INTRODUCTION
The central problem in cancer biology is to understand why tumor cells continue to survive and proliferate in situations where normal cells would arrest growth or apoptose. Although previous approaches have characterized cancer cells as autonomous entities and focused on the genetic mutations that contribute to tumor function, it is becoming increasingly clear that such models ignore the many ways in which cells interact with, and are regulated by, their surroundings. A different approach is to view cancer as a breakdown of the structural principles by which cells are organized within a tissue. In this review, we will provide some theoretical background for this approach, and describe some of our investigations in which we have found that either alterations in the microenvironment or altered perception of the microenvironment can cause normal cells to adopt tumorigenic behaviors.
We will present an overview of the critical components of the cellular microenvironment, focusing on the large macromolecules that comprise the extracellular matrix (ECM). Then, we will summarize the large variety of cell surface adhesion molecules that sense and respond to the ECM and to neighboring cells. Finally, we will describe some of our recent investigations, in which we have used transgenic mice and a three dimensional (3D) cell culture assay to model the cell–ECM and cell–cell interactions that function to create mammary epithelial tissue. These experiments have helped define some of the molecular mechanisms by which mammary cells sense and respond to their microenvironment, how perturbations of those mechanisms can lead to breast tumors, and how reversal of those perturbations may be an approach to reverting early stage cancers.
TISSUE ORGANIZATION: COMMUNICATING WITH THE MICROENVIRONMENT
The structure and function of normal tissues is determined by reciprocal dialogue that is mediated, in part, through interactions with the ECM (1). Extracellular matrix is a complex network of macromolecules that provides both architectural support to cells and contextual information to determine the correct response to a given set of stimuli (2). Cells respond to the milieu of soluble growth factors and cytokines in the context of their particular ECM, determining to proliferate, differentiate, arrest growth, or apoptose (3,4). In addition, ECM can act as a reservoir of other factors that are released from the network after proper activation. The composition of ECM varies considerably both within and between tissues (5,6), and changes temporally, as tissues adapt to changing conditions (7,8). Normal adherent cells must contact the ECM to avoid inappropriate growth or apoptosis, a phenomenon known as anchorage dependence (9). Malignant cells are thought to be anchorage independent, and although they continue to respond and contribute to the ECM, they develop into unstructured tumors by subverting the normal processes of tissue organization (10).
As more than 80% of human cancers are derived from epithelial cells, this cell type has been particular focus of cancer investigations. The basement membrane (BM) is the specialized ECM that separates epithelial cells from the surrounding stroma. Normal BM is an organized network composed of laminin, collagen type IV, nidogen/entactins, and proteoglycans such as perlecan (11) (Fig. 1). Other BMs may contain these components and/or other types of collagens, fibronectin, or tenascins. Much of the information that controls development and differentiation of epithelial cells is mediated by changes in the BM (12). In turn, selective degradation of the BM is both a component of tissue remodeling and a characteristic of tumor development. Below, we briefly discuss some of the key components of ECM and the cell surface receptors that interact with it.
Figure 1.
Architecture of the basement membrane and cell surface connections. Basement membrane is composed of interconnected networks of collagen IV, laminin, nidogen/entactin, and proteoglycans such as perlecan. These connect to the cell through cell surface receptors such as integrins and dystroglycan. These, in turn, connect to the actin cytoskeleton through cytoplasmic adapter protein complexes.
COMPONENTS OF THE EXTRACELLULAR MATRIX: STRUCTURE AND FUNCTION
Laminins
Laminins, a family of at least 11 glycoproteins, form an integral part of the structural scaffolding of BMs (13). In this role, laminins are essential: transgenic mice that cannot express functional laminins have an embryonic lethal phenotype, do not assemble BMs, and have disorganized extracellular deposits of collagen IV and perlecan (14,15). Laminins have a cruciform structure composed of a heterotrimer assembled from α, β, and γ chain subunits (Fig. 2) and are simultaneously secreted and incorporated into cell-associated matrices (16). The localized distribution of various laminin isoforms conveys information to direct tissue organization (17), and cell polarization (18) through subdomains of the laminin protein complex. For example, the E3 globular domain of laminin-1 is an essential component for functional differentiation of mouse mammary epithelial cells (19,20).
Figure 2.

Components of the basement membrane. Presented in diagrammatic form to relative scale (scale bar = 50 nm).
Laminins are capable of self-polymerization (21), and the assembly of laminin networks is coordinated with the structural organization of the other components of the BM and the underlying cytoskeleton (22). Adherence to laminin matrices by epithelial cells is essential for maintaining normal tissue organization: disruption of selected laminin isoforms produces severe neuromuscular (23), developmental (24), and blistering (25) disorders, and tumors derived from epithelial cells have aberrant production of laminin-binding integrins (26).
Collagens
Collagens are the most abundant structural components of the ECM (11). The collagen structural motif is a trimer of α chains, folded into a coiled-coil triple helix (Fig. 2). To date, collagens have been classified into 20 different types, based on the structural motifs present in the α chain subunit, although considerable diversity exists even within collagen types. Type IV collagen, which is the principal component of basement membranes, can be composed of the six known α chains, giving 56 possible combinations that vary between tissues (27). Defects in one chain can prevent incorporation of others in a tissue specific manner (28), as in Alport syndrome, a hereditary disease characterized by lethal nephritis, deafness, and ocular dysfunction (29).
Nidogen/Entactin
Nidogens are another essential component of BMs, and two nidogen isoforms with overlapping functions have been identified (30). Nidogen acts to stabilize the connection between the collagen IV network and the laminin network. In this capacity, it is essential both for embryonic development (31) and for functional differentiation of mature tissues (32).
Proteoglycans
Proteoglycans are a distinct and highly diverse subset of glycoproteins containing glycosaminoglycan side chains (11) that play a critical role in strengthening and maintaining BMs and in wound repair (33). In epithelial BM, perlecan is the most abundant proteoglycan. Perlecan deficient mice initially assemble basement membranes normally, although regions susceptible to mechanical stress show progressive and dramatic deterioration leading to embryonic lethality (34).
Other proteoglycans have been shown to regulate growth factor responsiveness (33,35), and misregulation of GPI-linked proteoglycans has been observed in several cancers. Glypicans are GPI-linked heparan sulfate proteoglycans that contribute to enhanced growth factor action in pancreatic cancer (36). EXT1 and EXT2 are heparan sulfate transferases required for synthesis of glypicans which when defective, predispose individuals to bone tumors and other skeletal dysplasias (37,38).
Fibronectins and Tenascins
Fibronectin, normally a component of stromal ECM, contacts epithelial cells when the BM is broken down during remodeling, involution, or malignancy (39,40). Tenascins are antiadhesive components of the ECM expressed during embryogenesis, wound healing, and involution (7), as well as in pathological states including tumorigenesis and metastasis. Fibronectin and tenascin cause cell spreading, a necessary component of cell cycle progression (41), so increased expression of these molecules by tumors and tumor stroma may be a mechanism for increased proliferation.
EXTRACELLULAR MATRIX RECEPTORS
Integrins
General Properties
Integrins are cell surface adhesion molecules named for their ability to integrate the information present in the composition of the ECM and to mediate tissue-specific gene expression (42). Integrins convey signals across the plasma membrane in both directions: association of integrins with ECM ligands can transmit a conformational change on the cytoplasmic face (known as outside–in signaling), while interactions within the cytoplasmic domains can modulate the substrate binding affinity of the extracellular domains (inside–out signaling).
