Abstract
The matrix metalloproteinase stromelysin-1 plays a central role during mammary gland development and tumor progression. To gain insight into the regulation of stromelysin-1 gene expression, the murine stromelysin-1 promoter was cloned and transfected into mouse mammary epithelial cells displaying various degrees of malignancy. A reconstituted basement membrane inhibited stromelysin-1 promoter activity in functionally normal cells, had little effect on moderately malignant cells and up-regulated the promoter in highly malignant cells. Spreading of normal and malignant cells was reduced by a reconstituted basement membrane, compared to a plastic substratum. Preventing spreading by maintenance of cells in suspension culture, regulated stromelysin-1 promoter activity in a manner similar to that on a reconstituted basement membrane. Conversely, increasing spreading by augmenting substratum adhesivity up-regulated stromelysin-1 promoter activity in tumor cells. In cells with reduced spreading in the presence of reconstituted basement membrane and in suspension culture, actin stress fibers were replaced by cortical actin bundles. In tumor cells, but not in functionally normal cells, treatment with phorbol diesters also resulted in accumulation of cortical actin and increased stromelysin-1 promoter activity. Consistent with an epithelial-to-mesenchymal conversion, regulation of stromelysin-1 gene expression in highly malignant cells was similar to its regulation in mammary fibroblasts. We conclude that the switch in transcriptional regulation of stromelysin-1 expression that occurs during epithelial-to-mesenchymal transition and conversion to tumorigenicity is related to altered regulation of signals from the cytoarchitecture.
Keywords: Breast cancer, Matrix metalloproteinases, Promoter, Actin
1. Introduction
The extracellular matrix (ECM) contributes significantly to establishment, maintenance and disruption of the dynamic and reciprocal communication between cells and their microenvironment (Adams and Watt, 1993; Lochter and Bissell, 1995; Ashkenas et al., 1996). A number of genes critical for these processes are regulated by ECM (Adams and Watt, 1993; Jones et al., 1993; Weaver et al., 1997). The ECM-degrading matrix metalloproteinases (MMPs) (Birkedal-Hansen et al., 1993; Basbaum and Werb, 1996; Werb, 1997) degrade ECM and alter ECM-mediated signaling events during developmental processes that require ECM penetration such as neurite extension and epithelial branching morphogenesis (Ganser et al., 1991; Nordstrom et al., 1995; Lelongt et al., 1997). A critical role of MMPs in organizing tissue structure and function is also indicated by their increased expression in pathological conditions such as arteriosclerosis and cancer (MacDougall and Matrisian, 1995; Ye et al., 1996; Lochter et al., 1997a). We have focused on analyzing the role of stromelysin-1 (SL-1) in the mammary gland, because its regulated expression in breast tissue correlates with morphogenesis and functional differentiation (Talhouk et al., 1992; Witty et al., 1995; Lund et al., 1996), and its elevated expression correlates with loss of function, aberrant morphogenesis, tumor progression and invasion (Sympson et al., 1994, 1995; Lochter et al., 1997a,b, 1998; Thomasset et al., 1998). In the mammary gland, SL-1 mRNA is localized in the stroma, but it can be produced by epithelial cells in highly aggressive tumors (McDonnell and Matrisian, 1990; Witty et al., 1995). In culture, SL-1 is expressed at low levels by non-malignant mammary epithelial cells, whereas mammary carcinoma cells produce elevated levels (McDonnell and Matrisian, 1990; Lochter et al., 1997b). To elucidate the mechanisms underlying the differential regulation of SL-1 expression, we have analyzed how the murine SL-1 promoter is modulated during mammary epithelial tumor progression. In this study, we determined the role of ECM, cell shape and cytoskeletal organization in controlling SL-1 promoter activity.
2. Materials and methods
2.1. Promoter constructs
A genomic SuperCos I (Stratagene, La Jolla, CA, USA) cosmid library of the mouse SL-1 gene (gift from Dr John S. Mudgett, Merck Research Laboratories, Rahway, NJ, USA) was used to isolate an Eco RI-Pst I fragment encompassing approximately 1.3 kb of 5′-untranslated sequences and approximately 0.2 kb of the first exon of SL-1. The Eco RI-Pst I fragment was subcloned into Bluescript KS (Stratagene) and sequenced with the CircumVent sequencing kit (New England BioLabs, Beverly, MA, USA) according to the manufacturer's instructions. The 5′-untranslated sequences were then amplified by polymerase chain reaction (PCR) and subcloned into the Kpn I-Bgl II cloning sites of the pGL2 vector (Promega, Madison, WI, USA) harboring a luciferase reporter gene to give pSL-1-Luc. Likewise, the Rous sarcoma virus (RSV) promoter was inserted after PCR amplification into the Kpn I-Bgl II sites of the pGL2 vector. The vector containing the RSV promoter attached to a β-galactosidase reporter gene has been described (Li et al., 1992). Nested deletion mutagenesis of the SL-1 promoter was performed with the Erase-a-Base system (Promega) according to the manufacturer's instructions
2.2. Cell culture
SCp2 cells (Desprez et al., 1993), EpH4 cells (a gift from Dr Ernst Reichmann, Epalignes, Switzerland) (Reichmann et al., 1989), p2S7c and p2S10c cells (Lochter et al., 1997a), SCg6 cells (Desprez et al., 1993; Lochter et al., 1997b) and TCL1 cells (Lochter et al., 1997b) were routinely maintained and passaged in medium containing serum as described (Desprez et al., 1993; Lochter et al., 1997b). All assays were performed in chemically defined medium consisting of DMEM/F12, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium (added as ITS medium supplement, Sigma, St. Louis, MO, USA) and 50 μg/ml gentamicin (Life Technologies, Gaithersburg, MD, USA) (Lochter et al., 1997a).
