Summary
Nuclear organization, such as the formation of specific nuclear subdomains, is generally thought to be involved in the control of cellular phenotype; however, there are relatively few specific examples of how mammalian nuclei organize during radical changes in phenotype, such as those occurring during differentiation and growth arrest. Using human mammary epithelial cells in which growth arrest is essential for morphological differentiation, we show that the arrest of cell proliferation is accompanied by a reorganization of the telomere-associated protein, TIN2, into one to three large nuclear subdomains. The large TIN2 domains do not contain telomeres and occur concomitant with the continued presence of TIN2 at telomeres. The TIN2 domains were sensitive to DNase, but not RNase, occurred frequently, but not exclusively near nucleoli, and overlapped often with dense domains containing heterochromatin protein 1γ. Expression of truncated forms of TIN2 simultaneously prevented the formation of TIN2 domains and relaxed the stringent morphogenesis-induced growth arrest in human mammary epithelial cells. Here we show that a novel extra-telomeric organization of TIN2 is associated with the control of cell proliferation and identify TIN2 as an important regulator of mammary epithelial differentiation.
Keywords: Nuclear structure, Three-dimensional culture, Breast, Morphogenesis, Quiescence, Heterochromatin protein 1
Introduction
Changes in higher-order nuclear organization may be a key event in the control of cellular phenotypes, particularly the changes in phenotype that occur during development and differentiation (reviewed by Lelièvre et al., 2000; Müller and Leutz, 2001). In lower eukaryotes, telomeres are among the nuclear structures that have been shown to undergo higher-order organization, which is important for cell phenotype. Telomeres are the repetitive DNA sequence and specialized proteins that cap the ends of linear chromosomes, and prevent their recombination or degradation by DNA repair processes. Telomeres have long been recognized as important nuclear organizers and regulators of cell phenotype in yeast (Gotta and Gasser, 1996). Specifically, yeast telomeres and their associated proteins organize into clusters at the nuclear periphery, and this clustering is associated with the formation of chromatin domains that determine the pattern of gene expression (Maillet et al., 1996; Gotta et al., 1996). In the somatic cells of higher eukaryotes, however, telomeres are generally randomly distributed throughout the nucleus, and telomeric functions other than their crucial role in chromosome end protection have not been reported.
The structure and function of telomeres depend on the activities of telomere-associated proteins. In mammalian cells, the telomeric end structure is controlled by several telomere-associated proteins, including TRF1, TRF2 and TIN2 (van Steensel and de Lange, 1997; Kim et al., 1999; Kim et al., 2003a). TRF1 and TRF2 bind exclusively to the double-stranded telomeric repeat sequence (Chong et al., 1995; Bilaud et al., 1997), and as such constitute primary telomere-associated proteins. These proteins are thought to function by promoting a closed or capped end structure that protects the chromosome ends from being recognized as ‘broken’ DNA; these proteins are also thought to negatively regulate telomere length by limiting the access of telomerase, the reverse transcriptase that can add telomeric DNA repeats to chromosome ends de novo. TIN2 also participates in chromosome end protection (Kim et al., 2004) and negatively regulates telomere length, although it does not bind telomeric DNA directly (Kim et al., 1999). Rather, TIN2 binds TRF1 (Kim et al., 1999) and indirectly influences telomere structure, possibly by altering the conformation of TRF1 (Kim et al., 2003a). In addition, TIN2 binds the telomeric proteins TRF2 and PTOP, also known as PIP1 (Kim et al., 2004; Liu et al., 2004; Ye et al., 2004). Thus, TIN2 is a secondary telomere-associated protein. To date, yeast homologues of TIN2 have not been identified (Kim et al., 1999; Kim et al., 2003b), and the full range of TIN2 functions in mammalian cells is not yet known.
The functional differentiation of the mammary epithelium depends on the growth arrest and proper arrangement of the epithelial cells into glandular structures termed acini. Among the intracellular alterations that are crucial for mammary epithelial cell differentiation, the role of nuclear reorganization is the least well understood and has been only sporadically investigated. We have shown that acinar differentiation entails the redistribution of nuclear proteins such as heterochromatin-associated protein H3K9m, splicing factor SRm160, and the nuclear mitotic apparatus protein NuMA (Lelièvre et al., 1998; Plachot and Lelièvre, 2004). Conversely, we have demonstrated that altering the distribution of NuMA in acinar cells perturbs their differentiation (Lelièvre et al., 1998). These findings suggest that the spatial organization of nuclear components may play an important role in controlling the phenotype of mammalian cells.
Given the importance of telomere organization in controlling gene expression in yeast, and the importance of nuclear organization in the differentiation of human mammary epithelial cells (HMECs), we asked whether the organization of telomeres and/or their associated proteins was important for the control of mammary epithelial phenotypes. To do so, we used three-dimensional (3D) cell culture models that recapitulate many aspects of HMEC differentiation. We show that TIN2 undergoes a striking reorganization into large nuclear domains when HMECs arrest proliferation, a prerequisite for acinar differentiation. The formation of large TIN2 domains is not accompanied by clustering of telomeres or TIN2 binding partners, the telomeric proteins TRF1 and TRF2. In addition, both formation of large TIN2 domains and mammary-cell growth arrest are impaired upon expression of truncated forms of TIN2. Our findings reveal a higher-order nuclear organization associated with growth arrest and define a novel nuclear organizing principle in mammalian cells based on the distribution of telomere-associated proteins.
Materials and Methods
Cell culture and differentiation
HMT-3522 non-neoplastic (S1) HMECs (Briand et al., 1987) were cultured in serum-free H14 medium (GIBCO BRL, St Louis, MO) as described (Petersen et al., 1992). Cells of the HMEC cell line 184 (strain 184) cultured in MCDB 170 medium (Cambrex Biosciences, Walkersville, MD) as described (Hammond et al., 1984), are termed post-selection HMECs because they spontaneously overcame the p16-mediated cell-cycle arrest of primary HMECs (Yaswen and Stampfer, 2002). To induce differentiation, cells were cultured for 10 days on tissue culture surfaces coated with 40 μl/cm2 Matrigel™ (BD Biosciences, Bedford, MA), a laminin-rich extracellular matrix (ECM); and in culture medium containing 5% Matrigel™ (Plachot and Lelièvre, 2004). Culture in collagen I was performed as described (Weaver et al., 2002). Briefly, cells were embedded in a collagen mixture consisting of DMEM-F12, 0.1 M HEPES, 0.04 M NaHCO3, cellagen solution AC-5 (ICN Biomedicals, Aurora, OH) diluted 1:4, pH 7.4. Multicellular structures were removed from the collagen gel by incubating with collagenase D (Roche, Indianapolis, IN) for 30 minutes at 37°C.
