Abstract
The extracellular matrix (ECM) is a dominant regulator of tissue development and homeostasis. “Designer microenvironments” in culture and in vivo model systems have shown that the ECM regulates growth, differentiation, and apoptosis in murine and human mammary epithelial cells (MEC) through a hierarchy of transcriptional events involving the intricate interplay between soluble and physical signaling pathways. Furthermore, these studies have shown that these pathways direct and in turn are influenced by the tissue structure. Tissue structure is directed by the cooperative interactions of the cell–cell and cell–ECM pathways and can be modified by stromal factors. Not surprisingly then, loss of tissue structure and alterations in ECM components are associated with the appearance and dissemination of breast tumors, and malignancy is associated with perturbations in cell adhesion, changes in adhesion molecules, and a stromal reaction. Several lines of evidence now support the contention that the pathogenesis of breast cancer is determined (at least in part) by the dynamic interplay between the ductal epithelial cells, the microenvironment, and the tissue structure (acini). Thus, to understand the mechanisms involved in carcinogenesis, the role of the microenvironment (ECM as well as the stromal cells) with respect to tissue structure should be considered and studied. Towards this goal, we have established a unique human MEC model of tumorigenesis, which in concert with a three-dimensional assay, recapitulates many of the genetic and morphological changes observed in breast cancer in vivo. We are currently using this system to understand the role of the microenvironment and tissue structure in breast cancer progression.
Keywords: extracellular matrix, integrin, adhesion molecules, breast cancer, microenvironment
Introduction
Cell – extracellular matrix (ECM) interactions are known to play important roles during embryonic morphogenesis and are required for the maintenance for tissue homeostasis in the adult organism (for a review, see Stoker et al. 1990; Adams and Watt 1993; Martins-Green and Bissell 1995; Askhenas et al. 1996). For example, it is known that the ECM is involved in the initiation and induction of embryonic branching morphogenesis in the salivary and mammary glands and promotes lung, pancreas, and kidney development (Bernfield and Banerjee 1982; Ekblom et al. 1990; Koch et al. 1991; Silberstein et al. 1992; Hisaoka et al. 1993). Postnatally the ECM is intimately involved in the estrous cycle and pregnancy-associated regulation of mammary gland proliferation, differentiation, and apoptosis (Talhouk et al. 1992; Sympson et al. 1994), while alterations in ECM turnover and cell–ECM interactions have been shown to be vital for wound healing and during an inflammatory response (Hauser et al. 1993; Murray et al. 1993; Martins-Green and Bissell 1995). Although much has yet to be learned about how the microenvironment regulates these physiological processes, it has been shown that the ECM directs changes in gene expression via both biochemical and biomechanical signalling pathways (for review, see Roskelley et al. 1995). This has been shown to occur through integral cell-membrane ECM-specific receptors, including the well-described integrin heterodimers (Hynes 1992) and syndecans (Bernfield et al. 1993). Furthermore, it has been reported that cell shape and the internal cell architecture per se can dictate how these ECM-generated signals are integrated and how the cell or tissue will respond (Dhawan and Farmer 1994; Roskelley et al. 1994; Ingber et al. 1994, 1995). Because changes in gene expression also modulate the expression of ECM receptors, ECM molecules, and alter tissue architecture, this underscores the existence of a dynamic reciprocity between the cell, its tissue structure, and the microenvironment, as was predicted more than a decade ago by Bissell et al. (1982) (Fig. 1).
Fig. 1.
Tissue structure, as determined by the dynamic interactions between the cell and the extracellular matrix (ECM), via its cell adhesion molecules (CAMs), as an integrator of function in the mammary gland. The ECM by its effects on tissue structure and through the CAMs, positively influences tissue-specific gene expression and inhibits inappropriate branching, proliferation, apoptosis, and the development of cancer.
Consistent with its role in dictating cell fate, alterations in cell–ECM interactions and ECM components have been shown to be present in epithelial cancers (Lochter and Bissell 1995; Dedhar 1995). Thus, perturbations in the expression and signalling of integrins have been described for kidney, skin, lung, mammary, and salivary tumors, while well-characterized changes in ECM proteins have been reported for breast, colon, and ovarian cancer (Petersen et al. 1992; Grant et al. 1994; Kibbey et al 1994; Cannistra et al. 1995; Zutter et al. 1995; Lochter and Bissell 1995; Berdichevsky et al. 1994; see also H.K. Kleinman, unpublished). Although it has now been accepted that these alterations are important features of tumors in vivo, both their role in the genesis of malignancies and an understanding of the events leading to their disturbance have yet to be determined. What is now becoming apparent, however, is that the dynamic interaction between the cell and its microenvironment is a major contributor to tumor development. We have attempted to illustrate this point in the following short review. We briefly describe what is presently known about changes in cell–ECM interactions and tissue structure associated with the various stages of breast cancer in vivo and reported for nonmalignant and malignant mammary epithelial cells (MECs) in culture. We also described a unique MEC model system of breast cancer, which in concert with a three-dimensional reconstituted basement membrane (BM) assay, is now being used to clarify many of the questions regarding the role of the ECM and its receptors in tumor progression. By emphasizing the relationship between cell–ECM interactions and changes in tissue morphology, it is the intention of this review to highlight an interactive structural paradigm of breast cancer aetiology: that there exists a dynamic interaction between the normal cell, its microenvironment, and the tissue structure (acini), which reciprocally influence each other to dictate the tissue phenotype and behavior. The cancer cell must subvert the regulatory mechanisms governing this polar, three-dimensional structure to be free to grow and metastasize.
The importance of the microenvironment in the regulation of mammary epithelial cell gene expression
The epithelial component of the postpubertal breast consists of a monolayer of luminal epithelial cells, which are in contact at their basal surfaces with myoepithelial cells and hence with a laminin-rich BM. This simple polarized cell bilayer, which is embedded in a stroma and surrounded by myoepithelial cells, is organized into branching epithelial ducts, which contain a central lumen and terminate in multiple lobuloalveoli or acini (Ronnov-Jessen et al. 1996). The luminal epithelial cells characteristically express the simple epithelial keratins 7, 8, 18, and 19 and the polymorphic epithelial mucin (PEM) but not vimentin or keratins 5 or 14. Also a small percentage of these cells express the estrogen receptor. The myoepithelial cells, on the other hand, express keratins 5 and 14 as well as α-actin and vimentin (Taylor-Papadimitriou et al. 1989, 1992). It is the luminal epithelial cells of the ducts and lobuloalveoli that give rise to the vast majority of cancers of the breast. The stroma of the human breast, which is composed of fat tissue, interstitial or interlobular dense connective tissue, intralobular loose connective tissue, and blood vessels, accounts for greater than 80% of the breast tissue volume (Drife 1979, 1986). During the estrous cycle and following pregnancy this secretory gland undergoes developmental cycles of proliferation, differentiation, and programmed cell death, which involve striking changes to both the stroma and the resident epithelial cells. The most dramatic changes occur during pregnancy and lactation when a fully functional mammary gland is reached. This pregnancy-associated differentiation is preceded by a peak of epithelial cell proliferation and branching morphogenesis, which culminates in the development of mature alveoli (Neville and Daniel 1987). Following cessation of lactation, ECM remodelling and BM loss ensue, and the mammary gland involutes by the process of programmed cell death (Strange et al. 1992; for review, see Tenniswood et al. 1994). Subsequently the gland returns to its nulliparous state.
Much of what is currently known about the role of the ECM in the regulation of mammary gland gene expression has been obtained from studies that have used animal models or their isolated cells and derivative cell lines. This has included studies using different murine strains and transgenic mouse models, which have examined the changes in tissue morphology and gene expression associated with the various developmental stages of the breast, such as occurs during pregnancy, lactation, and following involution (Daniel and Silberstein 1987; Strange et al. 1992, Talhouk et al. 1992; Sympson et al. 1994; Boudreau et al. 1995b; Neuenschwander et al. 1996; for review, see Lelievre et al. 1996). In addition to determining the important molecules and key events necessary for these stages, the in vivo studies have served to emphasize the necessity for a strict regulation of the changes in cell–ECM interactions and ECM turnover during these processes and have highlighted the vital contribution of the stroma. Thus, it is now clear that the development and function of the mammary epithelium depends on systemic hormonal interactions (Topper and Freeman 1980) and on Local cues from the surrounding mesenchyme or stroma, the basement membrane, and soluble cytokines (Sakakura et al. 1982; Daniel and Silberstein 1987; Chen and Bissell 1989; Streuli et al. 1991; for review see Howlett and Bissell 1993). For example, the branching morphogenesis that occurs during pregnancy is under the direction of and is dependent on ovarian hormones and factors provided by the adipocytes of the fat pad stroma (Daniel and Silberstein 1987). Furthermore, subsequent data has shown the important contribution of the stromal fibroblasts during this process. There is now evidence that stromal tissue and resident stromal fibroblasts can mediate the growth regulatory activities of oestrogen (Haslam 1986, 1991), epidermal growth factor, transforming growth factor α (Snedeker et al. 1991), and transforming growth factor β-1 (Silberstein et al. 1990; Robinson et al. 1991). Not surprisingly then, loss of regulated control of these interactions has deleterious effects. Thus, it was shown that transgenic CD-1 mice inappropriately expressing stromeylsin-1, an ECM degrading metalloproteinase, which is under the control of a mammary gland specific promoter, exhibited excessive branching morphogenesis while in the resting state and underwent unscheduled premature apoptosis during pregnancy (Sympson et al. 1994; Boudreau et al. 1995b). Moreover, four independent lines of these mice were shown to have a dramatically increased incidence of tumors (Sympson et al. 1995). Thus far, these mice have been shown to exhibit reactive stromas and phenotypic abnormalities ranging from severe hyperplasia, resembling a lactational phenotype, to overt adenocarcinomas (Sympson et al. 1995). It is not unreasonable, therefore, to suggest that when the tightly regulated processes governing mammary gland development become uncoupled, and tissue homeostasis is compromised, the process of mammary tumorigenesis can ensue.
Primary murine MEC cultures have provided an important model system for understanding the influence of the microenvironment on the expression of the differentiated phenotype and have thereby revealed the existence of a hierarchy of differentiation-specific gene expression. Thus, when plated on tissue culture plastic or attached type I collagen gels, freshly isolated murine MECs were shown to acquire a flat cuboidal morphology, but failed to differentiate and express milk protein genes, even in the presence of lactogenic hormones. In contrast, when plated on floating collagen gels these cells were now able to express β-casein, an early class of milk protein (Emerman and Pitelka 1977). Finally, when grown in the presence of a reconstituted, laminin-rich BM matrix these cells differentiated to form mature, spherical structures, reminiscent of the alveolar structures they were derived from in vivo, and were capable of the vectorial secretion of all classes of milk proteins (Barcellos-Hoff et al. 1989). These studies have also been used to illustrate the role of stromal factors as regulators of epithelial cell morphology and proliferation. For example, it was previously reported that normal ductal outgrowth and end bud formation did not occur if mammary epithelial tissue was isolated and cultured within collagen gels (Yang et al. 1980; Richards et al. 1982). Yet recently, using such collagen gel systems, it was shown that secreted stromal molecules such as scatter factor and heregulin were capable of promoting the outgrowth of primary murine MECs (Yang et al. 1995).
