Abstract
The microenvironment influences gene expression so that the behavior of a cell is largely determined by its interactions with the extracellular matrix, neighboring cells, and soluble local and systemic cues. We describe the essential roles of context and organ structure in directing mammary gland development and differentiated function and in determining the response to oncogenic insults, including mutations. We expand on the concept of “dynamic reciprocity” to present an integrated view of development, cancer, and aging and posit that genes are like the keys on a piano: Although they are essential, it is the context that makes the music.
Keywords: tissue context, differentiation, mammary gland, microenvironment, morphogenesis, stromal-epithelial interactions
INTRODUCTION
The function of an organ relies upon the organ’s constituent cell types and overall organization. It is the obvious uniqueness of this structure that distinguishes, e.g., a breast from a kidney and that directs the cells within the former to make milk and within the latter to filter blood and make urine; this is so despite the fact that they share an identical genome. But whereas tissue specificity is a certainty, there is little compelling evidence for the concept of terminal differentiation except in organs in which differentiation is defined by cell death or loss of nuclei. The instability and plasticity of the differentiated state (Bissell 1981, Blau & Baltimore 1991) allow phenotypic evolution to occur over the lifetime of a cell, tissue, organ, and organism to ensure adaptability and survival. The differentiated phenotype accomplishes this while being both (a) robust (stable to minor perturbations; the breast almost never turns into a kidney in vivo) and (b) labile, or responsive to external influences. With regard to the latter characteristic, given the appropriate cues, a resting mammary gland can easily be coaxed into a spectacular reversible functional differentiation program during pregnancy, corneal epithelium can be induced to sprout feathers or hair (Coulombre & Coulombre 1971, Ferraris et al. 1994), and aggressive carcinoma cells can be tamed to form normal tissues by changing their microenvironment (Mintz & Illmensee 1975) or to revert to a normal phenotype simply by changing microenvironmental signaling (Weaver et al. 1997, 2002). The interactions between a cell and its surroundings thus determine its pattern of gene expression and resultant differentiated phenotype despite the fact that the blueprint of the genome does not change. Here we describe what we know about the process of tissue specificity from the point of view of the mammary gland (our experimental organism), but the fundamentals of the issues discussed extend far beyond this organ. In the end, the unit of functional differentiation is the organism itself.
TISSUE ARCHITECTURE IS BOTH A CONSEQUENCE AND A CAUSE (THE END AND THE BEGINNING)
Dynamic Reciprocity Redux
The structure of a tissue or organ is critical for its function. Loss of tissue architecture is a prerequisite for, and one of the defining characteristics of, most cancers. Conversely, normal organ architecture can act as a powerful tumor suppressor, preventing malignant phenotypes even in cells stricken with gross genomic abnormalities (Mintz & Illmense 1975, Howlett et al. 1995, Weaver et al. 1997, Wang et al. 2002, Kirshner et al. 2003). But if organ function and homeostasis are driven by organ architecture, and if every cell in every organ carries the same genetic information, then how are tissue-specific form and function achieved? Elegant work by early developmental biologists, some of which is described below, inspired us to postulate that tissue-specific function is achieved by interactions between the cell and its surrounding extracellular matrix (ECM), a model dubbed dynamic reciprocity (Bissell et al. 1982). According to this model, the dynamic bidirectional cross talk from the ECM with the cell membrane (Bornstein et al. 1982) is extended to the broad realm of gene expression by connecting ECM–ECM receptor interactions to the cytoskeleton and to the nuclear matrix and chromatin and back again (reproduced in Figure 1a). An important feature of this model was that it took the then-evolving work of the role of ECM in development as that of a possible scaffold to a view of ECM as an integral determinant of tissue specificity itself. Most importantly, the work of a number of laboratories has provided substantial evidence for the essential components of the model in the intervening years. Although the original depiction of dynamic reciprocity dealt mainly with the role of the ECM, the cellular microenvironment also clearly includes adhesive and soluble paracrine signals from neighboring cells, distant tissues, and systemic cues (see Figure 1b for updated model). As such, organ structure and consequently organ function are determined by the dynamic and reciprocal interactions between the organ’s constituent tissues, the structure and function of which are determined by the dynamic and reciprocal interactions between the cells and ECM comprising a given tissue. And lest we forget, each organ is choreographed to function in a dynamic scenario with other organs and is of little use when removed from the greater context of the organism.
Figure 1.
(a) The original model of dynamic reciprocity, or the minimum required unit for tissue-specific functions. N, nucleus; MT, microtubules; IF, intermediate filaments; MF, microfilaments; C, collagen. Reprinted from Bissell et al. (1982) with permission from Elsevier. (b) A more complete view of dynamic reciprocity.
Tissue Interactions in Development
Every organ is composed of tissues derived from the embryonic germ layers: endoderm (which becomes epithelium of the lungs and digestive organs), mesoderm (which generates bone, muscle, and mesenchymal connective tissue), and ectoderm (which gives rise to the nervous system and epithelium of the skin and its derivatives, including the mammary gland). Epithelial and mesenchymal components interact during development to direct tissue morphogenesis (the physical creation of normal tissue architecture) and differentiation (acquisition of tissue-specific functions). That tissue development is not cell autonomous but is instead instructed by the surrounding environment was hypothesized as early as 1817 (Pander 1817). However, it was first demonstrated a century later by the elegant experiments of Ethel Browne, and Hans Spemann and Hilde Mangold, who used hydra and amphibian embryos, respectively (Browne 1909, Spemann 1918, Spemann & Mangold 1924). The famous organizer experiment showed that certain regions of the embryo could direct the development of adjacent groups of cells into specific tissues (Spemann 1918, Spemann & Mangold 1924).
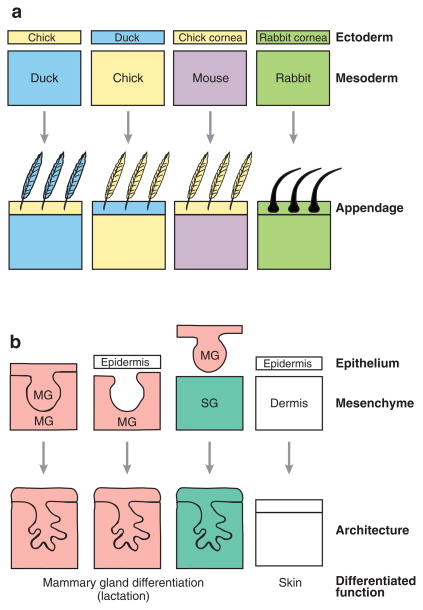
These early studies preceded a flurry of work over the next 80 years, demonstrating in many systems that cells derived from the different germ layers carry on an extensive cross talk to direct tissue development. Studies of vertebrate skin (Figure 2a) revealed that the identity, location, and pattern of development of ectodermal epidermal appendages (e.g., hair follicles in mammals and scales and feathers in birds) are determined by the dermis (a mesodermal derivative). Using tissue recombination techniques developed in the 1950s, Saunders and colleagues found that thigh mesoderm inserted beneath the ectoderm of an embryonic chick wing induced the wing to form leg feathers instead of flight feathers (Cairns & Saunders 1954, Saunders & Gasseling 1968). Chimeric feathers were occasionally found at the border of the graft site, demonstrating the specificity of the mesodermal signal. In similar studies recombining skin tissues from chick and duck, duck mesoderm instructed chick ectoderm to form feathers anatomically shaped like those of a duck; the converse was also true (Dhouailly 1967, 1970). In perhaps the most striking example, mesoderm from a mouse (which normally would induce mouse ectoderm to form hair follicles) was combined with corneal epithelium from a chick (which normally would become an appendage-free transparent surface), resulting in feather development (Coulombre & Coulombre 1971).