Synchronization of cells of the HMEC strain 184
184 HMECs were synchronized as described (Stampfer et al., 1993). Briefly cells were cultured in MEGM medium (Clonetics, La Jolla, CA) supplemented with transferrin (5 μg/ml) and isoproterenol (10−5M) (Sigma, St Louis, MO) and seeded at 50% confluence. Cells were allowed to recover from plating for 24 hours, rinsed twice in PBS, and then given MEGM lacking EGF and supplemented with 8 μg/ml EGF blocking antibody (MAb 225, American Type Culture Collection hybridoma clone HB-8508). After 48 hours, cells were either processed for immunostaining (G0 phase) or released from growth arrest by washing twice with PBS and replacing the medium with MEGM supplemented with 25 ng/ml EGF and then immunostained at 16 and 20 hours (S and G2-M phases).
Retroviral infections
Production of retroviruses that express wild-type or truncated TIN2 proteins has been described (Kim et al., 1999). We added a C-terminal V5 epitope tag by PCR to create TIN2-V5 and cloned the fragment into the same retroviral vector (pLXSN). Proliferating S1 or 184 HMECs (25–30% confluent) were infected for 6 hours each on three consecutive days with viruses expressing either TIN2-V5, TIN2-13, myc-TIN2-15 (Kim et al., 1999), hTERT (Counter et al., 1998) or GFP, and selected in 200 μg/ml G418 (Invitrogen Life Technologies, Carlsbad, CA). hTERT expression was verified by TRAP assay (Chemicon, Temecula, CA), GFP expression was verified by fluorescence microscopy, and mutant and wild-type TIN2 expression was verified by western analysis, immunofluorescence and analysis of telomere length.
Western blot analysis
Total protein extracts were prepared in Laemmli buffer containing 2% sodium dodecyl sulfate. For TIN2 and TIN2-13 expression analysis, 30 μg protein were separated on 4–12% polyacrylamide gradient gels (Invitrogen), and transferred to a nitrocellulose membrane. The membrane was blocked and incubated with rabbit polyclonal anti-TIN2 antibody that recognizes both full-length TIN2 and N-terminally truncated TIN2-13, followed by secondary antibody, as described (Kim et al., 1999). Horseradish peroxidase-conjugated secondary antibody was detected by enhanced chemiluminescence (Amersham, Piscataway, NJ).
Analysis of telomere length
Genomic DNA was isolated from HMECs S1 cells infected with insertless vector, TIN2-13, or TIN2-15 constructs, digested with HinfI and RsaI, and analyzed by Southern blotting using a telomere (TTAGGG)3 probe as described (Harley et al., 1990; Kim et al., 1999). Mean terminal restriction fragment lengths were determined using a phosphorimager (Amersham) and Imagequant software (Harley et al., 1990).
Immunofluorescence analysis
Monolayer or 3D cultures in four-well chamber slides (Nalge Nunc International, Naperville, IL) were either permeabilized with 0.5% peroxide and carbonyl-free Triton X-100 (Sigma) in cytoskeleton buffer (100 mM NaCl, 300 mM sucrose, 10 mM PIPES, pH 6.8, 5 mM MgCl2, 1 mM pefabloc, 10 μg/ml aprotinin, 250 μM NaF) prior to fixation in 4% paraformaldehyde (Sigma), or fixed with 4% paraformaldehyde prior to immunostaining (Lelièvre et al., 1998). In some experiments, 3D cultures were embedded in sucrose, frozen in Tissue-Tek OCT (Sakura Finetek, Torrance, CA) and 5–20 μm frozen sections were cut and used for immunostaining. Primary antibodies were mouse monoclonal anti-PML (Santa Cruz Biotechnology, Santa Cruz, CA), anti-TRF1 (Oncogene Research Products, San Diego, CA), anti-TRF2 (Imgenex, San Diego, CA), anti-nucleolin (Santa Cruz Biotechnology), anti-HP 1γ (Chemicon), anti-c-myc (clone 9E10, Roche) and rabbit polyclonal anti-TIN2 (Kim et al., 1999). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) or propidium iodide.
Normal human breast biopsies were obtained from women undergoing reduction mammoplasty for cosmetic reasons. The use of human material has been reviewed by the Regional Scientific-Ethical Committees for Copenhagen and Frederiksberg, Denmark and approved with reference (KF) 01–161/98. Tissue cryosections were dried for 15 minutes at room temperature, incubated for 5 minutes in 0.5% Triton X-100 in PBS and fixed in 2% paraformaldehyde. After blocking with 10% goat serum, sections were incubated overnight with TIN2 antibody or pre-immune serum from the same rabbit (1:100 in PBS with 10% goat serum) and then for 60 minutes with FITC-conjugated anti-rabbit IgG. Sections were counterstained with 1 μg/ml propidium iodide.
Immunocytochemistry and fluorescence in situ hybridization (FISH)
We used a modification of a FISH assay established to preserve initial immunocytochemical staining (Lansdorp et al., 1996). Immunocytochemistry was performed as described above. Following secondary antibody incubation and washing, cells were post-fixed with 4% paraformaldehyde in PBS, washed three times for 5 minutes each with PBS, dehydrated in ethanol and air-dried. An 18-mer biotinylated-(C3TA2)3 peptide-nucleic acid (PNA) probe (Applied Biosystems, Framingham, MA) was hybridized as described (Lansdorp et al., 1996). Following hybridization, samples were incubated with 0.5 μg/ml fluorescein-conjugated streptavidin, counterstained with DAPI, and mounted in anti-fade medium (Vector Laboratories, Burlingame, CA).
Growth analysis
Cells cultured in 3D for 6 or 10 days were assayed for 5-bromo-2-deoxyuridine (BrdU) incorporation using a commercial labeling and detection kit (Roche). The BrdU-labeling index was determined by scoring 200–400 DAPI-stained cells for BrdU positivity in four independent experiments. In parallel experiments, acini diameters were measured using a scaled eye-piece.
DNase I and RNase A treatments
Cells were permeabilized using 0.5% peroxide and carbonyl-free Triton X-100 (Sigma) in the presence of protease and phosphatase inhibitors in cytoskeleton buffer (100 mM NaCl, 300 mM sucrose, 10 mM PIPES, pH 6.8, 5 mM MgCl2), then incubated with 130 μg/ml DNase I (Worthington Biochemical Corporation, Lakewood, NJ) or 100 μg/ml DNase-free RNase A (Roche) in the presence of protease and phosphatase inhibitors in cytoskeleton buffer for 30 minutes at 37 °C. Cells were then fixed as described for immunostaining.
Results
TIN2 organizes into large nuclear domains during HMEC acinar differentiation
To understand whether and how telomeres and/or their associated proteins might influence mammalian cell phenotypes, we followed the localization of telomeres and primary and secondary telomere-associated proteins during the morphological differentiation of HMECs in 3D culture. The differentiation of HMECs under these conditions is accompanied by an arrest of cell proliferation (Petersen et al., 1992; Lelièvre et al., 1998), chromatin remodeling and changes in gene expression (Bissell et al., 2003; Plachot and Lelièvre, 2004). We initiated our studies using the non-neoplastic human breast epithelial cell line, HMT-3522 (S1), which forms tissue-like acini when cultured in 3D in the presence of Matrigel™. During the 10-day morphogenesis process, S1 cells proliferate for 5–6 days, then arrest growth, deposit an endogenous basement membrane, and polarize around a central lumen (Petersen et al., 1992).
