Abstract
Historically, the study of normal human breast function and breast disorders has been significantly impaired by limitations inherent to available model systems. Recent improvements in human breast epithelial cell lines and three-dimensional (3-D)3 culture systems have contributed to the development of in vitro model systems that recapitulate differentiated epithelial cell phenotypes with remarkable fidelity. Molecular characterization of these human breast cell models has demonstrated that normal breast epithelial cell behavior is determined in part by the precise interplay that exists between a cell and its surrounding microenvironment. Recent functional studies of integrins in a human model system provide evidence to support the idea that the structural stability afforded by integrin-mediated cell-extracellular matrix interactions is an important determinant of normal cellular behavior, and that alterations in tissue structure can give rise to tumorigenic progression.
Keywords: Microenvironment, human breast cancer, extracellular matrix, mammary gland, tumorigenesis
Introduction
In order to perform systematic analyses of complex biological problems, it is useful to develop tractable model systems that pare the subject in question down to its most essential components. In the past, this strategy involved the characterization of homogeneous cell populations propagated outside the body as monolayer cultures. Although this approach has been successful in elucidating certain universal principles of cell survival and growth, it has generally ignored the fact that within an organism, each cell exists in the context of a complex extracellular milieu, or microenvironment. In this brief review, we discuss the importance of tissue microenvironment in the preservation of normal cell function. In particular, we focus on a number of recent studies performed in human breast cells illustrating that both the structural and biochemical cues provided by the tissue microenvironment play critical roles in the suppression of tumorigenic phenotypes.
The Significance of Microenvironment In Vivo and in Culture Models
How is a tissue microenvironment defined? In addition to soluble factors that are widely recognized for their role in growth control, microenvironments are comprised of other cell types as well as the insoluble glycoproteins of the extracellular matrix (1–3). Within a given tissue, these microenvironmental factors cooperate to provide both the biochemical signals and structural constraints that are ultimately required to dictate the cellular behaviors appropriate for the tissue in question. Collectively, these extracellular cues influence intracellular programs of gene expression that, in turn, result in fundamental alterations in the composition of the microenvironment itself. This feedback mechanism, described previously by the term “dynamic reciprocity” (1), is now considered to be intimately coupled with the regulation of cellular differentiation, proliferation and survival (4–6). Therefore, in order to establish and maintain tissue-specific functions, cells residing within a tissue must achieve a homeostatic steady state with their surrounding microenvironment. Destabilization of this steady state can induce normal behavioral changes, such as those that are observed during embryogenesis or during wound healing (7,8). Alternatively, inappropriate alterations of cell-microenvironmental interplay can result in aberrant cellular behavior, as is observed during tumor progression (9).
In the last several decades, technological advances in cell culture methodologies have allowed us to begin to address the complexities inherent to tissue-specific function at the cellular level. Before the significance of microenvironment was fully appreciated, cell culture systems were developed in the absence of added extracellular matrix components. However, because cells grown in two-dimensional (2-D) monolayers bear only a limited phenotypic resemblance to their in vivo counterparts, these models were largely inadequate for the study of more complicated questions, such as the regulation of tissue specificity and the maintenance of normal tissue function.
In response to the limitations inherent to 2-D models, cell culture strategies experienced a rebirth of sorts as many investigators, starting with Michaelopoulos and Pitot (10) and Emmerman and Pitelka (11), began to redefine their model cell systems in the context of appropriate three-dimensional (3-D) microenvironments. In many cases, these strategies involved culturing cells on gels composed of extracellular matrix materials that were similar in composition to the matrix normally found associated with the cells in vivo. Using this approach, many cell types were found to assume a more or less normal cellular architecture and exhibit gene expression profiles that were reflective of an authentic differentiated phenotype (2, 12). For example, effective stratification of epidermal skin cells is reproduced in cultures supplemented with collagen I gel and other growth regulators (13-15). Likewise, axon outgrowth of astrocytic cells is optimized in a laminin-rich microenvironment (16, 17). Another laminin-rich matrix derived from Englebreth Holm Swarm (EHS) tumors supports differentiation of a variety of cell types, including hepatocytes (18–21), capillary-forming endothelial cells (22–25), bone-forming osteoclasts (26, 27) and epithelial cells from the kidney (28). As we will describe later, EHS 3-D culture systems are particularly useful in recapitulating the differentiated behavior of mammary gland epithelial cells.
The use of 3-D Microenvironments in the Study of Normal and Aberrant Mammary Gland Function
Unlike most other cells, breast epithelial cells in adult females experience recurrent episodes of regulated growth, differentiation and apoptosis (5, 9, 29). These specialized behaviors, most dramatically observed during cycles of pregnancy, lactation and involution, make the breast an excellent system in which to study the mechanisms regulating cellular differentiation. Interest in breast cell function has also intensified in response to a heightened awareness of human breast disorders, such as cancer. In fact, recent statistical reports show that breast cancer is the most common cancer among women in the Western world and that approximately 1 out of every 8 women will be diagnosed with breast cancer if she lives long enough (30, 31). These alarming statistics underscore the urgency of developing pertinent model systems to use in addressing the molecular basis of human breast cancer progression.
Given the ethical concerns and technical limitations associated with using human subjects in biological investigations, our understanding of the human breast draws significantly from studies performed in mammary tissues of the mouse (see comparative scheme in Fig. 1). In both humans and mice, the mammary gland is a complex structure populated by a variety of cell types, including fibroblasts, adipocytes, epithelial cells and cells of the vasculature (9, 29, 32). Myoepithelial and luminal epithelial cells are the two major types of epithelial cells in the mammary gland; together with components of the stroma they are responsible for generating the network of branching ducts that are embedded in the fibrous collagen-rich stromal matrix of the gland. Luminal epithelial cells, which display a characteristic polarized morphology in situ, form a continuous layer that constitutes the lining of each duct and alveolus. The basal surface of the luminal epithelial cell layer, in turn, is surrounded by a discontinuous layer of myoepithelial cells and a thin laminin-rich basement membrane. Each ductal branch terminates in a group of small spherical lobules called acini or alveoli.
Fig. 1.