For analysis of promoter activity, cellular morphology and actin organization, cells were maintained in chemically defined medium on tissue culture plastic substrata (Falcon, Beckton Dickinson, Franklin Lakes, NJ, USA) or on reconstituted basement membrane (rBM) (Matrigel, Collaborative, Bedford, MA, USA) gels that were prepared as described (Barcellos-Hoff et al., 1989; Lochter et al., 1997b). In some cases, rBM was added to the culture medium at a final protein concentration of 150–200 μg/ml. Lamininentactin (Sigma), tenascin-C (Life Technologies), thrombospondin (Life Technologies), type I collagen (Collagen Corporation, Fremont, CA, USA), type III collagen (Sigma), type IV collagen (Collaborative) and type VII collagen (Sigma) were added to the culture medium at final concentrations of 50 μg/ml, except for tenascin-C, which was used at 20 μg/ml. Progesterone was used at a final concentration of 1 mM (Sigma), 17β-estradiol (Sigma) at 1 nM, 3,3′,5′-triiodo-l-thyronine (Sigma) at 1 μM, L-thyroxine (Sigma) at 1 μM, prolactin (Sigma) at 3 μg/ml, transforming growth factor-β1 (TGFβ, R&D Systems, Minneapolis, MN, USA) at 1 ng/ml, epidermal growth factor (EGF, Sigma) at 50 ng/ml, hepatocyte growth factor (HGF, Collaborative) at 20 ng/ml, keratinocyte growth factor (KGF, Collaborative) at 50 ng/ml, and basic fibroblast growth factor (bFGF, Collaborative) at 50 ng/ml. Phorbol 12-myristate-13-acetate (PMA, Sigma), was added to the culture medium at final concentrations indicated in the text. Dihydrocytochalasin B was used at 5 μM. For cells maintained in suspension culture, wells were coated with 2 mg/ml poly (2-hydroxyethyl methacrylate) (polyHEMA, Sigma) as described (Roskelley et al., 1994). Poly-L-lysine was diluted in water and coated as described (Lochter et al., 1997b) at concentrations indicated in Fig. 6. As judged by the MTT assay (Mosmann, 1983), cell viability was not affected by any of the treatments used in this study (not shown). When glass coverslips were used instead of plastic substrata, coverslips were pretreated with 10 μg/ml poly-L-lysine as described (Lochter et al., 1997b).
Fig. 6.

Regulation of cell shape and SL-1 promoter activity in SCg6 cells by poly-L-lysine. (a) Micrographs of toluidine blue-stained SCg6 cells maintained for 2 days on tissue culture plastic substrata that were either not coated (w/o PLL), or coated with 25 μg/ml poly-L-lysine (with PLL). Note the markedly increased spreading of cells maintained on substrata coated with poly-L-lysine, compared to non-coated substrata. (b) SCg6 cells were maintained for 2 days on tissue culture plastic substrata coated with increasing concentrations of poly-L-lysine (PLL). Luciferase activity was measured and normalized with values obtained for cells maintained on substrata not coated with poly-L-lysine set to 100. Means from three independent experiments ±S.D. are shown.
2.3. Transfection
Transfection of cell lines was carried out with lipofectin reagent (Life Technologies) according to the manufacturer's instructions. For transient transfections, 150 000 cells were plated into dishes 3.5 cm in diameter and incubated for 24 h with 1 ml OptiMEM (Life Technologies) containing 8 μl lipofectin and 1 μg pSL-1-Luc or RSV promoter in pGL2, or 0.75 μg pSL-1-Luc and 0.75 μg RSV-β-galactosidase vector. The transfection medium was replaced with chemically defined medium and promoter activity was assayed two days later. For stable transfections, 1.2×106 cells maintained in dishes 10 cm in diameter were incubated for 24 h with 5 ml OptiMEM containing 40 μl lipofectin, 0.5 μg SV40neo vector (Schmidhauser et al., 1992) and 5 μg pSL-1-Luc or RSV promoter in pGL2. For transfections with two promoter constructs, 3.75 μg SL-1 promoter in pGL2, 3.75 μg RSV-β-galactosidase vector and 0.5 μg SV40neo vector were used. After transfection, cells were cultured in serum-containing medium and selected 2 days later by addition of 200 μg/ml Geneticin (Life Technologies). Surviving cells were pooled and expanded in medium containing serum.
2.4. Isolation of primary mammary fibroblasts
Fibroblasts were isolated from mammary glands of 70-day-old virgin mice. Abdominal glands were dissected, minced with scalpel blades and incubated in DMEM/F12 (Life Technologies) containing 5% fetal bovine serum, 2 mg/ml collagenase A (Boehringer Mannheim, Indianapolis, IN, USA) and 2 mg/ml trypsin (Life Technologies) for 30 min at 37°C on a rotary shaker at 100 rev./min. Cells were then centrifuged for 5 min at 80×g and the supernatant containing the fat tissue was discarded. Pelleted cells were resuspended in DMEM/F12 containing 40 Kunitz units deoxyribonuclease I (Sigma) per ml medium and incubated for 2 min at room temperature. Epithelial cells that were present in epithelial organoids were then separated from single cells by 3 to 4 centrifugations for 45 s at 80×g in DMEM/F12. The obtained single cell suspension was collected by centrifugation for 10 min at 80×g and resuspended in medium containing 5% fetal bovine serum, 5 μg/ml insulin and 50 μg/ml gentamicin (Sigma). Cells were then plated, grown to confluency and characterized for presence of fibroblasts and epithelial cells by dual immunofluoresence staining according to published procedures (Lochter et al., 1997a). In these cell populations, 97.6±5.28% (mean from three independent cell preparations) of cells expressed the mesenchymal marker protein vimentin and were devoid of cytokeratins, indicating that they were fibroblasts. All remaining cells (2.4±1.79%) were epithelial, as judged by synthesis of cytokeratins and lack of vimentin expression.
2.5. Assays for luciferase and β-galactosidase activity
To measure activity of the luciferase reporter gene, cells were plated at a density of 10 000 cells per well in 96-well multiwell plates and maintained for 2 days in culture, unless indicated otherwise. Cells were then lysed with 1 mM dithiothreitol and 1% (w/v) Triton X-100 in 100 mM sodium phosphate buffer (pH 7.5). Luciferase activity was assayed with a luminometer (Wallac, Gaithersburg, MD, USA) after addition of an equal volume of 150 μg/ml beetle luciferin (Promega), 9 mM adenosine-triphosphate, 20 mM MgCl2, 10 mM potassium phosphate buffer (pH 7.5) and 50 mM Hepes (pH 7.4). Activity of β-galactosidase was analyzed with a luminometer using the Galacto-Light Plus kit (TROPIX, Bedford, MA, USA), according to the manufacturer's instructions.
2.6. MTT assay
To determine the number of viable cells a tetrazolium salt-based assay was used with modifications from the original procedure (Mosmann, 1983). Twenty microliters of 5 mg/ml dimethylthiazolyl-diphenyltetrazolium bromide (MTT, Sigma) in PBS was added to 100 μl of culture medium of cells maintained for 2 days on rBM gels in 96-well tissue culture plates (Falcon, Beckton Dickinson, Franklin Lakes, NJ, USA). Cells were incubated with MTT for 1 h at 37°C, then the culture medium was removed, the metabolized MTT solubilized with 50 μl dimethyl sulfoxide, and absorbance measured at 570 nm.