To date, integrins have been found as heterodimeric combinations of 17 α subunits and eight β subunits that interact noncovalently in a restricted manner to create more than 20 heterodimeric family members expressed in a cell- and tissue-specific manner (43). The specific α/β pairing determines the ligand-binding specificity of the integrin. Both α and β subunits consist of large extracellular ligand-binding domains, transmembrane sequences, and relatively shorter cytoplasmic tails. Extensive structural information is available for integrin subdomains, although inherent flexibility has so far prevented a complete structural analysis (44).
Some integrins display specificity for short sequences; the most studied of these is the tripeptide arginine–glycine–aspartate (RGD), which is present in a variety of ECM proteins (45). Other integrins recognize conformational structures composed of different amino acids (45). A particular cell may express multiple types of integrins with overlapping specificity, i.e., a particular integrin may bind to multiple ligands, as whenαvβ3 binds to laminin, collagen, fibronectin, and tenascin C (46) or, a component of the ECM may associate with multiple integrins, as when laminin associates with α1β1, α2β1, α3β1, α6β1, α7β1 and α6β4 (43,47). The significance of this functional redundancy is unknown, although one can presume that this has evolved to allow rapid responsiveness to changes in the microenvironment.
Integrin Activation
Association of integrins with ECM ligands initiates the assembly of adapter proteins to form focal adhesion complexes that link to the cytoskeleton (48). In cultured adherent cells grown in the presence of serum factors, integrins first associate with talin and α-actinin, then with tensin, vinculin, and paxillin to recruit actin filaments (49) (Fig. 1). The reorganization of actin by focal adhesion complexes, in turn, causes integrin clustering and strengthens cell–ECM binding in a positive feedback mechanism. Clustered aggregates of focal adhesion complexes can be visualized by electron microscopy as focal adhesions, electron-dense structures at the cell–ECM boundary (50). This process physically links the cytoskeleton to the ECM (22), so that perturbations in the ECM are rapidly transduced to the interior of the cell and vice versa. Cytosolic enzymes that modulate cytoskeletal structure also affect the organization of the ECM. In this way, cells that contact each other are organized into a functional tissue that is able to respond to environmental changes as a unit.
The organization of the actin network has been shown to be controlled within the cell by the Rho family of small GTPases, consisting of Rho, Rac and Cdc42, each of which have been shown to control different aspects of cytoskeletal structure in fibroblasts (39). Rho controls the generation of stress fibers, thick bundles of actin filaments that connect to focal adhesions. Rac controls the formation of lamellipodia, thin actin sheets at the edges of cells that can lift up and fold backwards. Cdc42 controls the formation of filopodia, thin actin bundles at the cell surface that produce narrow protrusions (51). The complexity of the roles played by the Rho GTPases in epithelial cell shape is an active topic of investigation (52,53).
The binding activity of integrins to the ECM is also controlled by intracellular signals that modulate ligand associative properties in a process known as affinity maturation (54). Current models suggest that integrins exist on the cell surface in a low-affinity conformation, and require interaction with specific cytoplasmic components to become activated (55). Affinity maturation is generally mediated by heterotrimeric G-protein-coupled receptors of the Ras family (56) or through cytosolic modulation of cytoskeletal structure by the Rho family of GTPases (57,58).
Integrin Signaling
Ligation of integrins to ECM directly activates signal transduction pathways through kinases present in focal adhesion complexes. These include tyrosine kinases such as focal adhesion kinase (FAK), integrin-linked kinase (ILK), and members of the Src kinase family, as well as serine–threonine kinases of the Abl family. Among these, FAK has been a particular target of investigation. In normal cells, FAK is activated by cell adhesion and rapidly deactivated on cell detachment, and FAK signals through numerous pathways to mediate anchorage-dependent survival and proliferation (59). Induction of constitutively active FAK in normal cells leads to transformation and anchorage-independent growth, while overexpression of FAK in transformed cells restores anchorage dependency (60).
Focal adhesion kinase associates with Src (61) or c-Jun NH2 terminal kinase (JNK) (62,63) to activate the mitogen-activated protein kinase (MAPK) pathway. Anchorage dependence is mediated through both MAPK signaling and cytoskeletal reorganization, processes that are functionally separable, at least in cultured cells grown on plastic substrata: artificial clustering of integrins without occupation of the integrin ligand-binding site activated MAPK signaling without cytoskeletal reorganization, and simultaneous clustering of integrins with ligand binding-site association resulted in cytoskeletal reorganization even in the presence of MAPK inhibitors (56).
We now know that integrins also modulate the activity of growth factor-dependent signaling. In normal cells, optimal activation of receptors for insulin, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular – endothelial growth factor (VEGF) all require proper ECM context (64). Integrins can potentiate growth factor activation pathways by associating with receptors or components of receptor-signal transduction pathways or by directly or indirectly initiating signal transduction pathways (65,66). Through adhesion-mediated regulation of growth factor signaling, cells respond properly to the milieu of positive and negative signals from growth factors and cytokines.
Non-integrin Extracellular Matrix Receptors
Other cell-surface receptors have been shown to be involved in sensing the ECM. The syndecans, a family of transmembrane heparan sulfate proteoglycans, are expressed on all adherent cells (67) in a highly regulated cell-type and developmental-stage-specific manner (68). Syndecans are involved in the formation of focal adhesion and stress fibers, modulation of integrin function, and in response to mechanical stresses (69,70). A role for syndecans in mammary tumorigenesis was revealed by investigations of syndecan-1-deficient transgenic mice, which are resistant to Wnt-1-induced tumorigenesis (71).
Dystroglycan, originally isolated from skeletal muscle (72), now appears to act in all tissues as another receptor for laminin spanning the membrane and connecting to the cytoskeleton (73,74). In contrast to integrins and syndecans, so far only a single form of dystroglycan has been discovered in mammals. Dystroglycan is generated as a single polypeptide and cleaved into the extracellular α subunit and the transmembrane β subunit (75). α-Dystroglycan binds to laminin-1 (76) and to the proteoglycans, perlecan and agrin (77,78). At the cytoplasmic surface of epithelial cells, α-dystroglycan associates with actin filaments through isoforms of utrophin and dystrophin (75).
Dystroglycan acts both as a receptor of ECM signaling and in the assembly of BMs. Mice lacking dystroglycan do not survive past day 6 gestation, corresponding with defects in the Reichert’s membrane, a specialized BM that separates embryonic from maternal tissue (79). Embryoid bodies lacking dystroglycan assembled nonfunctional basement membranes with disorganized and patchy laminin-1, collagen type IV, and perlecan (80). Reintroduction of dystroglycan by adenovirus infection restored the ability of the embryoid bodies to organize laminin-1.
CELL–CELL ADHESION: SENSING THE NEIGHBORS
The principal molecule responsible for the formation of direct contacts between epithelial cells is E-cadherin (81). This function is essential, as transgenic mice lacking E-cadherin display defective development at the stage of formation of the trophectoderm, the first epithelium (82,83). The extracellular domain of E-cadherin is composed of five subunits with repeating internal sequence homology and calcium binding motifs (84) (Fig. 3). Adhesion is mediated in the presence of calcium ions by E-cadherin clustering and lateral homoassociation in a zipper-like mechanism to produce adherens junctions. Removal of calcium from the extracellular environment reverses this process and releases the cell–cell adherence (85).
Figure 3.