To understand the mechanism by which the ECM elicited its effects on breast tissue structure and gene expression, a series of studies have been conducted (which are still ongoing) using a number of immortalized murine MEC lines (e.g., CID-9, Scp2, EPH4), in conjunction with designer microenvironments. This has included the use of different extracellular matrices such as rat tail collagen, fibronectin, purified laminin and laminin fragments, as well as a reconstituted laminin-rich BM matrix and entails the manipulation of the cell or the microenvironment such as by the use of matrix overlay assays, the preclustering of epithelial cells with poly HEMA, the use of floating collagen versus rigid gels and specialized filter systems, as well as alterations of the hormonal or growth factor milieu. From these studies it has thus far been deduced that changes in cell shape and biochemical cascades, initiated by a laminin-containing BM and through laminin-specific integrin receptors, are necessary for mammary-specific gene expression and that loss of either of these cues will lead to apoptosis (for reviews see Roskelley et al. 1995; Boudreau and Bissell 1996; Lelievre et al. 1996). In addition, these studies have also shown that mammary gland acinar tissue structure dictates the greatest stringency of all for differentiation-specific gene expression and repression of programmed cell death (Roskelley et al. 1994; Boudreau et al. 1996).
Recapitulation of the normal phenotype of human mammary epithelial cells in culture
Based on the observation that cell–ECM interactions and acinar tissue structure are necessary for the expression of the fully differentiated phenotype of murine MECs, it was predicted that similar pathways would influence human MEC differentiation and morphogenesis. Not surprisingly then, analogous to murine MECs, human MECs in culture were shown to rely on both tractional forces within the gel and on laminin-induced biochemical events for the expression of the fully differentiated phenotype. Thus, when human MECs were cultured in floating collagen gels as opposed to rigid gels, the cells formed ducts with branched cylindrical structures containing a central lumen that was surrounded by polarized epithelial cells, similar to human luminal epithelial cells in vivo (Yang et al. 1980; Berdichevsky and Taylor-Papadimitriou 1991). However, under these conditions the cells did not form correctly polarized acinarlike structures comparable with those observed in vivo and instead grew duct-like extensions (Foster et al. 1983; Rudland et al. 1989). This ability to undergo rudimentary morphogenesis in collagen gels was subsequently shown to depend upon α2β1 integrins (Berdichevsky et al. 1992). Further studies by Howlett et al. (1995), showed that when non-malignant immortalized human MECs were embedded within collagen gels, although they formed spherical structures, these colonies were inversely polarized and did not deposit and organize an endogenous BM. In contrast, when normal primary human MECs or immortalized nonmalignant human MECs were cultured within a laminin-rich reconstituted BM, they formed alveolarlike structures composed of polarized luminal epithelial cells and deposited an endogenous BM (Petersen et al. 1992; Howlett et al. 1995). Interestingly, these structures growth arrested to form acinar structures of a similar size and with similar cell numbers to that of the terminal ductal lobular units in vivo, despite the presence of numerous growth factors, both in the medium and within the matrix (Fig. 2). When laminin-specific cell–ECM interactions were prevented by the use of either α3 or β1 integrin function-blocking antibodies, nonmalignant immortalized human MECs failed to proliferate and underwent apoptosis, despite the presence of other cell–ECM interactions and an abundance of growth factors (Howlett et al. 1995). However, when these cells were cultured within collagen gels, they were now dependent upon α2β1 integrin collagen-specific signalling for growth and survival, despite the continued presence of α3β1 laminin receptors (Howlett et al. 1995). This illustrates an inherent existing plasticity of integrin-mediated signalling in these epithelial cells. Thus, by the use of three-dimensional matrix assays it has now become possible to begin to understand the morphological and biochemical mechanisms underlying human MEC differentiation.
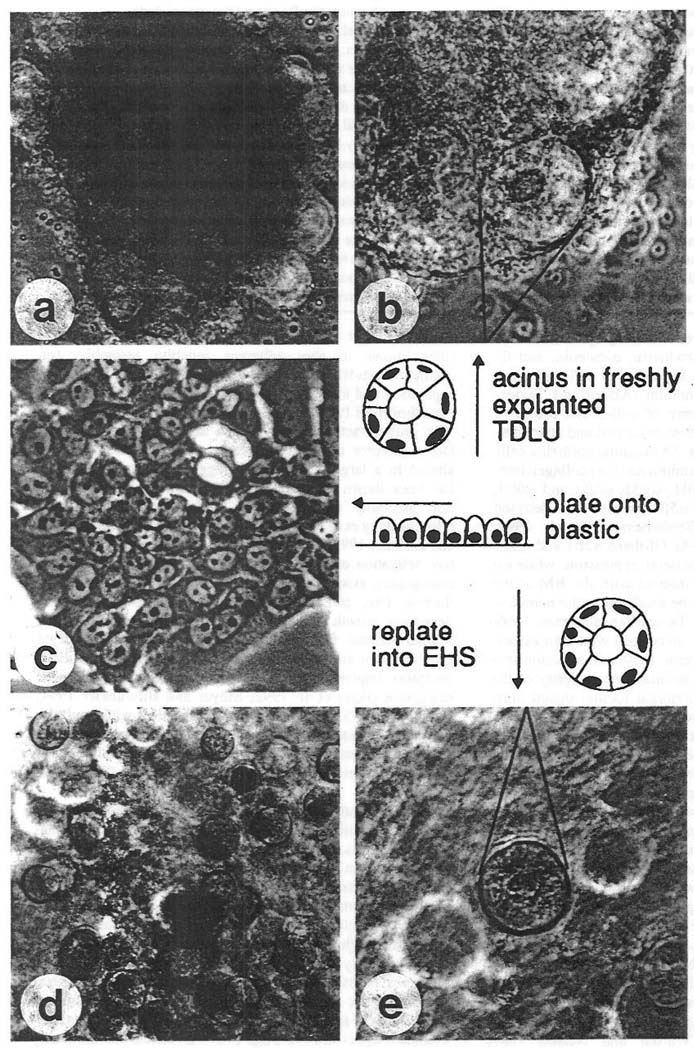
Fig. 2.
Phase-contrast micrographs of normal primary breast epithelial cells. (a) Freshly explanted terminal duct lobular unit at low magnification (×100). (b) Single acinus in focus at a higher magnification (×250). (c) Second passage cells in monolayer culture (×250). (d) Fully developed spheres in the EHS matrix (×100). (e) Single sphere at higher magnification, (x250). (Reproduced with permission from Petersen et al. 1992.)
Changes in cell adhesion and tissue structure are associated with breast cancer progression in vivo
Loss of tissue organization is one of the first visible changes seen in the development of breast cancer. Not surprisingly, then, pathologists diagnose breast biopsies primarily by recognizing altered relations between the ductal cells and their microenvironment (fig. 3). Identification of alterations in these interactions has permitted the following diagnoses: ductal hyperplasia (or proliferative fibrocystic changes), atypical ductal hyperplasia, intraductal carcinoma (or ductal carcinoma in situ; DCIS), and invasive (or infiltrating) ductal carcinoma.
Fig. 3.
Photo-contrast micrographs of tissue sections stained with hematoxylin and eosin of normal human breast tissue (a), ductal epithelial hyperplasia (b), atypical ductal hyperplasia (c), ductal carcinoma in situ (DCIS) (d), DCIS with invasive ductal carcinoma (e), and a fine needle aspirate of a ductal carcinoma (f). (a) In the normal breast, multiple cell types contribute to the organization of the ducts. On the innermost part of the duct (small arrows) are the ductal epithelial cells. External to the ductal cells are myoepithelial cells (arrowheads) derived from terminal differentiation of the ductal cells. The abundant collagen and other extracellular matrix material of the stroma (appearing fibrillar) wraps concentrically around the ducts. The stroma is arranged concentrically around the entire lobule (delineated by long arrows) and then around each individual acinus (×200). (b) In ductal epithelial hyperplasia, the ductal cells have grown towards the center of the duct and formed small bridges or arches across the lumen of the duct. The ductal cells that grow in their native environment next to the basal lamina (long thin arrows) have a more active nuclear appearance than the cells farthest from the basal lamina (long thick arrows). The activity of the nucleus is gauged on the basis of the degree of heterochromatin formation. The presence of abundant heterochromatin in the central-most ductal cells predicts that gene transcription has decreased in these cells compared with the cells toward the basal lamina zone (×400). Residual myoepithelial cells (small arrows) are still present. (c) In atypical ductal hyperplasia, the ductal cells are able to grow away from the basal lamina microenvironment (delineated by long, thin arrows) with almost no effect on their degree of activation. Furthermore, the myoepithelial cells become sparse. Note the presence of some cells with slightly more heterochromatin and less cytoplasm than the rest of the cells (compare curved arrow with open arrow cells). Also, the ductal cells focally have a shared polarity in a few areas of the duct (see the lower leftmost bridge of cells just below the curved arrow) (×200). (d) The defining feature of ductal carcinoma is the apparent complete trophic independence of the ductal cells from the basal lamina zone (delineated by short arrows). Thus, the ductal cells can grow away from the basal lamina zone into the center of the duct (arrow) and maintain identical nuclear and cytoplasmic features compared with the cells next to the native basal lamina zone (×400). (e) On the right is a focus of DCIS in which the original concentric wrapping of stromal collagen is intact, the outline of the duct is smooth, and the fibroblasts next to the DCIS (curved arrow) appear inactive since the nucleus is small and heterochromatic. In contrast, ragged groups of infiltrating ductal cells without the normal organized wrapping of collagen is seen on the left side. The invasive cells appear able to activate the stromal fibroblasts since the fibroblasts next to the invasive ductal cells show euchromatic nuclei (straight arrow) with occasional nucleoli. This diagnostic apparent tropic interaction between infiltrating cancer cells and the stroma is called desmoplasia (×400). (f) Ductal cells strip away from the stroma in fine needle aspirates and invasion is difficult to diagnose, although these cells maintain an active (abnormal) nuclear appearance in three dimensions, this includes lack of shared cell–cell interactions an polarity, microheterogeneity in DNA content, indicated by variation in hematoxylin staining, sharp nuclear membrane infoldings (curved arrow), large nucleoli (straight arrow), and chromatin asymmetry. Magnification: a & c, ×100; b, d, & e, ×200; f, ×1000.
In the normal, postpubertal, nonlactating female breast the luminal epithelial cells form a single row on the innermost part of the duct, while the myoepithelial cells lie between the ductal cells and the basal lamina (basement membrane) (Fig. 3a). While the myoepithelial cells are mitotically inactive and are thought to arise from the terminal differentiation of the ductal cells, the ductal luminal epithelial cells retain the potential to proliferate (Ferguson 1988; Sapino et al. 1990). Studies have shown that the luminal epithelial ductal cells can proliferate in response to a number of signals and that these mitotic events can occur anywhere along the ducts. However, there does appear to be a zone of relatively higher proliferation in the terminal ductule units (Russo et al. 1978; Sapino et al. 1990). What defines normal breast tissue behavior and histology is the maintenance of normal interactions between the ductal cells and the other cellular and molecular constituents of their microenvironment (Fig. 3a). Thus, while normal ductal and alveolar luminal epithelial cells may proliferate greatly during pregnancy, the ductal cells maintain their polarized relation to the myoepithelium and BM microenvironments without stratification, and there is no increase in their number relative to the other cellular components (Fig. 3a).