2.7. Histochemistry and visualization of stress fibers
To analyze cellular morphology, cells maintained in chemically defined medium on tissue culture plastic substrata or on rBM gels were fixed for 15 min with 2.5% glutaraldehyde in PBS, washed with water and stained with toluidine blue O (Sigma) as described (Lochter et al., 1991). Cells maintained in suspension culture were photographed without fixation or staining.
To visualize actin stress fibers, cells maintained on glass coverslips were fixed with 2% paraformaldehyde in PBS for 5 min and washed four times for 5 min with PBS containing 50 mM glycine. After one more wash with PBS, cells were incubated with 0.4% (w/v) Triton X-100 and 10% fetal bovine serum in PBS for 1 h, and subsequently with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma) in PBS for 1 h. Cells were then rinsed four times with PBS and once with water before mounting with Vectashield (Vector Laboratories, Burlingame, CA, USA). To visualize actin microfilaments in cells maintained in suspension cultures, cells were allowed to attach to glass coverslips for 40 min prior to fixation.
2.8. Zymograms
For zymography of conditioned medium, 106 cells were plated in 1 ml of chemically defined medium into dishes 3.5 cm in diameter that were either not treated, or coated with rBM or polyHEMA. Medium was collected 2 days later and casein zymography was performed as described (Fisher and Werb, 1995; Lochter et al., 1997b).
3. Results
3.1. Regulation of SL-1 promoter activity by ECM switches during tumor progression
To prepare a promoter–reporter construct, a genomic EcoR I-Pst I fragment encoding 1.3 kb of 5′-untranslated sequences of the mouse SL-1 gene was cloned from a cosmid library. The SL-1 promoter sequence was then attached to a luciferase reporter gene (pSL-1-Luc) and transfected into the functionally normal mouse mammary epithelial cell line SCp2 and the mouse mammary carcinoma cell line SCg6. Analogous to the ECM-response element in the β-casein promoter (Myers et al., 1998), SL-1 promoter activity could only be measured after stable transfection (not shown). In contrast, RSV promoter-luciferase and RSV promoter-β-galactosidase reporter gene constructs were active in both cell lines in transient transfection assays (Myers et al., 1998, and not shown). Therefore, all experiments were conducted with cells that were stably transfected with promoter–reporter constructs.
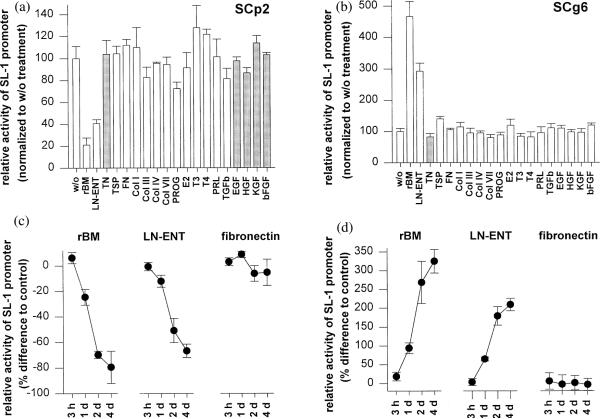
SL-1 mRNA is expressed in functionally normal SCp2 cells when they are cultured on a plastic substratum, but not on rBM (Lochter et al., 1997b). In contrast, SL-1 mRNA is increased in malignant SCg6 cells cultured on rBM. In both SCp2 and SCg6 cells, the SL-1 promoter construct pSL-1-Luc displayed a regulation similar to endogenous SL-1 mRNA (Fig. 1). In SCp2 cells maintained on top of a gel of rBM, SL-1 promoter activity was down-regulated as compared to a plastic substratum, whereas in SCg6 cells, the SL-1 promoter was up-regulated on rBM (Fig. 1a,b). Similar results were obtained when rBM or laminin-entactin were added to the culture medium of cells maintained on plastic (Fig. 1c,d). The effects of rBM and laminin-entactin on regulation of the SL-1 promoter were observed after 1 day and became maximal after 2 days (Fig. 1c,d). In neither SCp2 nor SCg6 cells, pSL-1-Luc was regulated by other ECM constituents, hormones and growth factors. These included tenascin-C, thrombospondin, fibronectin, types I, III, IV and VII collagen, progesterone, estradiol, triiodothyronine, thyroxine, prolactin, TGF-β, EGF, HGF, KGF and bFGF (Fig. 1a,b). These results demonstrate that basement membrane and its key components, laminin-entactin, are specific and important regulators of SL-1 gene expression in mouse mammary epithelial cells at the transcriptional level.
Fig. 1.
Regulation of SL-1 promoter activity in SCp2 and SCg6 cells by rBM and laminin-entactin. (a,b) SCp2 (a) and SCg6 (b) cells were maintained for 2 days on rBM gels or tissue culture plastic in medium without supplements (w/o), or on tissue culture plastic in medium supplemented with laminin-entactin (LN-ENT), tenascin (TN), thrombospondin (TSP), fibronectin (FN), type I collagen (Col I), type III collagen (Col III), type IV collagen (Col IV), type VII collagen (Col VII), progesterone (PROG), estradiol (E2), triiodothyronine (T3), thyroxine (T4), prolactin (PRL), TGFβ, EGF, HGF or KGF, Luciferase activity was measured and results normalized with values obtained for cells maintained on plastic in the absence of ECM, growth factors or hormones (w/o) set to 100. Means from three independent experiments ±S.D) are shown. Grey bars indicate values that have been corrected for cell number by using the MTT method. Tenascin decreased cell number in both SCp2 and SCg6 cells, whereas some growth factors increased cell number in SCp2, but not in SCg6 cells. (c,d) SCp2 (c) and SCg6 (d) cells were maintained for 3 h, 1 day (1 d), 2 days (2 d) and 4 days (4 d) on tissue culture plastic in medium supplemented with rBM, laminin-entactin (LN-ENT) or fibronectin. Values are expressed as percent decrease (c) or increase (d) of SL-1 promoter activity in cells maintained in the presence of ECM, compared to cells maintained in the absence of ECM. Means from three independent experiments ±S.D) are shown.
To determine whether the difference in SL-1 regulation in SCp2 and SCg6 cells was a general property of functionally normal and malignant cells, we examined the regulation of SL-1 promoter activity by rBM in a series of mammary epithelial cell lines representing different stages of tumor progression. We stably transfected the SL-1 promoter-reporter construct into another functionally normal mouse mammary epithelial cell line (EpH4), into another highly malignant mouse mammary carcinoma cell line (TCL1) which, like SCg6 cells, forms invasive tumors within 1 week after injection into mammary glands of immunocompromised mice (Lochter et al., 1997b), and into p2S7c and p2S10c cells that display only some of the characteristics of a malignant phenotype in culture, including the ability to grow in soft agarose (Lochter et al., 1997a,b, 1998), but form slow-growing tumors with a latency period that is several weeks longer than that observed with SCg6 and TCL1 cells (Sternlicht et al., 1999).