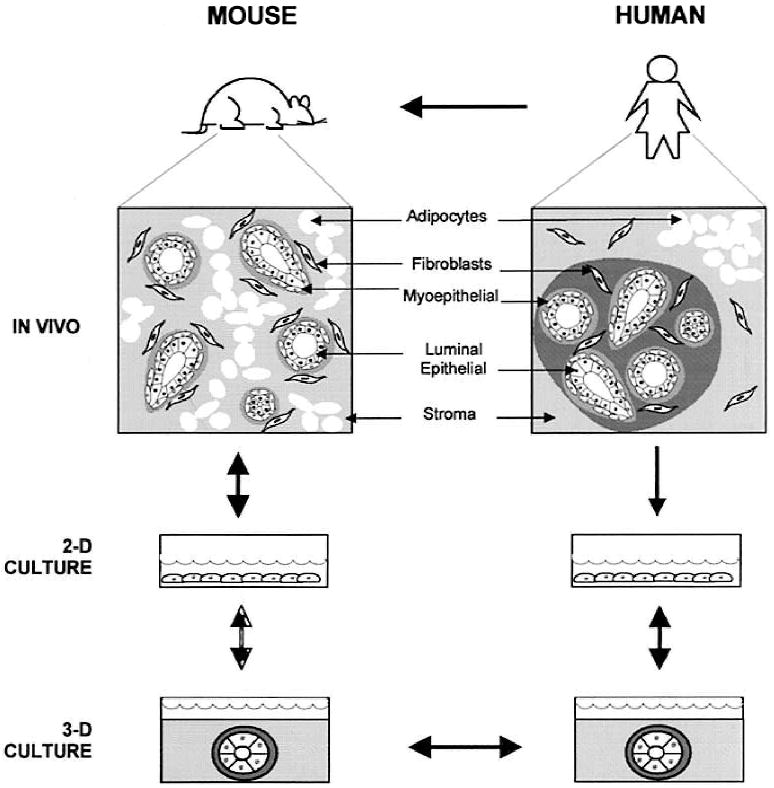
Modeling human breast function from studies of mouse mammary gland. As in humans, the mouse mammary tissue is comprised of multiple cell types, including luminal epithelial and myoepithelial cells, adipocytes, and stromal fibroblasts. Although mouse and human mammary tissue vary somewhat with respect to overall organization, the double-layered structure of the branching ducts and ductules is preserved in both organisms. In light of these fundamental similarities, it is not surprising that human and mouse epithelial cell types display similar behaviors in 3-D basement membrane (EHS) cultures: both cell types undergo morphogenesis to form spherical alveolar structures that are similar to acini in vivo. In effect, this observation bridges the gap between studies in human and mouse and thereby justifies the use of the mouse as a model of aspects of human breast function. Partially adapted from Rønnov-Jessen et al. (9).
In order to understand the process of mammary gland differentiation in molecular detail, we and others have examined the behavior of mouse mammary epithelial cells grown in culture, and have shown that differentiated mammary epithelial cell function can be effectively reproduced in the context of 3-D culture microenvironments. When grown as monolayers on rigid substrata, such as attached collagen I gels, luminal epithelial cells extracted from mouse mammary glands do not differentiate. However, once presented with a physiologically relevant substratum, such as a laminin-rich 3-D EHS gel or a floating collagen I gel, these same cells aggregate, assume classical columnar morphologies, and assemble into 3-D structures that are highly reminiscent of alveolar structures in vivo (11, 33, 34). Upon stimulation with lactogenic hormones, cells found in these cultured acinar structures express milk-specific proteins that are vectorially secreted into the central lumen (3, 5, 35). Collectively, these studies demonstrate that the structural and biochemical cues afforded by the 3-D EHS matrix are necessary and sufficient to allow for the expression of a differentiated epithelial cell phenotype in culture (4, 5, 36).
Given the striking similarities between mammary gland tissues from humans and mice, it was reasonable to predict that luminal epithelial cells derived from normal human breast would also display a differentiated phenotype in 3-D cultures (see model in Fig. 1). Such a finding would be of critical importance in establishing that observations made in the mouse system are likely to be of direct relevance to our understanding of the biology of human breast function. Moreover, because the 3-D culture assay allows for the expression of a cell's differentiated phenotype, cells that fail to differentiate, as would be the case with human breast malignancies, might also be clearly elucidated in this assay. Such an advance would be invaluable since phenotypic traits that are routinely used to distinguish visually between normal and malignant cells in vivo are masked in 2-D cultures. In fact, when grown in monolayers, normal cells are highly plastic and express many characteristics displayed by tumor cells in vivo (37, 38).
Due to recent advances in cell isolation and culture techniques, a variety of human breast epithelial cells lines are now available for study in 3-D culture. The development of human luminal epithelial cell lines has been of particular interest since the majority of breast cancers identified to date have apparently originated from luminal epithelial cell layers (9, 39, 40). Although readily available from both breast reduction surgeries and milk fluids, human breast cells are notoriously difficult to grow and maintain outside of the body (41–44). Primary human luminal epithelial cell cultures have been successfully cultured, but after only a few passages these cells undergo replicative senscence. Primary cultures that do persist tend to be heterogeneous and are prone to overgrowth by resident fibroblasts (45). Given the difficulties of working with primary cultures of normal breast cells, some investigators have concentrated on developing immortalized breast epithelial cell lines that display “normal” phenotypes in culture. This approach has been hampered by the fact that, unlike rodent cells, human cells rarely immortalize spontaneously (9, 44). As a result, only a few spontaneously immortalized human breast epithelial cell lines have been isolated and characterized thus far, including HMT-3522 (45), MCF-10A (46), and HME50 (47). Although these cell lines most likely harbor genetic lesions that predispose them to cancerous behavior, they contain diploid chromosomal arrays and are nontumorigenic. Moreover, while HMT-3522 and MCF-10A cells fail to express the luminal cell protein CK 19, they retain a number of characteristic luminal epithelial cell markers, including CK 18, suggesting that these cells exhibit a more or less “normal” differentiated phenotype. HBL-100 cells are also often used as a “normal” cellular control. However, detailed examinations indicate that these cells contain a near triploid chromosomal content, give rise to tumors in late passages in nude mice, and display elements of the transforming virus, SV40 (48, 49). Malignant human breast epithelial lines have been successfully generated by using extrinsic xenobiotic factors, such as oncogenic viruses (i.e., SV40, HPV 16, HPV 18)(9, 50, 51), chemical agents such as benzo(a)pyrene and asbestos (44, 52), and clinical doses of γ-irradiation (53). These treatments can result in the production of human breast cell lines that exhibit immortalized growth patterns and/or overtly malignant behaviors (9).