We immunostained proliferating and differentiated S1 cells for telomere-associated proteins including TIN2, Ku, ATM, TRF2 and TRF1. Among these proteins, only TIN2 showed a dramatic redistribution upon completion of acinar differentiation (Fig. 1A).
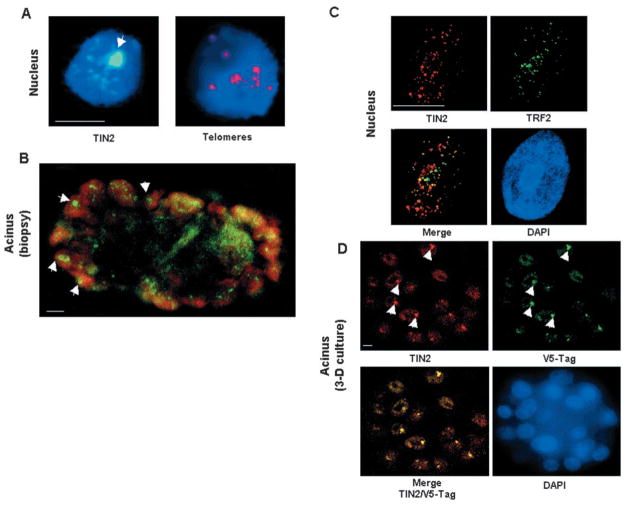
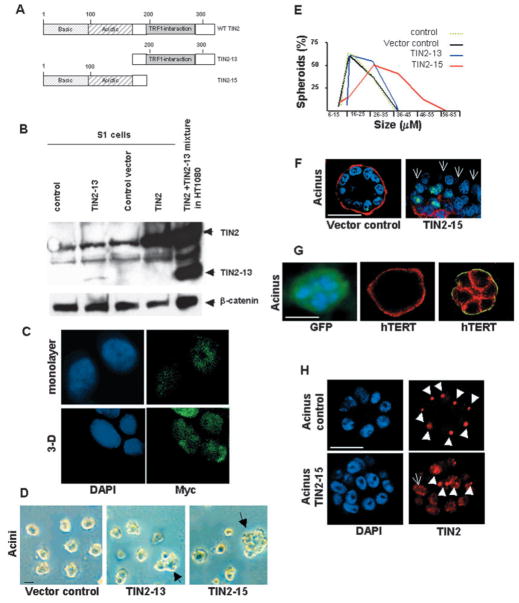
Fig. 1.
Large TIN2 domains are present in HMECs organized into acini. (A) Immunostaining for TIN2 (green) and PNA FISH for telomeres (red) in S1 acini in 3D culture. (B) Immunostaining for TIN2 (green) in a biopsy from normal breast tissue. (C) Dual immunostaining for TIN2 (red) and TRF2 (green) in the nuclei of proliferating 184 HMECs. Colocalization of TIN2 and TRF2 appears yellow. (D) Dual immunostaining for TIN2 (red) and V5 (green) in the nuclei of acini formed by 184 HMECs expressing V5-tagged TIN2 in 3D culture. Nuclei are counterstained with DAPI (blue) in A, C and D, and propidium iodide (red) in B. Images in B and D are confocal sections of acini containing several nuclei. Arrowheads indicate large TIN2 domains. Bar, 5 μm.
In human fibroblasts, TIN2 localizes exclusively to telomeres, which appear randomly distributed as small foci throughout the nucleus (Kim et al., 1999). TIN2 showed a similar random punctate pattern in the nuclei of proliferating S1 cells cultured as monolayers. However, when S1 cells underwent acinar differentiation in 3D culture, TIN2 reorganized into large domains (Fig. 1A). Each nucleus contained one to three large TIN2 domains that coexisted with the small foci seen in monolayer cultures. The small TIN2 foci probably corresponded to telomeres, which remained dispersed throughout the nuclei after differentiation, as determined by separate staining for telomeres using a PNA telomeric probe or immunostaining for primary telomere-associated proteins TRF1 or TRF2. It was not possible to co-stain 3D cultures for telomeres and TIN2 owing to high non-specific signals from the Matrigel™ after fixation. However, sectioning of 3D culture of acini followed by FISH using the telomeric PNA probe showed that the telomeres did not cluster after differentiation (Fig. 1A). Thus, TIN2 remained at telomeres upon differentiation, but additionally clustered into large nuclear domains. These domains did not result from the clustering of either telomeres or the TIN2 telomeric binding partners TRF1 and TRF2.
We detected large TIN2 domains in >80% of the nuclei present in S1 acini. We detected TIN2 only as small foci that overlapped with telomere-binding protein TRF2 in proliferating finite life span HMECs, strain 184 (Hammond et al., 1984; Yaswen and Stampfer, 2002) (see Fig. 1C). However, similarly to S1 cells, large TIN2 domains were observed in the majority of 184 cells that arrested proliferation and underwent morphological differentiation in 3D culture (see Fig. 1D, discussed below). Moreover, we detected large TIN2 domains in biopsies from normal human breast tissue, where many of the epithelial cells in the acini showed clustered TIN2 immunostaining (Fig. 1B). Thus, the formation of large TIN2 domains was not unique to S1 cells, and was not restricted to cultured cells.
To confirm that the large domains recognized by our affinity-purified antibody indeed correspond to TIN2, we expressed a C-terminally V5-epitope-tagged TIN2 protein in 184 HMECs using retroviral transduction. We allowed the cells to form acini in 3D culture, then dually stained with V5 and TIN2 antibodies. The antibodies showed >95% colocalization, identifying TIN2 in both small foci and large domains (Fig. 1D). We conclude that TIN2 organizes into large domains when HMECs undergo morphological differentiation.