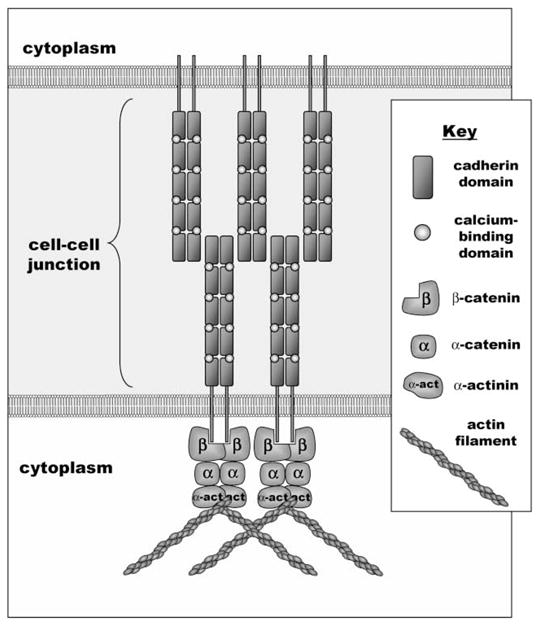
Components of E-cadherin-mediated adherens junctions. E-cadherin is a cell surface molecule composed of five repeating motifs that contain calcium-binding domains. In the presence of extracellular calcium, E-cadherin molecules homodimerize and then associate with E-cadherin dimers on adjacent cells in a zipper-like mechanism. This process is coincident with association with actin filaments through cytoplasmic adapters including the catenins and α-actinin.
Like integrins, cadherins connect to the actin cytoskeleton through a complex of cytosolic adaptor proteins. In general, cadherins may interact with either β-catenin or plakoglobin, which in turn binds α-catenin. Actin filaments can associate directly with α-catenin or indirectly, through adapter proteins such as α-actinin, vinculin, or ZO-1 (81). The Rho GTPase pathway also intersects with cadherins: cell–cell attachment activates Cdc42 (86), and IQGAP1, an effector of Cdc42 and Rac1 binds to β-catenin and disassociates cell–cell contacts (87). Integrin signaling can also modulate cadherin function through the adaptor integrin-linked kinase (ILK), as constitutive activation of ILK results in down-modulation of E-cadherin and an epithelial-to-mesenchymal transition (88).
Cadherins also interact with signal transduction pathways that impact on cell proliferation and differentiation (89). Cell–cell adhesion potentiates paracrine signaling and cadherin-mediated tight junction formation is essential for segregation of membrane receptors between apical and basolateral surfaces (90). Additionally, cadherins may indirectly participate in signaling processes by sequestering β-catenin into adherens junctions. When not sequestered, β-catenin activates gene expression through the lymphoid enhancer factor/T-cell specific factor (LEF/TCF) pathway (91). This pathway is essential for Wnt signaling, a mechanism for regulating epithelial cell proliferation in adult and embryonic tissues that is often dysregulated in colon carcinoma (92).
Numerous studies have correlated the loss of E-cadherin with the development of cancer (93). Decreased E-cadherin function is a component of epithelial-to-mesenchymal conversion, invasive tumor growth, and metastasis, and loss of E-cadherin is a negative prognostic marker (94). Similarly, altered expression of E-cadherin cytosolic adaptor proteins in pathologic conditions can also predispose to malignancies (95).
Until recently, these correlations were only suggestive of an underlying mechanism. Now a causal role for the loss of E-cadherin in the acquisition of invasiveness has been described in a transgenic mouse model (96): forced expression of E-cadherin during pancreatic β-cell tumorigenesis arrested tumor development, while forced expression of a dominant negative E-cadherin gene produced premature invasiveness and metastasis. Loss of E-cadherin is not always associated with increased tumor potential, however, as increased E-cadherin is associated with early stages in primary ovarian carcinomas (97). This exception proves the rule: all signaling and signaling molecules eventually need to be understood in the context of tissue and organs.
COMING TOGETHER: CELLULAR INTERACTIONS THAT DEFINE TISSUE FUNCTION IN THE MAMMARY GLAND
Integration of Signaling is Essential for Normal Function
It is clear that cells are organized in tissues by multiple signaling pathways that are integrated biochemically and physically by cell interaction with the ECM. The challenge is to understand how this comes about. To investigate the mechanisms of tissue organization and the ways in which these mechanisms break down during tumorigenesis, we have developed an assay that approximates the normal cell–ECM and cell–cell interactions in the mammary gland acini of both mice and humans (98). Our culture system uses a reconstituted basement membrane derived from the Engelbreth–Holm–Swarm (EHS) tumor (99), a mixture of laminin-1, collagen IV, and nidogen. When mouse mammary cells are cultured within this BM, nonmalignant cells organize to form physical structures that resemble the alveoli of the lactating mammary gland. As described below, this assay can be used further to distinguish between normal and malignant human breast cells, to characterize the mechanisms that define normal mammary epithelial architecture, and to characterize the defects that cause the aberrations of behavior in tumors.
Differentiation of Non-malignant Mammary Epithelial Cells
Grown on conventional tissue culture plastic substrata, mouse mammary epithelial cells grow in a flattened morphology and express little or no milk proteins, despite the presence of hormones (100). In contrast, cells grown on EHS matrix in the presence of lactogenic hormones form physical structures reminiscent of the lactating mammary gland (101) and organize into ducts and hollow structures that resemble functional alveoli. Milk proteins are apically secreted into central lumen, and the basal surfaces are encased by a distinct basal lamina (102,103). A hierarchical model of functional mammary epithelium was established in which both cell–cell and cell–ECM interactions provide signals for cytoskeletal organization and deposition of BM (104). The latter, in turn, signals for expression of some milk proteins. The morphological changes were found to be a necessary prerequisite for the biochemical activation of other milk protein genes, including β-casein and WAP (105,106).
TUMORIGENESIS AS A BREAKDOWN OF CELL–CELL AND CELL–ECM COMMUNICATION
Nature vs. Nurture: An Origin for Malignant Cells
Many investigations have characterized cancer as the consequence of genetic mutations that inactivate tumor-suppressor genes or activate oncogenes (107,108) and view cancer progression as the accumulation of genetic defects in overlapping pathways, each providing a progressive evolutionary advantages to the malignant progeny (109,110). Such approaches have focused attention on the viewpoint of malignant cells as independent entities and on genetic instability as the principal motivating force driving tumor progression (111,112). Although it is certain that genetic instability is a principal feature of cancer (113), this viewpoint of cancer as a collection of renegade cells ignores the multiple interconnected interactions that define the role of cells within a tissue. Furthermore, many of the characterized tumor-suppressors and proto-oncogenes have been found to act as intermediates of signal transduction pathways that exist to communicate information from the extracellular microenvironment into the cell (114). As these signaling pathways are connected in a web of co-dependencies and competitive influences, defects in one pathway can lead to dysregulation of many others. In such cases, the inappropriate behavior of tumor cells can be viewed as the consequence of aberrant perception of microenvironmental stimuli. Furthermore, just as normal tissue structure and function are highly dependent on reciprocal communications between cells and their microenvironment, so imbalances in these communications can contribute to the malignant behavior of a tumor. These imbalanced communications can be caused by mutations of genes encoding critical transduction intermediates, but can also be epigenetic, caused by aberrations in the microenvironment.
Aberrations in the Extracellular Matrix Cause Cancer
If the ECM is instrumental in normal development and differentiation, then a remodeling of its composition can lead to alterations in the function and structure of the organs (115). Therefore, ECM remodeling is a potent mechanism of cell regulation. In the mammary gland, the massive apoptosis of milk-secreting epithelial cells during the post-lactational regression phase is triggered by degradation of BM by matrix metalloproteinases (MMPs) (116). Similarly, MMP-mediated remodeling occurs in many tissues and in diverse activities such as wound healing and angiogenesis (117). Matrix metalloproteinases also play roles in invasion, tumor angiogenesis, and metastasis (118,119).