At all times the whole ductal system is embedded in and dynamically interacting with a collagen-rich stromal matrix, within which reside periductal fibroblasts, periductal blood vessels, and adipocytes. From histologic sections, it is clear that this stromal compartment is organized with respect to the ducts and lobules (Fig. 3a). The normal periductular fibroblasts are arranged parallel to the basal lamina of the ducts and lobules, in turn these fibroblasts synthesize, secrete, and organize collagen and ECM molecules with a clear concentric orientation to the ducts and lobules. The integrity of this ductal system is maintained by the coordinated activity and function of the cell–cell and cell–ECM adhesion systems. MEC homotypic interactions are mediated predominantly by the adherens junctions proteins, of which E cadherin, α-catenin, and β-catenin are the most abundantly expressed at cell–cell junctions in the breast luminal epithelium (Alford and Taylor-Papadimitriou 1996). Of the array of cell–ECM receptors known to exist in the breast, the best described and characterized are the integrin heterodimers. The luminal epithelial cells of the human breast express the laminin and (or) collagen integrin receptors: α1β1, α2β1, α3β1, αvβ1, α6β1 and α6β4, although the fibronectin receptor α5β1 has also been detected (Zutter et al. 1990, 1993; Berdichevsky et al. 1994; Bergstraesser and Weitzman 1994). Of these α2β1 and α3β1 have been shown to have a basolateral expression, while α6 and β4 are present where cells interact with the BM at the basal surface, and are thought to be localized to the hemidesmosomal junctions (Alford and Taylor-Papadimitriou 1996; Borradori and Sonnenberg 1996). In concert with their associated cytoskeletal and plaque proteins, these two adhesion systems mediate the assembly and maintain the integrity of the mammary epithelial ducts and terminal lobular ductal units throughout the developmental programs of the breast.
There is an accummulating body of evidence that suggests that integrin and adherens receptor pathways dynamically interact and synergize with growth factor pathways to tightly regulate epithelial tissue development. The best evidence for this has been provided by the coordinated expression and associated functions of cadherins and integrins observed during keratinocyte differentiation (Hodivala and Watt 1994; Braga et al. 1995; Hotchin et al. 1995) and myodifferentiation (Sastry et al. 1996), although there is now striking evidence for a functional interaction between these pathways during human MEC morphogenesis (Weaver et al. 1997). This implies that a breakdown in normal tissue architecture may be precipitated by changes in any one or more of these pathways or interactions. In support of this argument, adherens junction assembly has been shown to be a plastic event, which can be disrupted by several growth factors and oncogenes and modified by kinases and phosphatase inhibitors (Warren and Nelson 1987; D’Souza and Taylor-Papadimitriou 1994; Hoschuetzky et al. 1994; Ochiai et al. 1994; Kinch et al. 1995). Thus, molecules known to alter adherens junction assembly, for example c-erb-B2, have also been shown to inhibit integrin expression and alter integrin function (D’Souza and Taylor-Papadimitrious 1994; Ochiai et al. 1994; Mainiero et al. 1996), Also growth factor pathways, such as the epidermal growth factor receptor signalling cascade, which is known to be altered in a large percentage of aggressive breast cancers, has been shown to modulate both cell–cell adherens junction assembly and cell–ECM molecules and pathways (Hoscheutzky et al. 1994; Lichtner et al. 1995; Rohde-Schulz and Lichtner 1995; Narita et al. 1996). Furthermore, constitutive activation of intracellular signalling proteins (often by oncogenes), associated with integrin-mediated signal transduction (src, p21ras) are capable of uncoupling ECM-dependent growth and survival (Dedhar 1995). Interestingly, several stromal factors such as heregulin and hepatocyte growth factor are known to induce the activity of the ERB receptors, implying a role for stromally induced cell adhesion disruption (Peles et al. 1992; Meyer and Birchmeier 1994; Lupu et al. 1995; Yang et al. 1995). Consistent with a role for stromally mediated cell adhesion modulation, prostaglandin E2 receptor antagonism, an adipocyte-derived factor, was shown to repress the expression of α3 integrins (Zhang and Fulton 1994).
A large number of breast lesions are classified as benign and include proliferative fibrocystic changes, ductal hyperplasia, epitheliosis, and intraductal papillomas. Whether these perturbations in normal breast homeostasis constitute premalignant precursor changes capable of progressing to frank malignancy remains controversial. At the very least lesions predict a twofold increased risk of subsequent invasive carcinoma, although the carcinomas occur with approximately equal frequency in either breast (Page et al. 1978; Page 1987). However, biopsied patients shown to have marked proliferative fibrocystic changes do have a slight (twofold) increased relative risk of subsequent development of invasive ductal carcinoma (Page et al. 1978; Page 1987). Evidence that these may be precursors to invasive carcinomas include the observation of single or multiple clonal cytogenetic abnormalities in 11 of 15 short-term cultures of proliferative ductal lesions (Dietrich et al. 1995). Interestingly, these proliferative lesions contained similar karyotypic abnormalities as was previously reported for invasive breast carcinoma (Dietrich et al. 1995). Moreover, similarities in the loss of heterozygosity patterns exist between these proliferative lesions and concommittent intraductal or invasive carcinomas (O’Connell et al. 1994), while about 25% of proliferative lesions show grossly aneuploid DNA content suggestive of genomic instability (Visscher et al. 1993).
Morphologically these benign lesions are extremely heterogenous. This probably reflects the multiplicity of genetic, trophic, cell adhesion effects, and stromal interactions responsible for these changes. These lesions are generally divided into “proliferative fibrocystic changes,” “intraductal hyperplasia,” or “epitheliosis” and “intraductal papillomas.” The former group comprises the majority of diagnosed benign lesions of the breast. Ductal hyperplasias are generally characterized by an increase in the relative number of luminal epithelial cells, such that they now consist of a multilayer of luminal epithelia that are disproportionately increased in relation to the other cells of their mammary gland microenvironment. The luminal epithelial cells in this group of ductal proliferations still retain the ability to generate terminally differentiated myoepithelial cells and exhibit a partial trophic and survival dependence on the basal lamina. Thus, although the luminal epithelial cells stratify, those cells distal to the basal lamina have a less active cytologic appearance (Fig. 3b). The term “active cytologic appearance” is defined by pathologists as abundant euchromatin, prominent nucleoli, and commensurate cytoplasmic basophilia (reflecting increased numbers of polyribosomes), often with a Golgi zone present (Frost 1986). Furthermore pathologists distinguish these benign lesions from malignant proliferations, by the tendency for these luminal epithelial cells to orient themselves in a similar direction spatially and form attentuated bridges and slit-like spaces. This suggests they have retained their characteristic luminal cell polarity or at least the ability to respond to an microenvironmentally or structurally generated orientation cue. Consistent with this observation, the few studies that have examined cell adhesion systems in these lesions have shown that they retain the full expression of, and a normal distribution for, all of the adherens junction and integrin molecules (Bergstraesser and Weitzman 1994; Gui et al. 1995). Interestingly, when cultured in three-dimensional BM matrices, immortalized cells generated from these lesions are capable of growth arresting and recapitulating normal breast cytoarchitecture, implying that an altered in vivo breast microenvironment might be at least partially responsible for the expression of the proliferative phenotype (Petersen et al. 1992; Howlett et al. 1995; Weaver et al. 1997). Consistent with this hypothesis, it has been shown that growth factor responsiveness, which is dependent on cell–ECM interactions, can be modulated by different matrices, potentially yielding a higher proliferative response to a given growth factor (Elliott et al. 1992). In addition, there is considerable histological evidence that the ductal/myoepithelial compartment can interact with the stromal compartment, particularly the so-called specialized perilobular fibroblasts. A mutually beneficial interaction between fibroblasts and the ductal/myoepithelial population has been proposed by Rosai (1994) to produce phyllodes tumors (related to fibroadenoma). Phyllodes are viewed as neoplasms of perilobular fibroblasts that induce the non-neoplastic ductal or myoepithelial compartment to expand. The development of papillomas also appears to display a trophic interaction between ductal/myoepithelial cells and periductal fibroblasts. In this case, however, the neoplastic population has been shown to be the ductal/myoepithelial cells. However, it remains to be determined whether subtle differences in epithelial cell responsiveness or alterations in microenvironmental factors exist in this condition and how they might precipitate the development of these ductal hyperproliferative diseases. In this regard, the stroma in these mammary glands is also affected by fibrocystic intraductal hyperplasia and has been shown to expand with the epithelial proliferation. This stromal reaction often results in a fibrosis that extends into the loose intralobular connective tissue and distorts the terminal ductal lobular unit (Ronnov-Jessen et al. 1996).
Atypical ductal hyperplasia (ADH) is a marker of breast cancer risk and may be a precursor to intraductal carcinoma. Its occurrence predicts a four-fold increased relative risk for the development of invasive ductal adenocarcinoma (Page et al. 1978; Page 1987; Dupont et al. 1993). The morphological features of ADH can overlap with either the proliferative ductal lesions or carcinoma in situ (Mariuzzi et al. 1994). What distinguishes these lesions from the proliferative, non-atypical ductal lesions is that the cells now appear to be nearly independent of the trophic and survival influences of their microenvironment. For example, they have similar cytological features whether they are adjacent to the basal lamina or not (Fig. 3c). Furthermore, they are no longer strictly polarized and appear to be nearly independent of the orientation of their neighboring cells. However, they may still retain the rudiments of responsiveness to their microenvironments, and by definition the area of proliferation usually does not extend over 2 mm (Tavassoli 1992). Unfortunately, there is scant to negligible immunocytological or biochemical data available concerning the status of adhesion molecules and the stromal microenvironment in this preneoplastic disease.
Ductal carcinoma in situ is distinguished from invading malignancy in that the relation of the luminal epithelial cells with the periductal stroma is unaltered. The ductal epithelial cells in intraductal carcinoma or ductal carcinoma in situ have completely lost both their tropic and survival dependence on the basal lamina and grow equally well at any polarity, randomly within the duct. Furthermore, they are characterized by the almost complete absence of adjacent myoepithelial cells. Thus, the cells in the duct have a completely disordered spatial orientation and appear cytologically similar to each other, possibly reflecting clonal expansion (Fig. 3d). However, they are sometimes characterized by a microheterogeneity in DNA content indicating genomic instability, which can be detected by nuclear staining or FACS analysis (Fuji et al. 1996) and is correlated with nuclear structural abnormalities (Fig. 3f). In vivo studies suggest that the epithelial cells of in situ carcinomas express the full complement of luminal-specific integrin receptors. However, their localization is usually severely disrupted, and there is evidence for diffuse cytoplasmic staining, loss of membrane localization, and some decreased expression (Berdichevsky et al. 1994; Bergstraesser and Weitzmann 1994; Gui et al. 1995). The only published exception is α1β1, a collagen IV receptor, which was shown to be absent in the majority of in situ carcinomas examined in one study (Bergstraesser and Weitzmann 1994). Furthermore, another Study reported a decrease in the expression of α6/β4 integrins and suggested this was an early critical change in mammary tumor progression (Jones et al. 1992). Also Natali et al. (1992) reported perturbations in α6β4 localization in in situ carcinomas, and while some peripheral staining was observed around the primary tumor nests in 70% of lesions, this was associated with loss of polarity and morphological disorganization. The few studies that have examined the presence and state of cadherins in breast cancer in situ have found their localization to be perturbed, with evidence of diffuse cytosolic staining suggestive of disruption of cytoskeletal interactions (Gamallo et al. 1993; Sommers 1996).