TIN2 domains are frequently perinucleolar and associated with HP 1γ
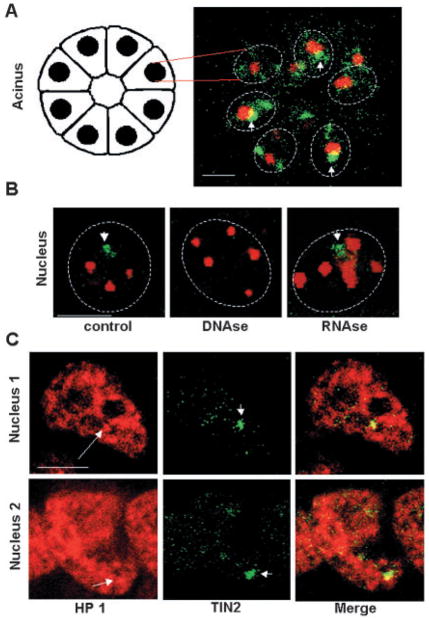
To determine whether large TIN2 domains overlap with other nuclear structures, we performed dual immunostaining for TIN2 and the nucleolar protein nucleolin. Whereas TIN2 domains did not colocalize with nucleoli, they frequently (>55%) were perinucleolar (Fig. 2A). As nucleoli are sites of intense RNA metabolism and are also linked to gene silencing (Olson et al., 2002), we asked whether integrity of the large TIN2 domains depended on intact RNA- or DNA-rich structures. We used PML as a control because its distribution into distinct domains is not dramatically altered upon RNase A or DNase I treatment (Szekely et al., 1999). TIN2 domains did not substantially overlap with PML domains, which contain proteins that participate in a variety of cellular processes including transcription (Borden, 2002). Moreover, RNase A treatment left both the PML and TIN2 domains intact (Fig. 2B), but DNase I treatment markedly and selectively eliminated the large TIN2 structures (Fig. 2B). This finding raised the possibility that the large TIN2 domains associate with chromatin, as disappearance of protein domains following DNase I treatment is considered a good indicator that such domains are part of DNA-rich regions (Szekely et al., 1999). This possibility was supported by staining acini for both TIN2 and heterochromatin protein 1γ (HP 1γ), which is known to participate in chromatin packaging and gene silencing (Li et al., 2002) (Fig. 2C). HP 1γ was widely but unevenly distributed throughout the nucleus, showing areas of relatively light staining, as well as regions of dense focal staining. The majority (80%) of large TIN2 domains colocalized with dense focal HP 1γ staining. These findings suggest that TIN2 may participate in organizing chromatin during breast acinar differentiation, a possibility we are currently investigating in greater detail.
Fig. 2.
Large TIN2 domains are often perinucleolar and colocalized with dense HP 1γ foci. (A) Dual immunostaining for TIN2 (green) and nucleolin (red) in S1 acini in 3D culture. The image is a confocal section of an acinus containing several nuclei, illustrated by the drawing on the left. (B) Dual staining for TIN2 (green) and PML (red) in S1 acini. 3D cultures were untreated (control) or treated for 30 minutes with DNase I (DNase) or RNase A (RNase) prior to immunostaining. (C) Dual immunostaining for HP 1γ (red) and TIN2 (green) in S1 acini. Arrows indicate HP 1γ domains that overlap with large TIN2 domains, arrowheads indicate large TIN2 domains and dashed lines delineate the nuclear periphery. Bar, 5 μm.
Formation of large TIN2 domains coincides with arrest of cell proliferation
Morphological differentiation into acini entails an arrest of cell proliferation, in addition to the formation of a polarity axis (Petersen et al., 1992; Weaver et al., 1997; Lelièvre et al., 1998). To determine whether the formation of large TIN2 domains was associated with polarity or growth arrest, we cultured S1 cells in collagen I, rather than the laminin-rich Matrigel™. Under these conditions, the cells arrest proliferation, form multicellular structures of sizes similar to the acini formed in Matrigel™, but the cells inversely polarize (Gudjonsson et al., 2002; Weaver et al., 2002). Large TIN2 domains were present in the majority of nuclei when S1 cells were cultured for 10 days in collagen I (Fig. 3A). Thus, formation of large TIN2 domains did not depend on acinar polarity.
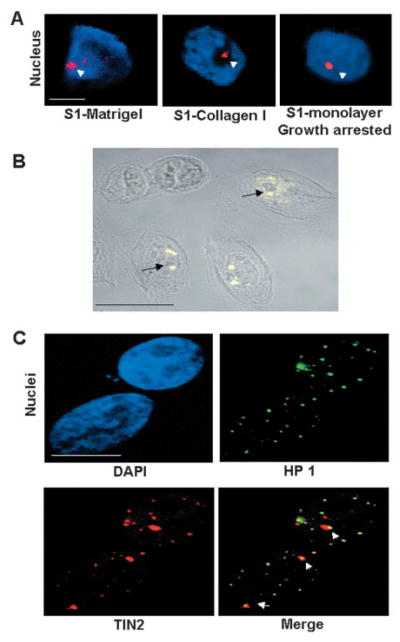
Fig. 3.
Formation of large TIN2 domains in growth-arrested cells is independent of the differentiation status. (A) Large TIN2 domains (arrowheads) revealed by immunostaining (red) in correctly polarized S1 cells cultured in 3D laminin-rich ECM (S1-Matrigel), S1 cells cultured in 3D collagen I (S1-Collagen I) that display altered polarity, and growth-arrested (EGF-deprived) S1 cells cultured as a monolayer on plastic. Nuclei are counterstained with DAPI (blue). (B) Immunostaining for TIN2 (yellow) in growth-arrested 184 cells cultured as a monolayer on plastic. The superimposed phase-contrast image shows the nucleoli as dark gray subnuclear structures. Arrows indicate large TIN2 domains located next to nucleoli. (C) Dual immunostaining for HP 1γ (green) and TIN2 (red) in growth-arrested 184 cells cultured as a monolayer on plastic. Arrowheads indicate overlapping (yellow) HP 1 staining and large TIN2 domains. Nuclei were counterstained with DAPI (blue). Bar, 5 μm.
We next asked whether growth arrest was necessary for the formation of large TIN2 domains. We cultured S1 and 184 HMECs as subconfluent monolayers in a defined medium, then arrested proliferation by providing medium lacking EGF. After 3 days, most of the cells withdrew from the cell cycle (Lelièvre et al., 1998) (not shown), and TIN2 formed large domains in about 80% of the nuclei (Fig. 3A,B). S1 cells also arrested proliferation upon reaching confluence, even in the presence of EGF, and TIN2 formed large domains in the majority of confluent S1 cells (not shown). Thus, the formation of large TIN2 domains was associated with growth arrest, rather than an absence of EGF or acinus formation per se.
Consistent with our observations in 3D cultures, the TIN2 domains that formed upon growth arrest in monolayer cultures frequently localized adjacent to nucleoli (Fig. 3B) and partially or totally overlapped with HP 1γ domains (Fig. 3C). To more definitively determine the relationship between large TIN2 domains and telomeres, we co-stained growth-arrested S1 monolayer for telomeres and TIN2. Although occasional telomeric foci could be observed within a large TIN2 domain, most of the large TIN2 domains were devoid of telomeres and most of the telomeres were outside the large TIN2 domains (Fig. 4A). Dual staining for TIN2 and TRF1 or TRF2 likewise showed that only TIN2 formed large domains in growth-arrested cells (Fig. 4A), and that the majority of TRF1 and TRF2 focal staining was excluded from these domains. The foci-like distribution of TIN2 outside large domains overlapped with TRF2, a marker of telomeres, indicating that TIN2 remains at telomeres in growth-arrested cells but additionally forms large domains outside the telomeres (Fig. 4B).
Fig. 4.
