Abstract
In order to understand why cancer develops as well as predict the outcome of pharmacological treatments, we need to model the structure and function of organs in culture so that our experimental manipulations occur under physiological contexts. This review traces the history of the development of a prototypic example, the three-dimensional (3D) model of the mammary gland acinus. We briefly describe the considerable information available on both normal mammary gland function and breast cancer generated by the current model and present future challenges that will require an increase in its complexity. We propose the need for engineered tissues that faithfully recapitulate their native structures to allow a greater understanding of tissue function, dysfunction, and potential therapeutic intervention.
Keywords: Basement membrane, Mammary gland, Microenvironment, Morphogenesis, Therapy, Tissue structure
1. Introduction
Much progress has been made in the development of therapies to treat breast cancer, as evidenced by a decrease in overall deaths during the 1990s [1]. Nevertheless, many potential drug candidates are lost in the transition from animal models to clinical trials [2]. Furthermore, the number of drug candidates far exceeds the number of patients in whom to test them, even if the drugs were found initially to be effective in mice. Therefore, it is clear that we need alternative models of human disease to aid in the identification and verification of therapeutic targets. Unfortunately, there are almost no techniques that allow the maintenance of organs ex vivo long enough to permit the necessary molecular biological investigations. As such, an enormous potential exists in the use of three-dimensional (3D) cell culture models as surrogates for tissues [3]. During the past three decades, investigations from a number of fields have converged to build simple yet effective models of the mammary gland, and have identified key molecular, structural, and mechanical cues important for maintaining its function and architecture in culture. In doing so, they have also provided insights into the fundamental differences between normal and malignant cells, the genetic and epigenetic changes involved in neoplastic transformation, and the potential strategies for treating cancer.
In the first half of this review, we provide a historical perspective of the design and evolution of the 3D models of the mammary gland acinus, outlining the key events and relevant papers that brought us to where we are now. We describe how these models are being used to understand the signals that regulate the normal function of mammary epithelial cells, and what we have learned about how those signals are disrupted in cancer. In the second half of this review, we describe questions that continue to be addressed using current models. We then discuss how these models may fall short of describing the physiology of the mammary gland and of breast cancer, and suggest some possible improvements to address questions of greater complexity.
2. The mammary gland
The functional secretory unit of the mammary gland is a highly organized branched ductal network of epithelial cells surrounded by myoepithelium and basement membrane (BM) embedded in a complex stroma (Fig. 1A). In the human, 10–15 ducts arise from the nipple, course through the mammary fat pad, and terminate in clusters of alveoli that are referred to as terminal ductal lobular units (TDLU). Each duct serves one specific lobule of the breast. Lobules are separated by thick connective tissue septae that contain nerves, blood vessels, and lymphatics. Although the mammary epithelium (anlage) is specified in the embryo, the mammary gland is unique amongst organs in that most of the branching morphogenesis required to develop the ductal tree occurs postnatally during puberty. Full functional differentiation of the gland occurs during pregnancy and lactation, leading to further branching of the ductal network to create a system of ducts that collect the milk produced by alveoli. After weaning, the entire gland undergoes involution, wherein mammary epithelial cells die and are replaced by large amounts of adipose tissue and mesenchyme that support the lobules in the non-pregnant adult [4,5]. Greater than 90% of human mammary carcinomas arise from the ductal epithelium [6]. It is, therefore, of great interest to understand (1) how the many signals involved in the intricate architectural regulation of the breast come together to control the organization and function of normal mammary epithelial cells, and (2) how these regulatory cues are disrupted and circumvented in cancer.
Fig. 1.
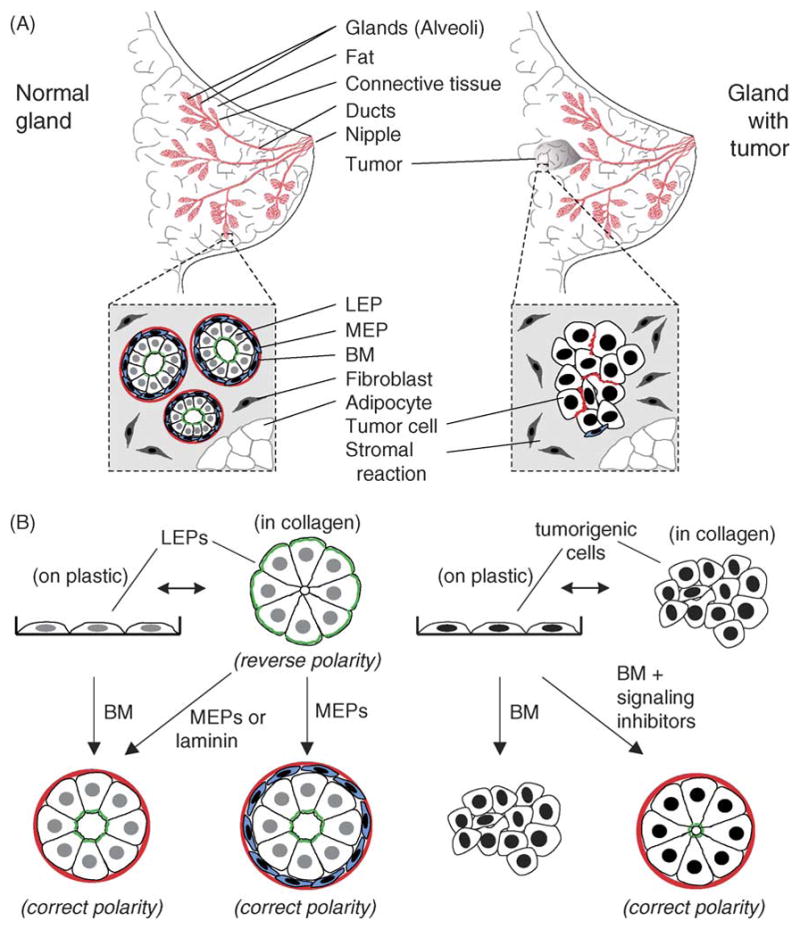
Structure and 3D model of mammary gland. (A) Luminal epithelial cells (LEP) forming the acinus are regulated by homotypic interactions with neighboring luminal cells, by heterotypic interactions with myoepithelial cells (MEP), and by interactions with the BM. The bi-layered structure is surrounded by a complex BM and a stroma comprised of stromal ECM, fibroblasts, and adipocytes, as well as nerves, blood, and lymphatic vessels (not depicted). This structure is disrupted in tumors. Additionally, myoepithelial cells surrounding the tumor have altered function [105,130]. (B) (left) In the 3D model of the normal mammary gland acinus, human or mouse mammary epithelial cells cultured in or on laminin-1-rich, malleable BM will form spherical structures with a central lumen. Addition of lactogenic hormones to mouse cells leads to the synthesis and vectorial secretion of milk proteins into the lumen (milk production by non-malignant human epithelial cells has not yet been achieved). Luminal epithelial cells fail to organize into an acinus when cultured in 3D collagen gels unless they receive signals from myoepithelial cells, which synthesize the laminin components of BM. (right) Oncogenic insults prevent tumorigenic epithelial cells from forming the acinus; these tumorigenic structures can be functionally reverted by restoring normal signaling pathways [52]. (B) modified from [131]. Red, BM; blue, MEPs; white/grey, LEPs; green, sialomucin.