Invasive or infiltrating ductal carcinoma (Fig. 3e), which represents the most common type of breast cancer, is a heterogenous disease with more than one phenotype often appearing within the same lesion (Petersen et al. 1995). Aside from disproportionate increases in luminal epithelial cell numbers and their complete trophic independence, these cells demonstrate a loss of cell–cell interactions, which is associated with increased invasiveness (for review see Takeichi 1993; Sommers 1996). Thus in several invasive carcinomas, cadherin and or catenin expression is often reduced or absent, and these changes are dependent on the grade, type, and differentiation state of the tumor (Gamallo et al. 1993; Oka et al. 1993; Lipponen et al. 1994). In general there is a good inverse correlation between E-cadherin expression and tumor grade or degree of differention (Sommers 1996). However, loss of expression and tumor grade or degree of differentiation (Sommers 1996). However, loss of expression need not be a prerequisite for the genesis of the invasive phenotype. There are several examples of aggressive, metastatic breast tumors that express the full complement of adherens junction proteins. Instead these tumors have lost the ability to assemble stable adherens junctions, probably via the action of one or more of the multitude of factors and pathways that have been shown to modulate the assembly of these junctions (see previous section on adhesion systems in the normal breast) (as for example shown by Matsuyoshi et al. 1992). Consistent with their invasive character, the luminal epithelial cells of these lesions have often disrupted the basement membrane and can be seen infiltrating the surrounding stroma (see Fig. 3f) (Flug and Kopf-Maier 1995). Thus, Barsky et al. (1983) reported that profound changes occurred with the destruction and continuity of the epithelial BM during the transition from benign to invasive carcenomos, such that irregular defects, thinning, fragmentation, and disruption were observed near foci of microinvasions. Here again there are exceptions, so that, in some invasive carcinomas, less than 1% of the cells have been reported to invade and metastasize into the surrounding stroma (Poste and Fidler 1980), while there are cases of benign lesions in which extreme changes in the stromal tissue and loss or disruption of the BM are present yet there is no invasion. Likewise there are reports of a continuous BM existing around continuous infiltrating carcinomas, such as has been observed for squamous-cell carcinoma of the head and neck (Gusterson et al. 1984). Invasiveness then likely involves a number of factors and circumstances that interact in concert to promote metastasis. This has been reported to include changes in the synthesis and secretion of metalloproteinases, BM protein synthesis, and alterations in the stroma (McDougall and Matrisian 1995). For example, in infiltrating ductal carcinoma there is usually a dramatic loss of basement membrane components, often associated with downregulation of lamina expression and less frequently collagen IV (Lochter and Bissell 1995). Moreover, this disease is characterized by a pronounced degree of desmoplasia and is often referred to as “scirrhous carcinoma” (Fig. 3e). Desmoplasia is the common host response to epithelial tumor invasion and is classically described as fibroblast proliferation in conjunction with overproduction of collagens (Sapino et al. 1988; Barsky et al. 1982; Ronnov-Jessen et al. 1996). A characteristic feature of this reaction is the appearance of activated myofibroblasts (Ronnov-Jessen et al. 1995, 1996). The origin of these myofibroblasts was shown to be the resident stromal fibroblasts, whose myogenic conversion was found to be contingent on the activity and conversion capacity of the resident tumorigenic MECs, thereby providing evidence for a dynamic interaction between these two cellular components in the process of this disease (Ronnov-Jessen et al. 1995). Associated with the desmoplasia there is often an increased stromal content of type I collagens, vitronectin, proteoglycans, and glycosaminoglycans, particularly hyaluronic acid and chrondroitin sulfate, and the inappropriate expression of such proteins as thrombospondin (Lochter and Bissell 1995). As such the influence of the ductal microenvironment exhibits many of the changes observed during wound healing, albeit in an uncontrolled fashion (Yeo et al. 1991). For example, during wound healing, fibrin, fibronectin, and tenascin are transiently present, while in contrast in invasive breast tumors these molecules are always present (Clezardin et al. 1993; Lochter and Bissell 1995; Ronnov-Jessen et al. 1996). Considering these striking stromal changes in the neoplastic breast, it is not unreasonable to suggest that this tissue component plays an integral role in the pathogenesis of the disease. Thus it has been hypothesized that “disruption of the self-regulating, dynamic, and reciprocal interaction between the stroma and epithelium in the adult could lead to various proliferative disorders, including neoplasia” (Ronnov-Jessen et al. 1996). In this regards, it is noteworthy that skin fibroblasts cultured from breast cancer patients and their cancer-free relatives displayed an abnormal phenotype, including growth characteristics and the secretion of a soluble migration-stimulating factor, a molecule shown to promote fibroblast migration, that is not synthesized by normal fibroblasts (Schor et al. 1985, 1988).
Several groups have provided evidence that many adhesion molecules including integrins, E cadherin, and its complement of plaque proteins may be downregulated, lost, disorganized, or overexpressed in invasive carcinoma of the breast (Zutter et al. 1990, 1993; Berdichevsky et al. 1994; Glukhova et al. 1995; Sommers 1994, 1996). However, the great heterogeneity among studies, patients within studies, and the type of carcinoma (infiltrating ductal vs. lobular), makes its difficult to derive any definitive conclusions. Generally, however, it may be stated that the degree and heterogeneity of alterations in these molecules increases with increasing tumor grade and aggressiveness.
Infiltrating carcinoma has a more marked phenotype, and an increased heterogeneity with respect to its cell adhesion molecule expression, than its in situ carcinoma counterpart. In these types of lesions the integrins are most frequently markedly disorganized and show a loss of polar distribution, and several studies have reported decreased overall expression or the complete loss of one or more specific integrin heterodimers (Zutter et al. 1990, 1993; Howlett et al. 1995; Glukhova et al. 1995). For example, a comparative study of integrin expression in three mammary tumor cell lines and a nonmalignant MEC line showed loss of expression of β4 integrin and decreased or disorganized expression of α2, α3, α6, and β1 integrins (Howlett et al. 1995). Bergestraesser and Weitzmann (1994) examined tissue sections and parallel primary cultures of several invasive MEC tumor specimens and concluded that the integrin tissue distribution was disturbed and expression was decreased in the majority of cases examined. Interestingly, they reported that several integrin receptors shown to be decreased or absent in vivo were induced in culture, suggesting in vivo factors or the in situ tissue structure may be dynamically modulating integrin expression. Several groups have reported α2 and or α3 integrin expression to be lost both in vivo and in culture, and one group was able to show that reexpression of α2 integrins in a highly malignant, metastatic breast cancer cell line was able to confer repression of tumorigenicity in vivo and loss of anchorage-free growth in culture (Zutter et al. 1990, 1993, 1995 Pignatelli et al. 1991; Berdichevsky et al. 1994). However, not all invasive mammary tumors loose their integrin expression, and examples of specific or relative integrin overexpression do exist. This in turn may reflect the general course of tumor development, such as has been described for breast tumors with a more aggressive clinical course and with a poorer overall prognosis (Taylor-Papadimitriou 1992). Thus Berdichevsky et al. (1994), reported that several aggressive, metastatic, vimentin-positive mammary tumors and tumor cell lines, with basal cell characteristics, retained full expression of most of their integrins with the exception of β4. More provocatively, while decreased expression of α6 integrins have been reported in vivo and in several mammary tumor cell lines in culture (Glukhova et al. 1995; Howlett et al. 1995), these integrins were shown to be retained by 30% of malignant breast tumors in vivo (Natali et al. 1992), and high α6 expression was strongly correlated with decreased patient survival (Friedrichs et al. 1995). Furthermore, overexpression of α6 integrin has been shown to correlate with increased metastasis in prostate cancer (Cress et al. 1995) and is associated with a more aggressive tumor phenotype (D’Ardenne et al. 1991; Rossen et al. 1994). In this regards, Shaw et al. (1996) reported that overexpression of a dominant-negative β4 integrin receptor repressed the metastatic potential of an aggressive highly metastatic breast cell line in culture. These effects were attributed to the ability of this manipulation to decrease the formation of α6β1 integrin heterodimers and thereby prevent its activity. Other integrin receptors that have been shown to exhibit striking changes in invasive breast cancer include the specific loss of α5β1 as well as an increase in the expression of αvβ3 (Zutter et al. 1990, 1993; Bergestraesser and Weitzmann 1994). Finally, while many breast tumors may retain the expression of several integrins, their function may be altered by transcriptional or posttranslational modifications (Leppa et al. 1995), or they may be rendered nonfunctional by other secreted proteins such as episialin (Wesseling et al. 1995) or osteopontin (Denhardt, and Chambers 1994). Unfortunately the role of integrin changes in breast cancer has mainly focused on their contribution to invasiveness and metastasis. To date there are no studies that have examined the relevance of alterations in the adherens junction cell–cell adhesion system and related this to alterations in cell–ECM or trophic factors. Furthermore, the role of ECM receptors in relation to the growth and morphological perturbations characteristic of breast cancer have yet to be studied. This may be due in part to the lack of relevant, physiological culture systems in which to study these features.
Changes in cell adhesion and tissue structure are associated with malignancy in culture
Based on the accumulated body of evidence showing that the ECM is a dominant regulator of tissue structure and gene regulation, it was hypothesized that the ability to sense and respond correctly to cues generated by the microenvironment was the function of a new class of tumor suppressor genes (Petersen et al. 1992). More specifically, the striking basement membrane alterations, stromal reaction, and perturbations in integrins and tissue architecture observed in vivo in mammary tumors led to the prediction that breast cancer was a disease of an aberrant microenvironment and (or) inappropriate microenvironmental responsiveness (Petersen et al. 1992; Ronnov-Jessen et al. 1996). It was thereafter determined that cell–ECM responsiveness could be the basis for an assay to distinguish normal, nonmalignant MECs from tumorigenic MECs (Petersen et al. 1992). Thus, it was demonstrated that cells from reduction mammoplasties and nonmalignant cell lines were able to growth arrest, deposit an endogenous BM, and form polarized, organotypic spheres resembling acini in situ. In contrast, primary breast carcinomas and tumorigenic breast cell lines formed large, loose, and unpolarized colonies of cells and failed to organize a BM or growth arrest (Petersen et al. 1992). Similarly, three-dimensional collagen gel assays were also used to distinguish between various SV-40 immortalized tumorigenic and nonmalignant human MECs (Shearer et al. 1992). The reconstituted BM studies of primary benign and tumorigenic MECs by Bergestraesser and Weitzmann (1994) have confirmed these observations. This assay system has since been used to reveal a latent function for the putative metastasis suppressor gene nm-23, whose altered expression is associated with poor clinical progress and aggressive tumor behavior in vivo (Barnes et al. 1991). Thus overexpression of nm-23 in the highly aggressive, metastatic mammary tumor cell line, MDA MB 435 was shown to reduce the metastatic potential of the parential cell line in nude mice (Leone et al. 1993). Using this three-dimensional assay it was shown that, while the parental cell line formed large, disorganized colonies and failed to growth arrest or deposit an organized BM, the transfectants were able to growth arrest and basally deposit a laminin and collagen IV containing BM (Fig. 4) (Howlett et al. 1994). This assay system is now being used to uncover subtle phenotypic and biochemical differences between normal and tumorigenic MECs. For example while a normal nonmalignant MEC line was shown to be entirely dependent upon β1-integrin signaling events for proliferation and survival in three-dimensional cultures, tumorigenic MEC lines were shown to be refractory (Howlett et al. 1995). However, while these studies are useful for identifying important pathways and molecular differences between normal and tumorigenic MECs the use of model systems that mimic the stages of mammary tumorigenesis are critical for understanding the early changes and subtle differences responsible for this process.