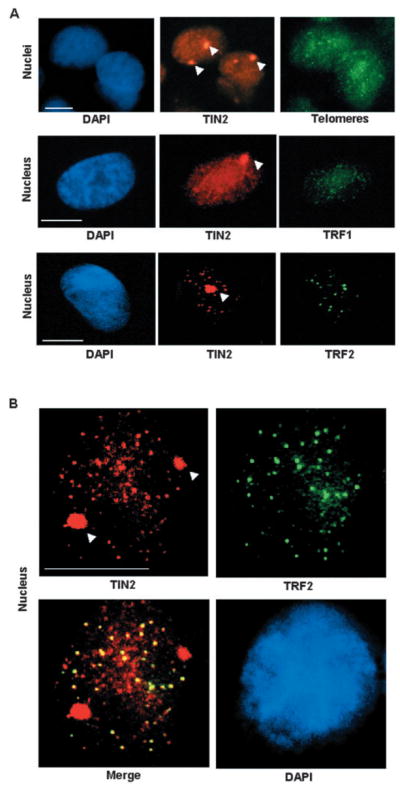
Formation of large TIN2 domains is independent of telomeres and TRF proteins. (A) HMECs (strain 184) were cultured as a monolayer on plastic and growth-arrested before fixation and dual staining for TIN2 (red) and telomeres (PNA FISH; green) (upper panels), TIN2 (red) and TRF1 (green) (middle panels), and TIN2 (red) and TRF2 (green) (lower panels). (B) Higher magnification images of dual staining for TIN2 (red) and TRF2 (green) showing colocalization at small foci (yellow), indicating telomeric localization. Nuclei were counterstained with DAPI (blue). Arrowheads indicate large TIN2 domains. Bar, 5 μm.
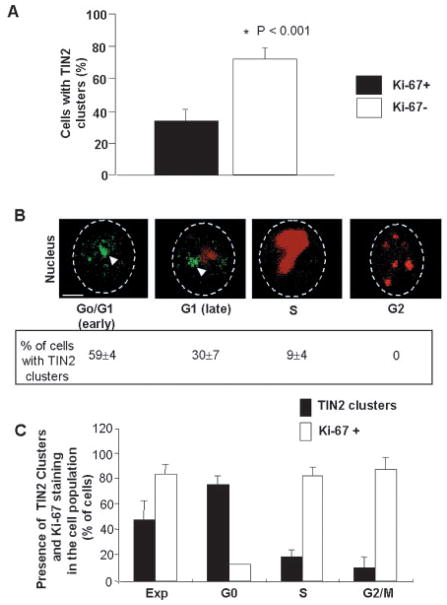
To more accurately define the cell-cycle dependence of TIN2 domain formation, we co-stained S1 cells for TIN2 and Ki-67, a cell proliferation marker that displays distinct nuclear distributions depending on the phase of the cell cycle (Braun et al., 1988). In proliferating cells (day 3 of 3D culture or monolayers in the presence of EGF), ~30% of cycling (Ki-67 positive) cells displayed large TIN2 domains. By contrast, ~80% of non-cycling (Ki-67 negative) cells displayed large TIN2 domains (Fig. 5A). Examination of the Ki-67 staining pattern and TIN2 distribution showed that large TIN2 domains were present primarily during the G0 and G1 phases of the cell cycle, whereas only a few cells in S phase, and virtually no cells in the G2 and M phases, had these large domains (Fig. 5B). To confirm that large TIN2 domains form primarily in G0 and G1, we synchronized 184 HMECs in monolayer culture by incubating in medium lacking EGF and supplemented with EGF blocking antibody. We immunostained the cells for Ki-67 and TIN2 while growth-arrested, as well as 16 and 20 hours after release from growth arrest, which corresponded to the mid-S and G2-M phases of the cell cycle, respectively, as confirmed by Ki-67 staining. The majority of 184 cells displaying large TIN2 domains were negative for Ki-67 (Fig. 5C). Thus, TIN2 formed large domains primarily when cells were quiescent.
Fig. 5.
Formation of large TIN2 domains correlates with exit from the cell cycle. (A) Percentage of S1 cells with large TIN2 domain(s) and Ki-67 staining. S1 cells were cultured in 3D for 3 days to obtain a mixed population of cycling Ki-67 positive (Ki-67+) and growth-arrested Ki-67 negative (Ki-67−) cells, and then fixed and dual immunostained for TIN2 and Ki-67. Shown is the percentage of cells containing large TIN2 domains (TIN2 clusters) and Ki-67 positive (filled bars) or negative (open bars) staining. Error bars show the s.e.m. for three different experiments. *P<0.001 when compared with Ki-67+ cells. (B) Dual immunostaining for TIN2 (green) and Ki-67 (red) in S1 cells after 3D culture for 3 days. The different phases of the cell cycle were identified by the pattern of Ki-67 staining. The percentage of cells showing large TIN2 domains (TIN2 clusters) in each phase of the cell cycle is given below each panel, and is the mean±s.e.m. of three different experiments. Arrowheads indicate large TIN2 domains and dashed lines delineate the nuclear periphery. (C) Histogram of the percentage of synchronized 184 HMECs with large TIN2 domains (TIN2 clusters) as a function of the cell cycle. Nuclei showing large TIN2 domains were counted as a percentage of total nuclei (revealed by DAPI counterstaining) during exponential (EXP), G0, S and G2-M phases of the cell cycle. Percentages of Ki-67-positive nuclei in each phase are shown. Bar, 2.5 μm.
Truncated forms of TIN2 prevent formation of large TIN2 domains and growth arrest
To determine whether the change in TIN2 organization is related to the status of growth in HMECs, we infected S1 cells with retroviruses expressing either N-terminally (TIN2-13)- or C-terminally (TIN2-15) truncated forms of TIN2 (Fig. 6A). These mutants interfere with the telomere length control function of wild-type TIN2 in a dominant-negative fashion (Kim et al., 1999). Viruses lacking an insert (vector control), expressing green fluorescent protein (GFP) or expressing hTERT (catalytic subunit of human telomerase) served as controls for the effects of infection, expression of an ectopic protein, and expression of a telomeric protein that does not interact with TIN2, respectively. Western analysis confirmed expression of TIN2-13 (Fig. 6B). However, because our polyclonal antibody was raised against an N-terminally truncated protein, TIN2-15 was undetectable by western analysis. In addition, detection of TIN2-15 in protein extracts using an antibody against the myc-epitope tag was marginal, indicating that TIN2-15 is unstable or the anti-myc antibody does not detect this protein readily on western blots. However, TIN2-15 was detectable in cell nuclei in monolayer and 3D cultures by anti-myc immunostaining (Fig. 6C). To confirm the expression of both truncated proteins, we performed Southern blot analysis using a telomeric probe to assess the effect of TIN2-13 and TIN2-15 on telomere length (Kim et al., 1999). Cells infected with either TIN2-13 or TIN2-15 constructs showed a 27% and 46% increase in mean telomere length, respectively, compared with vector control after seven population doublings, indicating that both TIN2 mutants were expressed and biologically active.
Fig. 6.