3. Historical perspective: what has been done so far?
3.1. Creation of 3D models of the mammary gland acinus
Development of current 3D models of the mammary gland acinus began almost 30 years ago as a matter of necessity, and proceeded stepwise from the careful observations and clever experimental manipulations of a number of investigators. As with epithelia from many tissues, early attempts to culture and maintain rodent mammary epithelial cells capable of generating physiologically relevant behaviors in response to differentiating cues were met with failure. The cells could be grown and propagated on tissue culture plastic, but this standard method of cell culture failed to maintain mammary epithelial cells that could form acini and produce milk in response to lactogenic hormones [7]. Cultivation of mammary cells in monolayer cultures did not irreversibly damage their potential for differentiation, however, since the cells were able to differentiate into an apparently normal tubular network when subsequently transplanted into the cleared mammary fat pads of mice [8]. Culture conditions were therefore developed to encourage the differentiation of these cells. Inspired by studies showing rat liver differentiation on floating collagen gels [9], Emerman and Pitelka demonstrated that mouse mammary epithelial cells would functionally differentiate under similar conditions [10]. The cells became polar and resumed their native shape, assembled a BM visible by electron microscopy, and arranged themselves such that myoepithelial cells were located on the basal side of the epithelial cells but on the luminal side of the BM, as they are in vivo; importantly, culturing the cells on thick floating collagen I gels in the presence of lactogenic hormones enabled production of some milk proteins [11]. Our laboratory showed that floating these gels could indeed lead to dramatic de novo synthesis and secretion of many milk proteins [12,13]. The induction of milk protein was later shown to be due to the formation of an endogenous BM [14].
Around the same time, investigators trying to understand the biochemistry of the extracellular matrix (ECM) itself were making their own breakthroughs. Non-collagenous ECM proteins, including fibronectin and laminin, were purified, allowing subsequent analysis of their effects on cellular functions [15–17]. Several studies suggested that these molecules, as well as the collagens, were important for embryonic induction and morphogenesis in many tissues [18–20]. Early on, it was postulated [21] and then shown experimentally that specific cell surface ECM receptors were required to convey the biochemical and mechanical stimuli into a cellular and nuclear response [22–25]. It was later realized that many of the ECM receptors formed a large, well-conserved family, renamed integrins [26], which are now understood to play an important role in the regulation of nearly all tissues (for review, see [27]). Additional evidence began to accumulate that the ECM was more than simply an architectural scaffold. Changes in cell shape that were shown to be causal in the regulation of proliferation and other functions [28–30] were found to be affected by, or due to, the ECM in a number of cellular lineages [22,31–34]. The concept that the ECM might regulate chromatin structure and thus the final expression of some genetic information [21] began to gain support; it was thus realized that the cellular (micro)environment was important for regulating tissue-specific gene expression [7,20].
But ECM is not a single entity: the nature and composition of the ECM molecules used as substrata were found to be important in determining growth, differentiation, and survival of mammary epithelial cells. BM containing collagen IV and laminin yielded better expression of mammary-specific functions in epithelial cells than those containing collagen I or fibronectin [33,35]. BM-like gels were isolated, characterized, and found to stimulate functional differentiation in a number of organs [36], exemplified by up-regulation of both transcription and secretion of milk proteins in primary mammary epithelial cells and cell lines [34,35,37,38]. It was determined that laminin-1 was the important molecule in BM for inducing expression of β-casein [39], and that the BM exerted part of its effects by causing the cells to round up [22,34]. In understanding the requirement for laminin-1, it was then understood that the differentiation of mammary epithelial cells cultured in floating collagen gels was a secondary response to cell shape changes which allowed cells to deposit their own laminin-rich BM [10,14].
The discovery of the first ECM-response element in the promoter of the β-casein gene [40,41] paved the way for studies of the relationship between the ECM and nuclear and chromatin structure. Initial studies suggested that chromatin context and histone acetylation/deacetylation were altered by addition of ECM [42,43]. The effects of laminin-1 described above were shown to be transmitted through changes in cell shape to alterations in supramolecular structure of the nucleus [44], and through genome-wide changes in methylation [45]. Clearly, many milk protein genes respond transcriptionally to the presence of correct ECM molecules but some, such as whey acidic protein, have more stringent requirements not only for a polarized structure, but also for a closed acinar structure [46,47]. It should be noted that ECM impacts gene expression both positively and negatively: whereas β-casein is induced by BM, transforming growth factor (TGF)-β is suppressed by BM in mammary epithelial cells [48], and there are other examples [49].