Figure 2.
The dramatic effect of tissue-tissue interactions. (a) Embryonic ectoderm/mesoderm recombination experiments determined that the identity of the mesoderm dictated the identity of the ectodermal appendage. (b) Epithelial/mesenchymal recombination experiments determined that the identity of the mesenchyme dictated the architecture of the developing epithelium. When mammary gland (MG) epithelium is recombined with salivary gland (SG) mesenchyme, the resulting structure can still produce milk, although the epithelial tree resembles a salivary gland. Panel b adapted from Parmar & Cunha (2004).
Ectoderm can play an instructive role during development as well. In vertebrates, the mesenchyme of the outgrowing limb is surrounded by a dorsal rim of ectoderm, the apical ectodermal ridge (AER). When the AER is removed, the limb fails to develop properly; when the AER covering the eventual wing is grafted onto the stump of a growing leg, the region develops wing parts (Saunders 1948). The mechanisms underlying induction by AER, skin mesoderm, and Spemann’s organizer have been studied extensively (reviewed recently in Wolpert 1998, Capdevila & Izpisua Belmonte 2001, Niehrs 2004) and involve common paracrine signaling molecules, including members of the fibroblast growth factor, transforming growth factor (TGF)-β, Wnt, and hedgehog families.
The Impressionable Epithelium
Some of the clearest examples of the importance of epithelial-mesenchymal interactions in morphogenesis and differentiation have come from recombination experiments using isolated tissues from the mammary gland and other organs (Figure 2b). Whereas mammary epithelium recombined with mammary mesenchyme develops a typical mammary tree, recombination with salivary gland mesenchyme generates structures resembling the salivary epithelial tree (Kratochwil 1969, Sakakura et al. 1976). Conversely, mammary mesenchyme can induce epithelial cells from other tissues to build a lactation-competent gland (Cunha et al. 1995). These experiments demonstrated that even adult cells retain a capacity for alternative modes of morphogenesis and differentiation. The importance of reciprocal interactions between epithelium and mesenchyme and the identification of the molecular mediators have now been demonstrated for several organs, including the lung, kidney, prostate, and salivary and mammary glands (reviewed in Hieda & Nakanishi 1997, Cardoso 2001, Marker et al. 2003, Parmar & Cunha 2004, Yu et al. 2004). The molecular players involved in epithelial-mesenchymal interactions during mammary gland development are detailed in Table 1; similar roles for many of these molecules have been found in the development of other organs.
Table 1.
Epithelial-mesenchymal interactions in the mammary gland
| Signaling by stroma |
Signaling by epithelium |
|||
|---|---|---|---|---|
| Stromal ligand/cue | Epithelial receptor | Epithelial ligand/cue | Stromal receptor | |
| During ductal development/puberty | HGF | cMet | Amphiregulin | EGFR (ErbB1) |
| IGF-I | IGF-I receptor | TGF-β | TGFβR-I, -II | |
| Activin/inhibin B | Activin receptors | PTHrP | PTHrP receptor | |
| Epimorphin | Unknown | |||
| MMP-2, -3, -9, -11 | N/a | |||
| During alveolar development/pregnancy | Neuregulin | ErbB3/ErbB4 | ||
| Activin/inhibin B | Activin receptors | |||
| KGF (FGF-7) | FGFR2-IIIb | |||
| Epimorphin | Unknown | |||
| MMP-3 | N/a | |||
Tissue interactions are thus a major source of information regulating tissue-specific activation of genes leading to the proper development of cells, tissues, and organs (Wessells 1977). As an example, Figure 3 depicts reciprocal interactions between the cells and tissues that comprise the adult mammary gland and between the mammary gland and other organs. As alluded to above and discussed in depth below (see section on Three-Dimensional Models of Mammary Gland Development), the morphogenesis of the mammary epithelium is regulated by its interactions with mesenchymal cells. During branching morphogenesis of mammary and other organs, nerves, blood vessels, and epithelium grow out simultaneously in intimately interacting trees (Coughlin 1975, Gebb & Shannon 2000). The details of these presumed communications have yet to be uncovered for the mammary gland, but in skin, peripheral nerves determine the pattern of arterial branching by stimulating localized secretion of vascular endothelial growth factor (VEGF) (Mukouyama et al. 2002). Additionally, the kinetics of development and functional differentiation (milk synthesis and secretion) are controlled by influences external to the epithelium, including pituitary and ovarian hormones, and mechanical cues from suckling at the nipple, which activates contraction of the myoepithelial cells.
Figure 3.
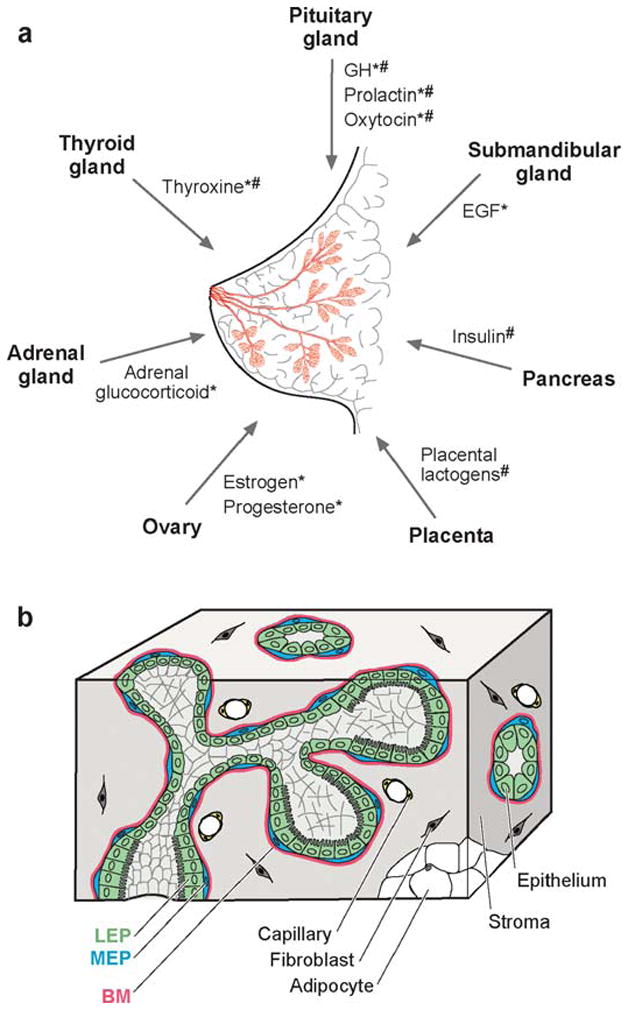
The structure and function of the mammary gland are influenced by communication with distant organs and between constituent tissues. (a) The human breast is a bilayered epithelial ductal tree (pink) embedded in a complex stroma. Signals released from distant organs influence ductal and acinar morphogenesis during puberty (*) and pregnancy (#) (reviewed in Hovey et al. 2002). (b) The epithelium consists of a layer of luminal epithelial cells (LEP) surrounded by myoepithelial cells (MEP) and basement membrane (BM). The epithelium is surrounded by a fibrous stromal compartment and adjacent fatty stroma. Molecular details of epithelial-mesenchymal interactions are described in Table 1.