We have investigated stromelysin-1 (SL-1), an MMP that plays an important role in both development and regression of the normal mammary gland and is found in mammary carcinomas. In mammary epithelial cells cultured on ECM, exogenous expression of SL-1 led to degradation of basement membrane, attempted re-entry into the cell cycle, and apoptosis (120,121). We also found that the stroma of mammary glands of transgenic mice that overexpressed SL-1 inappropriately (122) showed features of pre-neoplastic lesions (123) that eventually led to full malignancy (124,125). These changes were inhibited by crossing these mice with another strain that expressed an MMP inhibitor (126,127). The SL-1, expressed initially at low levels in the mammary epithelial cells, was subsequently produced at much higher levels in stromal fibroblasts (123), suggesting that disruption of ECM integrity led to a self-sustaining tumorigenic state. Consistent with this hypothesis, cultured epithelial cells containing an inducible SL-1 transgene (128,129) formed tumors that eventually became independent of transgene expression (125). A similar mechanism has been observed for the metalloproteinases matrilysin (130) and stromelysin-3 (131,132). Matrix metalloproteinases inhibitors are currently under test in clinical trials, although early results have been disappointing; MMPs may alternatively benefit the host or the tumor, depending on tumor stage and other microenvironmental factors (133).
Increased Signaling from Growth Receptors Causes Cancer
Signals from the microenvironment control both cell morphology and gene expression, and these pathways are coupled in tissues and in cells in 3-D (134). When cultured on inappropriate ECM, normal signaling patterns become decoupled from control of morphology (135). This effect can obscure phenotypic differences between different types of cells, and even between normal and tumor cells. When grown on conventional tissue culture plastic, few differences are observed between normal human mammary epithelial cells and malignant breast cancer cells, either in morphological appearance or in biochemical characteristics (136). When cultured on appropriate ECM, however, normal cells functionally differentiate, as demonstrated by growth arrest, structural organization, and polarized secretion of BM components. Malignant cells, on the other hand, continue to proliferate, forming amorphous structures (136).
These distinct behaviors suggested to us that growth on 3-D ECM could be used to dissect the mechanisms associated with malignancy. To avoid potentially artifactual differences specific to cell lines, we used a human breast cancer progression series. Derived originally from a human reduction mammoplasty (137), HMT-3522 cells were repeatedly passaged, acquiring numerous chromosomal abnormalities (138–140). Once they also lost the ability to sense the cues from the BM, they acquired tumorigenic potential when injected into immunocompromised mice (141). The functionally normal, early passage (S1) cells and the tumorigenic, late passage (T4-2) cells were similar in appearance when cultured on plastic substrata, but very different when cultured inside or on BM. As S1 and T4-2 cells respond to their microenvironment differently, we examined the differences in ECM receptor expression between the two cell lines. Normal human mammary epithelial cells express four α/β heterodimers: α1β1, α2β1, α3β1, and α6β4; all four of which bind laminin, while α1β1 and α2β1 also bind collagen (135). Analysis of the S1 and T4-2 cells revealed that the tumorigenic cells expressed both an increased level of β1 integrins and a anomalous pattern of expression (142). Integrin expression is often aberrant during tumor progression (143,144).
To test the hypothesis that increased signaling from β1 integrins was contributing to the tumorigenic behavior, the relative activity of this subunit was attenuated by function blocking antibodies. This treatment resulted in conversion to an organized phenotype in the 3-D BM and a reduction in tumorigenicity when transplanted into mice. The reverted T4-2 cells arrested proliferation, organized into acini, and organized a basement membrane (142). This reversion was also accompanied by down modulation of the endogenous level of the epidermal growth factor receptor (EGFR). Subsequently, a bi-directional cross-modulation of β1 integrin and the EGFR through the MAPK pathway was revealed by experiments in which inhibition of any of these components also led to functional reversion of the T4-2 cells (145). Thus, normalization of ECM signaling by reestablishing balance in signaling can produce an almost normal phenotype in culture and in vivo without changing the genotype (142).
Decreased Signaling from Pro-differentiation Receptors Causes Cancer
Much of the integrin literature has focused on the pro-growth properties of integrins, as normal cells require integrin signaling in order to avoid apoptosis or growth arrest (64), but it is clear that cell differentiation is also dependent on contact with ECM. Some have speculated that tissue organization with E-cadherin may produce anti-growth effects to counter integrin signaling (64). We have found that functional differentiation of mammary epithelial cells can occur in the absence of cell–cell contact if BM is present (104). We dissected the ECM signals for differentiation of mammary epithelial cells, and discovered a requirement for contact with BM laminin through two integrins and one nonintegrin ECM receptor (146). Recently, we have discovered that this nonintegrin receptor is dystroglycan, and we are beginning to find evidence that dystroglycan may play a general role in inducing cell polarization and growth arrest (Muschler et al., submitted). Surveys of tumorigenic and non-tumorigenic cell lines have revealed that inability to organize when cultured on BM correlates with loss of cell surface dystroglycan. The hypothesis that dystroglycan signaling is complementary to integrin signaling for producing differentiation is very attractive due to the wide tissue distribution of dystroglycan (74,79). According to this hypothesis, the disorganized and tumorigenic growth of T4-2 cells could be caused by increased MAPK signaling initiated by β1 integrins and insufficient relative signaling from dystroglycan or other growth inhibiting matrix receptors.
Thus, the pathways to organized differentiation or disorganized malignancy may be mediated by a balance between pro-growth and pro-differentiation signaling; pathways that are interconnected and self-reinforcing.
CONCLUSIONS
Cells respond to their microenvironment through ECM adhesion molecules. These interactions lead to activation of signaling cascades that impact on many other signaling pathways as well, modulating the responses to soluble growth factors and the connections to neighboring cells. In normal cells, these interacting pathways control tissue organization and development; when dysfunctional, these pathways can lead to cancer. Using transgenic and cell culture models, we have modeled aspects of both the productive and destructive interactions in breast cells. We have shown that inappropriate expression of ECM-remodeling metalloproteinases cause tissues to become disorganized, and that sustained disorganization can lead to tumorigenesis. Using cancer cell lines, we have found that misregulation of ECM-sensing adhesion molecules can be an important component of tumorigenesis, and that reasserting balance to the signaling pathways in tumor cells can revert the malignant phenotype. Thus, a balance in the network of cell–ECM interactions can determine the tumorigenic potential of a cell population, and in this way, these mediators of tissue architecture constitute a class of tumor suppressors. These networked interactions can be self-reinforcing: perturbation of normal signaling can produce tumorigenesis, while normalization of tumor signaling can lead to functional reversion. A better understanding of these pathways may lead to the next generation of cancer treatments aimed not necessarily at killing the cancer, but at restoring the natural order and consequent tissue function.
Acknowledgments
This work was supported by the Director, Office of Biological and Environmental Research (contract DE-AC03-76SF00098) of the U.S. Department of Energy and by NIH grant CA-57621 to M.J.B. D. Radisky was supported by a Distinguished Hollaender postdoctoral fellowship DE-AC03-SF00098. J. Muschler was supported by a California Breast Cancer Research Program grant #6KB-0130.