The current model of the mammary gland acinus is depicted in Fig. 1B. Functionally normal mouse or human mammary epithelial cells embedded in laminin-rich BM will form a polarized structure that resembles the normal mammary gland acinus; this polarized “tissue” responds to lactogenic hormones by producing and secreting milk proteins vectorially into the apical lumen [38]. In this way, cells are receiving cues from neighboring cells and the ECM that allow them to replicate functions seen in the mammary gland. What has thus emerged over the past three decades is not simply a cell culture model, but rather a paradigm—that tissue-specific functions can be recapitulated in the laboratory by providing cells with appropriate microenvironmental cues that allow the rebuilding of tissue-specific form. Further, because they are easily amenable to experimental manipulations, these physiologically relevant 3D models allow us to study molecular mechanisms underlying tissue specificity.
3.2. The 3D model of the mammary gland acinus as applied to cancer
Having a model of normal tissue form and function enables one to study how that tissue is disrupted in disease. We and other investigators have found that normal and cancer-derived mammary epithelial cells can be distinguished from each other using the 3D assay described above for rodent cells [38,50]. When cultured within a laminin-rich BM, normal mammary epithelial cells from reduction mammoplasty, as well as cell lines, form polarized acini with central lumina as they growth arrest, whereas carcinoma-derived mammary epithelial cells are unable to organize and instead form disordered colonies that continue to grow [50] (Fig. 1B). The same differences in morphology are found when we compare the behavior of normal and spontaneously tumorigenic clones derived from a human breast cancer progression series [51]. Although it can be difficult to distinguish normal and cancer-derived cells in two-dimensional (2D) cultures, placing the cells in 3D BM reveals not only these gross differences in their morphology, but also differences in their growth rates [50], and in the identity, quantity, and pattern of the proteins they express (for review, see [52]).
The ability of normal cells to differentiate into acini requires signaling through specific integrins, which were shown to play a role not only in growth regulation but also in apoptosis [53,54]. Tumorigenic cells were found to express abnormally high levels of a number of integrins, including β1-integrin, compared to normal epithelial cells when cultured in 3D BM; remarkably, inhibiting β1-integrin signaling to the level present in normal tissue with a function-blocking antibody caused the tumor cells to functionally “revert” to a normal phenotype [55] (Fig. 1B). Reverted cells were essentially indistinguishable from normal cells: they formed functionally polarized acini, reorganized their actin cytoskeletal networks, reformed cell–cell adhesions, down-regulated cyclin D1, up-regulated p21cip1, and stopped growing [55]. Pretreatment with the function-blocking antibody against β1-integrin also dramatically reduced the formation of tumors by the malignant cells when injected into mice. Additional work from our group and others has demonstrated that antagonizing one or two of the many pathways that are de-regulated or up-regulated in the tumorigenic cells will not only cause them to revert [56–59,133], but in some cases will also “normalize” the expression and activation levels of a number of the other signaling pathways. Importantly, these pathways are regulated reciprocally only in 3D and not in 2D cultures [52].
Just as tissue structure and function are connected reciprocally and dynamically, restoring normal tissue structure to tumor cells can restore normal tissue function, even when the cells contain considerable underlying genomic aberrations (reviewed in [52,60]). Therefore, phenotype could dominate genotype in malignant cells as it does in normal tissues (the genes in different tissues and organs are identical, yet the phenotypes are totally distinct). This paradigm has been used successfully in the clinic to treat leukemia [61], and the elucidation of the microenvironmental cues that regulate expression of genetic lesions in other types of tumors is an active area of investigation.
Conversely, the structure of the normal acinus can be disrupted by over-expression of known oncogenes [62–67]. For example, the exogenous expression of an activated form of the epidermal growth factor receptor ErbB2 was found to re-initiate proliferation in the acini, causing the normally hollow lumina to fill up with cells, a phenotype reminiscent of the early stages of breast cancer [64]. And active ErbB2 in conjunction with TGF-β increased the invasiveness of a mammary epithelial cell line, mirroring changes occurring in advanced neoplastic disease and metastasis [66]. Therefore, oncogenic assaults in appropriate experimental contexts will recapitulate the phenotypic consequences of similar assaults in the breast. Although these data are not surprising, they do allow us to begin to “connect the dots” between known genetic and epigenetic aberrations and the structural changes that occur during the progression to cancer.
4. What to do now?
The 3D model of the mammary gland acinus has evolved over the past three decades. Accordingly, our view of the “unit of function” of the mammary gland has evolved as well. In the early stages of the model, we considered the unit of function to be the cell plus its surrounding ECM [21]. As our understanding of the model advanced, it became clear that the synthesis of most milk proteins required cell–cell interactions and closure of the acinus around a central lumen. Thus, the unit of function (in this case, milk production) is greater than the cell plus its ECM environment. We proposed in 1987 that the unit was the gland itself [68,69]. Indeed, it is the formation of this differentiated structure that allows one to study the differences between normal and cancerous phenotypes. We now consider the unit of differentiated function to be the entire organ. It is important to recognize that the evolution of this model and the philosophy behind it were influenced not only by advances in developmental biology, but also by the remarkable technological advances in cell and molecular biology occurring at the time. In this light—and in light of recent progress in genomics, proteomics, bioinformatics, nanotechnology, and engineering—we believe it is time for the model to evolve yet again. In this section of the review, we highlight gaps in our knowledge amenable to study using the current model. We then propose several ways to build upon the model to address even more complex questions in mammary gland biology and breast cancer.
4.1. Breaking it down … (or milking the acinus for all its worth!)
What questions remain to be answered by 3D models of the mammary gland acinus? We know that both a laminin-rich BM and lactogenic hormones are required for functional differentiation, but how do the cells coordinately regulate the information from these ECM and hormonal cues? Signaling from laminin through β1-integrin regulates phosphorylation of the prolactin receptor [70], thus allowing prolactin to regulate the DNA binding activity of the Stat5 transcription factor [71]. Stat5 activity is required for the expression of many milk proteins [41,72–74], and its activation by prolactin requires cytoskeletal integrity [75]. Recent studies have also demonstrated that the collagen IV and laminin components of the BM signal through α2-, α6-, and β1-integrins to alter the expression of the estrogen receptor ER-α [76]. This regulation may be disrupted in cancer cells [77]. Further work is needed to identify the pathways activated by integrin ligation to regulate these hormonal receptors (and vice versa), and how those pathways are disrupted in malignancy. The ECM itself is under hormonal control in the mammary gland [78,79], and further work is required also to understand this reciprocal relationship in mammary gland development and breast cancer.