As was the case in SCp2 cells, pSL-1-Luc was up-regulated in EpH4 cells in response to rBM, whereas as in SCg6 cells, it was up-regulated in TCL1 cells in response to rBM (Fig. 2). pSL-1-Luc activity in p2S7c and p2S10c cells was unaffected by rBM (Fig. 2). Thus, regulation of SL-1 promoter activity by rBM was correlated with how far the cell lines were from functionally normal behavior, with down-regulation of promoter activity in functionally normal SCp2 and EpH4 cells, no measurable regulation in the moderately malignant p2S7c and p2S10c cells, and up-regulation in highly malignant SCg6 and TCL1 cells.
Fig. 2.
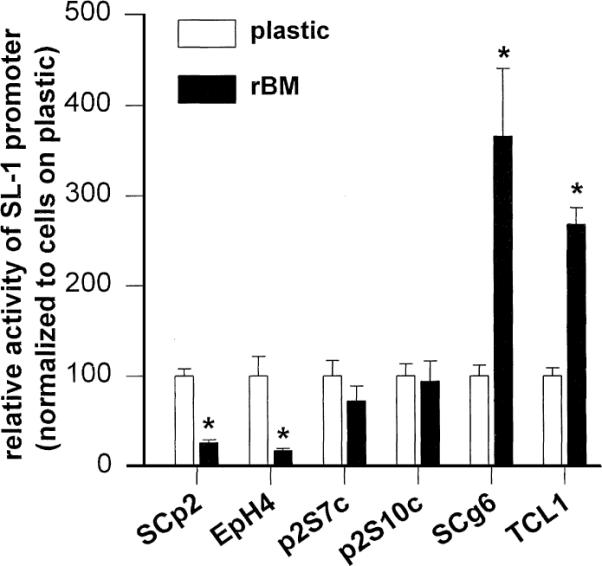
Regulation of SL-1 promoter activity by rBM in progressively more malignant cells. Activity of the SL-1 promoter in functionally normal SCp2 and EpH4 cells, in moderately malignant p2S7c and p2S10c cells, and in highly malignant SCg6 and TCL1 cells. Luciferase activity was analyzed 2 days after plating cells onto tissue culture plastic (white bars) or rBM substrata (black bars). Results are normalized with values obtained for cells maintained on plastic set to 100. Means from three independent experiments ±S.D. are shown. Asterisks indicate significant differences (P≤0.05, Student's t-test) in SL-1 promoter activity between cells maintained on plastic and rBM.
3.2. Regulation of SL-1 promoter activity by cell shape
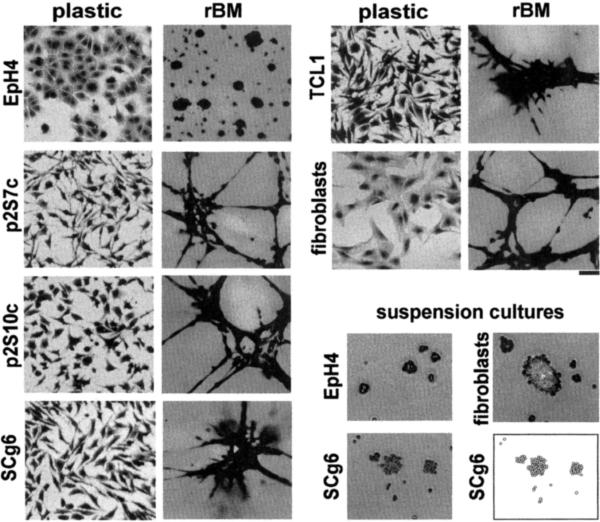
The morphology and function of mammary epithelial cells is strikingly different between cells maintained on tissue culture plastic and on substrata such as floating collagen and rBM (Li et al., 1987; Medina et al., 1987; Roskelley et al., 1994; Emerman and Pitelka, 1977). Because MMP expression in other cell types has been shown to be responsive to cell shape changes (Unemori and Werb, 1986; MacDougall and Kerbel, 1995; Tomasek et al., 1997; Kheradmand et al., 1998), we next examined the potential involvement of cell shape in modulating SL-1 promoter activity. SCp2 cells (Desprez et al., 1993; Lochter et al., 1997a) (not shown) and EpH4 cells (Reichmann et al., 1989) (Fig. 3) form flat, cobblestone-like monolayers when plated onto tissue culture plastic, whereas the tumor cell lines display a mesenchymal, scattered morphology, similar to fibroblasts prepared from the mouse mammary gland (Fig. 3). This observation is consistent with the fact that all four tumor cell lines examined here have undergone an epithelial-to-mesenchymal conversion (Lochter et al., 1997a,b), a process which is often associated with malignant progression in mammary cells (Birchmeier et al., 1996; Gilles and Thompson, 1996). When plated on top of rBM, non-malignant cells organized into multicellular aggregates of round cells (Fig. 3). In contrast, the malignant cell lines formed large cell aggregates that were interconnected by bundles of elongated cells, similar to the morphology that mammary fibroblasts adopt under these culture conditions (Fig. 3).
Fig. 3.
Morphology of mammary epithelial cell lines and fibroblasts cultured on different substrata. Micrographs of toluidine blue-stained EpH4 cells, p2S7c cells, p2S10c cells, SCg6 cells, TCL1 cells and mammary fibroblasts maintained for 2 days on tissue culture plastic or rBM substrata, or in suspension culture. Note the differences in morphologies of cells cultured on plastic and rBM substrata, and between EpH4 and carcinoma cells. In suspension culture all cell lines form multicellular aggregates. Individual SCg6 cells represented in the micrograph of cells maintained in suspension culture are also depicted in a cartoon. Bar, 50 μm.