References
- 1.Bissell MJ, Hall HG, Parry G. How Does Extracellular Matrix Direct Gene Expression? J Theor Biol. 1982;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 2.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin Signaling and Cell Growth Control. Curr Opin Cell Biol. 1998;10(2):220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 3.De Archangelis A, Georges-Labouesse E. Integrin and ECM Functions: Roles in Vertebrate Development. Trends Genet. 2000;16(9):389–395. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson E, Fässler R. Insights into Extracellular Matrix Functions from Mutant Mouse Models. Exp Cell Res. 2000;261(1):52–68. doi: 10.1006/excr.2000.5042. [DOI] [PubMed] [Google Scholar]
- 5.Tsai LH. Stuck on the ECM. Trends Cell Biol. 1998;8(7):292–295. doi: 10.1016/s0962-8924(98)01296-3. [DOI] [PubMed] [Google Scholar]
- 6.Streuli C. Extracellular Matrix Remodeling and Cellular Differentiation. Curr Opin Cell Biol. 1999;11(5):634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 7.Jones FS, Jones PL. The Tenascin Family of ECM Glycoproteins: Structure, Function, and Regulation During Embryonic Development and Tissue Remodeling. Dev Dyn. 2000;218(2):235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Ingber DE. The Structural and Mechanical Complexity of Cell-Growth Control. Nat Cell Biol. 1999;1(5):E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 9.Gumbiner BM. Cell Adhesion: The Molecular Basis of Tissue Architecture and Morphogenesis. Cell. 1996;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 10.Radisky D, Hagios C, Bissell MJ. Tumors Are Unique Organs Defined by Abnormal Signaling and Context. Semin Cancer Biol. 2000;11(2):87–95. doi: 10.1006/scbi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 11.Aumailley M, Gayraud B. Structure and Biological Activity of the Extracellular Matrix. J Mol Med. 1998;76(3–4):253–265. doi: 10.1007/s001090050215. [DOI] [PubMed] [Google Scholar]
- 12.Wessels NK. Tissue Interactions and Development. Benjamin-Cummings; Menlo Park, California: 1977. [Google Scholar]
- 13.Aumailley M, Smyth N. The Role of Laminins in Basement Membrane Function. J Anat. 1998;193(1):1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miner JH, Cunningham J, Sanes JR. Roles for Laminin in Embryogenesis: Exencephaly, Syndactyly, and Placentopathy in Mice Lacking the Laminin α5 Chain. J Cell Biol. 1998;143(6):1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of Basement Membranes After Targeting the LAMC1 Gene Results in Embryonic Lethality Due to Failure of Endoderm Differentiation. J Cell Biol. 1999;144(1):151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colognoto H, Yurchenco PD. Form and Function: The Laminin Family of Heterotrimers. Dev Dyn. 2000;218(2):213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Virtanen I, Gullberg D, Rissanen J, Kivilaakso E, Kiviluoto T, Laitenen LA, Lehto VP, Ekblom P. Laminin α1-Chain Shows a Restricted Distribution in Epithelial Basement Membranes of Fetal and Adult Human Tissues. Exp Cell Res. 2000;257(2):298–309. doi: 10.1006/excr.2000.4883. [DOI] [PubMed] [Google Scholar]
- 18.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and Function of Laminins in the Neuromuscular System of Developing, Adult, and Mutant Mice. J Cell Biol. 1997;139(6):1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz APN, Roskelley C, Bissell MJ. Laminin Mediates Tissue-Specific Gene Expression in Mammary Epithelia. J Cell Biol. 1995;129(3):591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slade MJ, Coope RC, Gomm JJ, Coombes RC. The Human Mammary Gland Basement Membrane Is Integral to the Polarity of Luminal Epithelial Cells. Exp Cell Res. 1999;247(1):267–278. doi: 10.1006/excr.1998.4340. [DOI] [PubMed] [Google Scholar]
- 21.Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-Assembly of Laminin Isoforms. J Biol Chem. 1997;272(50):31525–31532. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- 22.Colognato H, Winkelmann DA, Yurchenco PD. Laminin Polymerization Induces a Receptor–Cytoskeleton Network. J Cell Biol. 1999;145(3):619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant Differentiation of Neuromuscular Junctions in Mice Lacking s-Laminin/Laminin β2. Nature. 1995;374(6519):258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 24.Miner JH, Cunningham J, Sanes JR. Roles of Laminin in Embryogenesis: Exencephaly, Syndactyly, and Placentopathy in Mice Lacking the Laminin α5 chain. J Cell Biol. 1998;143(6):1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan MC, Lee K, Mihashita Y, Carter WG. Targeted disruption of the LAMA3 Gene in Mice Reveals Abnormalities in Survival and Late Stage Differentiation of Epithelial Cells. J Cell Biol. 1999;134(6):559–572. doi: 10.1083/jcb.145.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziober BL, Lin CS, Kramer RH. Laminin-Binding Integrins in Tumor Progression and Metastasis. Semin Cancer Biol. 1996;7(3):119–128. doi: 10.1006/scbi.1996.0017. [DOI] [PubMed] [Google Scholar]
- 27.Petetclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Hudson BG, Brooks PC. New Functions for Non-collagenous Domains of Human Collagen Type IV. J Biol Chem. 2000;275(11):8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 28.Kalluri R, Cosgrove D. Assembly of Type IV Collagen. J Biol Chem. 2000;275(17):12719–12724. doi: 10.1074/jbc.275.17.12719. [DOI] [PubMed] [Google Scholar]
- 29.Jais JP, Kneibelmann B, Giatras I, de Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Tryggvason K, Martin P, Hertz JM, Schroder C, Sanak M, Krejcova S, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC. X-linked Alport Syndrome: Natural History in 195 Families and Genotype–Phenotype Correlations in Males. J Am Soc Nephrol. 2000;11(4):649–657. doi: 10.1681/ASN.V114649. [DOI] [PubMed] [Google Scholar]
- 30.Kohfeldt E, Sasaki T, Göhring W, Timpl R. Nidogen-2: A New Basement Membrane Protein With Diverse Binding Properties. J Mol Biol. 1998;282(1):99–109. doi: 10.1006/jmbi.1998.2004. [DOI] [PubMed] [Google Scholar]
- 31.Mayer U, Kohfeldt E, Timpl R. Structural and Genetic Analysis of Laminin–Nidogen Interaction. Ann NY Acad Sci. 1998;857:130–142. doi: 10.1111/j.1749-6632.1998.tb10113.x. [DOI] [PubMed] [Google Scholar]
- 32.Pujuguet P, Simian M, Liaw J, Timpl R, Werb Z, Bissell MJ. Nidogen-1 Regulates Laminin-1-Dependent Mammary-Specific Gene Expression. J Cell Sci. 2000;113(5):849–858. doi: 10.1242/jcs.113.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of Cell Surface Heparan Sulfate Proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 34.Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan Maintains the Integrity of Cartilage and Some Basement Membranes. J Cell Biol. 1999;147(5):1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santra M, Eichstetter I, Iozzo RV. An Anti-oncogenic Role for Decorin. J Biol Chem. 2000;275(45):35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- 36.Kleeff J, Ishiwata T, Kumbasar A, Friess H, Büchler MW, Lander AD, Korc M. The Cell-Surface Heparan Sulfate Proteoglycan Glypican-1 Regulates Growth Factor Action in Pancreatic Carcinoma Cells and is Overexpressed in Human Pancreatic Cancer. J Clin Invest. 1998;102(9):1662–1673. doi: 10.1172/JCI4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick C, Duncan G, Goutsos KT, Tufaro F. The Putative Tumor Suppressors EXT1 and EXT2 Form a Stable Complex that Accumulates in the Golgi Apparatus and Catalyzes the Synthesis of Heparan Sulfate. Proc Natl Acad Sci USA. 2000;97(2):668–673. doi: 10.1073/pnas.97.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick C, Duncan G, Tufaro F. New Perspectives on the Molecular Basis of Hereditary Bone Tumors. Mol Med Today. 1999;5(11):481–486. doi: 10.1016/s1357-4310(99)01593-2. [DOI] [PubMed] [Google Scholar]