Aside from inducing signals through integrins, we also know that BM affects the structure of the nucleus, but again, we do not fully understand how. The laminin-dependent activation of the β-casein gene requires its stable integration into the genome [42,80]. This suggests that ECM-dependent genes are regulated in the context of chromatin organization. The expression of the β-casein gene can be modulated by altering the organization of histones, since agents that deacetylate histones activate the β-casein response element but suppress the endogenous expression of β-casein [42]; ECM also induces histone deacetylation [43]. How does laminin cause these structural changes in the nucleus? We hypothesize that some of the signals linking laminin to the nucleus arise through bi-directional connections between the cytoskeleton and the nuclear matrix, a model known as dynamic reciprocity [21,80]. Linkages between the cell-surface plasma membrane and the nucleus have been demonstrated to occur through the actin and intermediate filament cytoskeletal networks in other systems [81–83]. Laminin-induced cell rounding is required but not sufficient for β-casein expression [34,84]; nevertheless, changes in cell shape are known to lead directly to changes in the expression of some milk proteins [29] as well as alterations in nuclear structure [85]. Thus, laminin induces part of its effects on the nucleus indirectly by rounding up the cells. Many unanswered questions remain: How does laminin induce cell rounding? How are the resulting changes in nuclear architecture translated into changes in gene expression? And does cell rounding affect differentiation by mechanisms other than those that occur through changes in nuclear structure? The answers to these questions are currently being pursued in both mammary epithelial cells and a number of other cell types [86,87]. What is clear from these studies is that the cytoskeleton indeed plays a crucial role in connecting the ECM and other signals to the regulation of gene expression in the nucleus [21,132].
The mammary gland model described here appears to be useful for examining the processes that are involved in the formation of a tissue. In this regard, we are beginning to understand how the acinus forms in 3D cultures. Cell–cell and cell–ECM interactions lead to apical–basal polarity followed by apoptosis [88]. Cell death clearly plays an important role (for review, see [89]). In the absence of stimuli from integrin-mediated adhesion to the ECM, mammary epithelial cells undergo apoptosis [54,90,91]; in the 3D model, cells in the center of the forming acinus are isolated from the BM and subsequently die through caspase-mediated apoptosis and autophagy to allow cavitation of the lumen [92–94]. Understanding the signaling pathways leading to lumen formation and the establishment of apical–basal polarity in the acinus are active areas of study in many laboratories and many systems. However, interesting as these data are, it must be remembered that the mammary gland does not create itself from single cells isolated in a 3D gelatinous space, but instead grows out from a pre-existing rudimentary ductal tree. As such, it is unclear whether the same processes that create the lumen in culture are important in the breast, and therefore, any pathway implicated by the model needs to be verified in the appropriate contextual setting in vivo.
4.2. Cancer models: a reality check
Finally, we need to critically interrogate our 3D cell culture models with pathologists’ views of cancer progression. That is, invasive human breast cancers are derived from existing premalignant lesions [95], but the majority of our current culture models of cancer at best compare normal cells to genetically unrelated cells that have been isolated from tumors and propagated for numerous generations on tissue culture plastic. Do we see the same changes in 3D cultures? How do we model the intervening steps? The breast cancer progression series derived from HMT-3522 cells by Briand et al. [96] and Petersen and co-workers [51], and used as described above is the first human breast cancer model to examine premalignancy in a genetically relevant context without the use of dominant oncogenes. While it has already answered a few questions and has the potential to answer a large number of questions, it only describes one set of genomic changes possible in progression to breast cancer. In addition to developing more cell lines relevant for the study of cancer progression, we need to develop models where the rest of the microenvironment surrounding the cancer cells can be recreated. There are significant changes in the composition of the ECM in premalignant lesions leading to cancer. For example, desmoplasia and fibrosis are some of the early stromal reactions that occur and appear to predispose the affected tissues to cancer [97–101]. How are these changes brought about and how can we study them? To do the latter, we believe it is important to model the context as well as the cancer cells.
4.3. Building it up …
What refinements and additions do we need to make to the existing 3D model of the mammary gland acinus? Functional differentiation is dictated by the complexity of the tissue architecture achieved in culture [52]. Although the luminal epithelial cell is the main component of the breast and is responsible for the majority of mammary carcinomas, the organ is comprised of a number of additional cell types likely involved in regulating both normal function and progression to malignancy (see Fig. 1). In this regard, we will not fully understand how the mammary gland maintains tissue-specificity until we develop culture models that faithfully recapitulate its full structural and functional complexity. Early tissue recombination experiments revealed the importance of the stromal compartment for specifying the eventual architecture and differentiated function of the implanted epithelial cells [102,103]. More recent experiments by Runswick et al. [104] and Gudjonsson et al. [105] have shown that the epithelial bilayer (the acinus) can be rebuilt by co-culturing purified luminal epithelial cells with myoepithelial cells in 3D cultures (Fig. 1B). Myoepithelial cells derived from normal glands could direct normal luminal epithelial polarity but those derived from 75% of breast cancers could not [105]. Understanding the cell–cell interactions that regulate the function of the mammary gland is important for understanding tissue-specificity, especially since the myoepithelium is now considered to possess tumor-suppressive properties [105,106]. Having incorporated myoepithelial cells into the 3D model, we can begin to introduce the other cell types and observe their effects on epithelial cell function. In addition to myoepithelial cells, we need to investigate the roles of the stromal components and blood vessels in the normal gland and in cancer since interactions with the stroma regulate not only the normal phenotype, but also the malignant behavior [98,107–111]. Finally, the mammary gland undergoes repeated cycles of remodeling in the adult during the pregnancy cycles. To understand how this impacts the probability for developing cancer, we need to define and investigate the role of the stem cell compartment and the ECM remodeling enzymes expressed in the breast [112].