Fig. 4.
Immunohistochemical staining of type IV collagen and lamin expressed by nm23-H1 gene-transfected MDA-MB-435 clone H1-177 cells (A) and normal HMT-3522 breast epithelial cells. (B) Arrows show localization of type IV collagen at the basal surface of spheres formed by nm-23 gene-transfected cells and normal breast cells. Insets show similar localization but less intense staining of laminin for H1-177 cells (inset of panel A) and for reference HMT-3522 cells (inset of panel B). Note the absence of collagen IV deposition by the untransfected parental MDA-MB-435 cells (C) and control transfectants clone C-100 (D) (original magnification ×400; inset original magnification ×320). (Reproduced with permission from Howlett et al. 1994.)
A unique human breast cancer cell culture model of mammary tumor progression
Recognizing the necessity for unraveling the early critical molecular events during mammary epithelial cell transformation, investigators have developed progression model systems of breast cancer (Weaver et al. 1997). This includes the establishment and characterization of several SV-40 immortalized luminal epithelial cell lines generated from breast milk exudates, ranging from nonmalignant phenotypes to overt malignancy (Bartek et al. 1991; Shearer et al. 1992), a cell series of immortalized, preneoplastic transformed human MECs, generated by HPV E6 and E7 oncogenic transformation of cells from mammary reduction mammoplasties and their transformed irradiated counterparts and metastatic derivatives (Wazier et al. 1994, 1995; Band 1995), a xenograft model of MCF10A transformation (Miller et al. 1993), frozen organ cultures of primary MECs and their chemically derived immortalized and oncognenically transformed counterparts (Stampfer and Bartley 1985; Clarke et al. 1988; Stampfer and Yaswen 1993), and a growth factor manipulated model of human mammary tumorigenesis derived from a spontaneously immortalized cell line originating from fibrocystic disease (Briand et al. 1987). The HMT-3522 breast cancer cell series was established from a breast biopsy of a woman with benign fibrocystic disease (Briand et al. 1987) and propagated in a chemically defined medium for greater than 400 passages, without becoming malignant (Nielsen et al. 1989, 1994; Briand et al. 1996). When EGF was removed from passage 118 of these cells they became tumorigenic after an additional 120 passages (Briand et al. 1996). Not surprisingly, continuous passage on tissue culture plastic led to a number of mutations and amplifications in both the EGF-dependent and EGF-independent cell lines, including a p53 point mutation and a c-myc amplication by passage 59–60, as well as other deletions and rearrangements in subsequent passages (Madsen et al. 1992; Moyret et al. 1994; Nielsen et al. 1989, 1994; Briand et al. 1996). This cell line series then comprises the first culture model of spontaneous breast cancer progression. Recently we have begun studying selected cell passages (S-1 epidermal growth factor (EGF) dependent passages 50, 110, and 175; S-2 premalignant EGF independent passage 215; and T4-2 tumorigenic passage 25) from the breast cancer model system in concert with our three-dimensional reconstituted basement membrane assay system, to understand how cell–ECM interactions might influence the genesis of breast cancer. Analogous to in vivo breast cancer progression, as these cells progress towards malignancy their growth factor dependency declines, such that the premalignant cells exhibit EGF independance contingent on the activity of an autocrine loop (Madsen et al. 1992), while the tumor cells are completely EGF independent (Briand et al. 1996). Consistent with these changes, the proliferation rates also increase along the series (not shown). However, morphologically when these cells are grown as monolayers, only small phenotypic changes can be distinguished between the cell passages (Figs. 5a–5e). In contrast, after they have been cultured for 10 days within a reconstituted basement membrane matrix these cells demonstrate striking differences in their phenotypes (Figs. 5f–5j). This early passage EGF-dependent cell lines S-1 passage 50, 110, and 175 all growth arrest and undergo mammary epithelial cell morphogenesis within 6 or 7 days of culture. However, while the earlier cell passages form structures reminiscent of acini in vivo, passage S-l 175 consistency form acinar structures that are typically 40% larger than the earlier passages, possibly reflecting subtle changes in their cell cycle control mechanisms. In contrast, the tumorigenic cells fail to respond appropriately to cues from the basement membrane and form large disorganized, multilayered colonies that fail to growth arrest. Furthermore, these colonies now show features of invasiveness. Interestingly the premalignant cells exhibit a distinct, heterogeneous phenotype, and while all of the colonies formed are disorganized, only a subgroup of these cells continue to proliferate to eventually form large cyst-like structures with stratification, reminiscent of atypical ductal hyperplasia in vivo with some features of carcinoma in situ. These changes in phenotype can be demonstrated by examining the actin microfilaments of the various cell passages in three-dimensional cultures, by staining of cryosections with FITC phalloidin. Thus, while early passage cell lines form well organized spheroids, the premalignant and tumor cell colonies formed exhibit profoundly disorganized cytostructures (Fig. 6). These results imply that an alteration in the cellular responsiveness to the ECM precedes malignancy. Furthermore, they demonstrate that loss of ECM responsiveness is reflected by changes in the cytoarchitecture and tissue structure. Importantly, only the tumorigenic cell line is able to form continuously growing tumors in nude mice. Ongoing studies are now underway to begin to unravel the interacting pathways and events responsible for these changes.
Fig. 5.
Characterization of the HMT-3522 human breast cancer cell model. Phase-contrast micrographs of nonmalignant S-1 cell passages 50 (a and f), 110 (b and g), 175 (c and h), premalignant S-2 cells passage 215 (d and i), and tumorigenic T4-2 passage 25 (e and j) viewed directly on top of plastic or collagen type I (a–e) or inside EHS (f–j) for morphology. There are very few distinguishable morphological differences between these cell passages when they are grown as monolayers (a–e). However, S-1 cell passages 50 and 110 (f and g) arrest growth and form spherical structures, reminiscent of true acini in vivo (see Fig. 2), when cultured in EHS. The S-1 passage 175 cells also growth arrest; however, they form spherical structures that are 40% larger than the two earlier S-1 cell passages. In contrast, when grown in three dimensional BM cultures, the T4-2 cells do not growth arrest and instead form large irregular colonies (j). Distinct from both the S-1 and T4-2 cells, the premalignant S-2 cells are heterogeneous and form growth-arrested colonies as well as larger tightly packed, continuously growing colonies (i). All cultures were analyzed after 10–12 days of culturing either as monolayers or inside EHS (×400).
Fig. 6.
Characterization of actin microfilament organization in the HMT-3522 human breast cancer cell model after culture in three dimensions. Confocal fluorescence microscopy images of 5 µm cryosections of S-1 passage 50, 104, and 173; S-2 passage 215; and T4-2 passage 25 cells stained for F actin with phalloidin (Texas red). The S-1 nonmalignant cell passages 50, 104, and 173 all show organized filamentous F-actin, although the T4 colonies have disorganized, hatched bundles of actin. Similar to the T4 colonies, the S-2 premalignant colonies also have disorganized actin, yet there is no evidence of hatched bundles. All cultures were analyzed after 20–30 days inside EHS (×600).
Conclusions and future perspectives
In this review the progression of mammary carcinoma has been presented as a linear sequence of events; however, it should be emphasized that the development of breast cancer is stochastic with an unpredictable evolution that lacks a predetermined time scale or a prerequisite series of stages. Thus, the time course of breast tumor evolution can be quite long, taking anywhere from 5 to 30 years to develop, or it may appear suddenly and progress rapidly (Holt et al. 1993). Furthermore, while the appearance of atypical and proliferative ductal lesions can be viewed as potential precursors to infiltrating ductal carcinoma, many breast cancers arise without prior detection of fibrosis, while the existence of proliferative ductal hyperplasia does not necessarily preclude the development of cancer (Rosen 1993). Interestingly, the increased risk of developing breast cancer in patients diagnosed with atypical ductal hyperplasia is equally high in both breasts, and this has been attributed to an existing genetic predisposition. However, hereditary breast cancer accounts for only 5% of diagnosed cases, and only 25% of women who get breast cancer have known genetic and exposure-associated risk factors. Thus, while there is sufficient evidence to emphasize the genetic component of this disease, the concept that breast cancer arises simply via the accumulation of a number of critical genetic mutations is no longer sufficient to adequately explain the pathogenesis of this disease. For example, this simplistic perspective does not clarify the origins of the heterogenous responses to curative treatments, nor does it explain the aggressive nature of the disease in some individuals and benign course in others. Neither does it explain the sudden appearance of diffuse metastatic disease in some women after years of tumor dormancy (Love 1996).
Considering the growing body of evidence for the dominant role of the microenvironment on gene expression, it seems more likely that breast cancer results from a complex interaction between the cell, its tissue microenvironment, and the inherent tissue structure. Thus, it is postulated that breast cancer is a disease that represents the loss of normal tissue homeostasis and consists of both an epithelial contributor and a microenvironmental component that predict the course of the disease, with overt infiltrating carcinoma and metastasis representing the extreme deviant endpoint. The concept that the microenvironment is an important contributor to structural homeostasis explains the characteristic genomic heterogeneity and instability and the widely diverging clinical course observed for this disease, as well as why breast cancer takes so long to develop even in individuals with inherited susceptibility. It also provides an explanation for the poorly understood phenomena of spontaneous breast tumor reversion and the sporadic occurrence of an auxiliary nodal metastasis in which a true ductal carcinoma in situ, complete with a central necrosis and a surrounding intact basement membrane, is recapitulated (Barsky et al. 1994).