- 39.Shoenwaelder SM, Burridge K. Bidirectional Signaling Between the Cytoskeleton and Integrins. Curr Opin Cell Biol. 1999;11(2):274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 40.Sechler JL, Corbett SA, Wenk MB, Schwarzbauer JE. Modulation of Cell-Extracellular Matrix Interactions. Ann N Y Acad Sci. 1998;857:143–154. doi: 10.1111/j.1749-6632.1998.tb10114.x. [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Ingber DE. The Structural and Mechanical Complexity of Cell-Growth Control. Nat Cell Biol. 1999;1(5):E131–E137. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto S, Katz BZ, Lafrenie RM, Yamada KM. Fibronectin and Integrins in Cell Adhesion, Signaling, and Morphogenesis. Ann NY Acad Sci. 1998;857:119–129. doi: 10.1111/j.1749-6632.1998.tb10112.x. [DOI] [PubMed] [Google Scholar]
- 43.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand Binding to Integrins. J Biol Chem. 2000;275(29):21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 44.Leahy DJ. Implications of Atomic-Resolution Structures for Cell Adhesion. Annu Rev Cell Dev Biol. 1997;13:363–393. doi: 10.1146/annurev.cellbio.13.1.363. [DOI] [PubMed] [Google Scholar]
- 45.Ruoslahti E. RGD and Other Recognition Sequences for Integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 46.Boudreau NJ, Jones PL. Extracellular Matrix and Integrin Signalling: the Shape of Things to Come. Biochem J. 1999;339(3):481–488. [PMC free article] [PubMed] [Google Scholar]
- 47.Giancotti FG. Complexity and Specificity of Integrin Signaling. Nat Cell Biol. 2000;2(1):E13–E14. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- 48.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and Actin Filaments: Reciprocal Regulation of Cell Adhesion and Signaling. J Biol Chem. 2000;275(30):22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 49.Critchley DR. Focal Adhesions—the Cytoskeletal Connection. Curr Opin Cell Biol. 2000;12(1):133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 50.Sastry SK, Burridge K. Focal Adhesions: A Nexus for Intracellular Signaling and Cytoskeletal Dynamics. Exp Cell Res. 2000;261(1):25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 51.Scita G, Tenca P, Frittoli E, Tocchetti A, Innocenti M, Giardina G, Di Fiore PP. Signaling from Ras to Rac and Beyond: Not Just a Matter of GEFs. EMBO J. 2000;19(11):2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitz AAP, Govek EE, Böttner B, Van Aelst L. Rho GTPases: Signaling, Migration, and Invasion. Exp Cell Res. 2000;261(1):1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- 53.Braga V. Epithelial Cell Shape: Cadherins and Small GTPases. Exp Cell Res. 2000;261(1):83–90. doi: 10.1006/excr.2000.5050. [DOI] [PubMed] [Google Scholar]
- 54.Hughes PE, Pfaff M. Integrin Affinity Modulation. Trends Cell Biol. 1998;8(9):359–364. doi: 10.1016/s0962-8924(98)01339-7. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez C, Clark K, Burrows L, Schofield NR, Humphries MJ. Regulation of The Extracellular Ligand Binding Activity of Integrins. Front Bioscience. 1998;3:D684–D700. doi: 10.2741/a313. [DOI] [PubMed] [Google Scholar]
- 56.Aplin AE, Howe A, Alahari SK, Juliano RL. Signal Transduction and Signal Modulation by Cell Adhesion Receptors: the Role of Integrins, Cadherins, Immunoglobulin-Cell Adhesion Molecules, and Selectins. Pharmacol Rev. 1998;50(2):197–263. [PubMed] [Google Scholar]
- 57.Hall A. Rho GTPases and the Actin Cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz MA, Shattil SJ. Signaling Networks Linking Integrins and Rho Family GTPases. Trends Biochem Sci. 2000;25(8):388–391. doi: 10.1016/s0968-0004(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 59.Renshaw MW, Price LS, Schwartz MA. Focal Adhesion Kinase Mediates the Integrin Signaling Requirement for Growth Factor Activation of MAP Kinase. J Cell Biol. 1999;147(3):611–618. doi: 10.1083/jcb.147.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cary LA, Guan JL. Focal Adhesion Kinase in Integrin-Mediated Signaling. Front Biosci. 1999;4:D102–D113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- 61.Fincham VJ, James M, Frame MC, Winder SJ. Activated ERK/MAP Kinase Is Targeted to Newly-Forming Cell-Matrix Adhesions by Integrin Engagement and v-Src. EMBO J. 2000;19(12):2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oktay M, Wary KK, Dans M, Birge R, Giancotti FG. Integrin-mediated Activation of Focal Adhesion Kinase Is Required for Signaling to Jun NH2-terminal Kinase and Progression through the G1 Phase of the Cell Cycle. J Cell Biol. 1999;145(7):1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almeida EAC, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. atrix Survival Signaling: From Fibronectin via Focal Adhesion Kinase to c-Jun NH2-terminal Kinase. J Cell Biol. 2000;149(3):741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giancotti FG, Ruoslahti E. Integrin Signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 65.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin Signaling and Cell Growth Control. Curr Opin Cell Biol. 1999;10(2):220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 66.Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL. Regulation of STAT3 by Direct binding to the Rac1 GTPase. Science. 2000;290(5489):144–147. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- 67.Rapraeger AC. Syndecan-regulated Receptor Signaling. J Cell Biol. 2000;149(5):995–997. doi: 10.1083/jcb.149.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carey DJ. Syndecans: Multifunctional Cell-Surface Co-Receptors. Biochem J. 1997;327(1):1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmerman P, David G. The Syndecans, Tuners of Transmembrane Signaling. FASEB J. 1999;13(suppl):S91–S100. doi: 10.1096/fasebj.13.9001.s91. [DOI] [PubMed] [Google Scholar]
- 70.Woods A, Couchman JR. Integrin Modulation by Lateral Association. J Biol Chem. 2000;275(32):24233–24236. doi: 10.1074/jbc.R000001200. [DOI] [PubMed] [Google Scholar]
- 71.Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 Is Required for Wnt-1-Induced Mammary Tumorigenesis in Mice. Nat Genet. 2000;25(3):329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 72.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a Glycoprotein Component of the Dystrophin Complex in Dystrophic Muscle. Nature. 1990;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 73.Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of Dystroglycan in Normal Adult Mouse Tissues. J Histochem Cytochem. 1998;46(4):449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- 74.Hemler ME. Dystroglycan Versatility. Cell. 1999;97(5):543–546. doi: 10.1016/s0092-8674(00)80764-3. [DOI] [PubMed] [Google Scholar]
- 75.Henry MD, Campbell KP. Dystroglycan Inside and Out. Curr Opin Cell Biol. 1999;11(5):602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 76.Andac Z, Sasaki T, Mann K, Brancaccio A, Deutzmann R, Timpl R. Analysis of Heparin, α-Dystroglycan, and Sulfatide Binding to the G Domain of the Laminin α1 Chain by Site-Directed Mutagenesis. J Mol Biol. 1999;287(2):253–264. doi: 10.1006/jmbi.1999.2606. [DOI] [PubMed] [Google Scholar]