How do the repeated cycles of remodeling in the breast change the biology of the tissue? Epidemiological findings indicate that nulliparous women have a higher incidence of breast cancer than multiparous women, especially if the latter bear children at a young age, and that increase in parity is associated with a decreased risk for breast cancer, with each additional birth conferring an additional decrease in risk [113]. Why are the epithelial cells of young multiparous women less prone to neoplastic alterations? Does repeated remodeling of the stromal microenvironment play a role in this phenomenon, and if so, how? We lack appropriate culture models to begin to investigate these changes. We could build models of the branched ductal tree that respond to hormonal cues by stimulating the bursts of acinar growth and milk secretion during pregnancy and lactation, followed by the apoptotic cell death and remodeling during involution [114]. This will require us to functionally link our model of the mammary gland acinus with models of mammary gland branching morphogenesis [115–117] in such a way as to recreate both the structure and the temporal differentiation of the gland. Finally, we need to include the stem cells and the mammary fat pad and the function of adipocytes in the gland’s development and remodeling [111,118,119].
4.4. Towards an engineered breast …
Future strategies to recreate the mammary gland (and, indeed, other tissues and organs) in culture will likely be driven by interdisciplinary collaborations. Recent advances in nanotechnology, materials science, and biomedical engineering are unleashing the potential to directly control the microenvironment at multiple levels and scales (Fig. 2). That is, we should have the capability to construct designer tissues in which the chemistry, geometry, and mechanics at every scale—from the nanometer to the centimeter—are controlled precisely. Not only will these strategies allow us to replicate the gland in culture, but such control also gives us the ability to answer questions not amenable to study in vivo using traditional approaches and materials. We still have a very limited understanding of the nature of the ECM signals sensed by mammary epithelial cells. Signaling from laminin-1 through both integrin and non-integrin (e.g., dystroglycan) receptors is required for acinus formation and differentiation [14,22,39,84], but the role of other BM molecules in the differentiation process is unclear. For example, it is not clear why laminin-1 can replace myoepithelial cells in conferring polarity to luminal epithelial cells, but laminin-5 and -10/11 cannot [105]. Some studies suggested that nidogen-1 augments the effects of laminin-1 on functional differentiation of mammary epithelial cells [120]; but the effects of nidogen-1 require the presence of its collagen IV-binding domain, suggesting that cells can sense the differences between individual molecules and an intact BM. Indeed, cells cultured on plastic make copious amounts of BM molecules, but they appear to need a malleable substratum to deposit an intact BM [14]. The BM is a complex mixture of large interacting glycoproteins, each of which contains many cellular binding domains; thus, unraveling the roles of the different molecules is not a trivial problem. However, we can begin to understand the BM/ECM requirement in more detail by taking advantage of recently developed synthetic scaffolds in which the adhesive moieties, proteolytic sites, and mechanical properties are defined precisely [121,122], and by using gels made of purified ECM molecules with inserted or deleted domains (Fig. 2A) [123]. These strategies have been used to study neurite outgrowth and angiogenesis for tissue engineering applications [124,125]. Although they have yet to be applied to mammary gland or cancer biology in detail, studies using biosynthetic and recombinant matrices offer us an additional level of control not available in conventional ECM formats.
Fig. 2.
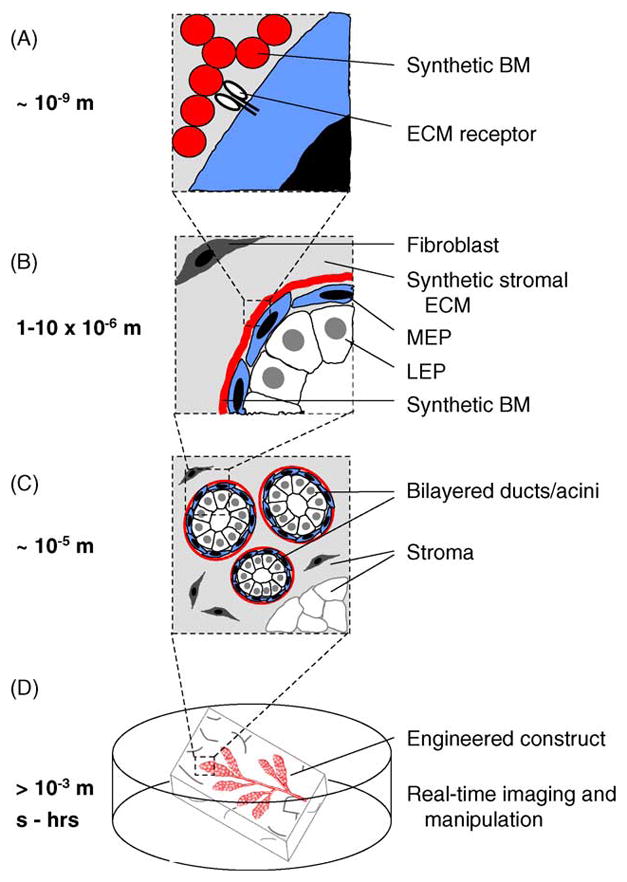
The tissue-engineered breast. New strategies should enable the control of the microenvironment at the nano-, micro-, and macro-scales with temporal precision. (A) Synthetic and recombinant ECM polymers impart cues sensed directly by cell-surface receptors. (B–C) Microfabricated constructs control the positions of multiple cell types with respect to each other and the ECM with micrometer precision across large areas of tissue. (D) Engineered breast tissues that can be visualized and manipulated in real time.
Advances in biological microfabrication are enabling investigators to control the architecture of the microenvironment and the position of cells in three dimensions with micrometer precision. Biomedical engineers are developing a wide variety of techniques to recreate tissue architecture, including photolithographic- and CAD-based techniques that allow rapid prototyping and freeform design (reviewed in [126]). These strategies are currently being pursued to engineer many tissues, including liver, pancreas, and bone. Proper rebuilding of the breast will require the ability to control or direct the position of many types of cells with respect to each other (Fig. 2B and C); recent innovations in laser- and electrophoretic-guided cell deposition offer a few possible approaches [127,128]. The ability to control the positions of cells a priori in 3D culture opens up a tantalizing array of questions to pursue: What is the relationship between the vascular network and the ductal tree? Do adipocytes within the mammary fat pad influence morphogenesis or development of cancer? Does a tumor that forms in one lobule of the mammary gland influence the behaviors of the cells in adjacent or distant lobules?