Equipped with a variety of human breast cell types, from both primary and immortalized populations, the Petersen and Bissell laboratories performed a series of experiments to classify the phenotypes of normal and cancerous human breast epithelial cells in a 3-D basement membrane culture assay (38). These studies showed that normal human mammary epithelial cells, whether from primary or spontaneously immortalized cell populations, were capable of adopting a differentiated phenotype in 3-D, EHS matrices (Fig. 2). After 7–10 days in culture, normal cells became growth-arrested and formed spherical colonies that corresponded in size and cell number to freshly explanted acini. Within each sphere, the component cells adopted classical polarized epithelial morphologies: the apical surfaces of the cells cooperated in the formation of a central lumen and cell nuclei assumed a basal position. As would be expected for a functionally differentiated mammary gland, sialomucin expression was restricted to the apical surfaces of the cells (Fig. 2A), whereas basement membrane deposition occurred basally (Fig. 2C). Milk protein production, another widely recognized indicator of mammary gland development, was not observed in 3-D EHS cultures of human cell lines such as HMT-3522 cells; this is not surprising given the fact that HMT-3522 cells were derived from a resting mammary gland and therefore lacked the hormonal and tissue-dependent cues necessary to prime and promote the lactating phenotype. Malignant human breast cells also displayed characteristic phenotypes in 3-D culture. Instead of forming organized acini, tumorigenic cells, whether from biopsy cultures or from established cell lines, formed cell aggregates with large diameters and large numbers of cells (38). Moreover, cells within these aggregates continued to proliferate and lacked polarity and an organized basement membrane (Fig. 2B and 2D). Remarkably, even subtle changes in breast epithelial cell behaviors can be detected in the context of the 3-D EHS assay. For example, while HBL-100 cells (described earlier) formed sphere-like clusters and deposited a type IV collagen-containing basement membrane, the cell clusters grown in 3-D did not experience the growth arrest that is characteristically observed with normal cells. Collectively, therefore, these results demonstrate that the 3-D basement membrane assay has the capacity not only to distinguish between normal and overtly malignant breast cell phenotypes, but also to reveal subtle behavioral anomalies that may be characteristic of cells in the early stages of tumor progression. Furthermore, these findings suggest that the ability to sense basement membrane appropriately and to form 3-D organotypic structures may be the function of a novel class of “suppressor” genes that are lost as cells become malignant.
Fig. 2.
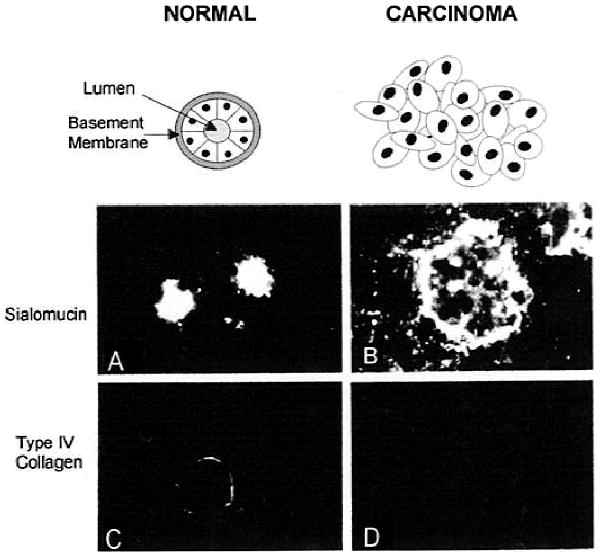
The 3-D basement membrane assay allows for the expression of normal and malignant phenotypic traits by human breast cells. Primary cultures of normal breast epithelial cells (A and C) or breast carcinoma colonies were grown in 3-D EHS matrices for 7–10 days and were processed for immunofluore scence staining with antibodies directed against sialomucin (a marker for apical cell surfaces) (A and B) or against type IV collagen (a marker for basement membrane) (C and D). The staining here demonstrates that while the normal breast epithelial cells grown in 3-D are capable of forming organized spheres with central lumena and basally-deposited basement membranes, their tumorigenic counterparts fail to undergo polarized morphogenesis and do not deposit endogenous basement membrane-like material. Reproduced from Petersen et al. (38).
The function of one tumor suppressor gene, called nm23-H1, that is dramatically reduced in aggressive breast carcinomas, has already been tested in the basement membrane assay. Previous studies showed that reexpression of nm23-H1 in the human breast carcinoma cell line, MDA-MB-435, reduced the metastatic potential of the cells in vivo (54). To examine the role of nm23-H1 in breast epithelial cell differentiation and tumorigenic behavior further, the phenotypes of two human MDA-MB-435 breast carcinoma cell populations, one of which had been mock transfected, and one of which had been transfected with a vector encoding nm23-H1, were assayed in 3-D EHS gels (55). In contrast to the mock transfectants that displayed disorganized phenotypes in 3-D culture, the nm23-H1 overexpressing cells regained several aspects of a normal cellular phenotype, including the formation of organized acinus-like spheres, basal deposition of basement membrane and growth-arrest. These findings revealed for the first time that the nm23-H1 gene product functions in cellsto preserve normal cell phenotype, perhaps by allowing cells to respond appropriately to the surrounding microenvironment.
Human Breast Cancer Progression Models and the HMT-3522 Series
Functional comparisons between unpaired normal and tumorigenic cell types are limited by the possibility that observed differences may be due to variability inherent to the individual cell types and not due to differences resulting from malignancy. Therefore, recent efforts have focused upon generating human breast tumor progression models in which both nonmalignant or tumorigenic cells have been derived from a single cell lineage. For example, one recently reported progression series is comprised of four distinct breast tumor primary cell lines derived from the same patient, each of which corresponds to a discrete stage of malignancy (56). Using this cell series, Liu and colleagues demonstrated a loss in p53 expression resulting from a frameshift mutation that occurred relatively early in the breast progression series (57). Another human breast cell model was generated by exposing normal breast epithelial cells in culture to clinical doses of γ-irradiation (53). Using this model, Liu and colleagues showed that γ-irradiated cell populations assumed tumorigenic phenotypes and that tumorigenic behavior correlated with the loss of p53, as well as transformation-dependent down-regulation of NES-1, a novel protein with potential serine protease activity (58).
Although acute exposures to environmental factors, such as radiation, may accelerate the onset of breast cancer disease, it is widely believed that, in many cases, breast cancer is induced by more subtle changes, such as hormonal imbalances, that develop as an organism matures and ages (44, 51, 59). Recently, a human breast cancer progression series, originating from the HMT-3522 cell line mentioned above, was generated to approximate the onset of cancer in the absence of exposure to xenobiotic factors (Fig. 3) (45, 60). HMT-3522 cells were originally derived from a reduction mammoplasty from a woman who later appeared to have had fibrocystic breast disease. At the time the culture was initiated, there was no detectable malignancy in the explanted tissue. The HMT-3522 cell line, unlike other human mammary epithelial cells, was established from a purified luminal epithelial cell population and has been cultured over a period of 10 years (i.e., 500+ passages) in a chemically defined medium containing insulin, transferrin, epidermal growth factor (EGF), hydrocortisone, estradiol, prolactin and selenite (45). This breast cell culture, which was initially very heterogeneous, gave rise to subsequent populations of cells that displayed a more or less uniform, polygonal shape. Every 10 passages, these cell cultures, referred to in the literature as S-1, were karyotyped and tested for tumorigenic behavior in nude mice. No tumors were detected for any of the cell populations grown under these conditions, despite the fact that cells from later passages were notably aneuploid (61) and carried a mutation in the p53 gene (62).