Of Terminal Differentiation and Molecular Vitalism
In this age of genomics and gene expression arrays, one could easily accept the argument that a cell’s status (for example, its identity and the identity of the tissue and organ in which it resides) could be inferred mainly by examining the genes that it expresses. Although this may very well prove to be true, it is a fallacy to argue that therefore it is the genes themselves that determine and regulate the pattern of gene expression. Additionally, are the genes expressed the sole determinant of the status of a cell or how it may behave? The data from tissue recombination studies suggest that even differentiated cells retain a high degree of flexibility, or as Marc Kirschner and colleagues described so eloquently, an “interconvertible multi-statedness is a key aspect of multicellular self-organization” (Kirschner et al. 2000). This flexibility is apparent during tissue regeneration and repair and to a remarkable degree in organisms, such as the newt, that can regenerate entire organs and limbs even in the adult animal. That a differentiated cell (meaning, for example, a cell that has become a hepatocyte and functions within the context of the liver) can even respond to cues that direct the development of a different tissue to express muscle myosin (Chiu & Blau 1984, Blau et al. 1985) should have dispelled the notion that the process of differentiation locks cells into a particular fate without recourse. Indeed, cultured cells that invariably lose their differentiated phenotypes when grown in a petri dish can be induced to form both normal and diseased tissue structures when returned to the appropriate environment in vivo (DeOme et al. 1959, Daniel & DeOme 1965). Similarly, cells in culture can regain their differentiated phenotypes if the microenvironment of the culture vessel is tailored to mimic the cell’s normal microenvironment in vivo (reviewed in Bissell 1981 and below).
THREE-DIMENSIONAL MODELS OF MAMMARY GLAND DEVELOPMENT: RATIONALE AND EXAMPLES
The Structure of the Human Breast
The mammary gland is an excellent example of an organ, the development and differentiation of which require dynamic and reciprocal signaling between cells and their (micro)environment. Unlike other organs, the majority of mammary gland development occurs postnatally during puberty. In females, a surge of steroid hormones induces the anlage (the mammary ductal rudiment present at birth) to undergo a burst of branching morphogenesis. The mammary gland is composed of two tissue compartments, the ectodermally derived epithelium and the mesodermally derived stroma (depicted schematically in Figure 3). The bilayered epithelial tree consists of a central layer of luminal epithelial cells surrounded by a layer of myoepithelial cells and basement membrane (BM), a specialized laminin-rich form of ECM (lrECM). In humans, the epithelium (both luminal and myoepithelial) is surrounded by a loose intralobular connective tissue stroma and a denser interlobular stroma, which together account for 80% of the volume of the resting breast and house nerves, blood vessels, and lymphatics (Drife 1986). The ducts terminate in lobular structures known as terminal ductal lobular units (TDLUs), which give rise to alveolar buds during pregnancy that become secretory alveoli during lactation. Luminal epithelium is induced during lactation to produce and vectorially secrete milk into the ducts; milk is squeezed through the mammary tree to its opening at the nipple by concerted contraction of myoepithelial cells induced by suckling. Once lactation is terminated by cessation of suckling, the gland remodels during involution by the concerted action of hormones, metalloproteinases, and molecules involved in apoptosis (Talhouk et al. 1991, 1992; for a recent review, see Hennighausen & Robinson 2005).
Signaling by the Microenvironment
Interactions between luminal epithelial cells, ECM and its remodeling enzymes, and the other cells of the gland are critical for development and differentiation (Fata et al. 2004, Parmar & Cunha 2004). Myoepithelial cells secrete laminin-1 to build the BM that surrounds the epithelial compartment (Gudjonsson et al. 2002), direct the polarization of luminal epithelial cells (Runswick et al. 2001, Gudjonsson et al. 2002), and regulate morphogenesis of the ductal tree (Niranjan et al. 1995). Loss of these activities correlates with breakdown of normal mammary architecture and leads to tumor progression (reviewed in Adriance et al. 2005). During branching morphogenesis at puberty (Witty et al. 1995, Fata et al. 1999, Wiseman et al. 2003), and later during involution of the gland upon weaning (Talhouk et al. 1992, Lund et al. 1996), extensive breakdown and remodeling of the ECM occur via precise expression/activation/inhibition of matrix-degrading enzymes, especially members of the matrix metalloproteinase (MMP) family. Inappropriate expression of MMPs causes breakdown of the BM, disrupting functional differentiation (milk protein expression) of luminal epithelial cells (Sympson et al. 1994, Witty et al. 1995) and, in the case of MMP-3, leading to epithelial-to-mesenchymal transition (EMT), apoptotic cell death, genomic instability, induction of a reactive fibrotic stroma, and eventually tumor formation (Sympson et al. 1995; Alexander et al. 1996; Lochter et al. 1997; Thomasset et al. 1998; Sternlicht et al. 1999, 2000; Radisky et al. 2005). One mechanism by which destruction of BM leads to EMT and genomic instability is through increased levels of cellular reactive oxygen species, which upregulate expression of certain transcription factors and cause oxidative DNA damage (Radisky et al. 2005).
Proper development of the ductal tree depends on permissive and instructive cues from the stromal compartment. For example, both epithelial and stromal cells express estrogen receptor (ER)-α, and mammary glands from ER-α-knockout mice have a rudimentary underdeveloped ductal tree (Bocchinfuso & Korach 1997). Experiments recombining epithelium and stroma from wild-type and ER-α-knockout mice demonstrated that estrogen signaling is required in stromal cells during ductal morphogenesis (Cunha et al. 1997). Further experiments in culture revealed that, in response to estrogen, stromal fibroblasts produce hepatocyte growth factor (HGF), which acts in a paracrine role to induce growth of the epithelial tree (Zhang et al. 2002a). Reciprocal signaling from epithelium to the stroma is also required for the development of the gland. Epidermal growth factor receptor (EGFR) is required in the stromal compartment (Wiesen et al. 1999). The EGFR ligand, amphiregulin, is expressed on and cleaved from the surface of the epithelium by the cell-surface sheddase ADAM (a disintegrin and metalloproteinase)-17, presumably in response to estrogen signaling (Sternlicht et al. 2005). Consequently, mammary development is impaired in mice expressing signaling-defective EGFR (Fowler et al. 1995, Xie et al. 1997, Sebastian et al. 1998). These positive signals are balanced by negative cues, including TGF-β. Members of the TGF-β superfamily and their receptors are expressed throughout development of the gland (reviewed in Daniel et al. 2001, Serra & Crowley 2005). TGF-β in particular inhibits branching morphogenesis during puberty (Silberstein & Daniel 1987, Robinson et al. 1991, Pierce et al. 1993), blocks formation of alveoli and secretion of milk during pregnancy (Jhappan et al. 1993, Kordon et al. 1995, Siegel et al. 2003), and promotes apoptosis during involution (Nguyen & Pollard 2000, Gorska et al. 2003, Bailey et al. 2004).