In addition to the ability to recreate the structure of the mammary gland on the nano-, micro-, and macro-scales, recent advances in imaging, nanotechnology, and synthetic chemistry will enable us to expand the 3D models into the fourth dimension (Fig. 2D). Not only will we be able to image our engineered tissues in real time using two-photon and spinning disk confocal setups, but we will also be able to control the chemical and mechanical makeup of the tissues with spatial and temporal precision by incorporating caged fluorophores and “smart polymers” into the cells and surrounding ECM. Using biosynthetic polymers that incorporate moieties that respond to light, we can activate the polymerization, degradation, and controlled release of molecules including growth factors and morphogens contained within the polymeric network. Light can similarly be used to control the activity of single molecules both intra- and extracellularly using chromophore-assisted laser inactivation (CALI) [129]. Caged fluorophores and quantum dots will allow us to visualize changes occurring in real time as our engineered tissues respond to externally applied morphogenic, hormonal, and oncogenic signals and gradients. In short, we should be able to redefine how the “microenvironment” is studied in culture.
5. Conclusions
We have described the development of relatively simple but “functional” models of the mammary gland acinus that have allowed investigators to probe the tissue-specificity and the complex differences between normal tissue and breast cancer. The models take advantage of the principle that if the appropriate 3D microenvironmental cues are given, cells will respond by displaying their appropriate functional phenotypes in culture, both by elaborating necessary genes, including some crucial myoepithelial and stromal genes, and by responding to, and modifying, the surrounding 3D matrix. We have, therefore, proposed that a full understanding of the complexity of mammary gland development and breast cancer will require us to engineer a model of the mammary gland that takes into account its architectural and developmental complexity, and we have described a number of ways to build upon the current model to achieve that goal. We envision a future model of the breast that will recapitulate its development, regulation, and neoplastic transformation, and the role of stem cells in these processes in such a way as to allow both basic studies of tissue biology and applied analysis of potential targeted therapeutics leading to successful eradication of pain and morbidity associated with breast cancer.
Acknowledgments
We would like to thank Melissa Adriance and other members of the Bissell laboratory for helpful suggestions. This work was supported by grants from the Office of Biological and Environmental Research of the Department of Energy (DE-AC03-76SF00098, MJB), the National Cancer Institute (CA64786, MJB; CA57621, MJB with Zena Werb), and an Innovator award of the Breast Cancer Research Program (BCRP) of the Department of Defense (DOD; DAMD17-02-1-438) to M.J.B. C.M.N. was supported by a postdoctoral fellowship from the BCRP of the DOD (W81XWH-04-1-0582).
Abbreviations
- BM
basement membrane
- ECM
extracellular matrix
- LEP
luminal epithelial cell
- MEP
myoepithelial cell
- TDLU
terminal ductal lobular unit
- TGF-β
transforming growth factor-β
- 2D
two-dimensional
- 3D
three-dimensional
References
- 1.Mettlin C. Global breast cancer mortality statistics. CA Cancer J Clin. 1999;49:138–44. doi: 10.3322/canjclin.49.3.138. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN. Opportunities and challenges in the development of targeted therapies. Semin Oncol. 2004;31:21–7. doi: 10.1053/j.seminoncol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424:870–2. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 4.Osborne MP. Breast development and anatomy. Philadelphia: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 5.Russo J, Russo IH. Development pattern of human breast and susceptibility to carcinogenesis. Eur J Cancer Prev. 1993;2(Suppl 3):85–100. [PubMed] [Google Scholar]
- 6.Silberstein GB. Tumour-stromal interactions. Role of the stroma in mammary development. Breast Cancer Res. 2001;3:218–23. doi: 10.1186/bcr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- 8.Daniel CW, Deome KB. Growth of mouse mammary glands in vivo after monolayer culture. Science. 1965;149:634–6. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975;94:70–8. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- 10.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–28. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 11.Emerman JT, Enami J, Pitelka DR, Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci USA. 1977;74:4466–70. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–55. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EY, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci USA. 1985;82:1419–23. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–15. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada KM, Olden K. Fibronectins—adhesive glycoproteins of cell surface and blood. Nature. 1978;275:179–84. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO, Destree AT, Perkins ME, Wagner DD. Cell surface fibronectin and oncogenic transformation. J Supramol Struct. 1979;11:95–104. doi: 10.1002/jss.400110110. [DOI] [PubMed] [Google Scholar]
- 17.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin—a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–7. [PubMed] [Google Scholar]
- 18.Ekblom P, Alitalo K, Vaheri A, Timpl R, Saxen L. Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci USA. 1980;77:485–9. doi: 10.1073/pnas.77.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spooner BS, Faubion JM. Collagen involvement in branching morphogenesis of embryonic lung and salivary gland. Dev Biol. 1980;77:84–102. doi: 10.1016/0012-1606(80)90458-3. [DOI] [PubMed] [Google Scholar]
- 20.Hay ED. The cell biology of the extracellular matrix. New York: Plenum Press; 1981. [Google Scholar]
- 21.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 22.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–95. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 24.Ruoslahti E, Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:517–8. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- 25.Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, et al. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–82. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- 26.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–54. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 27.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–9. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 29.Farmer SR, Ben-Ze’av A, Benecke BJ, Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978;15:627–37. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- 30.Bissell MJ, Farson D, Tung AS. Cell shape and hexose transport in normal and virus-transformed cells in culture. J Supramol Struct. 1977;6:1–12. doi: 10.1002/jss.400060102. [DOI] [PubMed] [Google Scholar]
- 31.Rath NC, Reddi AH. Collagenous bone matrix is a local mitogen. Nature. 1979;278:855–7. doi: 10.1038/278855a0. [DOI] [PubMed] [Google Scholar]