Fig. 3.
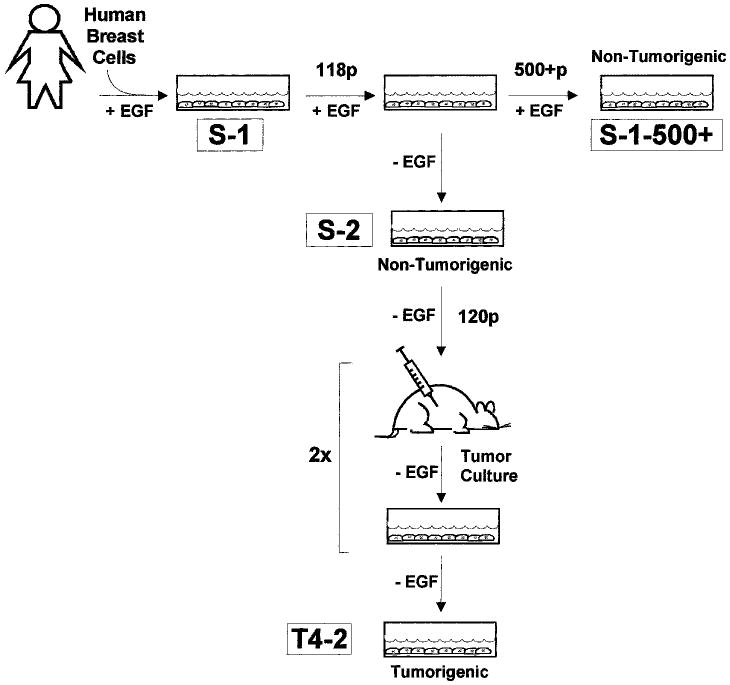
The HMT-3522 progression series. HMT-3522-S-1 cells, isolated from a reduction mammoplasty of a woman with fibrocystic disease, have been cultured in a chemically-defined medium containing EGF for over 500 passages (10 years) (45). Although in later passages these cells exhibit a significant number of chromosomal rearrangements, cells throughout the S-1 series fail to give rise to tumors in nude mice. S-1 cells from passage 118 have been grown in the absence of EGF (called S-2 cells). After 120 passages in EGF-free medium, these cells have given rise to tumors in mice (60). Cells propagated from these tumors are referred to as T4-2 cells.
One characteristic feature of the HMT-3522-S-1 cell series is that their growth is EGF-dependent. Because growth autonomy has been cited previously as a prerequisite for malignant conversion (63–65), Briand and coworkers reasoned that removal of EGF from the HMT-3522 growth medium might induce their malignant transformation (60). Therefore, S-1 cells from passage 118 were selected for growth in the absence of EGF. After a brief latent period, the EGF-free HMT-3522 cultures resumed growth, and gave rise to a series of cells referred to as HMT-3522-S-2 (or gt-1) (see Fig. 3). Initially, the behavior of the S-2 subline did not diverge significantly from that of the parental S-1 cell lines. However, after approximately 120 passages in EGF-free medium, these S-2 cells gave rise to tumors in nude mice. Characterization of the cells cultured from these mouse tumors, called HMT-3522-T4-2 (or mt-1) cells, revealed that the onset of tumorigenicity coincided with the acquisition of an extra copy of the short arm of chromosome 7 (trisomy 7p), a cytogenetic defect that has been associated with many solid tumors (66–68).
Although all cell culture systems are prone to artifactural discrepancies, the HMT-3522 cell series was generated in the absence of any known exogenous transforming agents and therefore represents a useful model in which to study the cellular and molecular aspects of breast cancer progression. The interpretation of data derived from the HMT-3522 cell series is further simplified by the fact that this cell series was propagated following uniform and controlled procedures in a single laboratory. Furthermore, as with breast cell malignancies in vivo, malignant behavior in the HMT-3522 progression series was manifest after an extensive period of time in culture; this slower progression is likely to be physiologically more relevant than xenobiotic-induced transformations, which give rise to full-blown tumors in a fraction of the time (51, 69). For these reasons, the HMT-3522 progression series appeared to be a particularly useful and tractable model system for use in evaluating the role of the tissue microenvironment in the multistep process of breast tumor development.
Studies of Integrin Function Reveal a Role for Tissue Structure in Breast Cancer Progression
One feature common to solid breast tumors in vivo is a notable disorganization of cellular architecture (32, 40, 70). Instead of displaying the polarized morphology that is typical of normal luminal epithelial cells, cancerous cells are apolar, they exhibit disorganized cell-cell junctions and distorted cell nuclei, and they fail to organize into ductal structures. It is widely appreciated that the integrity of epithelial cell shape and tissue architecture depends upon the molecular systems that mediate cell-cell and cell-substratum interactions (71, 72). Not surprisingly therefore, qualitative and quantitative changes in a number of adhesion molecules have been implicated in cancer progression [reviewed in (40)]. Integrin-dependent cell-substratum interactions in particular have been the focus of a number of recent investigations of human breast cancer progression.
Integrins comprise a family of over 20 heterodimeric transmembrane proteins, each of which contains both an α- and a β-subunit (73–75). Integrin heterodimers are displayed on the basolateral surfaces of epithelial cells where they function as receptors for glycoproteins of the insoluble extracellular matrix, such as laminin and collagen (6, 8, 72, 73). While engaged by extracellular ligands, integrin receptors associate intracellularly with components of the filamentous cytoskeleton, thereby establishing cytomechanical transmembrane linkages that influence overall cell shape. Integrin-matrix interactions also trigger intracellular signaling cascades that are similar to those stimulated in response to growth factors (73, 74, 76, 77). Because of their dual significance in the maintenance of overall cell homeostasis, integrin receptors have become prime targets for study of the progression of cancer in human breast.