The mesenchymal compartment also expresses morphogens, including epimorphin (Hirai et al. 1998, 2001; Simian et al. 2001) and members of the Wnt and notch families, that guide the development of the epithelial tree (Uyttendaele et al. 1998). That overexpression of epimorphin in the mammary gland leads to tumor development (Bascom et al. 2005) highlights the stroma’s importance in regulating conversion to the malignant phenotype, a concept introduced more than 100 years ago (Paget 1889). Normal stroma has tumor-suppressive properties, in contrast to stroma derived from breast cancer. Embryonic mammary mesenchyme can induce differentiation of mammary tumors (DeCosse et al. 1973). Conversely, human breast cancer xenografts produce significantly faster growing tumors when the cells are mixed with carcinoma-derived fibroblasts than when the cells are mixed with normal fibroblasts (Camps et al. 1990, van Roozendaal et al. 1996, Dong-Le Bourhis et al. 1997) or when they are injected into a previously irradiated stroma (Barcellos-Hoff & Ravani 2000). The latter effect is apparently due to irradiation-induced activation of TGF-β, which is the culprit in wound-induced tumors (Sieweke et al. 1990) and is known to lead to a fibrotic response in abnormal microenvironments by increasing synthesis of ECM molecules such as collagen I (Ehrhart et al. 1997). Increased tissue stiffness itself can promote malignant transformation by leading to deregulated integrin signaling (Paszek et al. 2005), and patients with such fibrotic lesions have a poor prognosis (Colpaert et al. 2001).
Breast carcinomas consist not only of the aberrant epithelial cells and stroma but also recruited blood vessels, activated fibroblasts, and infiltrating macrophages, lymphocytes, and leukocytes. Growing evidence points to recruitment of macrophages as important for breast tumor progression, with macrophage infiltration correlating with a poor prognosis (Leek et al. 1996, Goswami et al. 2005). Finally, alterations in the stroma are not solely due to changes in the constituent population of cells or deposition of ECM because stroma associated with breast tumors contains both genetic and epigenetic alterations (Deng et al. 1996, Washington et al. 2000, Allinen et al. 2004, Hu et al. 2005), and stromal fibroblasts in which the TGF-β type II receptor is inactivated stimulate the development of tumors in the adjacent epithelium (Bhowmick et al. 2004, Radisky & Bissell 2004). Clearly, the context in which an epithelial cell receives an oncogenic insult plays a large role in whether or not that cell generates a frank tumor, as shown in a number of earlier studies (for a review, see Kenny & Bissell 2003).
Organotypic Culture Models to Study Form, Function, and Dysfunction
Many of the details of microenvironmental signaling in the mammary gland have been uncovered using three-dimensional (3D) culture models (for historical overviews, see Bissell et al. 2003, 2005; Nelson & Bissell 2005). Differentiated mammary epithelial cell structure and function can be reproduced in culture when cells are given an appropriate microenvironment that recapitulates aspects of the above-described tissue structure. When grown on plastic substrata, human and rodent mammary epithelial cells flatten out and fail to respond to lactogenic cues; that is, they “forget” their mammary phenotype. However, when grown within a malleable lrECM, these same cells will assemble into polarized 3D acinar structures that resemble alveoli in vivo (Emerman & Pitelka 1977, Lee et al. 1985, Barcellos-Hoff et al. 1989, Aggeler et al. 1991). Cells that are not attached to BM undergo apoptosis (Boudreau et al. 1995), and apoptosis of cells in the center of the structures leads to the formation of hollow lumina (Blatchford et al. 1999, Debnath et al. 2002, Mills et al. 2004), a process similar to canalization of the ducts in vivo (Humphreys et al. 1996). When stimulated with lactogenic hormones, cultured acini of rodent epithelial cells express and secrete milk proteins into the central lumina (Emerman & Pitelka 1977; Lee et al. 1984, 1985; Streuli et al. 1995b). The binding of laminin-1 to integrin and other ECM receptors, now shown to include dystroglycan (M.L. Wier, M.L. Oppizzi, M.D. Henry, A. Onishi, K.P. Campbell, et al., manuscript submitted), causes changes in both cell shape and biochemical signaling to induce functional differentiation (Streuli et al. 1991, 1995b; Roskelley et al. 1994; Muschler et al. 1999). Even though milk appears to be expressed upon parturition with all protein constituents simultaneously, 3D culture studies have revealed that there is specificity in the regulation by microenvironmental context: Lactoferrin expression only requires cell rounding and β-casein can be expressed by single, rounded cells in contact with laminin, whereas the expression of whey acidic protein (WAP) requires formation of the polarized acinus (reviewed in Roskelley et al. 1995).
In addition to illuminating the processes of acinus formation and milk protein secretion, 3D culture models have been highly successful in recapitulating the epithelial remodeling and invasion central to the branching morphogenesis that builds the initial epithelial tree during puberty. Primary epithelial organoids or mammary epithelial cell lines cultured within gels of collagen I or lrECM can be induced to form branching structures by coculture with stromal fibroblasts or by exogenous addition of growth factors, such as HGF or epidermal growth factor (EGF) (Brinkmann et al. 1995, Soriano et al. 1995, Yang et al. 1995, Hirai et al. 1998, Niemann et al. 1998, Simian et al. 2001), or of cytokines, such as members of the tumor necrosis factor (TNF)-α family (Lee et al. 2000, Michaelson et al. 2005). Blocking either MMP activity or cell binding to epimorphin prevents branching (Hirai et al. 1998, Lee et al. 2000, Simian et al. 2001, Michaelson et al. 2005). To initiate a branch, epithelial cells must transiently loosen their interactions with neighboring cells and invade the surrounding ECM. Culture models of mammary and kidney epithelial branching have revealed that cells at the leading edge of branches undergo a transient or partial EMT (O’Brien et al. 2004; C.S. Chen, C.M. Nelson, S. Bennett, C. Gilles, Y. Hirai, et al., manuscript in submission)—one of many developmental processes frequently hijacked by cancer cells—which requires coordinate signaling from growth factors, MMPs, and epimorphin.
Recreating the microenvironment in culture also allows one to distinguish clearly between cells that do and do not differentiate (such as normal and tumorigenic breast cells), something difficult to achieve in traditional two-dimensional cultures. Whereas normal cells form polarized growth-arrested acini when cultured in 3D lrECM (Barcellos-Hoff et al. 1989), breast cancer cell lines or primary cells derived from carcinomas form highly disorganized and proliferative colonies reminiscent of tumors (Petersen et al. 1992, Weaver et al. 1995). Antagonizing one or more of the many pathways that are dysregulated in tumor cells causes them to functionally revert to a normal phenotype: The cells stop growing, form polarized acini, and are less tumorigenic when injected into nude mice (Howlett et al. 1995, Hirschi et al. 1996, Weaver et al. 1997, Wang et al. 1998, Kirshner et al. 2003, Liu et al. 2004, Park et al. 2006). Additionally, the activation levels of the other signaling pathways normalize to levels seen in nontumorigenic cells (for a review, see Bissell et al. 2005). These results demonstrate that tumorigenicity is context dependent, that tissue structure can be dominant over genotype, and that differentiation therapy, a concept used in treating some forms of leukemia, is a potentially powerful strategy for cancer therapy.
TISSUE SPECIFICITY IN THE MAMMARY GLAND AND BEYOND: CONTEXT IS ALL
From ECM to ECM-Response Elements
In the presence of a malleable laminin-rich substratum, mammary epithelial cells round up, organize into acinar structures, hollow out to form a central lumen, and secrete milk proteins, including β-casein, in response to lactogenic hormones. The laminin-induced expression of β-casein involves activation of an ECM-response element (ECM-RE) in the promoter of the β-casein gene (Schmidhauser et al. 1990, Schmidhauser et al. 1992, Myers et al. 1999) by β1-integrin-induced phosphorylation of the prolactin receptor, thus allowing prolactin to regulate the DNA-binding activity of the Stat5 transcription factor (Streuli et al. 1995a, Edwards et al. 1998). ECM-REs have been found in the promoter regions of several proteins, including those of αs1-casein (Jolivet et al. 2005), albumin (Liu et al. 1991), and TGF-β, which is regulated negatively by laminin (Streuli et al. 1993). Given that a multitude of ECM molecules is part of what comprises the microenvironment, we can imagine that the family of ECM-REs will be refined in the future to include, for example, laminin-response element, collagen-response element, and various combinations thereof. ECM also regulates the expression of tissue-specific transcription factors, such as mammary gland factor (MGF, or Stat5a) (Schmitt-Ney et al. 1991), which can thereby transduce context-dependent information indirectly by binding to the promoter regions of milk protein genes (Groner & Gouilleux 1995).