- 32.Sugrue SP, Hay ED. Response of basal epithelial cell surface and cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981;91:45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wicha MS, Lowrie G, Kohn E, Bagavandoss P, Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci USA. 1982;79:3213–7. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–82. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci USA. 1987;84:136–40. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–8. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 37.Medina D, Li ML, Oborn CJ, Bissell MJ. Casein gene expression in mouse mammary epithelial cell lines: dependence upon extracellular matrix and cell type. Exp Cell Res. 1987;172:192–203. doi: 10.1016/0014-4827(87)90105-4. [DOI] [PubMed] [Google Scholar]
- 38.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–35. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5′ sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA. 1990;87:9118–22. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of beta-casein gene expression. Mol Biol Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, et al. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol. 1998;18:2184–95. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pujuguet P, Radisky D, Levy D, Lacza C, Bissell MJ. Trichostatin A inhibits beta-casein expression in mammary epithelial cells. J Cell Biochem. 2001;83:660–70. doi: 10.1002/jcb.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lelievre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, et al. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci USA. 1998;95:14711–6. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298:122–32. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Chen LH, Bissell MJ. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1989;1:45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin CQ, Dempsey PJ, Coffey RJ, Bissell MJ. Extracellular matrix regulates whey acidic protein gene expression by suppression of TGF-alpha in mouse mammary epithelial cells: studies in culture and in transgenic mice. J Cell Biol. 1995;129:1115–26. doi: 10.1083/jcb.129.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol. 1993;120:253–60. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidhauser C, Casperson GF, Bissell MJ. Transcriptional activation by viral enhancers: critical dependence on extracellular matrix-cell interactions in mammary epithelial cells. Mol Carcinog. 1994;10:66–71. doi: 10.1002/mc.2940100203. [DOI] [PubMed] [Google Scholar]
- 50.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–8. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin Cancer Biol. 1995;6:175–84. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- 52.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–62. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108(Pt 5):1945–57. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- 54.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93:3509–13. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–45. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–6. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol. 2004;164:603–12. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirschi KK, Xu CE, Tsukamoto T, Sager R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996;7:861–70. [PubMed] [Google Scholar]
- 59.Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1-4S, a cell–cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci USA. 2003;100:521–6. doi: 10.1073/pnas.232711199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–95. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tallman MS, Nabhan C, Feusner JH, Rowe JM. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–67. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- 62.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–93. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spancake KM, Anderson CB, Weaver VM, Matsunami N, Bissell MJ, White RL. E7-transduced human breast epithelial cells show partial differentiation in three-dimensional culture. Cancer Res. 1999;59:6042–5. [PubMed] [Google Scholar]
- 64.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–92. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003;163:315–26. doi: 10.1083/jcb.200304159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, et al. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci USA. 2004;101:1257–62. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wrobel CN, Debnath J, Lin E, Beausoleil S, Roussel MF, Brugge JS, et al. CSF-1R activation promotes Src-dependent disruption of mammary epithelial architecture. J Cell Biol. 2004;165:263–73. doi: 10.1083/jcb.200309102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bissell MJ, Barcellos-Hoff MH. The influence of extracellular matrix on gene expression: is structure the message? J Cell Sci Suppl. 1987;8:327–43. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- 69.Bissell MJ, Hall HG. Form and function in the mammary gland: the role of the extracellular matrix. In: Nevell MC, Daniel CW, editors. The mammary gland: development, regulation, and function. New York: Plenum Publishing Corp; 1987. pp. 97–146. [Google Scholar]
- 70.Edwards GM, Wilford FH, Liu X, Hennighausen L, Djiane J, Streuli CH. Regulation of mammary differentiation by extracellular matrix involves protein-tyrosine phosphatases. J Biol Chem. 1998;273:9495–500. doi: 10.1074/jbc.273.16.9495. [DOI] [PubMed] [Google Scholar]
- 71.Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, Schindler C, et al. Stat5 as a target for regulation by extracellular matrix. J Biol Chem. 1995;270:21639–44. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- 72.Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, et al. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol. 2001;155:531–42. doi: 10.1083/jcb.200107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukhopadhyay SS, Wyszomierski SL, Gronostajski RM, Rosen JM. Differential interactions of specific nuclear factor I isoforms with the glucocorticoid receptor and STAT5 in the cooperative regulation of WAP gene transcription. Mol Cell Biol. 2001;21:6859–69. doi: 10.1128/MCB.21.20.6859-6869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gouilleux F, Wakao H, Mundt M, Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994;13:4361–9. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zoubiane GS, Valentijn A, Lowe ET, Akhtar N, Bagley S, Gilmore AP, et al. A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. J Cell Sci. 2004;117:271–80. doi: 10.1242/jcs.00855. [DOI] [PubMed] [Google Scholar]
- 76.Novaro V, Roskelley CD, Bissell MJ. Collagen-IV and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. J Cell Sci. 2003;116:2975–86. doi: 10.1242/jcs.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Novaro V, Radisky DC, Ramos Castro NE, Weisz A, Bissell MJ. Malignant mammary cells acquire independence from extracellular context for regulation of estrogen receptor alpha. Clin Cancer Res. 2004;10:402S–9S. doi: 10.1158/1078-0432.ccr-031209. [DOI] [PubMed] [Google Scholar]
- 78.Shyamala G, Barcellos-Hoff MH, Toft D, Yang X. In situ localization of progesterone receptors in normal mouse mammary glands: absence of receptors in the connective and adipose stroma and a heterogeneous distribution in the epithelium. J Steroid Biochem Mol Biol. 1997;63:251–9. doi: 10.1016/s0960-0760(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 79.Haslam SZ, Woodward TL. Reciprocal regulation of extracellular matrix proteins and ovarian steroid activity in the mammary gland. Breast Cancer Res. 2001;3:365–72. doi: 10.1186/bcr324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boudreau N, Myers C, Bissell MJ. From laminin to lamin: regulation of tissue-specific gene expression by the ECM. Trends Cell Biol. 1995;5:1–4. doi: 10.1016/s0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- 81.Pienta KJ, Coffey DS. Nuclear-cytoskeletal interactions: evidence for physical connections between the nucleus and cell periphery and their alteration by transformation. J Cell Biochem. 1992;49:357–65. doi: 10.1002/jcb.240490406. [DOI] [PubMed] [Google Scholar]