TIN2 controls growth arrest in mammary epithelial cells. (A) Schematic of wild-type TIN2 (WT TIN2), and N-terminally (TIN2-13) and C-terminally (TIN2-15) truncated forms of TIN2. (B) Expression of TIN2 and TIN2-13 in control and TIN2-13 expressing cells shown on western blots. Lanes contained cells used for infection (control), cells expressing TIN2-13 (TIN2-13), cells infected with empty vector (control vector), cells overexpressing wild-type TIN2 (TIN2), control HT1080 fibroblasts expressing exogenous TIN2 and TIN2-13 (TIN2 +TIN2− 13 mixture in HT1080). Arrows indicate the location of the respective bands for TIN2 and TIN2-13. β-catenin was used as a loading control. (C) TIN2-15 expression in monolayer and 3D culture shown by anti-myc immunostaining (green). Nuclei were counterstained with DAPI. Magnification ×1200. (D) Vector control, TIN2-13 and TIN2-15 infected S1 cells were cultured in 3D for 10 days. Shown are phase-contrast images of acini formed by vector control S1 cells and abnormal looking colonies formed by TIN2-13 and TIN2-15 S1 cells. The arrows indicate enlarged and/or irregular multicellular structures. (E) Non-infected S1 cells (control) and vector control, TIN2-13, or TIN2-15 infected S1 cells were cultured in 3D for 10 days. Acini were classified according to six diameter ranges (6–15 μm, 16–25 μm, 26–35 μm, 36–45 μm, 46–55 μm, 56–65 μm). Shown is the percentage of acini in each diameter range out of a total of 400 acini observed in each independent experiment. Three experiments were performed. (F) Immunostaining for the endogenous basement membrane component collagen IV (red) and Ki-67 (green) in vector control or TIN2-15 S1 cells cultured in 3D for 10 days. When proper morphogenesis occurs, acini are surrounded by a continuous basement membrane and >90% of the cells arrest proliferation. One nucleus positive for Ki-67 is seen out of ten nuclei in vector control; five nuclei positive for Ki-67 are seen out of 14 nuclei in TIN2-15. Arrows indicate the absence of collagen IV around part of the TIN2-15 colony. (G) GFP-S1 cells organized in an acinus (left panel). Immunostaining for the endogenous basement membrane component collagen IV (red) (central panel) and α6-integrin (green) and β-catenin (red) (right panel) in hTERT-expressing S1 cells cultured in 3D for 10 days. When proper morphogenesis occurs, in addition to the continuous basement membrane, acini display the localization of α6-integrin at the basal cell membrane (against the basement membrane) and β-catenin at cell-cell junctions. (H) Immunostaining for TIN2 (red) in control or TIN2-15 S1 cells. In the control acinus, eight of nine nuclei, identified by DAPI staining, have a large TIN2 domain (arrowheads). In the acinar structure formed by TIN2-15-expressing cells, four of thirteen nuclei show one or two large TIN2 domains (arrowheads) and one nucleus shows completely fragmented TIN2 domains (arrow). Bar, 25 μm.
In contrast to control cells, cells that expressed TIN2-13 or TIN2-15 formed heterogeneous and disorganized acini. TIN2-15 was more potent than TIN2-13 in this regard (Fig. 6D). TIN2-15-expressing cells formed acini that were up to fourfold larger than control acini, indicating a loss of growth control. In addition, very few of these acini showed basal localization of collagen IV, indicating loss of acinar polarity (Fig. 6E,F). GFP and hTERT-expressing S1 cells did not display any detectable alteration in acinar morphogenesis (Fig. 6G).
To further investigate the effects of TIN2 mutants on proliferation, we assessed cell cycle activity by Ki-67 immunostaining and BrdU incorporation. After 10 days in 3D culture, both TIN2-13- and TIN2-15-expressing S1 cells showed substantially more Ki-67 staining (Fig. 6F) than control cells. Moreover, the sharp drop in BrdU-positive cells usually observed between days 6 and 10 of acinus formation was less pronounced in TIN2-13-expressing cells, and essentially eliminated in TIN2-15-expressing cells (decrease in BrdU positive index: 64.6±8.8% in control, 52.98±4.4% in TIN2-13 and 16.6±9.8% in TIN2-15). Similarly, TIN2-15-expressing cells cultured as monolayers were resistant to EGF withdrawal-induced growth arrest, as indicated by the high number of BrdU-positive cells compared with controls after 3 days in medium lacking EGF (not shown). Thus, cells expressing mutant forms of TIN2 had a diminished capacity to respond to signals for growth arrest.
To determine whether the TIN2 mutants acted in a dominant-negative fashion to prevent the formation of large TIN2 domains, we examined the distribution of TIN2 in TIN2-13- and TIN2-15-expressing cells cultured in 3D for 10 days. Formation of large TIN2 domains was reduced sharply, approximately 3- and 4.5-fold respectively, in the aberrant acini formed by TIN2-13- and TIN2-15-expressing cells. Thus, nuclei with large TIN2 domains were observed in 80% of control cells, 27% of TIN2-13-expressing cells and 18% of TIN2-15-expressing cells. Within the disorganized acini, nuclei with prominent TIN2 domains could be seen alongside nuclei devoid of large TIN2 domains or showing only fragmented TIN2 domains (Fig. 6H). Expression of TIN2-15 was not accompanied by the formation of large domains containing TIN2-15 (see Fig. 6C). Thus, the TIN2 mutants greatly diminished formation of large TIN2 domains and prevented efficient growth arrest.
Discussion
Our findings provide the first evidence that a mammalian telomere-associated protein forms a novel nuclear structure, which is not associated with telomeres, and that formation of this structure is important for the growth arrest of HMECs under monolayer and 3D culture conditions. This protein, TIN2, associates with telomeres indirectly by binding to TRF1 (Kim et al., 1999) and TRF2 (Kim et al., 2004). We show here that TIN2 also forms large non-telomeric domains in a non-neoplastic human mammary epithelial cell line and a finite life span human mammary-cell strain. In addition, large TIN2 domains were detectable in normal human breast tissue, indicating that these structures do form in vivo.
The large TIN2 domains observed in mammary epithelial cells were not accompanied by clustering of telomeric DNA or the TIN2 binding partners TRF1 and TRF2 proteins. The clustering or aggregation of telomeric components has been described during spermatogenesis in mammalian cells (Zalensky et al., 1997). In male germ cells, clustering of telomeric DNA is mediated by a telomere-binding protein complex (hSTBP) that includes a variant of histone H2B but does not contain TRF1 or TRF2 (Gineitis et al., 2000). This higher organization of telomeric DNA is established during early meiosis and is proposed to be important for fertilization and early development (Zalensky et al., 1997). By contrast, we have found no evidence for higher-order telomere clusters (i.e. large domains that include telomeric DNA) in somatic mammalian cells, in agreement with previous studies (Ludérus et al., 1996) that used tumor-derived and non-transformed mammalian cells. However, these studies did not explore the effects of growth arrest or tissue differentiation on telomere organization. Our results show that although the striking phenotypic changes that accompany acinar morphogenesis do not alter telomere organization, acinar morphogenesis is accompanied by higher-order nuclear organization of the secondary telomere-associated protein, TIN2. Thus, in contrast to the clustering of telomeres in germ cells, our findings suggest that telomere components, but not telomeres, may cluster in somatic cells.