If an interactive paradigm for breast cancer etiology were correct, then expression of a tumor phenotype should in all probability be a plastic condition whose behavior can be modified by host microenvironmental – tumor cell interactions. This predicted tumor phenotype plasticity has been elegantly demonstrated by the seminal studies of Mintz and IllMensee (1975). In these experiments, embryonal carcinoma cells that were fused with normal blastocysts were able to give rise to phenotypically normal, genetically mosaic mice (for review see Mintz and Fleischman 1981). Another example of the modulatory role of the tissue microenvironment on the tumor phenotype was demonstrated by our previous work, in which active pp60src constructs attached to a lac-Z gene, packaged in a replication-defective virus, were injected into stage 24 chick embryos and subsequently appeared as apparently normal blue cells in a well-formed background of feathers and tissues (Stoker et al. 1990). Removal and culture of those cells without the tumor suppressor tissue microenviroment effects led to the rescue of their oncogenic properties (Boudreau et al. 1995). Keeping this in mind then, it seems inherently logical that to understand the origins and pathogenesis of this disease and to try to develop new thereapeutic approaches, it will be necessary to understand the role of the microenvironment both in the maintenance of the normal healthy breast and its involvement in the events preceding and promoting the development of breast cancer. To this end, reconstructed cell culture model systems will play a vital role, as has been demonstrated by the coculture model systems used by Ronnov-Jessen et al. (1995) to discern the origins of the myofibroblasts in the breast cancer associated stromal reaction.
Acknowledgements
This work is based on ideas and research supported by the Office of Health and Environmental Research of the U.S. Department of Energy (currently under contract DE-AC03-SF00098 to M.J.B.). More recent efforts have been partially funded by a grant from the National Institutes of Health (CA-64786 to M.J.B.). V.M.W. was briefly supported by a grant from the U.S. Department of Defense (94MM4558) and subsequently by the Canadian Medical Research Council and is presently funded by the Breast Cancer Fund of the State of California (BCRP University of California-IFB-0400). O.W.P. is funded by the Danish Cancer Society (No. 95-100-44), the Thaysen Foundation, the Novo Foundation, and the Danish Medical Research Council (No. 9503681). The authors thank Richard Schwarz for computer advice and support, Carolyn Larabell for the confocal images, and Jonathon Lakins for help with the figures.
Abbreviations
- ECM
extracellular matrix
- MEC
mammary epithelial cell
- DCIS
ductal carcinoma in situ
- BM
basement membrane
Contributor Information
V.M. Weaver, Ernest Orlando Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720, U.S.A..
A.H. Fischer, Department of Pathology, Emory University Hospital, 1364 Clifton Road NE, Atlanta, GA 30322, U.S.A.
O.W. Peterson, Structural Cell Biology Unit, Institute of Medical Anatomy, The Panum Institute, DK-2200 Copenhagen N, Denmark.
M.J. Bissell, Ernest Orlando Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720, U.S.A.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Alford D, Taylor-Papadimitriou J. Cell adhesion molecules in the normal and cancerous mammary gland. J. Mammary Gland Biol. Neoplasms. 1996;1:207–218. doi: 10.1007/BF02013644. [DOI] [PubMed] [Google Scholar]
- Ashkenas J, Muschler J, Bissell MJ. The extracellular matrix in epithelial biology: shared molecules and common themes in distant phyla. Dev. Biol. 1996;179 doi: 10.1006/dbio.1996.0317. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V. Preneoplastic transformation of human mammary epithelial cells. Semin. Cell Biol. 1995;6:185–192. doi: 10.1006/scbi.1995.0015. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes R, Masood S, Barker E, Rosengard AM, Coggin DL, Crowell T, King CR, Porter-Jordan K, Wargotz ES, Liotta LA, et al. Low nm23 protein expression in infiltrating ductal breast carcinomas correlates with reduced patient survival. Am. J. Pathol. 1991;139:245–250. [PMC free article] [PubMed] [Google Scholar]
- Barsky SH, Roa CN, Grotendorst GR, Liotta LA. Increased of type V collagen in desmoplasia of human breast carcinoma. Am. J. Pathol. 1982;108:276–283. [PMC free article] [PubMed] [Google Scholar]
- Barsky SH, Siegal GP, Janotta F, Liotta LA. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab. Invest. 1983;49:140–147. [PubMed] [Google Scholar]
- Barsky SH, Doberneck SA, Grossman DA, Love SM. Recapitulation of carcinoma in situ in auxillary nodal breast carcinoma metastasis: clinical and biological implications. Breast Cancer Res. Treat. 1994;23 Suppl:8. [Google Scholar]
- Bartek JJB, Kyprianou N, Lalani EN, Staskova Z, Shearer M, Chang S, Taylor-Papadimitriou J. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian vius 40 large tumor antigen with a recombinant retrovirus. Proc. Natl. Acad. Sci. U.S.A. 1991;88:3528–3524. doi: 10.1073/pnas.88.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky F, Taylor-Papadimitriou J. Morphological differentiation of hybrids of human mammary epithelial cell lines is dominant and correlates with the pattern of expression of intermediate filaments. Exp. Cell Res. 1991;194:267–274. doi: 10.1016/0014-4827(91)90364-z. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Gilbert C, Shearer M, Taylor-Papadimitriou J. Collagen-induced rapid morphogenesis of human mammary epithelial cells: the role of the alpha 2 beta 1 integrin. J. Cell Sci. 1992;102:437–446. doi: 10.1242/jcs.102.3.437. [DOI] [PubMed] [Google Scholar]
- Berdichevsky F, Wetzels R, Shearer M, Maringnone S, Ramaekers FCS, Taylor-Papadimitriou J. Integrin expression in relation to cell phenotype and malignant change in the human breast. Mol. Cell. Differ. 1994;2:255–274. [Google Scholar]
- Bergstraesser LM, Weitzman SA. Alterations in integrin and basement membrane protein expression by malignant breast cells. Int. J. Oncol. 1994;4:915–930. doi: 10.3892/ijo.4.4.915. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Banerjee SD. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev. Biol. 1982;90:291–305. doi: 10.1016/0012-1606(82)90378-5. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Hinkes MT, Gallo RL. Development expression of the syndecans: possible function and regulation. Development. 1993 Suppl.:205–212. [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim. Biophys. Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does extracellular matrix direct gene expression? J. Theor. Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Blashke RJ, Howlett AR, Desprez P-Y, Petersen OW, Bissell MJ. Cell differentiation by extracellular matrix components. Methods Enzymol. 1994;245:535–556. doi: 10.1016/0076-6879(94)45027-7. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr. Opin. Cell. Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Bissell MJ. Regulation of gene expression by the extracellular matrix. In: Comper WD, editor. Extracellular matrix. Vol. 2. Molecular Components and Interactions. Amsterdam, B.V: Harwood Academic Publishers; 1996. pp. 246–261. [Google Scholar]
- Boudreau N, Reddy ST, Stoker AW, Fairman C, Bissell MJ. The embryonic environment and the extracellular matrix suppress oncogenic transformation by rous sarcoma virus in the chick embryo. Mol. Cell. Differ. 1995a;3:261–274. [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science (Washington, D.C.) 1995b;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9309–9313. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VMM, Hodivala KJ, Watt FM. Calcium-induced changes in distribution and solubility of cadherins, integrins and their associated cytoplasmic proteins in human keratinocytes. Cell Adhes. Commun. 1995;3:201–215. doi: 10.3109/15419069509081287. [DOI] [PubMed] [Google Scholar]
- Briand P, Petersen OW, van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propogated in chemically defined medium. In Vitro Cell. Dev. Biol. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res. 1996;56:2039–2044. [PubMed] [Google Scholar]
- Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of beta 1 and alpha v beta 3 integrins in ovarian cancer. Gynecol. Oncol. 1995;58:216–225. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- Chen LH, Bissell MJ. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1989;1:45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Stampfer MJ, Milley R, O’Rourke E, Walen KH, Kriegler M, Kopplin J, McCormick F. Transformation of human mammary epithelial cells by oncogenic retroviruses. Cancer Res. 1988;48:4689–4694. [PubMed] [Google Scholar]
- Clezardin PL, Frappart M, Clerget C, Pechoux C, Delmas PD. Expression of thrombospondin (TSP1) and its receptors (CD36 and CD51) in normal, hyperplastic, and neoplastic human breast. Cancer Res. 1993;53:1421–1430. [PubMed] [Google Scholar]
- Cress AE, Rabinovitz I, Weiguo Z, Nagle RB. The α6β1 and α6β4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB. Postnatal development of the rodent mammary gland. In: Neville M, Daniel C, editors. The mammary gland: development, regulation and function. New York: Plenum Press; 1987. pp. 3–36. [Google Scholar]
- D’Ardenne AJ, Richman PI, Horton MA, McAuley AE, Jordon S. Coordinate expression of the α6 integrin laminin receptor sub-unit and laminin in breast cancer. J. Pathol. 1991;165:213–220. doi: 10.1002/path.1711650304. [DOI] [PubMed] [Google Scholar]
- Dedhar S. Integrin mediated signal transduction in oncogenesis: an overview. Cancer Metastasis Rev. 1995;24:165–172. doi: 10.1007/BF00690289. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Chambers AF. Overcoming obstacles to metastasis—defenses against host defenses: osteopontin (OPN) as a shield against attack by cytotoxic host cells. J. Cell. Biochem. 1994;56:48–51. doi: 10.1002/jcb.240560109. [DOI] [PubMed] [Google Scholar]
- Dhawan J, Farmer SR. Induction of collagen synthesis in response to adhesion and TGF beta is dependent on the actin-containing cytoskeleton. Adv. Exp. Med. Biol. 1994;358:159–168. doi: 10.1007/978-1-4615-2578-3_15. [DOI] [PubMed] [Google Scholar]
- Dietrich CU, Pandis N, Teixeira MR, Aardi G, Gerdes AM, Andersen JA, Heim S. Chromosome abnormalities in benign hyperproliferative disorders of epithelial and stromal breast tissue. Int. J. Cancer. 1995;60:49–53. doi: 10.1002/ijc.2910600107. [DOI] [PubMed] [Google Scholar]
- Drife JO. Evolution, menstruation, and breast cancer. In: Bulbrook RD, Taylor DJ, editors. Commentaries on research in breast diseases. New York: Liss; 1979. pp. 1–23. [Google Scholar]
- Drife JO. Breast development in puberty. In Endocrinology of the breast: basic and clinical aspects. In: Angeli A, Bradlow HL, Dogliotti L, editors. Ann. N.Y. Acad. Sci. No. 12. 1986. pp. 58–65. [DOI] [PubMed] [Google Scholar]
- D’Souza B, Taylor-Papadimitriou J. Overexpression of ERBB2 in human mammary epithelial cells signals inhibition of transcription of the E-cadherin gene. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7202–7206. doi: 10.1073/pnas.91.15.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont WD, Parl FF, Harmann WH, Brinton LA, Winfield AC, Worrell JA, Schuyler PA, Plummer WD. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993;71:1258–1265. doi: 10.1002/1097-0142(19930215)71:4<1258::aid-cncr2820710415>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ekblom M, Klein G, Mugrauer G, Fecker L, Deutzmann R, Timpl R, Ekblom P. Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell. 1990;60:337–346. doi: 10.1016/0092-8674(90)90748-4. [DOI] [PubMed] [Google Scholar]