ECM-induced formation of the polarized acinus affects signaling between epithelial cells. In response to laminin, mammary epithelial cells upregulate expression of several of the connexin gap junction proteins, enhancing gap junctional intercellular communication (GJIC) (El-Sabban et al. 2003). Inhibiting GJIC downregulates β-casein expression. That loss of connexin expression leads to and correlates with tumor progression and that reexpression of connexins can inhibit the metastatic phenotype highlight the importance of cell-cell communication in guiding and responding to tissue architecture (Carystinos et al. 2001). Indeed, disrupting tight junctions prevents the establishment of tissue polarity and disrupts the structure of already polarized cells, leading to neoplastic growth (reviewed in Itoh & Bissell 2003).
Aside from inducing signal transduction through integrins and determining tissue morphology, the microenvironment also affects the structure of the nucleus. Histone acetylation promotes chromosome decondensation and unfolding, increasing the accessibility to transcription factors and other regulatory machinery, thereby enhancing transcription (Jenuwein & Allis 2001). Activation of the ECM-RE in the promoter of the β-casein gene can be modulated by altering the organization of histones (Myers et al. 1998), and addition of laminin induces histone deacetylation in mammary epithelial cell lines (Pujuguet et al. 2001). Recent experiments have demonstrated that cell rounding by itself (independent of cell-ECM interactions) leads to histone modifications (J. Le Beyec, R. Xu, S.Y. Moonlee, C.M. Nelson, A. Rizki, and M.J. Bissell, unpublished data). Because the cytoskeleton appears to physically connect the ECM to the nucleus through ECM receptors (Maniotis et al. 1997), and because destruction of ECM by MMPs leads to genomic instability through alternative splicing of the Rac1 transcript (Radisky et al. 2005), it is tempting to speculate that the effects due to changes in cell morphology are transmitted to the nucleus through the cytoskeleton. Taken together, these data support and expand dynamic reciprocity (Figure 1a,b), whereby tissue specificity is determined and maintained by interactions of adhesion receptors with surrounding ECM and neighboring cells. These interactions activate downstream signaling pathways, in conjunction with altering cytoskeletal structure and cell and nuclear morphology, to modulate binding of transcription factors to the microenvironment-specific response elements of tissue-specific genes. The resulting changes in gene expression modify a panoply of signaling proteins produced by the cell, including ECM proteins and tissue-specific transcription factors, cementing the organ-specific phenotype.
Tissue Specificity Throughout Evolution
If context directs development, then do organs that develop similar structures do so using similar contextual cues? The answer, at least for the branched organs of placental mammals, appears to be a qualified “yes.” The pancreas, lung, kidney, prostate, and salivary and mammary glands all develop by branching morphogenesis, driven by epithelial-mesenchymal interactions involving stimulatory signaling in part from HGF and EGF, balanced by inhibitory signaling from members of the TGF-β family, and regulated by ECM and MMPs (reviewed in Davies 2002). This conservation of contextual signaling was first glimpsed in the tissue recombination experiments of the 1960s, discussed above (see section on Tissue Architecture Is Both a Consequence and a Cause). Interestingly, the epithelium in these organs is initially derived from different germ layers: endoderm in the pancreas and lung, mesoderm in the kidney, and ectoderm in the mammary gland. However, there are also major differences in the contexts under which each of these organs develops, which likely plays a role in the final tissue-specific architecture and function achieved. The pattern of branching of the lung is determined by embryonic patterning cues (Chuang & McMahon 2003), the kidney has its own growth factor [glial cell–derived neurotrophic factor (GDNF)], and the mammary gland develops uniquely in the context of puberty.
Although the mammary gland is a relatively recent evolutionary acquisition (Oftedal 2002), the similarities between its development and that of other, more ancient organs (such as the pancreas, which is present as a branched structure even in cartilaginous fish, of which the last common ancestor to mammals was 450 Mya) suggest that some of the above-described mechanisms for directing tissue specificity may be conserved (last reviewed in Ashkenas et al. 1996). Indeed, homologs of ECM proteins and integrins are present in many invertebrates. The nematode worm Caenorhabditis elegans expresses collagens and a β1-integrin homolog, βpat-3; mutations in the collagen IV homologs emb-9 and let-2 are embryonic lethal, suggesting the importance of BM in worm development (Kramer 1994). The fly Drosophila melanogaster expresses laminins, dystroglycan, and a number of α- and β-integrins, and similar to the mammary gland, dystroglycan is required for generation of apico-basal polarity in Drosophila epithelial cells (Deng et al. 2003). Hydra express laminins, collagens, MMPs, and a putative β1-integrin, which are required for proper epithelial morphogenesis during head and tentacle regeneration (Shimizu et al. 2002, Zhang et al. 2002b). Even the slime mold Dictyostelium discoideum expresses ECM during its multicellular slug phase and stalk development, which is regulated by a Stat transcription factor homolog (Shimada et al. 2004). ECM-REs are also evolutionarily conserved, at least functionally, if not in nucleotide sequence: Sea urchin embryonic development requires collagen-induced activation of a short promoter element in the LpS1 gene (Seid et al. 1997). Because cytoskeleton is, in general, conserved through different phyla (Muller et al. 2005), it is likely that cell and tissue context play an analogous role in the development, differentiation, and homeostasis of many organisms.
INTEGRATION
A fundamental property of all known (and therefore, presumably, successful) forms of life is the ability to adapt to changes in both the environment external to the organism and the internal (micro)environment. Terminal change—an inability to adapt—in all dynamic systems leads to equilibrium, which for living things is death. Dynamic reciprocity, then, is scalable both in time and space and is a mechanism by which single cells within tissues maintain homeostasis in spite of an uncertain environment over the organism’s lifetime. Tissue-specific context is thus important not only for development and differentiation but also as a protective mechanism against cancer and other diseases. However, as much as we might wish otherwise, tissue context is not static even in the adult, succumbing eventually to the effects of living: reactive oxygen species, carcinogens, diet, shrinking telomeres—in sum, the effects of aging (Hasty et al. 2003). The context of an old breast is not the same as that of a young breast. As menopause approaches, epithelial cells die off, the stromal compartment alters, the entire morphology of the organ changes. It is instructive to combine our vast knowledge of developmental biology with emerging concepts in tissue specificity so as to generate an integrated understanding of development, homeostasis, cancer, and aging.
The essence of what we have laid out here is that the integration of signaling hangs on the structure of an organ, for structure has information, a kind of information distinct from the genomic blueprint of the cell. When one considers all of the signaling pathways involved in differentiation, the complexity is staggering. There is clearly more than one way of integrating the same combination of signals into a phenotype (Bissell et al. 2003); this is precisely why development is so miraculously robust.
FUTURE DIRECTIONS: DECODING THE LANGUAGE OF FORM
Organ architecture is thus both a consequence and a cause for development, differentiation, and homeostasis. But how does the architecture of an organ (or tissue, or cell) make itself heard? We understand something about the alphabet (ECM, receptors, cytoskeleton, nuclear matrix, chromatin) and even less about the rules of grammar that turn random words into commands (activation of tissue-specific response elements). We believe that decoding this language requires abandoning the currently fashionable “molecule-centric” style of inquiry and adopting a more interdisciplinary approach that takes into account dynamic changes, spatial segregation of events, and tissue architecture.