Using a variety of cell culture manipulations, we determined that the important step for the formation of large TIN2 domains was exit from the cell cycle. The importance of the large TIN2 domains for the growth arrest of HMECs was evident from the behavior of cells expressing mutant forms of TIN2. Truncated forms of TIN2 prevented the formation of large TIN2 domains, and simultaneously interfered with the ability of cells to arrest proliferation both in 3D and monolayer culture. These data suggest that TIN2 reorganization is probably crucial for proper HMEC growth control and hence subsequent acinar differentiation. We do not yet know whether large TIN2 domain formation and its influence on growth arrest occur in multiple cell types, or are restricted to HMECs. Preliminary data suggest that large TIN2 domains do not form naturally in growth-arrested fibroblasts (unpublished data), raising the possibility that their formation is restricted to all or certain types of epithelial cells.
Although dual staining for TIN2 and telomeric DNA could not be achieved in 3D culture, dual staining on growth-arrested cells in monolayer culture showed that telomeres were not constituents of the large TIN2 domains. These data suggest that there is an extra-telomeric function for TIN2 upon growth arrest of HMECs. It is important to emphasize that the reorganization of TIN2 observed upon growth arrest in HMECs was not associated with loss of TIN2 from telomeres. Rather, the reorganization entailed a gain of TIN2 at mostly perinucleolar sites. The mechanisms that trigger recruitment of TIN2 to extra-telomeric domains in growth-arrested HMECs are not yet understood but may involve binding to as yet unknown partners. Our finding that the large TIN2 domains frequently associate with HP 1γ suggests that TIN2 may be recruited to extra-telomeric sites by chromatin-associated proteins. In support of this possibility, other HP 1 variants have been shown to interact with TRF1-PIN2 in mice and Ku70 in humans (Netzer et al., 2001; Song et al., 2001).
The role of TIN2 within HP 1-TIN2 domains remains to be deciphered. TIN2 domains were DNase sensitive, suggesting an association with chromatin, and they frequently associated with HP 1γ, a known heterochromatin-associated protein. Therefore, our current hypothesis is that large TIN2 domains may promote chromatin compaction in association with other chromatin components. Indeed the highly conserved HP 1 proteins are key transcriptional regulators and are critical for the functional and structural organization of the nucleus (Kellum, 2003). Notably, the binding of HP 1 variants (including HP 1γ) to many different proteins indicates a central role for this protein family in nuclear function. Thus, TIN2 may have the capability to organize chromatin, possibly in conjunction with HP 1, although so far TIN2 has only been shown to promote the compaction of telomeric chromatin in association with TRF1 (Kim et al., 2003a). The participation of large TIN2 domains in chromatin compaction is suggested further by the frequent localization of these domains to perinucleolar regions, where heterochromatin and/or regions of gene silencing have been located (Olson et al., 2002).
Chromatin remodeling is a key event in differentiation processes in both non-mammalian and mammalian cells (Müller and Leutz, 2001; Plachot and Lelièvre, 2004). Most interestingly, it was recently demonstrated that alterations in chromatin structure also precede exit from the cell cycle (Barbie et al., 2004): perinucleolar replication foci were shown to persist throughout S phase prior to exit from the cell cycle. In addition, pRB and histone deacetylase complexes localized to perinucleolar replication sites and thus, might be poised to establish repressive chromatin structures in the vicinity of the nucleolus. One possibility is that HP 1 intervenes at this step via its ability to propagate heterochromatin and hence help silence genes that promote cell proliferation. TIN2 may facilitate this process in HMECs by promoting chromatin compaction owing to its influence on a binding partner, similar to its effect on the compaction of telomeric DNA (Kim et al., 2003a). This could result in the formation of a highly dense chromatin structure capable of repressing gene activity. The expression of truncated forms of TIN2, one of which was shown to be defective in telomeric DNA compaction (Kim et al., 2003a), was sufficient to prevent the formation of large TIN2 domains and growth arrest. These results suggest that these TIN2 domains may have a repressive effect on genes that promote proliferation.
Whether telomere organization influences the formation of large extra-telomeric TIN2 domains as well as the function of TIN2 in these large domains is an exciting question. TIN2 is a critical regulator of telomere length, and TIN2-induced changes in telomere length may, in turn, affect telomere organization and the formation of large TIN2 domains. Indeed expression of truncated forms of TIN2 in S1 cells affected both telomere length and the formation of large TIN2 domains. The molecular tools currently available do not allow us to specifically target TIN2 located in the large perinucleolar domains and thus to assess whether the organization of large TIN2 domains is sufficient to control growth arrest. The identification of TIN2 binding partners within the large domains will be critical to address this question.
In yeast, clustering of telomeric DNA and associated proteins influences cellular phenotype; our findings indicate that mammary epithelial cell phenotypes are influenced by the organization of a secondary telomere-associated protein. It will be of interest to examine other differentiation systems for TIN2 organization and also for the organization of other telomere-associated proteins. Although telomeric sequences tend to be highly conserved across species, mammalian telomeric proteins show significantly greater divergence (Li et al., 2000; de Lange, 2004), suggesting that the telomeric proteins of higher organisms may have functions in addition to telomere protection and regulation of telomere length.
Acknowledgments
We thank Martha Stampfer and Paul Yaswen for 184 cells, Robert Weinberg for hTERT cDNA, Fritz Rank for breast biopsy samples and Tove Marianne Lund for technical assistance. We also thank Carol Prives, Sybille Galosy and Derek Radisky and in particular Zena Werb for critical reading of the manuscript and insightful comments. This work was supported by the Department of Energy, Office of Health and Environmental Research (DE-AC03 SF0098 to M.J.B. and J.C.), Walther Cancer Institute (WCI-110-114 to S.A.L.), Jim and Diane Robbers Foundation at the Purdue Cancer Center (S.A.L.), California Breast Cancer Research Program (88AV01 to S-H.K.), Danish Research Council and Novo Nordisk Foundation to O.W.P., National Institutes of Health (CA64786 to M.J.B. and AG09909 to J.C.), Department of Defense Breast Cancer Research Program (DAMD17-02-1-0438 to M.J.B.), California Breast Cancer Research Program (7FB0018 to P.K.), Joyce Fox Jordan Cancer Research Program at the Purdue Cancer Center (C.P.), Department of Energy, Undergraduate Laboratory Fellowship program (P.C.) and National Institutes of Health training grant (AG00266).