To change cell shape by other means, we prevented cell-to-substratum adhesion by coating plastic substrata with the non-adhesive compound polyHEMA (Folkman and Moscona, 1978; Roskelley et al., 1994). In such suspension cultures, all cell lines formed multicellular spheroids (Fig. 3, and not shown). Regulation of the pSL-1-Luc in both normal and malignant cells maintained in suspension culture was similar to its regulation by rBM (Fig. 4, compare with Fig. 2). Maintenance of cells in suspension did not affect pSL-1-Luc activity in p2S7c cells, and resulted in a slight up-regulation of SL-1 promoter activity in p2S10c cells (Fig. 4). Thus, SL-1 promoter activity was again regulated in the opposite direction by a change in cell shape in functionally normal and highly malignant cells, whereas the moderately malignant p2S7c and p2S10c cells cultured in suspension had an intermediate phenotype, with little or no effect on pSL-1-Luc. When 5′-nested deletions of the SL-1 promoter were stably transfected into SCp2 and SCg6 cells, approximately 1.2 kb of untranslated sequences could be removed without affecting promoter activity (not shown), suggesting that regulatory sequences around the TATA box, including the AP1 site, are sufficient to confer basic promoter activity as well as ECM- and shape-responsiveness.
Fig. 4.
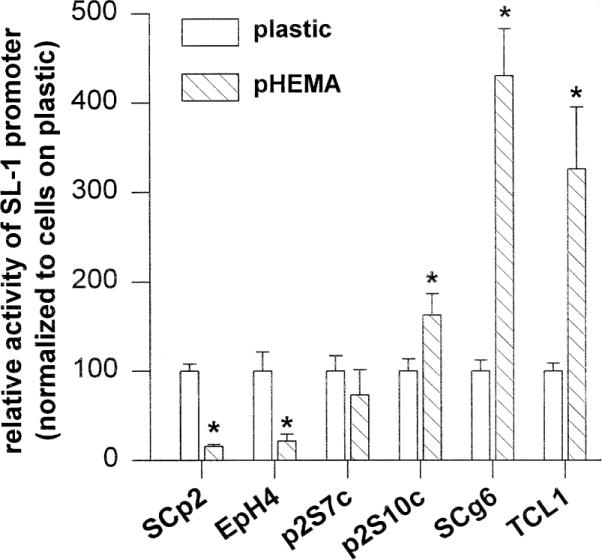
Regulation of SL-1 promoter activity in cells maintained in suspension culture. Activity of the SL-1 promoter in functionally normal SCp2 and EpH4 cells, in moderately malignant p2S7c and p2S10c cells, and in highly malignant SCg6 and TCL1 cells. Luciferase activity was analyzed 2 days after maintaining cells on tissue culture plastic (white bars) or in suspension culture (hatched bars, pHEMA). Results are normalized with values obtained for cells maintained on plastic set to 100. Means from three independent experiments ±S.D. are shown. Asterisks indicate significant differences (P≤0.05, Student's t-test) in SL-1 promoter activity between cells maintained on plastic and in suspension cultures.
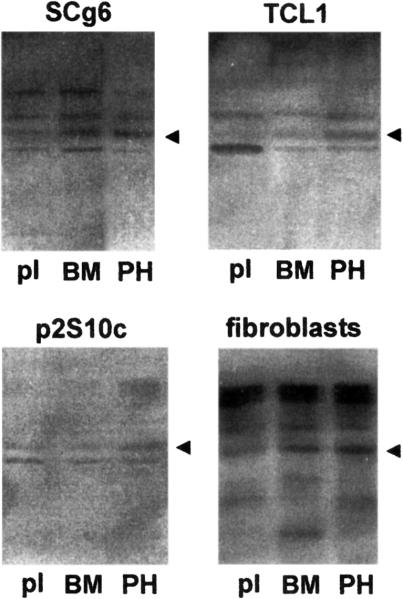
The expression of the endogenous SL-1 gene detected by casein substrate gel zymography paralleled the results on regulation of pSL-1-Luc. The 57 kDa caseinase corresponding to the latent SL-1 proenzyme (Lochter et al., 1997b) increased in intensity in SCg6 and TCL1 cells that were maintained on rBM or in suspension, compared to cells maintained on tissue culture plastic (Fig. 5). Because the malignant cells had undergone an epithelial-to-mesenchymal transition, we next asked whether the altered regulation of SL-1 gene expression represented a switch from an epithelial-type regulation to that seen in fibroblasts. Fibroblasts prepared from mammary glands of virgin mice displayed a regulation of SL-1 protein expression that was similar to SCg6 and TCL1 cells (Fig. 5).
Fig. 5.
Regulation of SL-1 protein expression in cells maintained on rBM and in suspension culture. SCg6 cells, TCL1 cells, p2S10c cells and mammary fibroblasts were maintained for 2 days on tissue culture plastic (pl) or rBM (BM) substrata, or in suspension culture (PH). Conditioned medium was then analyzed for the presence of SL-1 by casein substrate gel zymography. Negative images of zymograms are shown. The caseinolytic activity due to SL-1 is indicated by arrowheads.
We reasoned that if decreased cell spreading was the cause of modulation of SL-1 promoter activity, then an increase in cell spreading should also result in a modulation of SL-1 promoter activity, but in the opposite direction. SCg6 cells (Fig. 6a), but not SCp2 cells (not shown), could be forced to spread to a greater extent when plated on substrata coated with poly-L-lysine. SCg6 cells were less fusiform when maintained on poly-L-lysine-coated substrata compared to non-coated substrata (Fig. 6a), reminiscent of non-malignant mammary epithelial cells (see Fig. 3). Activity of pSL-1-Luc decreased in SCg6 cells with increasing concentrations of poly-L-lysine used for substratum-coating (Fig. 6b). These data confirm the inverse relationship between the degree of cell spreading and the level of SL-1 promoter activity in SCg6 cells. Thus, forced adaptation of a more `normal' morphology on plastic reduces SL-1 expression in mammary tumor cells.
3.3. Regulation of SL-1 promoter activity by ECM and cell shape correlates with organization of the actin cytoskeleton
Both SCp2 and SCg6 cells exhibit stress fibers on plastic, and cortical actin in the presence of rBM and in suspension culture (Fig. 7a). Thus, down-regulation of pSL-1-Luc activity in SCp2 cells and its up-regulation in SCg6 cells under these conditions are paralleled by the loss of stress fibers and accumulation of cortical actin.
Fig. 7.
Actin microfilament organization in SCg6 cells and SCp2 cells, and activity of SL-1 promoter in response to PMA. (a, b) SCg6 cells (a) and SCp2 cells (b) were maintained for 2 days on glass coverslips in the absence (control) or in the presence of rBM, or PMA used at a concentration of 400 nM. SCg6 cells maintained in suspension culture for 2 days (polyHEMA) were plated onto glass coverslips for 40 min allowing attachment, but not spreading of cell clusters. Cells were fixed, permeabilized and stained with FITC-conjugated phalloidin to detect polymerized actin. (c, d) SCg6 cells (c) and SCp2 cells (d) were maintained for 2 days on tissue culture plastic (white circles) or rBM (black squares) substrata and incubated with increasing concentrations of PMA. Luciferase activity was measured and normalized with values obtained for cells maintained on tissue culture plastic in the absence of PMA set to 100. Means from three independent experiments ±S.D. are shown.