- Elliott B, Ostman A, Westermark B, Rubin K. Modulation of growth factor responsiveness of murine mammary carcinoma cells by matrix interactions: correlation of cell proliferation and spreading. J. Cell. Physiol. 1992;152:292–301. doi: 10.1002/jcp.1041520210. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Ferguson DJP. An ultrastructural study of mitosis and cytokinesis in normal “resting” human breast. Cell Tissue Res. 1988;252:581–587. doi: 10.1007/BF00216645. [DOI] [PubMed] [Google Scholar]
- Flug M, Kopf-Maier P. The basement membrane and its involvement in carcinoma cell invasion. In: Hay E, editor. And overview of epithelio-mesenchymal transformation. New York: Karger; 1995. pp. 69–84. [PubMed] [Google Scholar]
- Foster CS, Smith CA, Dinsdale EA, Monaghan P, Neville AM. Human mammary gland morphogenesis in vitro: the growth and differentiation of normal breast epithelium in collagen gel cultures defined by electron microscopy, monoclonal antibodies and autoradiography. Dev. Biol. 1983;96:197–216. doi: 10.1016/0012-1606(83)90323-8. [DOI] [PubMed] [Google Scholar]
- Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA. High expression level of α6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995;55:901–906. [PubMed] [Google Scholar]
- Frost JK. An evaluation of the cellular morphologic expression of biologic behavior. Switzerland: Karger, Basel; 1986. The cell in health and disease. [PubMed] [Google Scholar]
- Fujii H, Szumel R, Marsh C, Zhou W, Gabrielson E. Genetic progression, histological grade, and allelic loss in ductal carcinoma in situ of the breast. Cancer Res. 1996;56:5260–5265. [PubMed] [Google Scholar]
- Gamallo CJP, Suarez A, Pizarro A, Navarro P, Quintanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am. J. Pathol. 1993;142:987–993. [PMC free article] [PubMed] [Google Scholar]
- Glukhova M, Koteliansky V, Sastre Z, Thiery JP. Adhesion systems in normal breast and in invasive breast carcinoma. Am. J. Pathol. 1995;146:706–716. [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Kibbey MC, Kinsella JL, Cid MC, Kleinman HK. The role of basement membrane in angiogenesis and tumor growth. Pathol. Res. Pract. 1994;190:854–863. doi: 10.1016/S0344-0338(11)80989-1. [DOI] [PubMed] [Google Scholar]
- Gui GPH, Wells CA, Browne PD, Yeomans P, Jordan S, Puddefoot JR, Vinson GP, Carpenter R. Integrin expression in primary breast cancer and its relation to axillary nodal status. Surgery. 1995;117:102–108. doi: 10.1016/s0039-6060(05)80236-3. [DOI] [PubMed] [Google Scholar]
- Gusterson BA, Warburton MJ, Mitchell D, Kraft N, Hancock WW. Invading squamous cell carcinoma can retain a basal lamina. Lab. Invest. 1984;51:82–87. [PubMed] [Google Scholar]
- Haslam SZ. Mammary fibroblast influence on normal mouse mammary epithelial cell responses to estrogen in vitro. Cancer Res. 1986;46:310–316. [PubMed] [Google Scholar]
- Haslam SZ. Stromal–epithelial interactions in normal and neoplastic mammary gland. In: Lippmann M, Dickson R, editors. Regulatory mechanisms in breast cancer. Hingham, Mass: Kluwer; 1991. pp. 401–420. [DOI] [PubMed] [Google Scholar]
- Hauser IA, Johnson DR, Madri JA. Differential induction of VCAM-1 on human iliac venous and arterial endothelial cells and its role in adhesion. J. Immunol. 1993;151:5172–5185. [PubMed] [Google Scholar]
- Hisaoka M, Haratake H, Hashimoto H. Pancreatic morphogenesis and extracellular matrix organization during rat development. Differentiation. 1993;53:163–172. doi: 10.1111/j.1432-0436.1993.tb00705.x. [DOI] [PubMed] [Google Scholar]
- Hodivala KJ, Watt FM. Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J. Cell Biol. 1994;124:589–600. doi: 10.1083/jcb.124.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoscheutzky H, Hermann A, Kemler R. β-Catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J. Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin NA, Gandarillas A, Watt FM. Regulation of cell surface β1 integrin levels during keratinocyte terminal differentiation. J. Cell Biol. 1995;128:1209–1219. doi: 10.1083/jcb.128.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AR, Bissell MJ. The influence of tissue microenvironment (stromal and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. [PubMed] [Google Scholar]
- Howlett AR, Petersen OW, Steeg PS, Bissell MJ. A novel function for the nm23-H1 gene: overexpression in human breast carcinoma cells leads to the formation of basement membrane and growth arrest. J. Natl. Cancer Inst. 1994;86:1838–1844. doi: 10.1093/jnci/86.24.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J. Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Dike L, Hansen L, Karp S, Liley H, Maniotis A, McNamee H, Mooney D, Plopper G, Sims J, Ning W. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int. Rev. Cytol. 1994;173:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Sun Z, Betensky H, Wang N. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J. Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- Jones JL, Critchley DR, Walker RA. Alteration of stromal protein and integrin expression in breast—a marker of premalignant change? J. Pathol. 1992;167:399–406. doi: 10.1002/path.1711670409. [DOI] [PubMed] [Google Scholar]
- Kibbey MC, Yamamura K, Jun SH, Grant DS, Kleinman HK. Enhancement of tumor growth by basement membrane: modulation of growth and angiogenesis by laminin-derived synthetic peptides. Cancer Treat. Res. 1994;71:267–275. doi: 10.1007/978-1-4615-2592-9_14. [DOI] [PubMed] [Google Scholar]
- Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of Ras-transformed breast epithelia. J. Cell. Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Wehrle-Haller B, Baumgartner S, Spring J, Brubacher D, Chiquet M. Epithelial synthesis of tenascin at tips of growing bronchi and graded accumulation in basement membrane and mesenchyme. Exp. Cell Res. 1991;194:297–300. doi: 10.1016/0014-4827(91)90368-5. [DOI] [PubMed] [Google Scholar]
- Lelievre S, Weaver VM, Bissell MJ. Extracellular matrix signaling from the cellular membrane skeleton to the nuclear skeleton: a model of gene regulation. Recent Prog. Hormone Res. 1996;15:417–432. [PMC free article] [PubMed] [Google Scholar]
- Leone A, Flatow U, Van Houtte K, Steeg PS. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization, and enzymatic activity. Oncogen. 1993;8:2325–2333. [PubMed] [Google Scholar]
- Leppa S, Heino J, Jalkanen M. Increased glycosylation of β1 integrins affects the interaction of transformed S115 mammary epithelial cells with laminin-1. Cell Growth Differ. 1995;6:853–861. [PubMed] [Google Scholar]
- Lichtner RB, Kaufmann AM, Kittmann A, Rohde-Schulz B, Walter J, Williams L, Ullrich A, Schirrmacher V, Khazaie K. Ligand mediated activation of ectopic EGF receptor promotes matrix protein adhesion and lung colonization of rat mammary adenocarcinoma cells. Oncogene. 1995;10:1823–1832. [PubMed] [Google Scholar]
- Lipponen P, Saarelainen E, Ji H, Aaltomaa S, Syrjanen K. Expression of E-cadherin (E-cd) as related to other prognostic factors and survival in breast cancer. J. Pathol. 1994;174:101–109. doi: 10.1002/path.1711740206. [DOI] [PubMed] [Google Scholar]
- Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in breast cancer. Semin. Cancer Biol. 1995;6:165–173. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- Love SM. Breast cancer: an interactive paradigm. Breast J. 1996;2:171–175. [Google Scholar]
- Lupu R, Cardillo M, Harris L, Hijazi M, Rosenberg K. Interaction between erbB-receptors and heregulin in breast cancer tumor progression and drug resistance. Semin. Cancer Biol. 1995;6:135–145. doi: 10.1006/scbi.1995.0016. [DOI] [PubMed] [Google Scholar]
- MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14:351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- Madsen MW, Lykkesfeldt AE, Laursen I, Nielsen KV, Briand P. Altered gene expression of c-myc, epidermal growth factor receptor, transforming growth factor-α, and c-erb-B2 in an immortlized human breast epithelial cell line, HMT-3522, is associated with decreased growth factor requirements. Cancer Res. 1992;52:1210–1217. [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti FG. The intracellular functions of alpha6beta4 integrin are regulated by EGF. J. Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariuzzi GM, Mariuzzi L, Mombello A, Santinelli A, Valli M, Rahal D, Thompson D, Bartels PH. Quantitative study of ductal breast cancer progression. Morphometric evaluation of the phenotypical changes occurring in benign and preinvasive epithelial lesions. Pathol. Res. Pract. 1994;190:1056–1065. doi: 10.1016/S0344-0338(11)80901-5. [DOI] [PubMed] [Google Scholar]
- Martins-Green M, Bissell MJ. Cell–ECM interactions in development. Semin. Dev. Biol. 1995;6:149–159. [Google Scholar]
- Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell–cell adhesion is perturbed by v-src tyrosine phosphorylation in meta-static fibroblasts. J. Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Distinct isoforms of neuregulin are expressed in mesenchymal and neuronal cells during mouse development. Proc. Natl. Acad. U.S.A. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ, Heppner GH. Xenograft model of progressive human proliferative breast disease. J. Natl. Cancer Inst. 1993;85:1725–1732. doi: 10.1093/jnci/85.21.1725. [DOI] [PubMed] [Google Scholar]
- Mintz B, Fleischman RA. Teratocarcinomas and other neoplasms as developmental defects in gene expression. Adv. Cancer Res. 1981;34:211–278. doi: 10.1016/s0065-230x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyret C, Madsen MW, Cooke J, Briand P, Theillet C. Gradual selection of a cellular clone presenting a mutation at codon 179 of the p53 gene during establishment of the immortalized human breast epithelial cell line HMT-3522. Exp. Cell Res. 1994;215:380–385. doi: 10.1006/excr.1994.1355. [DOI] [PubMed] [Google Scholar]
- Murray JS, Madri J, Pasqualini T, Bottomly K. Functional CD4 T cell subset interplay in an intact immune system. J. Immunol. 1993;150:4270–4276. [PubMed] [Google Scholar]
- Narita T, Kawakami-Kimura N, Sato M, Matsuura N, Higashiyama S, Taniguchi N, Kannagi R. Alteration of integrins by heparin-binding EGF-like growth factor in human breast cancer cells. Oncology. 1996;53:374–381. doi: 10.1159/000227591. [DOI] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Botti C, Mottolese M, Bigotti A, Segatto O. Changes in expression of α6β4 integrin heterodimer in primary and mestastatic breast cancer. Br. J. Cancer. 1992;66:318–322. doi: 10.1038/bjc.1992.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander S, Schwartz A, Wood TL, Roberts CT, Jr, Henninghausen L, LeRoith D. Involution of the lactating mammary gland is inhibited by the IGF system in a transgenic mouse model. J. Clin. Invest. 1996;97:2225–2232. doi: 10.1172/JCI118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MC, Daniel CW, editors. The mammary gland: development, regulation and function. New York: Plenum Press; 1987. [Google Scholar]
- Nielsen KV, Briand P. Cytogenic Analysis of in vitro karyotype evolution in a cell line established from nonmalignant human mammary epithelium. Cancer Genet. Cytogenet. 1989;39:103–118. doi: 10.1016/0165-4608(89)90236-7. [DOI] [PubMed] [Google Scholar]