- 77.Peng HB, Alie AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR. The Relationship Between Perlecan and Dystroglycan and Its Implication in the Neuromuscular Junction. Cell Adhes Comm. 1998;5(6):475–489. doi: 10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- 78.Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G Domains of Laminin α1 and α2 Chains and Perlecan to Heparin, Sulfatides, α-Dystroglycan and Several Extracellular Matrix Proteins. EMBO J. 1999;18(4):863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibrahgimov-Beskrovnaya O, Campbell KP. Dystroglycan Is Essential for Early Embryonic Development: Disruption of Reichert’s Membrane in Dag1-Null Mice. Hum Mol Gen. 1997;6(6):831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 80.Henry MD, Campbell KP. A Role of Dystroglycan in Basement Membrane Assembly. Cell. 1998;95(6):859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- 81.Steinberg MS, McNutt PM. Cadherins and Their Connections: Adhesion Junctions Have Broader Functions. Curr Opin Cell Biol. 1999;11(5):554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 82.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin Null Mutant Embryos Fail to Form a Trophectoderm Epithelium. Proc Natl Acad Sci USA. 1994;91(17):8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riethmacher D, Brinkmann V, Birchmeier C. A Targeted Mutation in the Mouse E-Cadherin Gene Results in Defective Preimplantation Development. Proc Natl Acad Sci USA. 1995;92(3):855–859. doi: 10.1073/pnas.92.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J. A New Crystal Structure, Ca2+ Dependence and Mutational Analysis Reveal Molecular Details of E-Cadherin Homoassociation. EMBO J. 1999;18(7):1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Troyanovski SM. Mechanism of Cell–Cell Adhesion Complex Assembly. Curr Opin Cell Biol. 1999;11:561–566. doi: 10.1016/s0955-0674(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 86.Kim SH, Li Z, Sacks DB. E-Cadherin-Mediated Cell–Cell Atachment Activates Cdc42. J Biol Chem. 2000;275(47):36999–37005. doi: 10.1074/jbc.M003430200. [DOI] [PubMed] [Google Scholar]
- 87.Kuroada S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsura Y, Yonehara S, Kaibuchi K. Role of IQGAP1, a Target of the Small GTPases Cdc41 and Rac1, in Regulation of E-Cadherin-Mediated Cell–Cell Adhesion. Science. 1998;281(5378):832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 88.Dedhar S. Cell–Substrate Interactions and Signaling Through ILK. Curr Opin Cell Biol. 2000;12(2):250–256. doi: 10.1016/s0955-0674(99)00083-6. [DOI] [PubMed] [Google Scholar]
- 89.Hazan RB, Norton L. The Epidermal Growth Factor Receptor Modulates the Interaction of E-Cadherin with the Actin Cytoskeleton. J Biol Chem. 1998;273(15):9078–9084. doi: 10.1074/jbc.273.15.9078. [DOI] [PubMed] [Google Scholar]
- 90.Vleminckx K, Kemler R. Cadherins and Tissue Formation: Integrating Adhesion and Signaling. Bio-Essays. 1999;21(3):211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 91.Ben-Ze’ev A, Shtutman M, Zhurinsky J. The Integration of Cell Adhesion with Gene Expression: The Role of β-Catenin. Exp Cell Res. 2000;261(1):75–82. doi: 10.1006/excr.2000.5045. [DOI] [PubMed] [Google Scholar]
- 92.Peifer M, Polakis P. WNT Signaling in Oncogenesis and Embryogenesis—A Look Outside the Nucleus. Science. 2000;287(5458):1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 93.Yap AS. The Morphogenetic Role of Cadherin Cell Adhesion Molecules in Human Cancer: A Thematic Review. Cancer Invest. 1998;16(4):252–261. doi: 10.3109/07357909809039774. [DOI] [PubMed] [Google Scholar]
- 94.Semb H, Christofori G. Insights From Model Systems: The Tumor-Suppressor Function of E-Cadherin. Am J Hum Genet. 1998;63(6):1588–1593. doi: 10.1086/302173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Christofori G, Semb H. The Role of the Cell-Adhesion Molecule E-Cadherin as a Tumor Suppressor Gene. Trends Biochem Sci. 1999;24(2):73–76. doi: 10.1016/s0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 96.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A Causal Role for E-Cadherin in the Transition from Adenoma to Carcinoma. Nature. 1998;392(6672):190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 97.Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, Somasiri A, Roskelley CD. E-Cadherin Induces Mesenchymal-to-Epithelial Transition in Human Ovarian Surface Epithelium. Proc Natl Acad Sci USA. 1999;96(11):6249–6254. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bissell MJ, Weaver VM, Lelièvre SA, Wang F, Petersen OW, Schmeichel KL. Tissue Structure, Nuclear Organization, and Gene Expression in Normal and Malignant Breast. Cancer Res. 1999;59(7 suppl):1757s–1764s. [PubMed] [Google Scholar]
- 99.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and Characterization of Type IV Procollagen, Laminin, and Heparan Sulfate Proteoglycan from the EHS Sarcoma. Biochemistry. 1982;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 100.Lee EYHP, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of Mouse Mammary Epithelial Cells with Collagen Substrata: Regulation of Casein Gene Expression and Secretion. Proc Natl Acad Sci. 1985;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a Reconstituted Basement Membrane and its Components on Casein Gene Expression and Secretion in Mouse Mammary Epithelial Cells. Proc Natl Acad Sci. 1987;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional Differentiation and Alveolar Morphogenesis of Primary Mammary Cultures on Reconstituted Basement Membrane. Development. 1989;105(2):223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Cytodifferentiation of Mouse Mammary Epithelial Cells Cultured on a Reconstituted Basement Membrane Reveals Striking Similarities to Development In Vivo. J Cell Sci. 1991;99(2):407–417. doi: 10.1242/jcs.99.2.407. [DOI] [PubMed] [Google Scholar]
- 104.Streuli CH, Bailey N, Bissell MJ. Control of Mammary Epithelial Differentiation: Basement Membrane Induces Tissue-Specific Gene Expression in the Absence of Cell–Cell Interaction and Morphological Polarity. J Cell Biol. 1991;115(5):1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roskelley CD, Desprez PY, Bissell MJ. Extra-cellular Matrix-Dependent Tissue-Specific Gene Expression in Mammary Epithelial Cells Requires Both Physical and Biochemical Signal Transduction. Proc Natl Acad Sci USA. 1994;91(26):12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin CQ, Dempsey PJ, Coffey RJ, Bissell MJ. Extracellular Matrix Regulates Whey Acidic Protein Gene Expression of TGF-α in Mouse Mammary Epithelial Cells: Studies in Culture and in Transgenic Mice. J Cell Biol. 1995;129(4):1115–1126. doi: 10.1083/jcb.129.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lengauer C, Kinzler KW, Vogelstein B. Genetic Instabilities in Human Cancers. Nature. 1998;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 108.Ingvarsson S. Molecular Genetics of Breast Cancer Progression. Semin Cancer Biol. 1999;9(4):277–288. doi: 10.1006/scbi.1999.0124. [DOI] [PubMed] [Google Scholar]
- 109.Turker MS. Estimation of Mutation Frequencies in Normal Mammalian Cells and the Development of Cancer. Semin Cancer Biol. 1998;8(6):407–419. doi: 10.1006/scbi.1998.0112. [DOI] [PubMed] [Google Scholar]
- 110.Cahill DP, Kinzler KW, Vogelstein B, Lengauer C. Genetic Instability and Darwinian Selection In Tumors. Trends Genet. 2000;15(12):M57–M60. [PubMed] [Google Scholar]
- 111.Sporn MB. The War on Cancer. Lancet. 1996;347(9012):1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- 112.Folkman J, Hahnfeldt P, Hlatky L. Cancer: Looking Outside the Genome. Nat Rev Mol Cell Biol. 2000;1(1):76–79. doi: 10.1038/35036100. [DOI] [PubMed] [Google Scholar]