To test whether the state of actin polymerization is coupled to regulation of SL-1 promoter activity in functionally normal and malignant cells, we disrupted actin microfilaments in SCp2 and SCg6 cells with dihydrocytochalasin B. Dihydrocytochalasin B reduced pSL-1-Luc activity by 24.3±4.7% in SCp2 cells on plastic and by 20.8±3.8% on rBM, whereas it inhibited pSL-1-Luc activity by 77.2±5.0% in Scg6 cells on plastic and by 92.0±3.7% on rBM. Loss of filamentous actin after treatment of cells with dihydrocytochalasin B was confirmed in both cell types and under both culture conditions by incubating cells with fluorescein-conjugated phalloidin (not shown). Thus, actin polymerization per se appears to play only a minor role in controlling the SL-1 promoter in functionally normal SCp2 cells. In contrast, actin polymerization appears to be a prerequisite for SL-1 promoter activity in SCg6 cells on plastic, and the presence of cortical actin rather than the loss of stress fibers may account for up-regulation of the SL-1 promoter in the presence of rBM. The phorbol ester PMA, which has been shown to affect actin organization in other cell types (Schliwa et al., 1984; Kellie et al., 1985; Werb et al., 1986; Delescluse et al., 1988), altered stress fibers to cortical actin in SCg6 cells, but not in SCp2 cells (Fig. 7a,b). Under these conditions, pSL-1-Luc activity in SCg6 cells on plastic was increased to a level similar to that on rBM (Fig. 7c). Treatment of SCg6 cells maintained on rBM with PMA under conditions where actin is already cortical, barely affected pSL-1-Luc activity (Fig. 7c). pSL-1-Luc activity was not affected in SCp2 cells by incubation with PMA (Fig. 7d). Taken together, our data suggest that actin organization is important in regulating the SL-1 promoter activity in malignant mammary cells.
4. Discussion
During tumor progression, epithelial cells undergo profound alterations in gene expression that eventually result in an invasive and metastatic tumor cell phenotype. In the mouse mammary gland, SL-1 plays a fundamental role both during normal development and tumor formation (Sympson et al., 1994, 1995; Witty et al., 1995; Lochter et al., 1997a, 1998; Sternlicht et al., 1999). In this study we have shown that the regulation of SL-1 promoter activity changes in mouse mammary epithelial cells as they progress towards tumorigenicity.
We found that the SL-1 promoter was down-regulated in functionally normal cell lines (SCp2 and EpH4) in response to rBM, but up-regulated in highly malignant cell lines (SCg6 and TCL1), indicating that the regulation of SL-1 expression described earlier (Lochter et al., 1997b) is at the transcriptional level. This regulation was selective for laminin-entactin, because a variety of other ECM molecules and growth factors were without effect on regulation of the SL-1 promoter in mammary epithelial cells. These included TGFβ and EGF, which have been reported to affect SL-1 promoter activity in other cell types (Kerr et al., 1988; McDonnell et al., 1990). The observation that rBM inhibits SL-1 promoter activity in functionally normal cells is physiologically relevant because normal mammary epithelial cells in vivo are in contact with a basement membrane, and do not express SL-1 mRNA (Witty et al., 1995; Thomasset et al., 1998). Indeed, in wound healing in vivo stromelysin-1 and other MMPs are up-regulated at the edge of the migrating epithelial sheet where the cells are no longer in contact with the basement membrane (Saarialho-Kere et al., 1994).
The increase in SL-1 promoter activity in SCg6 and TCL-1 cells in response to rBM, and the lack of an rBM effect in the moderately malignant cell lines p2S7c and p2S10c suggests that the switch from down-regulation to up-regulation of SL-1 promoter activity by rBM during tumor progression is progressive and correlates with the degree of malignancy of mammary carcinoma cells. This may be functionally significant for tumor cell invasion and metastasis formation, because epithelial and endothelial basement membranes may be breached by tumor cells that synthesize SL-1 (MacDougall and Matrisian, 1995; Lochter et al., 1997b). Thus, the difference in regulation of SL-1 expression by rBM in functionally normal and highly malignant cells may be instrumental for tumor cell aggressiveness. In light of our results showing that SL-1 can cause premalignant and malignant changes in mouse mammary epithelial cells both in culture and in vivo (Sympson et al., 1995; Lochter et al., 1997a, 1998; Thomasset et al., 1998), the loss of the propensity to respond to a basement membrane by inhibition of SL-1 expression may be a major event initiating tumor progression and invasion.
In contrasts to the finding of a rBM-responsive element in the β-casein promoter (Schmidhauser et al., 1992), we could not identify a separate element in the murine SL-1 promoter. Only 96 base pairs upstream of the putative mRNA cap site were required to confer both basal promoter activity and ECM-responsiveness. This region of the SL-1 promoter contains an AP1 site, previously shown to be important for growth factor regulation of SL-1 promoter activity in other cell types (Kerr et al., 1992). However, both SCp2 and SCg6 expressed high levels of AP1 binding activity regardless of whether they were maintained on plastic or rBM substrata. This suggests that the AP1 site is used by both functionally normal and malignant cells to confer basal SL-1 promoter activity, but that the ECM-responsiveness of the SL-1 promoter may be brought about by additional regulatory interactions.
The morphology of all cell lines tested was strikingly different between cells maintained on tissue culture plastic and rBM, with cells being less spread on rBM than on plastic. This suggested that cell spreading and cell shape may be important in regulating SL-1 promoter activity. When spreading was prevented by maintaining cells in suspension culture, regulation of the SL-1 promoter in functionally normal and malignant cells was similar to its regulation by rBM, indicating that the change in cell shape induced by rBM is sufficient to account for its activity. When spreading was increased by augmenting substratum adhesivity, there was a decrease in promoter activity in SCg6 cells. In contrast to the regulation of SL-1 expression by rBM and cell shape, integrins appeared to be required for general transcriptional activity of the SL-1 gene in SCg6 cells, because SL-1 promoter activity was blunted on plastic and rBM substrata as well as in suspension culture by function-perturbing antibodies against α1 and α2 integrins (Lochter et al., 1999). Thus, in mammary carcinoma cells, ECM and integrins appear to control both basal transcription and regulated expression of SL-1.
