Nichols PW, Zlotnikov G, Jones PA, Smith HS. Contiguous patches of normal human mammary epithelium derived from a single stem cell: Implications for breast carcinogenesis. Cancer Research. 1996;56:402–404. [PubMed] [Google Scholar]
- Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Molecular Endocrinology. 1993;7:1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. Journal of Biological Chemistry. 1994;269:16433–16442. [PubMed] [Google Scholar]
- Umekita Y, Enokizono N, Sagara Y, Kuriwaki K, Takasaki T, Yoshida A, Yoshida H. Immunohistochemical studies on oncogene products (EGF-R, c-erbB-2) and growth factors (EGF, TGF-alpha) in human breast cancer: their relationship to oestrogen receptor status, histological grade, mitotic index and nodal status. Virchows Archiv A. Pathological Anatomy and Histopathology. 1992;420:345–351. doi: 10.1007/BF01600214. [DOI] [PubMed] [Google Scholar]
- Unden AB, Sandstedt B, Bruce K, Hedblad M, Stahle-Backdahl M. Stromelysin-3 mRNA associated with myofibroblasts is overexpressed in aggressive basal cell carcinoma and in dermatofibroma but not in dermatofibrosarcoma. Journal of Investigative Dermatology. 1996;107:147–153. doi: 10.1111/1523-1747.ep12329541. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Molecular Endocrinology. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK. Prolactin involvement in breast cancer. Endocrine-Related Cancer. 1999;6:389–404. doi: 10.1677/erc.0.0060389. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. PNAS. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C, Gutman A, Nicholson R, Wasylyk B. The c-Ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoproteins. EMBO Journal. 1991;10:1127–1134. doi: 10.1002/j.1460-2075.1991.tb08053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazer DE, Chu Q, Liu XL, Gao Q, Safaii H, Band V. Loss of p53 protein during radiation transformation of primary human mammary epithelial cells. Molecular and Cellular Biology. 1994;14:2468–2478. doi: 10.1128/mcb.14.4.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochemistry and Cell Biology. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. Journal of Cell Biology. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated `C'-receptor and unique A-receptor messages. Molecular Endocrinology. 1990;4:1833–1840. doi: 10.1210/mend-4-12-1833. [DOI] [PubMed] [Google Scholar]
- Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Molecular Endocrinology. 1996;10:1379–1387. doi: 10.1210/mend.10.11.8923464. [DOI] [PubMed] [Google Scholar]
- Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. Journal of the National Cancer Institute. 1975;55:231–273. [PubMed] [Google Scholar]
- White R, Parker MG. Molecular mechanisms of steroid hormone action. Endocrine-Related Cancer. 1998;5:1–14. [Google Scholar]
- Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Molecular Biology of the Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, Rouyer N, Lutz Y, Adida C, Loriot M, Bellocq JP, Chambon P, Basset P. Stromelysin 3 belongs to a subgroup of proteinases expressed in breast carcinoma fibroblastic cells and possibly implicated in tumor progression. PNAS. 1993;90:1843–1847. doi: 10.1073/pnas.90.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TL, Xie JW, Haslam SZ. The role of mammary stroma in modulating the proliferative response to ovarian hormones in the normal mammary gland. Journal of Mammary Gland Biology and Neoplasia. 1998;3:117–131. doi: 10.1023/a:1018738721656. [DOI] [PubMed] [Google Scholar]
- Xie J, Haslam SZ. Extracellular matrix regulates ovarian hormone-dependent proliferation of mouse mammary epithelial cells. Endocrinology. 1997;138:2466–2473. doi: 10.1210/endo.138.6.5211. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yang J, Richards J, Guzman R, Imagawa W, Nandi S. Sustained growth in primary culture of normal mammary epithelial cells embedded in collagen gels. PNAS. 1980;77:2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu A, Dougherty C, Chen X, Guzman R, Nandi S. Estrogen and progesterone receptors can be maintained in normal human breast epithelial cells in primary culture and after transplantation into nude mice. Oncology Reports. 2000;7:17–21. doi: 10.3892/or.7.1.17. [DOI] [PubMed] [Google Scholar]
- Zajchowski DA, Sager R, Webster L. Estrogen inhibits the growth of estrogen receptor-negative, but not estrogen receptor-positive, human mammary epithelial cells expressing a recombinant estrogen receptor. Cancer Research. 1993;53:5004–5011. [PubMed] [Google Scholar]
- Zhang QX, Hilsenbeck SG, Fuqua SA, Borg A. Multiple splicing variants of the estrogen receptor are present in individual human breast tumors. Journal of Steroid Biochemistry and Molecular Biology. 1996;59:251–260. doi: 10.1016/s0960-0760(96)00120-3. [DOI] [PubMed] [Google Scholar]
- Zutter MM, Santoro SA, Painter AS, Tsung YL, Gafford A. The human alpha 2 integrin gene promoter. Identification of positive and negative regulatory elements important for cell-type and developmentally restricted gene expression. Journal of Biological Chemistry. 1994;269:463–469. [PubMed] [Google Scholar]
- Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. PNAS. 1995;92:7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]