Normal human breast epithelial cells display characteristic integrin profiles. In human breast, at least two β-subunits, β1 and β4, are coexpressed along with 4 different α-subunits, α1, α2, α3 and α6 (6, 40, 70). These subunits pair to give rise to 4 α/β heterodimers, α1β1,α2β1,α3β1 and α6β4 all of which bind to the extracellular matrix component, laminin; α1β1 and α2β1 also bind collagens (78). While β1-containing integrin heterodimers associate intracellularly with the actin cytoskeleton, α6β4 associates with hemidesmosome structures that link the plasma membrane with the intracellular intermediate filament network along the basolateral surface of the cell (6, 79). α6β4 integrin expression is characteristically restricted to myoepithelial cells in vivo (80, 81), however purified primary cultures of nonmalignant luminal epithelial cells and immortalized nonmalignant myoepithelial cell lines express significant levels of α6β4 integrins, which may facilitate their survival and their ability to express and organize an endogenous basement membrane, both of which contribute to the formation of 3-D structures in culture (82). Although studies of integrin profiles of breast cancer cells in vivo and in culture have been somewhat contradictory, it has been observed consistently that malignancy is accompanied by qualitative and/or quantitative alterations in integrin expression (40, 70, 83–85). For example, α2β1 integrin has been shown to be reduced in many breast tumors that retain a partial differentiated function while poorly differentiated tumors have been found to exhibit dramatically reduced α2β1 integrin expression (85–87).
In order to characterize further the causal relationships between alterations in integrin expression and localization and breast cancer progression, an analysis of integrin function was performed using the HMT-3522 cancer progression model described earlier in conjunction with the 3-D basement membrane assay (82). As would be expected for a normal breast epithelial cell, HMT-3522-S-1 cells grown in 3-D EHS gels were able to form organized spherical structures resembling acini in vivo (Fig. 4A and 4D) whereas tumorigenic HMT-3522-T4-2 cells continued growing into large disordered aggregates and failed to undergo morphogenesis or polarize (Fig. 4B and 4E). Although both S-1 and T4-2 cells displayed comparable qualitative integrin profiles, the integrins in these two cell types vary significantly with respect to expression levels and cellular distribution. Overall β4 and β1 integrin expression was higher in T4-2 cells than in S-1 cells; tumorigenic T4-2 cells also exhibited an inflated surface ratio of β1 to β4 integrins. Integrin distribution, which was restricted to the basal or basolateral surfaces of nonmalignant S-1 epithelial cells, is random and disorganized in the T4-2 tumor cells.
Fig. 4.
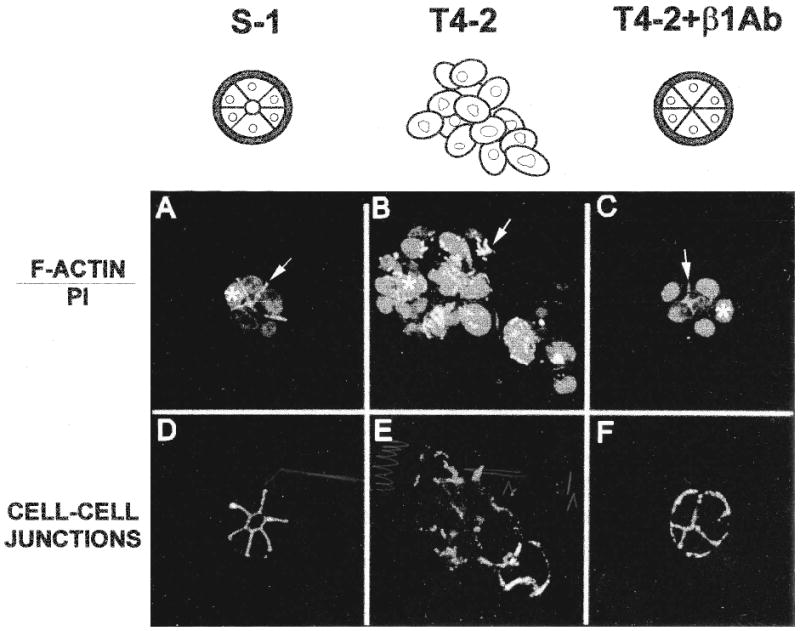
Inhibition of β1 integrin function reverts the tumorigenic phenotype of HMT-3522-T4-2 cells. Non-malignant S-1 (A and D) and tumorigenic T4-2 cells (B, C, E and F) were grown in 3-D EHS in the absence (A, B, D, and E) or in the presence (C and F) of the β1 integrin inactivating antibody, AIIB2. Immunofluorescence analysis of the resulting cultures demonstrates that inhibition of β1 integrin function in T4-2 cell populations causes reversion to a “normal” phenotype: (A-C) Cortical F-Actin organization (see bright staining indicated by arrows) and nuclear morphology (see light grey areas denoted by asterisks) are restored to normal. (D-F) Cell-cell junctions (i.e., E-cadherin and catenin localization) are reassembled. In addition to these structural rearrangements, the reverted T4-2 cells became growth arrested, exhibited normalized integrin expression patterns, yielded reduced tumorigenicity in nude mice (not shown). Reproduced in black and white from an original color figure published by Weaver et al. (82).
To address the possibility that the observed increase in β1 integrin levels was responsible for the tumorigenic behavior displayed by the T4-2 cells, experiments were performed in which β1 integrin inhibitory antibodies were used to attenuate β1 integrin function. Remarkably, treatment of T4-2 cells with this antibody, or with its corresponding Fab fragments, resulted in a dramatic reversion of the malignant phenotype in 3-D EHS matrix cultures (Fig. 4C and 4F) (82). Specifically, after 10 days in culture with the antibody, T4-2 cells formed organized acini and growth was arrested. Within each acinus, cells assumed a “normal” polarized morphology: they deposited an organized endogenous basement membrane, formed normal cell-matrix and cell-cell junctions, reorganized their intracellular cytoskeletal networks and displayed reduced tumorigenicity in nude mice. Moreover, overall integrin localization was restored.
The importance of integrins in breast tumor progression was additionally reinforced by the fact that inhibition of α6β4 integrin function had dramatic effects on the morphogenic behavior of nonmalignant S-1 cells in 3-D EHS matrix cultures (82). When cultured with anti-β4 or anti-α6 integrin function-perturbing antibodies, S-1 cells failed to undergo morphogenesis and form organized acinar structures, but instead assembled into large continuously growing disorganized aggregates, similar to those observed with the T4-2 tumor colonies. A role for the α6β4 integrin in mammary epithelial cell morphogenesis has also been reported by Stahl and colleagues (88). Thus the α6β4 integrin pathway can influence both morphogenesis and growth regulation in mammary epithelial cells.