References
- Barbie DA, Kudlow BA, Frock R, Zhao J, Johnson BR, Dyson N, Harlow E, Kennedy BK. Nuclear reorganization of mammalian DNA synthesis prior to cell cycle exit. Mol Cell Biol. 2004;24:595–607. doi: 10.1128/MCB.24.2.595-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilaud T, Brun C, Ancelin K, Koering CE, Laroche T, Gilson E. Telomeric localization of TRF2, a novel human telobox protein. Nat Genet. 1997;17:236–239. doi: 10.1038/ng1097-236. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;6:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden KL. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol Cell Biol. 2002;22:5259–5269. doi: 10.1128/MCB.22.15.5259-5269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Papadopoulos T, Muller-Hermelink HK. Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56:25–33. doi: 10.1007/BF02889998. [DOI] [PubMed] [Google Scholar]
- Briand P, Petersen OW, van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- Chong L, van Steensel B, Broccoli D, Erdjument-Bromage H, Hanish J, Tempst P, de Lange T. A human telomeric protein. Science. 1995;270:1663–1667. doi: 10.1126/science.270.5242.1663. [DOI] [PubMed] [Google Scholar]
- Counter CM, Meyerson M, Eaton EN, Ellisen LW, Caddle SD, Haber DA, Weinberg RA. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- de Lange T. T-loops and the origin of telomeres. Nat Rev Mol Cell Biol. 2004;5:323–329. doi: 10.1038/nrm1359. [DOI] [PubMed] [Google Scholar]
- Gineitis AA, Zalenskaya IA, Yau PM, Bradbury EM, Zalensky AO. Human sperm telomere-binding complex involves histone H2B and secures telomere membrane attachment. J Cell Biol. 2000;151:1591–1598. doi: 10.1083/jcb.151.7.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta M, Gasser SM. Nuclear organization and transcriptional silencing in yeast. Experientia. 1996;52:1136–1147. doi: 10.1007/BF01952113. [DOI] [PubMed] [Google Scholar]
- Gotta M, Laroche T, Formenton A, Maillet L, Scherthan H, Gasser SM. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild type Saccharomyces cerevisiae. J Cell Biol. 1996;134:1349–1363. doi: 10.1083/jcb.134.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Rønnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to signal to luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SL, Ham RG, Stampfer MR. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci USA. 1984;81:5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB, Futcher C, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Kellum R. HP1 complexes and heterochromatin assembly. Curr Top Microbiol Immunol. 2003;274:53–77. doi: 10.1007/978-3-642-55747-7_3. [DOI] [PubMed] [Google Scholar]
- Kim SH, Beausejour C, Davalos AR, Kaminker P, Heo SJ, Campisi J. TIN2 mediates functions of TRF2 at human telomeres. J Biol Chem. 2004;279:43799–43804. doi: 10.1074/jbc.M408650200. [DOI] [PubMed] [Google Scholar]
- Kim SH, Han S, You YH, Chen DJ, Campisi J. The human telomere-associated protein TIN2 stimulates interactions between telomeric DNA tracts in vitro. EMBO Rep. 2003a;4:685–691. doi: 10.1038/sj.embor.embor872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Parrinello S, Kim J, Campisi J. Mus musculus and Mus spretus homologues of the human telomere-associated protein TIN2. Genomics. 2003b;81:422–32. doi: 10.1016/s0888-7543(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kaminker P, Campisi J. TIN2, a new regulator of telomere length in human cells. Nat Genet. 1999;23:405–412. doi: 10.1038/70508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, Raap AK, Tanke HJ. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- Lelièvre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, Bissell MJ. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci USA. 1998;95:14711–14716. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelièvre SA, Bissell MJ, Pujuguet P. Cell nucleus in context. Crit Rev Eukaryot Gene Expr. 2000;10:13–20. doi: 10.1615/critreveukargeneexpr.v10.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–483. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Kirschmann DA, Wallrath LL. Does heterochromatin protein 1 always follow code? Proc Natl Acad Sci USA. 2002;99:16462–16469. doi: 10.1073/pnas.162371699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Safari A, O’Connor MS, Chan DW, Laegeler A, Qin J, Songyang Z. PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol. 2004;6:673–680. doi: 10.1038/ncb1142. [DOI] [PubMed] [Google Scholar]
- Ludérus ME, van Steensel B, Chong L, Sibon OC, Cremers FF, de Lange T. Structure, subnuclear distribution, and nuclear matrix association of the mammalian telomeric complex. J Cell Biol. 1996;135:867–881. doi: 10.1083/jcb.135.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet L, Boscheron C, Gotta M, Marcand S, Gilson E, Gasser SM. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 1996;10:1796–1811. doi: 10.1101/gad.10.14.1796. [DOI] [PubMed] [Google Scholar]
- Müller C, Leutz A. Chromatin remodeling in development and differentiation. Curr Opin Genet Dev. 2001;11:167–174. doi: 10.1016/s0959-437x(00)00175-1. [DOI] [PubMed] [Google Scholar]
- Netzer C, Rieger L, Brero A, Zhang CD, Hinzke M, Kohlhase J, Bohlander SK. SALL1, the gene mutated in Townes-Brocks syndrome, encodes a transcriptional repressor which interacts with TRF1/PIN2 and localizes to pericentromeric heterochromatin. Hum Mol Genet. 2001;10:3017–3024. doi: 10.1093/hmg/10.26.3017. [DOI] [PubMed] [Google Scholar]
- Olson MO, Hingorani K, Szebeni A. Conventional and nonconventional roles of the nucleolus. Int Rev Cytol. 2002;219:199–266. doi: 10.1016/S0074-7696(02)19014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells [published erratum appears in Proc Natl Acad Sci USA (1993) 90, 2556] Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachot C, Lelièvre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298:122–132. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Szekely L, Kiss C, Mattsson K, Kashuba E, Pokrovskaja K, Juhasz A, Holmvall P, Klein G. Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin- associated nuclear bodies. J Gen Virol. 1999;80:2889–2900. doi: 10.1099/0022-1317-80-11-2889. [DOI] [PubMed] [Google Scholar]
- Song K, Jung Y, Jung D, Lee I. Human Ku70 interacts with heterochromatin protein 1alpha. J Biol Chem. 2001;276:8321–8327. doi: 10.1074/jbc.M008779200. [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Pan CH, Hosoda J, Bartholomew J, Mendelsohn J, Yaswen P. Blockage of EGF receptor signal transduction causes reversible arrest of normal and immortal human mammary epithelial cells with synchronous reentry into the cell cycle. Exp Cell Res. 1993;208:175–188. doi: 10.1006/excr.1993.1236. [DOI] [PubMed] [Google Scholar]
- van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three- dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Lelièvre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen P, Stampfer MR. Molecular Changes accompanying senescence and immortalization of cultured human mammary epithelial cells. Int J Biochem Cell Biol. 2002;34:1382–1394. doi: 10.1016/s1357-2725(02)00047-x. [DOI] [PubMed] [Google Scholar]
- Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 2004;18:1649–1654. doi: 10.1101/gad.1215404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalensky AO, Tomilin NV, Zalenskaya IA, Teplitz RL, Bradbury EM. Telomere-telomere interactions and candidate telomere binding protein(s) in mammalian sperm cells. Exp Cell Res. 1997;232:29–41. doi: 10.1006/excr.1997.3482. [DOI] [PubMed] [Google Scholar]