SUMMARY POINTS.
Development, differentiation, and homeostasis are controlled by cell-cell interactions, cell-ECM interactions, ECM-degrading enzymes, and soluble cues (hormones, cytokines, and growth factors).
Malignant phenotype can be reverted without changing genotype. Thus phenotype can be dominant over genotype.
Signaling pathways are context dependent.
Maintenance of homeostasis requires maintenance of form.
Acknowledgments
We apologize to those whose work could not be cited owing to space limitations and have cited reviews where possible. Our work was supported by grants from the Office of Biological and Environmental Research of the Department of Energy (DE-AC03-76SF00098 and a Distinguished Fellow Award to M.J.B.), the National Cancer Institute (CA64786 to M.J.B.; CA57621 to Zena Werb and M.J.B.), and the Breast Cancer Research Program of the Department of Defense (Innovator Award DAMD17-02–1-438 to M.J.B. and postdoctoral fellowship W81XWH-04–1-0582 to C.M.N.).
- Microenvironment
local and systemic constituents surrounding a cell, including ECM, other cells, and soluble factors released locally or transmitted from other organs, such as hormones
- ECM
extracellular matrix
- Morphogenesis
the process of development by which an organ achieves its final structure
- Mesenchyme
mass of connective tissue, mainly derived from mesoderm, in embryonic and developing organs that usually develops into the stroma
- BM
basement membrane
- lrECM
laminin-rich ECM
- TDLU
terminal ductal lobular unit
- EMT
epithelial-to-mesenchymal transition
- MMP
matrix metalloproteinase
- ECM-RE
ECM-response element
Contributor Information
Celeste M. Nelson, Email: cmnelson@lbl.gov.
Mina J. Bissell, Email: mjbissell@lbl.gov.
LITERATURE CITED
- Adriance MC, Inman JL, Petersen OW, Bissell MJ. Myoepithelial cells: Good fences make good neighbors. Breast Cancer Res. 2005;7:190–97. doi: 10.1186/bcr1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99(Pt. 2):407–17. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–77. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Ashkenas J, Muschler J, Bissell MJ. The extracellular matrix in epithelial biology: shared molecules and common themes in distant phyla. Dev Biol. 1996;180:433–44. doi: 10.1006/dbio.1996.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JP, Nieport KM, Herbst MP, Srivastava S, Serra RA, Horseman ND. Prolactin and transforming growth factor-β signaling exert opposing effects on mammary gland morphogenesis, involution, and the Akt-forkhead pathway. Mol Endocrinol. 2004;18:1171–84. doi: 10.1210/me.2003-0345. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–35. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–60. [PubMed] [Google Scholar]
- Bascom JL, Fata JE, Hirai Y, Sternlicht MD, Bissell MJ. Epimorphin overexpression in the mouse mammary gland promotes alveolar hyperplasia and mammary adenocarcinoma. Cancer Res. 2005;65:8617–21. doi: 10.1158/0008-5472.CAN-05-1985. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. Clearly demonstrates, using genetic means, the role of the stroma in cancer development. [DOI] [PubMed] [Google Scholar]
- Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:1–14. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Mian IS, Radisky D, Turley E. Tissue-specificity: Structural cues allow diverse phenotypes from a constant genotype. In: Müller GB, Newman SA, editors. Origination of Organismal Form: Beyond the Gene in Developmental and Evolutionary Biology. Vol. 7. Cambridge, MA: MIT Press; 2003. pp. 103–17. [Google Scholar]
- Blatchford DR, Quarrie LH, Tonner E, McCarthy C, Flint DJ, Wilde CJ. Influence of microenvironment on mammary epithelial cell survival in primary culture. J Cell Physiol. 1999;181:304–11. doi: 10.1002/(SICI)1097-4652(199911)181:2<304::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Blau HM, Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991;112:781–83. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Pavlath GK, Hardeman EC, Chiu C-P, Silberstein L, et al. Plasticity of the differentiated state. Science. 1985;230:758–66. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–34. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- Bornstein P, McPherson J, Sage H. Synthesis and secretion of structural macromolecules by endothelial cells in culture. In: Nossel H, Vogel H, editors. Pathobiology of the Endothelial Cell, P and S Biomedical Sciences Symposia. Vol. 6. New York: Academic; 1982. pp. 215–28. [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–93. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol. 1995;131:1573–86. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne E. The production of new hydranths in hydra by the insertion of small grafts. J Exp Zool. 1909;7:1–37. [Google Scholar]
- Cairns JM, Saunders JW. The influence of embryonic mesoderm on the regional specification of epidermal derivatives in the chick. J Exp Zool. 1954;127:221–48. [Google Scholar]
- Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, et al. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci USA. 1990;87:75–79. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Izpisua Belmonte JC. Patterning mechanisms controlling vertebrate limb development. Annu Rev Cell Dev Biol. 2001;17:87–132. doi: 10.1146/annurev.cellbio.17.1.87. [DOI] [PubMed] [Google Scholar]
- Cardoso WV. Molecular regulation of lung development. Annu Rev Physiol. 2001;63:471–94. doi: 10.1146/annurev.physiol.63.1.471. [DOI] [PubMed] [Google Scholar]
- Carystinos GD, Bier A, Batist G. The role of connexin-mediated cell-cell communication in breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2001;6:431–40. doi: 10.1023/a:1014787014851. [DOI] [PubMed] [Google Scholar]
- Chiu CP, Blau H. Reprogramming cell differentiation in the absence of DNA synthesis. Cell. 1984;37:879–87. doi: 10.1016/0092-8674(84)90423-9. [DOI] [PubMed] [Google Scholar]
- Chuang PT, McMahon AP. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 2003;13:86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Colpaert C, Vermeulen P, Van Marck E, Dirix L. The presence of a fibrotic focus is an independent predictor of early metastasis in lymph node-negative breast cancer patients. Am J Surg Pathol. 2001;25:1557–58. doi: 10.1097/00000478-200112000-00016. [DOI] [PubMed] [Google Scholar]
- Coughlin MD. Early development of parasympathetic nerves in the mouse submandibular gland. Dev Biol. 1975;43:123–39. doi: 10.1016/0012-1606(75)90136-0. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Metaplastic induction of scales and feathers in the corneal anterior epithelium of the chick embryo. Dev Biol. 1971;25:464–78. doi: 10.1016/0012-1606(71)90042-x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Christov K, Guzman R, Nandi S, et al. Mammary phenotypic expression induced in epidermal cells by embryonic mammary mesenchyme. Acta Anat (Basel) 1995;152:195–204. doi: 10.1159/000147698. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Hom YK, Cooke PS, Taylor JA, Lubahn DB. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- Daniel CW, DeOme KB. Growth of mouse mammary glands in vivo after monolayer culture. Science. 1965;149:634–36. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Robinson S, Silberstein GB. The transforming growth factors β in development and functional differentiation of the mouse mammary gland. Adv Exp Med Biol. 2001;501:61–70. doi: 10.1007/978-1-4615-1371-1_7. [DOI] [PubMed] [Google Scholar]
- Davies JA. Do different branching epithelia use a conserved developmental mechanism? Bioessays. 2002;24:937–48. doi: 10.1002/bies.10161. [DOI] [PubMed] [Google Scholar]
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- DeCosse JJ, Gossens CL, Kuzma JF, Unsworth BR. Breast cancer: induction of differentiation by embryonic tissue. Science. 1973;181:1057–58. doi: 10.1126/science.181.4104.1057. [DOI] [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–59. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, et al. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–84. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- DeOme KB, Faulkin LJJ, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–20. [PubMed] [Google Scholar]
- Dhouailly D. Analysis of the factors in the specific differenciation of the neoptile feathers in the duck and chicken. J Embryol Exp Morphol. 1967;18:389–400. [PubMed] [Google Scholar]
- Dhouailly D. The determination of specific differentiation of neoptile and teleoptile feathers in the chick and the duck. J Embryol Exp Morphol. 1970;24:73–94. [PubMed] [Google Scholar]