Minimally, these results demonstrate that the misregulation of cellular integrins may play a causal role in tumorigenesis in the human breast. However by demonstrating that, given the appropriate structural cues, T4-2 cells, which harbor a number of prominent chromosomal abnormalities, display normal cellular behavior while nonmalignant S-1 cells can be forced to behave like tumor cells, these results support the much broader interpretation that tissue structure can influence cellular behavior and thus the tissue phenotype may be construed as the ultimate determinant of genotypic expression. Interestingly, Deng and colleagues recently demonstrated that, although breast cells found adjacent to neoplastic breast lesions in situ displayed normal cellular morphologies, these cells often harbored chromosomal rearrangements (i.e., loss of heterozygosity; LOH), some of which were also found in the adjacent tumor (89). Thus, even in vivo, these mutations were silent when constrained by a normal tissue phenotype. The observations made with the HMT-3522 cell series may help to explain the results obtained in a number of previously described studies. For example, several decades ago, Mintz and Ilmensee showed that chimeric mouse embryos, comprised of both normal and teratocarcinoma cells, gave rise to animals that retained the tumor cells but exhibited no detectable developmental abnormalities or neoplastic tumors (90, 91). A similar phenomenon was observed when oncogenic v-src was used to transform chicken cells in ovo (92, 93). Although v-src did not give rise to tumor-like growth in early chick embryos, these same infected cells, when explanted and grown in culture, underwent dedifferentiation and became transformed. Collectively, these findings reinforce the concept that the tumorigenic phenotype can be suppressed when cells are placed in a milieu where they can assume a normal tissue architecture.
Future Human Breast Culture Models
While use of the 3-D basement membrane assay has proven to be effective in the characterization of a single human breast cell type in culture, these studies have largely ignored the fact that, in vivo, no single cell type is an island. Rather within a tissue, many different cell types contribute to the overall character of the microenvironment. Therefore, the challenge facing us now is to take the design of microenvironment one step further and incorporate multiple cell types into a single model system. This type of approach will be essential, not only for understanding the dynamic interplay that normally exists between different cells of the breast, but also for understanding aberrant behaviors associated with invasive breast cancer. Towards this end, it has already been demonstrated that tumor cells grown alone in type I collagen matrices form noninvasive spheres, but that co-culture of the same tumor cells with a complex stromal cell population results in cultured lesions that look like invasive tumors in situ (9, 69). Moreover, this co-culture strategy has allowed us to understand that the appearance of myofibroblasts in the stroma of the breast, a general wound-response observed early and during the stromal reaction to neoplasia, is due in part to the conversion of resident fibroblasts (69). Based on these results, it is reasonable to predict that similar co-culture manipulations performed with normal cell types could be used to model the multicellular composition found within a normal breast. The use of such multicellular culture model systems will enable us to perform experiments to determine how the structural cues are relayed to luminal epithelial cells in the absence of EHS matrix. How is alveolar function regulated in vivo? What is the role of myoepithelial cells and fibroblasts in preserving normal tissue structure? How and when is this structure lost during the progression to malignancy? Ultimately, the systematic development of multicellular breast cultures could provide us with tractable model systems for use in the search for effective breast cancer therapies.
Conclusions
In order to understand the molecular basis of normal breast function and breast cancer progression, it is important to generate meaningful in vitro model systems that allow us to take a reductionist view of each component tissue type. Given recent advances in breast cell models as well as the development of tissue culture conditions that effectively recapitulate differentiated cell behaviors, we are now poised to unravel the complexities of breast tissue function. Already these improvements have facilitated the development of phenotypic assays for use in the characterization of normal and overtly malignant breast cell phenotypes as well as phenotypic intermediates that may be reflective of discrete stages in breast cancer progression. Functional assays in these human breast cell systems have revealed the importance of integrin-dependent signaling and tissue structure in suppressing a tumorigenic phenotype. In light of these findings, it remains to be determined whether other anomalous cellular behaviors, such as senescence, may also be directly attributable to alterations in structural cues from the microenvironment. The fact that stromal components of many tissues, including the breast, experience time-dependent changes makes this an attractive hypothesis for future consideration.
Acknowledgments
This work was supported by the U.S. Department of Energy, Office of Biological and Environmental Research (contract DE-AC03-76SF00098 to MJB) and by the National Institutes of Health (grants CA64786 and CA57621 to MJB). Additional support was received from the U.S. Department of Energy, Office of Biological and Environmental Research (an Alexander Hollaender Distinguished Postdoctoral Fellowship to KLS; administered by the Oak Ridge Institute for Science and Education) and by the Breast Cancer Fund of the State of California (BCRP University of California-IFB-0400 to VMW).
Footnotes
Abbreviations: two-dimensional (2-D); three-dimensional (3-D); Englebreth Holm Swarm matrix (Matrigel) (EHS matrix); epidermal growth factor (EGF).