- 82.Fey EG, Wan KM, Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984;98:1973–84. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Mol Biol Cell. 1999;10:2817–28. doi: 10.1091/mbc.10.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sims JR, Karp S, Ingber DE. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J Cell Sci. 1992;103(Pt 4):1215–22. doi: 10.1242/jcs.103.4.1215. [DOI] [PubMed] [Google Scholar]
- 86.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 87.Thomas CH, Collier JH, Sfeir CS, Healy KE. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci USA. 2002;99:1972–7. doi: 10.1073/pnas.032668799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Brien LE, Zegers MM, Mostov KE. Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–7. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 89.Zahir N, Weaver VM. Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev. 2004;14:71–80. doi: 10.1016/j.gde.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 90.Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, et al. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109(Pt 3):631–42. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- 91.Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol. 2000;149:431–46. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blatchford DR, Quarrie LH, Tonner E, McCarthy C, Flint DJ, Wilde CJ. Influence of microenvironment on mammary epithelial cell survival in primary culture. J Cell Physiol. 1999;181:304–11. doi: 10.1002/(SICI)1097-4652(199911)181:2<304::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 93.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 94.Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci USA. 2004;101:3438–43. doi: 10.1073/pnas.0400443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allred DC, Mohsin SK. Biological features of premalignant disease in the human breast. J Mammary Gland Biol Neoplasia. 2000;5:351–64. doi: 10.1023/a:1009573710675. [DOI] [PubMed] [Google Scholar]
- 96.Briand P, Nielsen KV, Madsen MW, Petersen OW. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawal. Cancer Res. 1996;56:2039–44. [PubMed] [Google Scholar]
- 97.Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in breast cancer. Semin Cancer Biol. 1995;6:165–73. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 98.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–73. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petersen OW, Lind Nielsen H, Gudjonsson T, Villadsen R, Ronnov-Jessen L, Bissell MJ. The plasticity of human breast carcinoma cells is more than epithelial to mesenchymal conversion. Breast Cancer Res. 2001;3:213–7. doi: 10.1186/bcr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–88. [PubMed] [Google Scholar]
- 101.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 102.Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–41. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- 103.Cunha GR. Role of mesenchymal-epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer. 1994;74:1030–44. doi: 10.1002/1097-0142(19940801)74:3+<1030::aid-cncr2820741510>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 104.Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–30. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- 105.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res. 1997;3:1949–58. [PubMed] [Google Scholar]
- 107.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–46. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–6. [PubMed] [Google Scholar]
- 109.Schor SL, Schor AM. Phenotypic and genetic alterations in mammary stroma: implications for tumour progression. Breast Cancer Res. 2001;3:373–9. doi: 10.1186/bcr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci. 2004;117:1495–502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- 111.Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, et al. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol. 1998;153:457–67. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 114.Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–82. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, Naundorf H, et al. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998;143:533–45. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J Cell Biol. 1998;140:159–69. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–31. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bartley JC, Emerman JT, Bissell MJ. Metabolic cooperativity between epithelial cells and adipocytes of mice. Am J Physiol. 1981;241:204–C208. doi: 10.1152/ajpcell.1981.241.5.C204. [DOI] [PubMed] [Google Scholar]
- 119.Cases S, Zhou P, Shillingford JM, Wiseman BS, Fish JD, Angle CS, et al. Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development. 2004;131:3047–55. doi: 10.1242/dev.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pujuguet P, Simian M, Liaw J, Timpl R, Werb Z, Bissell MJ. Nidogen-1 regulates laminin-1-dependent mammary-specific gene expression. J Cell Sci. 2000;113(Pt 5):849–58. doi: 10.1242/jcs.113.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caplan MR, Schwartzfarb EM, Zhang S, Kamm RD, Lauffenburger DA. Effects of systematic variation of amino acid sequence on the mechanical properties of a self-assembling, oligopeptide biomaterial. J Biomater Sci Polym Ed. 2002;13:225–36. doi: 10.1163/156856202320176493. [DOI] [PubMed] [Google Scholar]
- 122.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–8. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 123.Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14:551–8. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 124.Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. J Biol Chem. 2000;275:6813–8. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- 125.Zisch AH, Zeisberger SM, Ehrbar M, Djonov V, Weber CC, Ziemiecki A, et al. Engineered fibrin matrices for functional display of cell membrane-bound growth factor-like activities: study of angiogenic signaling by ephrin-B2. Biomaterials. 2004;25:3245–57. doi: 10.1016/j.biomaterials.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 126.Tsang VL, Bhatia SN. Three-dimensional tissue fabrication. Adv Drug Deliv Rev. 2004;56:1635–47. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 127.Odde DJ, Renn MJ. Laser-guided direct writing of living cells. Biotechnol Bioeng. 2000;67:312–8. doi: 10.1002/(sici)1097-0290(20000205)67:3<312::aid-bit7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 128.Voldman J. BioMEMS: building with cells. Nat Mater. 2003;2:433–4. doi: 10.1038/nmat936. [DOI] [PubMed] [Google Scholar]
- 129.Wang FS, Jay DG. Chromophore-assisted laser inactivation (CALI): probing protein function in situ with a high degree of spatial and temporal resolution. Trends Cell Biol. 1996;6:442–5. doi: 10.1016/s0962-8924(96)40005-8. [DOI] [PubMed] [Google Scholar]
- 130.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 131.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–88. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. MMP-3 induced Rac1b stimulates formation of ROS, causing EMT and genomic instability. Nature. 2005 doi: 10.1038/nature03688. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Howlett AR, Petersen OW, Steeg PS, Bissell MJ. A novel function for the nm23-H1 gene: overexpression in human breast carcinoma cells leads to the formation of basement membrane and growth arrest. J Natl Cancer Inst. 1994;86:1838–44. doi: 10.1093/jnci/86.24.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]