- Dong-Le Bourhis X, Berthois Y, Millot G, Degeorges A, Sylvi M, et al. Effect of stromal and epithelial cells derived from normal and tumorous breast tissue on the proliferation of human breast cancer cell lines in coculture. Int J Cancer. 1997;71:42–48. doi: 10.1002/(sici)1097-0215(19970328)71:1<42::aid-ijc9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Drife JO. Breast development in puberty. In: Angeli A, Bradlow HL, Dogliotti L, editors. Endocrinology of the Breast: Basic and Clinical Aspects. New York: New York Acad. Sci; 1986. pp. 58–65. [DOI] [PubMed] [Google Scholar]
- Edwards GM, Wilford FH, Liu X, Hennighausen L, Djiane J, Streuli CH. Regulation of mammary differentiation by extracellular matrix involves protein-tyrosine phosphatases. J Biol Chem. 1998;273:9495–500. doi: 10.1074/jbc.273.16.9495. [DOI] [PubMed] [Google Scholar]
- Ehrhart EJ, Segarini P, Tsang ML, Carroll AG, Barcellos-Hoff MH. Latent transforming growth factor β1 activation in situ: quantitative and functional evidence after low-dose γ-irradiation. FASEB J. 1997;11:991–1002. doi: 10.1096/fasebj.11.12.9337152. [DOI] [PubMed] [Google Scholar]
- El-Sabban ME, Sfeir AJ, Daher MH, Kalaany NY, Bassam RA, Talhouk RS. ECM-induced gap junctional communication enhances mammary epithelial cell differentiation. J Cell Sci. 2003;116:3531–41. doi: 10.1242/jcs.00656. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–28. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Fata JE, Leco KJ, Moorehead RA, Martin DC, Khokha R. Timp-1 is important for epithelial proliferation and branching morphogenesis during mouse mammary development. Dev Biol. 1999;211:238–54. doi: 10.1006/dbio.1999.9313. [DOI] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris C, Chaloin-Dufau C, Dhouailly D. Transdifferentiation of embryonic and post-natal rabbit corneal epithelial cells. Differentiation. 1994;57:89–96. doi: 10.1046/j.1432-0436.1994.5720089.x. [DOI] [PubMed] [Google Scholar]
- Fowler KJ, Walker F, Alexander W, Hibbs ML, Nice EC, et al. A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc Natl Acad Sci USA. 1995;92:1465–69. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebb SA, Shannon JM. Tissue interactions mediate early events in pulmonary vasculogenesis. Dev Dyn. 2000;217:159–69. doi: 10.1002/(SICI)1097-0177(200002)217:2<159::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gorska AE, Jensen RA, Shyr Y, Aakre ME, Bhowmick NA, Moses HL. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-β receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am J Pathol. 2003;163:1539–49. doi: 10.1016/s0002-9440(10)63510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- Groner B, Gouilleux F. Prolactin-mediated gene activation in mammary epithelial cells. Curr Opin Genet Dev. 1995;5:587–94. doi: 10.1016/0959-437x(95)80027-1. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–59. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–25. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Hieda Y, Nakanishi Y. Epithelial morphogenesis in mouse embryonic submandibular gland: its relationships to the tissue organization of epithelium and mesenchyme. Dev Growth Differ. 1997;39:1–8. doi: 10.1046/j.1440-169x.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J Cell Biol. 1998;140:159–69. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Radisky D, Boudreau R, Simian M, Stevens ME, et al. Epimorphin mediates mammary luminal morphogenesis through control of C/EBPβ. J Cell Biol. 2001;153:785–94. doi: 10.1083/jcb.153.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Xu CE, Tsukamoto T, Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996;7:861–70. [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Vonderhaar BK. Establishing a framework for the functional mammary gland: from endocrinology to morphology. J Mammary Gland Biol Neoplasia. 2002;7:17–38. doi: 10.1023/a:1015766322258. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108(Pt. 5):1945–57. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Hu M, Yao J, Cai L, Bachman KE, van den Brule F, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- Humphreys RC, Krajewska M, Krnacik S, Jaeger R, Weiher H, et al. Apoptosis in the terminal endbud of the murine mammary gland: a mechanism of ductal morphogenesis. Development. 1996;122:4013–22. doi: 10.1242/dev.122.12.4013. [DOI] [PubMed] [Google Scholar]
- Itoh M, Bissell MJ. The organization of tight junctions in epithelia: implications for mammary gland biology and breast tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8:449–62. doi: 10.1023/B:JOMG.0000017431.45314.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, et al. Targeting expression of a transforming growth factor β1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J. 1993;12:1835–45. doi: 10.1002/j.1460-2075.1993.tb05832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet G, Pantano T, Houdebine LM. Regulation by the extracellular matrix (ECM) of prolactin-induced αs1-casein gene expression in rabbit primary mammary cells: role of STAT5, C/EBP, and chromatin structure. J Cell Biochem. 2005;95:313–27. doi: 10.1002/jcb.20397. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–95. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Gerhart J, Mitchison T. Molecular “vitalism”. Cell. 2000;100:79–88. doi: 10.1016/s0092-8674(00)81685-2. A superb synthesis of the organizing principles of biology. [DOI] [PubMed] [Google Scholar]
- Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1–4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci USA. 2003;100:521–26. doi: 10.1073/pnas.232711199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGFβ1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47–61. doi: 10.1006/dbio.1995.1060. [DOI] [PubMed] [Google Scholar]
- Kramer JM. Genetic analysis of extracellular matrix in C. elegans. Annu Rev Genet. 1994;28:95–116. doi: 10.1146/annurev.ge.28.120194.000523. [DOI] [PubMed] [Google Scholar]
- Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Lee EY, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci USA. 1985;82:1419–23. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–55. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Hwang JJ, Murphy G, Ip MM. Functional significance of MMP-9 in tumor necrosis factor-induced proliferation and branching morphogenesis of mammary epithelial cells. Endocrinology. 2000;141:3764–73. doi: 10.1210/endo.141.10.7697. [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–29. [PubMed] [Google Scholar]
- Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol. 2004;164:603–12. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, DiPersio CM, Zaret KS. Extracellular signals that regulate liver transcription factors during hepatic differentiation in vitro. Mol Cell Biol. 1991;11:773–84. doi: 10.1128/mcb.11.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–72. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–93. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253:165–74. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Michaelson JS, Cho S, Browning B, Zheng TS, Lincecum JM, et al. Tweak induces mammary epithelial branching morphogenesis. Oncogene. 2005;24:2613–24. doi: 10.1038/sj.onc.1208208. [DOI] [PubMed] [Google Scholar]
- Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci USA. 2004;101:3438–43. doi: 10.1073/pnas.0400443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–89. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Muller J, Oma Y, Vallar L, Friederich E, Poch O, Winsor B. Sequence and comparative genomic analysis of actin-related proteins. Mol Biol Cell. 2005;16:5736–48. doi: 10.1091/mbc.E05-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of labor among the α6β4 integrin, β1 integrins, and an E3 laminin receptor to signal morphogenesis and β-casein expression in mammary epithelial cells. Mol Biol Cell. 1999;10:2817–28. doi: 10.1091/mbc.10.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, et al. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol. 1998;18:2184–95. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15:342–52. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AV, Pollard JW. Transforming growth factor β3 induces cell death during the first stage of mammary gland involution. Development. 2000;127:3107–18. doi: 10.1242/dev.127.14.3107. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–34. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, et al. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998;143:533–45. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, et al. HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development. 1995;121:2897–908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, Mostov KE. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev Cell. 2004;7:21–32. doi: 10.1016/j.devcel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7:225–52. doi: 10.1023/a:1022896515287. [DOI] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growth in cancer of the breast. Lancet. 1889;1:571–73. [PubMed] [Google Scholar]