References
- 1.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression. J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 2.Stoker AW, Streuli CH, Martins-Green M, Bissell MJ. Designer microenvironments for the analysis of cell and tissue function. Curr Opin Cell Biol. 1990;2:864–874. doi: 10.1016/0955-0674(90)90085-s. [DOI] [PubMed] [Google Scholar]
- 3.Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau N, Meyers C, Bissell MJ. From laminin to lamin: regulation of tissue-specific gene expression by the ECM. Trends Cell Biol. 1995;5:1–4. doi: 10.1016/s0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- 5.Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signaling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–7474. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenas J, Muschler J, Bissell MJ. The extracellular matrix in epithelial biology: Shared molecules and common themes in distant phyla. Devel Biol. 1996;180:433–444. doi: 10.1006/dbio.1996.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gumbiner B. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 9.Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Michaelopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Exp Cell Res. 1975;94:70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- 11.Emmerman JT, Pitelka DR. Maintenance and identification of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman HK, Luckenbill-Edds L, Cannon FW, Sephel GC. Use of extracellular matrix components for cell culture. Anal Biochem. 1987;166:1–13. doi: 10.1016/0003-2697(87)90538-0. [DOI] [PubMed] [Google Scholar]
- 13.Kopan R, Traska G, Fuchs E. Retinoids as important regulators of terminal differentiation: Examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987;105:427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopan R, Fuchs E. The use of retinoic acid to probe the relation between hyperproliferation-associated keratins and cell proliferation in normal and malignant epidermal cells. J Cell Biol. 1989;109:295–307. doi: 10.1083/jcb.109.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watt FM. Terminal differentiation of epidermal keratinocytes. Curr Opin Cell Biol. 1989;1:1107–1115. doi: 10.1016/s0955-0674(89)80058-4. [DOI] [PubMed] [Google Scholar]
- 16.Kleinman HK, Ogle RC, Cannon FB, Little CD, Sweeny TM, Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci USA. 1988;85:1282–1286. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH, Fawcett JW. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci. 1995;108:1307–1315. doi: 10.1242/jcs.108.3.1307. [DOI] [PubMed] [Google Scholar]
- 18.DiPersio CM, Jackson DA, Zaret KS. The extracellular matrix coordinately modulates liver transcription factors and hepatocyte morphology. Mol Cell Biol. 1991;11:4405–4414. doi: 10.1128/mcb.11.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Ze'ev A, Robinson GS, Bucher NLR, Farmer SR. Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci USA. 1988;85:2161–2165. doi: 10.1073/pnas.85.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. J Clin Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissell DM, Caron JM, Babiss LE, Friedman JM. Transcriptional regulation of the albumin gene in cultured rat hepatocytes. Mol Biol Med. 1990;7:187–197. [PubMed] [Google Scholar]
- 22.Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis. Cell. 1989;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- 23.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant DS, Tashiro KI, Seui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- 25.Grant DS, Kinsella JL, Kibbey MC, LaFlamme S, Burbelo PD, Goldstein AL, Kleinman HK. Matrigel induces thymosin β4 gene in differentiating endothelial cells. J Cell Sci. 1995;108:3685–3694. doi: 10.1242/jcs.108.12.3685. [DOI] [PubMed] [Google Scholar]
- 26.Gentili C, Bianco P, Neri M, Malpeli M, Campanile G, Castagnola P, Cancedda R, Cancedda FD. Cell proliferation, extracellular matrix mineralization, and ovotransferrin transient expression during in vitro differentiation of chick hypertrophic chondrocytes into osteoblast-like cells. J Cell Biol. 1993;122:703–712. doi: 10.1083/jcb.122.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990;63:437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- 28.Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci. 1995;108:2791–2800. doi: 10.1242/jcs.108.8.2791. [DOI] [PubMed] [Google Scholar]
- 29.Neville MC, Daniel CW, editors. The Mammary Gland. Plenum Press; New York: 1987. [Google Scholar]
- 30.Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel JJ, Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993;85:892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 31.Wolff MS, Collman GW, Barrett JC, Huff J. Breast cancer and environmental risk factors: Epidemiological and experimental findings. Ann Rev Pharmacol Toxicol. 1996;36:573–596. doi: 10.1146/annurev.pa.36.040196.003041. [DOI] [PubMed] [Google Scholar]
- 32.Weaver VM, Fischer AH, Petersen OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo. J Cell Sci. 1991;99:407–417. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- 34.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-β1 gene. J Cell Biol. 1993;120:253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lelièvre S, Weaver VM, Bissell MJ. Extracellular matrix signaling from the cellular membrane skeleton to the nuclear skeleton: A model of gene regulation. Recent Progress Hormone Res. 1996;51:417–432. [PMC free article] [PubMed] [Google Scholar]
- 37.Bissell MJ. The differentiated state of normal and malignant cells or how to define a normal cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- 38.Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor-Papadimitriou J, Lane EB. Keratin expression in the mammary gland. In: Neville MC, Daniel CW, editors. The Mammary Gland. Plenum Publishing; New York: 1987. pp. 181–215. [Google Scholar]
- 40.Alford D, Taylor-Papadimitriou J. Cell adhesion, molecules in the normal and cancerous mammary gland. J Mam Gland Biol Neoplasia. 1996;1:207–218. doi: 10.1007/BF02013644. [DOI] [PubMed] [Google Scholar]
- 41.Easty GC, Easty DM, Monaghan P, Ormerod MG, Neville AM. Preparation and identification of human breast epithelial cells in culture. Int J Cancer. 1980;26:577–584. doi: 10.1002/ijc.2910260509. [DOI] [PubMed] [Google Scholar]
- 42.Janss DH, Hillman EA, Malan-Shibley LB, Ben TL. Methods for the isolation and culture of normal human breast epithelial cells. In: Harris CC, Trump BR, Stoner GD, editors. Methods in Cell Biology. Academic; New York: 1980. pp. 107–134. [DOI] [PubMed] [Google Scholar]
- 43.Stampfer M, Hallowes RC, Hackett AJ. Growth of normal human mammary cells in culture. In Vitro. 1980;16:415–425. doi: 10.1007/BF02618365. [DOI] [PubMed] [Google Scholar]
- 44.Stampfer MR, Yaswen P. Culture systems for study of human mammary epithelial cell proliferation, differentiation and transformation. Cancer Surveys. 1993;18:7–34. [PubMed] [Google Scholar]
- 45.Briand P, Petersen OW, VanDeurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Devel Biol. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 46.Soule HD, Maloney TM, Wolman SR, Peterson WD, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF- 10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 47.Shay JW, Tomlinson G, Piatyszek MA, Gollahon LS. Spontaneous in vitro immortalization of breast epithelial cells from a patient with Li-Fraumeni syndrome. Mol Cell Biol. 1995;15:425–432. doi: 10.1128/mcb.15.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Fromontel CC, Nardeux PC, Soussi T, Lavialle C, Estrade S, Carloni G, Chandrasekaran K, Cassingena R. Epithelial HBL-100 cell line derived from milk of an apparently healthy woman harbors SV40 genetic information. Exp Cell Res. 1985;160:83–94. doi: 10.1016/0014-4827(85)90238-1. [DOI] [PubMed] [Google Scholar]
- 49.Gaffney EVA. A cell line (HBL-100) established from human breast milk. Cell Tissue Res. 1982;227:563–568. doi: 10.1007/BF00204786. [DOI] [PubMed] [Google Scholar]