- Nielsen KV, Madsen MW, Briand P. In vitro karyotype evolution and cytogenetic instability in the non-tumorigen ic human breast epithelial cell line HMT-3522. Cancer Genet. Cytogenet. 1994;78:189–198. doi: 10.1016/0165-4608(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Ochiai A, Akimoto S, Kanai Y, Shibata T, Oyama T, Hirohashi S. c-erb B-2 gene product associates with catenins in human cancer cells. Biochem. Biophys. Res. Commun. 1994;205:73–78. doi: 10.1006/bbrc.1994.2631. [DOI] [PubMed] [Google Scholar]
- O’Connell P, Pekkel V, Fuqua S, Osborne CK, Allred CD. Molecular genetic studies of early breast cancer evolution. Breast Cancer Res. Treat. 1994;32:5–12. doi: 10.1007/BF00666201. [DOI] [PubMed] [Google Scholar]
- Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takeichi M, Mori T. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–1701. [PubMed] [Google Scholar]
- Page DL. Diagnostic histopathology of the breast. New York: Churchill Livingstone; 1987. [Google Scholar]
- Page DL, Zwagg RV, Rogers LW, Williams LT, Walker WE, Hartmann WH. Relation between component parts of fibrocystic disease complex and breast cancer. J. Natl. Cancer Inst. 1978;61:1055–1063. [PubMed] [Google Scholar]
- Peles E, Bacus SS, Koski RA, Lu HS, Wen D, Ogden SG, Levy RB, Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Bissell MJ. The microenvironment of the breast: three-dimensional models to study the roles of the stoma and the extracellular matrix in function and dysfunction. Breast J. 1995;1:22–35. [Google Scholar]
- Pignatelli M, Hanby AM, Stamp GW. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumors. J. Pathol. 1991;165:25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature (London) 1980;283:139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Richards J, Guzman R, Konrad M, Yang J, Nandi S. Growth of mouse mammary gland end buds cultured in collagen gel matrix. Exp. Cell Res. 1982;141:433–443. doi: 10.1016/0014-4827(82)90231-2. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Silberstein GB, Roberts AB, Flanders KC, Daniel CW. Regulated expression and growth inhibitory effects of transforming growth factor-β isoforms in mouse mammary gland development. Development. 1991;113:867–878. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- Rohde-Schulz B, Lichtner RB. EGF enhances attachment of metastatic rat mammary adenocarcinoma cell clone MTLn3 to fibronectin and collagen. Invasion Metastasis. 1995;15:1–10. [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer: recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J. Clin. Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol. Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- Rosai J. Ackerman’s surgical pathology. St. Louis, Mo: Mosby; 1996. [Google Scholar]
- Rosen PP. Proliferative breast ‘disease.’ An unresolved diagnostic dilemma. Cancer. 1993;71:3798–3807. doi: 10.1002/1097-0142(19930615)71:12<3798::aid-cncr2820711203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Desprez P-Y, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. U.S.A. 1994;19:12 378–12 382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr. Opin. Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen K, Dahlstrom KK, Mercurio AM, Wewer UM. Expression of the α6β4 integrin by squamous cell carcinomas and basal cell carcinomas: possible relation to invasive potential? Acta Dermato-Venereol. 1994;74:101–105. doi: 10.2340/0001555574101105. [DOI] [PubMed] [Google Scholar]
- Rudland PS, Hughes CM, Ferns SA, Warburton MJ. Characterization of human mammary cell types in primary cultures: immunofluorescent and immunocytochemical indicators of cellular heterogeneity. In Vitro Cell Dev. Biol. 1989;25:23–35. doi: 10.1007/BF02624407. [DOI] [PubMed] [Google Scholar]
- Russo J, Calaf G, Roi L, Russo IH. Influence of age and gland topography on cell kinetics of normal human breast tissues. J. Natl. Cancer Inst. 1978;78:413–418. [PubMed] [Google Scholar]
- Sakakura T, Sakagami Y, Nishizuka Y. Dual origin of mesenchymal tissue participating in mouse mammary gland embryogenesis. Dev. Biol. 1982;91:202–207. doi: 10.1016/0012-1606(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Sapino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and nonmalignant breast tissue. Int. Int. J. Cancer. 1988;41:707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- Sapino A, Macri L, Gugliotta P, Bussolati G. Evaluation of proliferating cell types in human and mouse mammary gland by a double immunostaining procedure. Acta Histochem. Suppl. 1990;40:81–84. [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Thomas DA, Muschler J, Horwitz AF. Integrin α subunit ratios, cycloplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J. Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor SL, Schor AM, Durning P, Rushton G. Skin obtained from cancer patients displays fetal-like behavior in collagen gels. J. Cell Sci. 1985;73:235–244. doi: 10.1242/jcs.73.1.235. [DOI] [PubMed] [Google Scholar]
- Schor SL, Schor AM, Grey A-M, Rushton G. Fetal and cancer patients fibroblasts produce an autocrine migration stimulating factor not made by normal adult cells. J. Cell Sci. 1988;90:391–399. doi: 10.1242/jcs.90.3.391. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Chao C, Wewer UM, Mercurio AM. Function of the integrin α6βl in metastatic breast carcinoma cells assessed by expression of a dominant-negative receptor. Cancer Res. 1996;56:959–963. [PubMed] [Google Scholar]
- Shearer M, Bartkova J, Bartek J, Berdichevsky F, Barnes D, Millis R, Taylor-Papadimitriou J. Studies of clonal cell lines developed from primary breast cancers indicate that the ability to undergo morphogenesis in vitro is lost early in malignancy. Int. J. Cancer. 1992;51:602–612. doi: 10.1002/ijc.2910510417. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Strickland P, Coleman S, Daniel CW. Epithelium dependent extracellular matrix synthesis in transforming growth factor-β-1 growth inhibited mouse mammary gland. J. Cell Biol. 1990;110:2209–2219. doi: 10.1083/jcb.110.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein GB, Flanders KC, Roberts AB, Daniel CW. Regulation of mammary morphogenesis: evidence for extracellular matrix-mediated inhibition of ductal budding by transforming growth factor-β-1. Dev. Biol. 1992;152:354–362. doi: 10.1016/0012-1606(92)90142-4. [DOI] [PubMed] [Google Scholar]
- Snedeker SM, Brown CF, DiAugustine RP. Expression and functional properties of transforming growth factor α and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 1991;88:276–280. doi: 10.1073/pnas.88.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL. The role of cadherin-mediated adhesion in breast cancer. J. Mammary Gland Biol. Neoplasms. 1996;1:219–229. doi: 10.1007/BF02013645. [DOI] [PubMed] [Google Scholar]
- Sommers CL, Gelmann EP, Kemler R, Cowin P, Byers SW. Alterations in beta-catenin phosphorylation and plakoglobin expression in human breast cancer cells. Cancer Res. 1994;54:3544–3552. [PubMed] [Google Scholar]
- Stampfer MR, Bartley JC. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo(a)pyrene. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MR, Yaswen P. Culture systems for study of human mammary epithelial cell proliferation, differentation and transformation. Cancer Surv. 1993;18:7–34. [PubMed] [Google Scholar]
- Stoker AW, Streuli CH, Martins-Green M, Bissell MJ. Designer microenvironments for the analysis of cell and tissue function. Curr. Opin. Cell Biol. 1990;2:864–874. doi: 10.1016/0955-0674(90)90085-s. [DOI] [PubMed] [Google Scholar]
- Stoker W, Hatier C, Bissell MJ. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelial. J. Cell Biol. 1990;1ll:217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development. 1992;115:498. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J. Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromeylysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Bissell MJ, Werb Z. Mammary gland tumor formation in transgenic mice overexpressing stromelysin-1. Semin. Cancer. Biol. 1995;6:159–163. doi: 10.1006/scbi.1995.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr. Opin. Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli FA. Pathology of the breast. Norwalk, Conn: Appleton & Lange; 1992. [Google Scholar]
- Taylor-Papadimitriou J. Normal cell lineages and the phenotype of the breast cancer cell. In: Goldhirsch A, editor. Endocine therapy of breast cancer V. New York: Springer-Verlag; 1992. pp. 3–16. [Google Scholar]
- Taylor-Papadimitriou J, Stampfer M, Bartek J, Lewis A, Boshell M, Lane EB, Leigh IM. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J. Cell Sci. 1989;94:403–413. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- Tenniswood M, Taillefer D, Lakins J, Guenette S, Mooibroek M, Daehlin L, Welsh J. Control of gene expression during apoptosis in hormone-dependent tissues. In: Tomei LD, Cope FO, editors. Apoptosis II. The molecular basis of cell death. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 283–311. [Google Scholar]
- Topper YJ, Freeman CS. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol. Rev. 1980;60:1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Visscher DW, Micale MA, Crissman JD. Pathological and biological relevance of cytophotometric DNA content to breast carcinoma genetic progression. J. Cell. Biochem. Suppl. 1993;17G:114–122. doi: 10.1002/jcb.240531122. [DOI] [PubMed] [Google Scholar]
- Warren SL, Nelson WJ. Nonmitogenic morphoregulatory action of pp60(v-src) on multicellular epithelial structures. Mol. Cell. Biol. 1987;7:1326–1337. doi: 10.1128/mcb.7.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazier DE, Chu Q, Liu ZL, Gao Q, Safaii H, Band V. Loss of p53 protein during radiation transformation of primary human mammary epithelial cells. Mol. Cell. Biol. 1994;14:2468–2478. doi: 10.1128/mcb.14.4.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazier DE, Liu ZL, Chu Q, Gao A, Band V. Immortalization of distinct human mammary epithelial cell types by human papilloma virus 16 E6 or E7. J. Cell Biol. 1995;92:3687–3691. doi: 10.1073/pnas.92.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin. Cancer Biol. 1995;6:175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in 3-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997 doi: 10.1083/jcb.137.1.231. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Richards J, Guzman R, Imagawa W, Nandi S. Sustained growth in primary cultures of normal mammary epithelial cells embedded in collagen gels. Proc. Natl. Acad. Sci. U.S.A. 1980;77:2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G, Weidner KM, Birchmeier C, Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in morphogenesis and differentiation of the mammary gland. J. Cell Biol. 1995;131:215–226. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo T-K, Brown L, Dvorak HF. Alterations in proteoglycan synthesis common to healing wounds and tumors. Am. J. Pathol. 1991;138:1437–1450. [PMC free article] [PubMed] [Google Scholar]
- Zhang SZ, Fulton AM. Modulation of integrin-laminin receptor function on mammary tumor cells by prostaglandin E2 receptor antagonism. Cancer Lett. 1994;85:233–238. doi: 10.1016/0304-3835(94)90280-1. [DOI] [PubMed] [Google Scholar]
- Zutter MM, Mazoujian G, Santoro SA. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am. J. Pathol. 1990;137:863–870. [PMC free article] [PubMed] [Google Scholar]
- Zutter MM, Krigman HR, Santoro SA. Altered integrin expression in adenocarcinoma of the breast analysis by in situ hybridization. Am. J. Pathol. 1993;142:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the α2β1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]