- Pander C. Beitrage zur Entwickelungsgeschichte des Huhnchens im Eye. Wurzburg: H.L. Bronner; 1817. p. 42. [Google Scholar]
- Park C, Zhang H, Pallavicini M, Gray JW, Baehner F, et al. β1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in 3D cultures and in vivo. Cancer Res. 2006;66:1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11:437–58. doi: 10.1677/erc.1.00659. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. Demonstrates the role of mechanical stress in the control of phenotype. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–68. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce DFJ, Johnson MD, Matsui Y, Robinson SD, Gold LI, et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-β1. Genes Dev. 1993;7:2308–17. doi: 10.1101/gad.7.12a.2308. [DOI] [PubMed] [Google Scholar]
- Pujuguet P, Radisky D, Levy D, Lacza C, Bissell MJ. Trichostatin A inhibits β-casein expression in mammary epithelial cells. J Cell Biochem. 2001;83:660–70. doi: 10.1002/jcb.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–27. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CW. Regulated expression and growth inhibitory effects of transforming growth factor-β isoforms in mouse mammary gland development. Development. 1991;113:867–78. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–82. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signaling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–47. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–30. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–41. doi: 10.1126/science.827022. Elegantly delineates the different roles of mesenchyme and epithelium in morphogenesis and differentiation. [DOI] [PubMed] [Google Scholar]
- Saunders JW. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Saunders JW, Gasseling MT. The origin of pattern and feather germ tract specificity. J Exp Zool. 1968;135:503–28. [Google Scholar]
- Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Extracellular matrix and hormones transcriptionally regulate bovine β-casein 5′ sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA. 1990;87:9118–22. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of β-casein gene expression. Mol Biol Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Ney M, Doppler W, Ball RK, Groner B. β-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991;11:3745–55. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, et al. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9:777–85. [PubMed] [Google Scholar]
- Seid CA, Ramachandran RK, George JM, Govindarajan V, Gonzalez-Rimbau MF, et al. An extracellular matrix response element in the promoter of the LpS1 genes of the sea urchin Lytechinus pictus. Nucleic Acids Res. 1997;25:3175–82. doi: 10.1093/nar/25.15.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Crowley MR. Mouse models of transforming growth factor β impact in breast development and cancer. Endocr Relat Cancer. 2005;12:749–60. doi: 10.1677/erc.1.00936. [DOI] [PubMed] [Google Scholar]
- Shimada N, Nishio K, Maeda M, Urushihara H, Kawata T. Extracellular matrix family proteins that are potential targets of Dd-STATa in Dictyostelium discoideum. J Plant Res. 2004;117:345–53. doi: 10.1007/s10265-004-0165-3. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Zhang X, Zhang J, Leontovich A, Fei K, et al. Epithelial morphogenesis in hydra requires de novo expression of extracellular matrix components and matrix metalloproteinases. Development. 2002;129:1521–32. doi: 10.1242/dev.129.6.1521. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor β signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA. 2003;100:8430–35. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-β. Science. 1990;248:1656–60. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-β. Science. 1987;237:291–93. doi: 10.1126/science.3474783. [DOI] [PubMed] [Google Scholar]
- Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–31. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108(Pt. 2):413–30. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- Spemann H. Uber die Determination der ersten Organanlagen des Amphibienembryonen. Zool Jahr Supp. 1918;15:1–48. [Google Scholar]
- Spemann H, Mangold H. Uber induktion von Embryonalagen durch Implantation Art-fremder Organisatoren. Arch Mikr Anat Entw Mech. 1924;100:599–638. [Google Scholar]
- Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–13. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–46. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–33. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–95. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, et al. Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995a;270:21639–44. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995b;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-β1 gene. J Cell Biol. 1993;120:253–60. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Bissell MJ, Werb Z. Mammary gland tumor formation in transgenic mice overexpressing stromelysin-1. Semin Cancer Biol. 1995;6:159–63. doi: 10.1006/scbi.1995.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–93. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–82. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–49. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, et al. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol. 1998;153:457–67. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttendaele H, Soriano JV, Montesano R, Kitajewski J. Notch4 and Wnt-1 proteins function to regulate branching morphogenesis of mammary epithelial cells in an opposing fashion. Dev Biol. 1998;196:204–17. doi: 10.1006/dbio.1998.8863. [DOI] [PubMed] [Google Scholar]
- van Roozendaal KE, Klijn JG, van Ooijen B, Claassen C, Eggermont AM, et al. Differential regulation of breast tumor cell proliferation by stromal fibroblasts of various breast tissue sources. Int J Cancer. 1996;65:120–25. doi: 10.1002/(SICI)1097-0215(19960103)65:1<120::AID-IJC20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, et al. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94(19):1494–503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–26. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington C, Dalbegue F, Abreo F, Taubenberger JK, Lichy JH. Loss of heterozygosity in fibrocystic change of the breast: genetic relationship between benign proliferative lesions and associated carcinomas. Am J Pathol. 2000;157:323–29. doi: 10.1016/S0002-9440(10)64543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol. 1995;6:175–84. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells NK. Tissue Interactions and Development. Vol. 276. Menlo Park, CA: Benjamin/Cummings; 1977. An excellent, concise review of the early material on epithelial-mesenchymal interactions, especially on mesoderm induction experiments. [Google Scholar]
- Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–44. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–33. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. Pattern formation in epithelial development: the vertebrate limb and feather bud spacing. Philos Trans R Soc London Ser B. 1998;353:871–75. doi: 10.1098/rstb.1998.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Paterson AJ, Chin E, Nabell LM, Kudlow JE. Targeted expression of a dominant negative epidermal growth factor receptor in the mammary gland of transgenic mice inhibits pubertal mammary duct development. Mol Endocrinol. 1997;11:1766–81. doi: 10.1210/mend.11.12.0019. [DOI] [PubMed] [Google Scholar]
- Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, et al. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995;131:215–26. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev. 2004;14:550–57. doi: 10.1016/j.gde.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Bennett JM, Smith KT, Sunil N, Haslam SZ. Estrogen mediates mammary epithelial cell proliferation in serum-free culture indirectly via mammary stroma-derived hepatocyte growth factor. Endocrinology. 2002a;143:3427–34. doi: 10.1210/en.2002-220007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fei K, Agbas A, Yan L, Zhang J, et al. Structure and function of an early divergent form of laminin in hydra: a structurally conserved ECM component that is essential for epithelial morphogenesis. Dev Genes Evol. 2002b;212:159–72. doi: 10.1007/s00427-002-0225-4. [DOI] [PubMed] [Google Scholar]