- 50.Band V, Zajchowski D, Kulesa V, Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci USA. 1990;87:463–467. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris CC. Human tissues and cells in carcinogenesis research. Cancer Res. 1987;47:1–10. [PubMed] [Google Scholar]
- 52.Stampfer MR, Bartley JC. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci USA. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wazer DE, Chu Q, Liu XL, Gao Q, Safaii H, Band V. Loss of p53 protein during radiation transformation of primary human mammary epithelial cells. Mol Cell Biol. 1994;14:2468–2478. doi: 10.1128/mcb.14.4.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leone A, Flatow U, VanHoutte K. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization, and enzymatic activity. Oncogene. 1993;8:2325–2333. [PubMed] [Google Scholar]
- 55.Howlett AR, Petersen OW, Steeg PS, Bissell MJ. A novel function for the nm23-H1 gene: overexpression in human breast carcinoma cells leads to the formation of basement membrane and growth arrest. J Natl Cancer Inst. 1994;86:1838–1844. doi: 10.1093/jnci/86.24.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Band V, Zajchowski D, Swisshelm K, Trask D, Kulesa V, Cohen C, Connolly J, Sager R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990;50:7351–7357. [PubMed] [Google Scholar]
- 57.Liu XL, Band H, Gao Q, Wazer DE, Chu Q, Band V. Tumor cell-specific loss of p53 protein in a unique in vitro model of human breast tumor progression. Carcinogenesis. 1994;15:1969–1973. doi: 10.1093/carcin/15.9.1969. [DOI] [PubMed] [Google Scholar]
- 58.Liu SL, Wazer DE, Watanabe K, Band V. Identification of a novel serine protease-like gene, the expression of which is down-regulated during breast cancer progression. Cancer Res. 1996;56:3371–3379. [PubMed] [Google Scholar]
- 59.Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Sem Cancer Biol. 1995;6:175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- 60.Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res. 1996;56:2039–2044. [PubMed] [Google Scholar]
- 61.Vang Nielsen K, Madsen MW, Briand P. In vitro karyotype evolution and cytogenetic instability in the nontumorigenic human breast epithelial cell line HMT-3522. Cancer Genet Cytogenet. 1994;78:189–199. doi: 10.1016/0165-4608(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 62.Moyret C, Madsen MW, Cooke J, Briand P, Theillet C. Gradual selection of a cellular clone presenting a mutation at Codon 179 of the p53 gene during establishment of the immortalized human breast epithelial cell line HMT-3522. Exp Cell Res. 1994;215:380–385. doi: 10.1006/excr.1994.1355. [DOI] [PubMed] [Google Scholar]
- 63.Madsen MW, Lykkesfeldt AE, Laursen I, Nielsen KV, Briand P. Altered gene expression of c-myc, epidermal growth factor receptor, transforming growth factor-α and c-erb-B2 in an immortalized breast epithelial cell line, HMT-3522 is associated with decreased growth factor requirements. Cancer Res. 1992;52:1210–1217. [PubMed] [Google Scholar]
- 64.Dairkee SH, Deng G, Stampfer MR, Waldman FM, Smith HS. Selective cell culture of primary breast carcinoma. Cancer Res. 1995;55:2516–2519. [PubMed] [Google Scholar]
- 65.Sporn MB, Roberts AB. Autocrine growth factors and cancer. Nature. 1985;313:747–751. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- 66.Johansson B, Heim S, Mandahl N, Mertens F, Mitelman F. Trisomy 7 in nonneoplastic cells. Genes, Chromosomes Cancer. 1993;6:199–205. doi: 10.1002/gcc.2870060402. [DOI] [PubMed] [Google Scholar]
- 67.Thompson F, Emerson J, Dalton W, Yang JM, McGee D, Villar H, Knox S, Massay K, Weinstein R, Bhattacharyya A, Trent J. Clonal chromosome abnormalities in human breast carcinomas. I. Twenty-eight cases with primary disease. Genes Chromosomes Cancer. 1993;7:185–193. doi: 10.1002/gcc.2870070402. [DOI] [PubMed] [Google Scholar]
- 68.Trent J, Yang JM, Emerson J, Dalton W, McGee D, Massay K, Thompson F, Villar H. Clonal chromosome abnormalities in human breast carcinomas. II. Thirty-four cases with metastatic disease. Genes Chromosomes Cancer. 1993;7:194–203. doi: 10.1002/gcc.2870070403. [DOI] [PubMed] [Google Scholar]
- 69.Rønnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. J Clin Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- 71.Eaton S, Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell. 1995;82:5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 72.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 73.Hynes RO. Integrins: Versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 74.Clark EA, Brugge JS. Integrins and signal transduction pathways: The road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 75.Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. doi: 10.1016/s0955-0674(97)80158-5. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: Emerging paradigms of signal transduction. Ann Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 77.Juliano RL, Varner JA. Adhesion molecules in cancer: The role of integrins. Curr Opin Cell Biol. 1993;5:812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 78.Zutter MM, Santoro SA. Widespread histologic distribution of the α2β1 integrin cell surface collagen receptor. Am J Pathol. 1990;137:113–120. [PMC free article] [PubMed] [Google Scholar]
- 79.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Heimer LMH, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, Garrod D. Integrin α6β4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones JCR, Kurpackus MA, Cooper HM. A function for the integrin α6β4 in the hemidesmosome. Cell Reg. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones JL, Royall JE, Critchley DR, Walker RA. Modulation of myoepithelial-associated α6β4 integrin in a breast cancer cell line alters invasive potential. Exp Cell Res. 1997;235:25–333. doi: 10.1006/excr.1997.3662. [DOI] [PubMed] [Google Scholar]
- 82.Weaver VM, Petersen WO, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones JL, Critchley DR, Walker RA. Alteration of stromal protein and integrin expression in breast—a marker of premalignant change. J Pathol. 1992;167:399–406. doi: 10.1002/path.1711670409. [DOI] [PubMed] [Google Scholar]
- 84.Koukoulis GK, Virtanen I, Korhonen M, Litinen L, Quaranta V, Gould VE. Immunohistochemical localization of integrins in the normal, hyperplastic and neoplastic breast: Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991;139:787–799. [PMC free article] [PubMed] [Google Scholar]
- 85.Zutter M, Mazoujian GM, Santoro SA. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol. 1990;137:863–870. [PMC free article] [PubMed] [Google Scholar]
- 86.Zutter MM, Krigman HR, Santoro SA. Altered integrin expression in adenocarcinoma of the breast: Analysis by in situ hybridization. Am J Pathol. 1993;142:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- 87.Berdichevsky F, Wetzels R, Shearer M, Martignone FC, Ramaekers S, Taylor-Papadimitriou J. Integrin expression in relation to cell phenotype and malignant change in the human breast. Mol Cell Differ. 1994;2:255–274. [Google Scholar]
- 88.Stahl S, Weitzman S, Jones JC. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110:55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- 89.Deng G, Lu Y, Zlotnikov G, Thor A, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- 90.Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mintz B, Fleischman RA. Teratocarcinomas and other neoplasms as developmental defects in gene expression. Adv Cancer Res. 1981;34:211–278. doi: 10.1016/s0065-230x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- 92.Stoker AW, Hatier C, Bissell MJ. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J Cell Biol. 1990;111:217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boudreau N, Reddy ST, Stoker AW, Fairman C, Bissell MJ. The embryonic environment and the extracellular matrix suppress oncogenic transformation by Rous sarcoma virus in the chick embryo. Mol Cell Differ. 1995;3:261–274. [Google Scholar]