- 113.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 114.McCormick F. Signalling Networks That Cause Cancer. Trends Biochem Sci. 2000;24(12):M53–M56. [PubMed] [Google Scholar]
- 115.Streuli C. Extracellular Matrix Remodelling and Cellular Differentiation. Curr Opin Cell Biol. 1999;11(5):634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 116.Werb Z. ECM and Cell Surface Proteolysis: Regulating Cellular Ecology. Cell. 1997;91(4):439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 117.Vu TH, Werb Z. Matrix Metalloproteinases: Effectors of Development and Normal Physiology. Genes Dev. 2000;14(17):2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 118.Lukashev ME, Werb Z. ECM Signalling: Orchestrating Cell Behavior and Misbehavior. Trends Cell Biol. 1998;8(11):437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 119.McCawley LJ, Matrisian LM. Matrix Metalloproteinases: Multifunctional Contributors to Tumor Progression. Mol Med Today. 2000;6(4):149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 120.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and Apoptosis in Mammary Epithelial Cells by Extracellular Matrix. Science. 1995;267(5199):891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boudreau N, Werb Z, Bissell MJ. Suppression of Apoptosis by Basement Membrane Requires Three-Dimensional Tissue Organization and Withdrawal From the Cell Cycle. Proc Natl Acad Sci USA. 1996;93(8):3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted Expression of Stromelysin-1 in Mammary Gland Provides Evidence for Proteinases in Branching Morphogenesis and the Requirement for an Intact Basement Membrane for Tissue-Specific Gene Expression. J Cell Biol. 1994;125(3):681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, Werb Z, Bissell MJ. Expression of Autoactivated Stromelysin-1 in Mammary Glands of Transgenic Mice Leads to a Reactive Stroma During Early Development. Am J Pathol. 1998;153(2):457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sympson CJ, Bissell MJ, Werb Z. Mammary Gland Formation In Transgenic Mice Overexpressing Stromelysin-1. Semin Cancer Biol. 1995;6(3):59–163. doi: 10.1006/scbi.1995.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The Stromal Proteinase MMP3/Stromelysin-1 Promotes Mammary Carcinogenesis. Cell. 1999;98(2):137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of Mammary Epithelial Cell Apotosis and Entactin Degradation by a tissue Inhibitor of Metallo-proteinases Transgene. J Cell Biol. 1996;135(6 pt 1):1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sternlicht MD, Bissell MJ, Werb Z. The Matrix Metalloproteinase Stromelysin-1 Acts as a Natural Mammary Tumor Promoter. Oncogene. 2000;19(8):1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. Misregulation of Stromelysin-1 Expression in Mouse Mammary Tumor Cells Accompanies Acquisition of Stromelysin-1-Dependent Invasive Properties. J Biol Chem. 1997;272(8):5007–5015. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- 129.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix Metalloproteinase Stromelysin-1 Triggers a Cascade of Molecular Alterations that Leads to Stable Epithelial-to-Mesenchymal Conversion and a Premalignant Phenotype in Mammary Epithelial Cells. J Cell Biol. 1997;139(7):1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rudolph-Owen LA, Chan R, Muller WJ, Matrisian LM. The Matrix Metalloproteinase Matrilysin Influences Early-Stage Mammary Tumorigenesis. Cancer Res. 1998;58:5500–5506. [PubMed] [Google Scholar]
- 131.Noel AC, Lefebvre O, Maquoi E, VanHoorde L, Chenard MP, Mareel M, Foidart JM, Basset P, Rio MC. Stromelysin-3 Expression Promotes Tumor Take in Nude Mice. J Clin Invest. 1996;97(8):1924–1930. doi: 10.1172/JCI118624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Masson R, Lefebvre O, Noel A, El Fahime M, Chenard MP, Wendling C, Kebers F, LeMeur M, Dierich A, Foidart JM, Basset P, Rio MC. In Vivo Evidence that the Stromelysin-3 Metalloproteinase Contributes in a Paracrine Manner to Epithelial Cell Malignancy. J Cell Biol. 1998;140(6):1535–1541. doi: 10.1083/jcb.140.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bergers G, Coussens LM. Extrinsic Regulators of Epithelial Tumor Progression: Metalloproteinases. Curr Opin Genet Devel. 2000;10(1):120–127. doi: 10.1016/s0959-437x(99)00043-x. [DOI] [PubMed] [Google Scholar]
- 134.Huang S, Ingber DE. Shape-Dependent Control of Cell Growth, Differentiation, and Apoptosis: Switching between Attractors in Cell Regulatory Networks. Exp Cell Res. 2000;261(1):91–103. doi: 10.1006/excr.2000.5044. [DOI] [PubMed] [Google Scholar]
- 135.Schmichel KL, Weaver VM, Bissell MJ. Structural Cues from the Tissue Microenvironment Are Essential Determinants of the Human Mammary Epithelial Cell Phenotype. J Mammary Gland Biol Neoplasia. 1998;3(2):201–213. doi: 10.1023/a:1018751124382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells. Proc Natl Acad Sci USA. 1992;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Briand P, Peterson OW, van Deurs B. A New Diploid Nontumorgenic Human Breast Epithelial Cell Line Isolated and Propagated in Chemically Defined Medium. In Vitro Cell Dev Biol. 1987;23(3):181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 138.Madsen MW, Lykkesfeldt AE, Laursen I, Nielsen KV, Briand P. Altered Gene Expression of C-Myc, Epidermal Growth Factor Receptor, Transforming Growth Factor-α, and C-Erb-B2 in an Immortalized Human Breast Epithelial Cell Line, HMT-3522, Is Associated with Decreased Growth Factor Requirements. Cancer Res. 1992;52(5):1210–1217. [PubMed] [Google Scholar]
- 139.Moyret C, Madsen MW, Cooke J, Briand P, Theillet C. Gradual Selection of a Cellular Clone Presenting a Mutation at Codon 179 of the p53 Gene During Establishment of the Immortalized Human Breast Epithelial Cell Line HMT-3522. Exp Cell Res. 1994;215(2):380–385. doi: 10.1006/excr.1994.1355. [DOI] [PubMed] [Google Scholar]
- 140.Nielsen KV, Madsen MW, Briand P. In Vitro Karyotype Evolution in a Cell Line Established from Nonmalignant Human Mammary Epithlium. Cancer Genet Cytogenet. 1994;78(2):189–199. doi: 10.1016/0165-4608(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 141.Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and Malignant Transformation of Human Breast Epithelial Cells Following Epidermal Growth Factor Withdrawal. Cancer Res. 1996;56(9):2039–2044. [PubMed] [Google Scholar]
- 142.Weaver VM, Peterson OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the Malignant Phenotype of Human Breast Cells in Three-Dimensional Culture and In Vivo by Integrin Blocking Antibodies. J Cell Biol. 1997;137(1):231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Varner JA, Cheresh DA. Integrins and Cancer. Curr Opin Cell Biol. 1996;8(5):724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 144.Mizejewski GJ. Role of Integrins in Cancer: Survey of Expression Patterns. Proc Soc Exp Biol Med. 1999;222(2):124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- 145.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal Interactions Between β1-Integrin and Epidermal Growth Factor Receptor in Three-Dimensional Basement Membrane Breast Cultures: A Different Perspective in Epithelial Biology. Proc Natl Acad Sci USA. 1998;95(25):14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of Labor among the α6β4 Integrin, β1 Integrins, and an E3 Laminin Receptor to Signal Morphogenesis and β-Casein Expression in Mammary Epithelial Cells. Mol Biol Cell. 1999;10(9):2817–2828. doi: 10.1091/mbc.10.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]