A role of cell shape in regulating gene expression in mammary epithelial cells has also been demonstrated for the homeobox gene Hoxa-1, which is negatively regulated in SCp2 and EpH4 cells by both rBM and maintenance of cells in suspension culture (Srebrow et al., 1998). Furthermore, a decrease in cell spreading is required for β-casein expression in SCp2 cells (Roskelley et al., 1994). Interestingly, as in the case of SL-1, the effect of rBM on Hoxa-1 and β-casein expression was apparent only after culture periods longer than 1 day (Roskelley et al., 1994; Srebrow et al., 1998).
Our data suggest that a switch from stress fibers to cortical actin, rather than the loss of stress fibers alone, is prerequisite for the increase in SL-1 promoter activity observed in highly malignant cells in response to rBM and for culture in suspension. On plastic, both SCp2 and SCg6 cells had elaborate stress fibers. Stress fibers were replaced by cortical actin when cells were maintained in suspension culture or in the presence of rBM. When actin stress fibers of SCg6 cells maintained on tissue culture plastic were converted into cortical actin by treatment of cells with PMA, SL-1 promoter activity was similar to that observed in cells maintained on rBM and in suspension culture. It is possible that PMA increases SL-1 promoter activity in SCg6 cells on plastic by activation of its AP-1 site, rather than by altering actin organization. However, this appears to be unlikely, because neither SCg6 cells maintained on rBM, nor SCp2 cells maintained on rBM or plastic showed appreciably altered SL-1 promoter activity in the presence of PMA. When cortical actin filaments were disrupted by treatment with dihydrocytochalasin B, SL-1 promoter activity was strongly inhibited in SCg6 cells. Our results described here support previous data on the importance of cell shape and the actin cytoskeleton in regulating expression and promoter activity of MMPs in other cell types (Matrisian et al., 1986; Unemori and Werb, 1986; Werb et al., 1986; Werb and Clark, 1989; MacDougall and Kerbel, 1995; Tomasek et al., 1997; Kheradmand et al., 1998). Moreover, the actin cytoskeleton acquires important new functions during progression of mammary epithelial cells from functionally normal to malignant, and it may participate in causing both high expression and altered regulation of SL-1 in malignant mammary cells.
Changes in cell shape and expression and function of cytoskeletal proteins may be of general importance during tumor progression for cells to acquire tumor cell-specific functions. These changes are part of a process in which epithelial cells lose their typical properties and acquire mesenchymal/fibroblastic characteristics (Birchmeier et al., 1996; Gilles and Thompson, 1996). For example, mammary cells gradually replace their epithelial cytokeratins by mesenchymal vimentin intermediate filaments (Birchmeier et al., 1996; Gilles and Thompson, 1996; Lochter et al., 1997a). This phenomenon appears to contribute to the invasive phenotype of mammary carcinoma cells, because over expression of vimentin increases cell motility and invasion (Hendrix et al., 1996, 1997). Furthermore, remodeling of the actin cytoskeleton through modulation of cytoskeletal regulatory molecules such as gelsolin (Dosaka-Akita et al., 1998) and members of the ezrin family (McClatchey et al., 1998) are frequent genetic changes seen during tumor progression.
Our data further support the acquisition of fibroblastic properties of mammary epithelial cells in the course of malignant conversion. Thus, mammary carcinoma cells behave like mammary fibroblasts morphologically and biochemically, not only on plastic, but also on rBM substrata. Moreover, the regulation of SL-1 expression by rBM and culture of cells in suspension were similar in highly malignant cells and fibroblasts, suggesting that the mechanism of regulation of gene expression in mammary carcinoma cells and fibroblasts bear striking similarities. The fibroblast-like shape that cells adopt during epithelial-to-mesenchymal conversion may play a significant role in this regulation of gene expression. That the moderately malignant cell lines, p2S7c and p2S10c cells, are intermediate in behavior between normal and highly malignant cells on rBM suggests that acquisition of `mesenchymal regulation' of SL-1 expression is not a sudden, all-or-none incidence during tumor progression, but involves successive changes in gene regulation. Therefore, ECM, cell shape and cytoskeletal organization and composition play distinct roles in regulating gene expression in mammary epithelial cells depending on the degree of epithelial and mesenchymal characteristics the cells display.
Acknowledgements
We are grateful to Dr John S. Mudgett and Merck Research Laboratories for gift of SL-1 genomic DNA, to Dr Ernst Reichman for providing EpH4 cells, and to Debbie Lam and Lana Spivak for excellent technical assistance. This work was supported by grants from the US Department of Energy, Office of Health and Environmental Research (contract DE-AC03-76-SF00098) and the National Cancer Institute (grant CA 57621), and by a postdoctoral fellowships from the European Molecular Biology Organization (A.L.) and the California Breast Cancer Research Program (A.L.). GenBank accession number for murine stromelysin-1 promoter: AF077676.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1187–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Ashkenas J, Muschler J, Bissell MJ. The extracellular matrix in epithelial biology: shared molecules and common themes in distant phyla. Dev. Biol. 1996;180:433–444. doi: 10.1006/dbio.1996.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr. Opin. Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, BrandSaberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat. 1996;156:216–227. doi: 10.1159/000147848. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Delescluse C, Bernard BA, Furstenberg G, et al. Effect of diterpene esters on actin cytoskeleton of SV40-transformed keratinocytes is not reproduced by diacylglycerols. Carcinogenesis. 1988;9:333–334. doi: 10.1093/carcin/9.2.333. [DOI] [PubMed] [Google Scholar]
- Desprez PY, Roskelley C, Campisi J, Bissell MJ. Isolation of functional cell lines from a mouse mammary epithelial cell strain: The importance of basement membrane and cell–cell interactions. Mol. Cell. Diff. 1993;1:99–110. [Google Scholar]
- Dosaka-Akita H, Hommura F, Fujita H, et al. Frequent loss of gelsolin expression in non-small cell lung cancers of heavy smokers. Cancer Res. 1998;58:322–327. [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Fisher SJ, Werb Z. Techniques for studying the catabolism of extracellular matrix components. In: Haralson MA, Hassel JR, editors. Extracellular Matrix: A Practical Approach. IRL Press Ltd; Oxford: 1995. pp. 261–281. [Google Scholar]
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Ganser GL, Stricklin GP, Matrisian LM. EGF and TGF influence in vitro lung development by the induction of matrix-degrading metalloproteinases. Int. J. Dev. Biol. 1991;35:453–461. [PubMed] [Google Scholar]
- Gilles C, Thompson E. The epithelial to mesenchymal transition and metastatic progression in carcinoma. Breast J. 1996;2:83–96. [Google Scholar]
- Hendrix MJ, Seftor EA, Chu YW, Trevor KT, Seftor RE. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996;15:507–525. doi: 10.1007/BF00054016. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Trevor KT. Experimental co-expression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am. J. Pathol. 1997;150:483–495. [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Schmidhauser C, Bissell MJ. Regulation of gene expression and cell function by extracellular matrix. Crit. Rev. Eukaryot. Gene Expr. 1993;3:137–154. [PubMed] [Google Scholar]
- Kellie S, Holme TC, Bissell MJ. Interaction of tumour promoters with epithelial cells in culture. An immunofluorescence study. Exp. Cell Res. 1985;160:259–274. doi: 10.1016/0014-4827(85)90174-0. [DOI] [PubMed] [Google Scholar]
- Kerr LD, Magun BE, Matrisian LM. The role of c-Fos in growth factor regulation of stromelysin/transin expression. Matrix Suppl. 1992;1:176–183. [PubMed] [Google Scholar]
- Ker LD, Olashaw NE, Matrisian LM. Transforming growth factor 1 and cAMP inhibit transcription of epidermal growth factor and oncogene-induced transin RNA. J. Biol. Chem. 1988;263:16999–17005. [PubMed] [Google Scholar]
- Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- Lelongt B, Trugnan G, Murphy G, Ronco PM. Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J. Cell Biol. 1997;136:1363–1373. doi: 10.1083/jcb.136.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AP, Myers CA, Kaminski DL. Gene transfer in primary cultures of human hepatocytes. In Vitro Cell Dev. Biol. 1992;28A:373–375. doi: 10.1007/BF02877062. [DOI] [PubMed] [Google Scholar]
- Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc. Natl. Acad. Sci. USA. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Bissell MJ. Involvement of extracellular matrix in breast cancer. Semin. Cancer Biol. 1995;6:165–173. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- Lochter A, Vaughan L, Kaplony A, Prochiantz A, Schachner M, Faissner A. J1/tenascin in substrate–bound and soluble form displays contrary effects on neurite outgrowth. J. Cell Biol. 1991;113:1159–1171. doi: 10.1083/jcb.113.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J. Cell Biol. 1997a;139:1862–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J. Biol. Chem. 1997b;272:5007–5015. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- Lochter A, Sternlicht MD, Werb Z, Bissell MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Ann. NY Acad. Sci. 1998;857:101–110. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- Lochter A, Nave M, Werb Z, Bissell MJ. α1 and β2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of stromelysin-1 expression. Mol. Biol. Cell. 1999;10:271–282. doi: 10.1091/mbc.10.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LR, Romer J, Thomasset N, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall JR, Kerbel RS. Constitutive production of 92-kDa gelatinase B can be suppressed by alterations in cell shape. Exp. Cell Res. 1995;218:508–515. doi: 10.1006/excr.1995.1185. [DOI] [PubMed] [Google Scholar]
- MacDougal JR, Matrisian LM. Contribution of tumor and stromal matrix metalloproteinases in tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14:351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- Matrisian LM, Leroy P, Ruhlmann C, Gesnel MC, Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol. Cell Biol. 1986;6:1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AL, Saotome I, et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly matastatic tumors. Genes Dev. 1998;12:1121–1133. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell S, Matrisian LM. Stromelysin in tumor progression and metastasis. Cancer Metastasis Rev. 1990;9:305–319. doi: 10.1007/BF00049521. [DOI] [PubMed] [Google Scholar]
- McDonnell SE, Kerr LD, Matrisian LM. Epidermal growth factor stimulation of stromelysin mRNA in rat fibroblasts requires induction of proto-oncogenes c-fos and c-jun and activation of protein kinase C. Mol. Cell Biol. 1990;10:4284–4293. doi: 10.1128/mcb.10.8.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D, Li ML, Oborn CJ, Bissell MJ. Casein gene expression in mouse mammary epithelial cell lines: dependence upon extracellular matrix and cell type. Exp. Cell Res. 1987;172:192–203. doi: 10.1016/0014-4827(87)90105-4. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;16:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Myers CA, Schmidhauser C, Mellentin-Michelotti J, et al. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol. Cell. Biol. 1998;18:2184–2195. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom LA, Lochner J, Yeung W, Ciment G. The metalloproteinase stromelysin-1 (transin) mediates PC12 cell growth cone invasiveness through basal laminae. Mol. Cell. Neurosci. 1995;6:56–68. doi: 10.1006/mcne.1995.1006. [DOI] [PubMed] [Google Scholar]
- Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J. Cell Biol. 1989;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl. Acad. Sci. USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J. Clin. Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M, Nakamura T, Porter KR, Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J. Cell Biol. 1984;99:1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin and extracellular matrix-dependent regulation of casein gene expression. Mol. Biol. Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebrow A, Friedmann Y, Ravanpay A, Daniel CW, Bissell MJ. Expression of HOXA-1 and HOXB-7 is regulated by ECM-dependent signals in mammary epithelial cells. J. Cell. Biochem. 1998;69:377–391. doi: 10.1002/(sici)1097-4644(19980615)69:4<377::aid-jcb1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinikel D, Bissell MJ, Werb Z. The Stromal Proteinase MMP3/stromelysin-1 promote mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Bissell MJ, Werb Z. Mammary gland tumor formation in transgenic mice over expressing stromelysin-1. Semin. Cancer Biol. 1995;6:159–163. doi: 10.1006/scbi.1995.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Co-ordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J. Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasset N, Lochter A, Sympson CJ, et al. Expression of autoactivated stromelyin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am. J. Pathol. 1998;153:457–466. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Halliday NL, Updike DL. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J. Biol. Chem. 1997;272:7482–7487. doi: 10.1074/jbc.272.11.7482. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J. Cell Biol. 1986;103:1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo using integrin blocking antibodies. J. Cell Biol. 1997;137:231–246. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: Regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Werb Z, Clark EJ. Phorbol diesters regulate expression of the membrane neutral metalloendopeptidase (EC 3.4.24.11) in rabbit synovial fibroblasts and mammary epithelial cells. J. Biol. Chem. 1989;264:9111–9113. [PubMed] [Google Scholar]
- Werb Z, Hembry RM, Murphy G, Aggeler J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J. Cell Biol. 1986;102:697–702. doi: 10.1083/jcb.102.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol. Biol. Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin-1 promoter which results in reduced gene expression. J. Biol. Chem. 1996;271:13055–13060. doi: 10.1074/jbc.271.22.13055. [DOI] [PubMed] [Google Scholar]