Summary
Nidogen-1 (entactin) acts as a bridge between the extracellular matrix molecules laminin-1 and type IV collagen, and thus participates in the assembly of basement membranes. To investigate the role of nidogen-1 in regulating cell-type-specific gene expression in mammary epithelium, we designed a culture microecosystem in which each component, including epithelial cells, mesenchymal cells, lactogenic hormones and extracellular matrix, could be controlled. We found that primary and established mesenchymal and myoepithelial cells synthesized and secreted nidogen-1, whereas expression was absent in primary and established epithelial cells. In an epithelial cell line containing mesenchymal cells, nidogen-1 was produced by the mesenchymal cells but deposited between the epithelial cells. In this mixed culture, mammary epithelial cells express β-casein in the presence of lactogenic hormones. Addition of either laminin-1 plus nidogen-1, or laminin-1 alone, to mammary epithelial cells induced β-casein production. We asked whether recombinant nidogen-1 alone could signal directly for β-casein. Nidogen-1 did not induce β-casein synthesis in epithelial cells, but it augmented the inductive capacity of laminin-1. These data suggest that nidogen-1 can cooperate with laminin-1 to regulate β-casein expression. Addition of full-length nidogen-1 to the mixed cultures had no effect on β-casein gene expression; however, a nidogen-1 fragment containing the laminin-1 binding domain, but lacking the type IV collagen-binding domain, had a dominant negative effect on β-casein expression. These data point to a physiological role for nidogen-1 in the basement membrane-induced gene expression by epithelial cells.
Keywords: Nidogen-1, Entactin, Basement membrane, Extracellular matrix, Tissue-specific gene expression, Epithelial-mesenchymal interaction, Mammary gland
Introduction
Tissue-specific gene expression in epithelial cells is achieved through integration of multiple and sustained signals from the cellular microenvironment. Mouse mammary epithelial cells isolated from mid-pregnant mammary glands form acinus-like structures if grown on a gel of reconstituted basement membrane (rBM), and differentiate to secrete milk proteins vectorially into a central lumen (Barcellos-Hoff et al., 1989; reviewed in Lin and Bissell, 1993). When these cells are cultured on tissue culture plastic, they rapidly acquire a flat morphology and no longer express milk proteins, even in the presence of lactogenic hormones. Extracellular matrix (ECM) signals also downregulate genes encoding proteins that stimulate cell proliferation such as Id-1, c-myc and cyclin D1, and those that are involved in apoptosis such as caspases (Desprez et al., 1995; Boudreau et al., 1995, 1996). ECM signaling is processed in a hierarchical fashion that is necessary for the subsequent correct processing of the complex microenvironmental cues (reviewed in Roskelley et al., 1995). When lactogenic hormones are present, laminin-1 is sufficient to provide the minimal signal that triggers β-casein gene expression by binding to integrins (Streuli et al., 1995). Loss of mammary tissue-specific function is also associated with loss of basement membrane (BM) in vivo. Involution of the mammary gland is accompanied by an upregulation of the net proteolytic activity, which disrupts BM, and ultimately leads to shrinkage of the milk-secreting alveoli as a result of programmed cell death. In transgenic mice engineered to express either increased or decreased proteolytic activities in their mammary gland, BM formation and degradation correlate with gain or loss of epithelial function, respectively (Sympson et al., 1994; Alexander et al., 1996; Thomasset et al., 1998).
In vivo, BM formation and remodeling take place through epithelial-mesenchymal interactions. This process can be recapitulated in cocultures of isolated epithelial and mesenchymal cells (Reichmann et al., 1989; Schuger et al., 1997; Smola et al., 1998; reviewed in Werb et al., 1996). BM is composed mainly of laminins, collagen type IV, heparan sulfate proteoglycans and nidogen-1, and forms a barrier that physically separates the epithelial and the mesenchymal compartments (Timpl, 1996). Not all the BM components originate from the epithelial cells, but their cell-specific gene expression is BM-dependent. Therefore, some mesenchymal components that are instrumental in BM formation are important for gene expression in epithelium. The assembly of a complete BM macromolecular network requires the cooperation of both epithelial and mesenchymal cells, some of the molecular components being synthesized by one cell type only (Simon-Assmann and Kedinger, 1993; Ekblom et al., 1994; Pujuguet et al., 1994; Dziadek, 1995; Timpl and Brown, 1996).
Nidogen-1 is made by mesenchymal cells and is incorporated into the epithelial BM. It is a sulfated glycoprotein of 150 kDa with three globular domains, G1, G2 and G3, and contains binding sites for other ECM molecules. A high affinity (Kd=0.5 nM) C-terminal binding site for laminin-1 (G3) and a central binding site for type IV collagen (G2) have been identified (Durkin et al., 1988; Fox et al., 1991; Reinhardt et al., 1993; Pöschl et al., 1996). Considering that type IV collagen does not bind strongly to laminin-1, the ability of nidogen-1 to assemble with these two components in ternary complexes implies that it plays a crucial role in organizing supramolecular BM networks (Timpl and Brown, 1996). Nidogen-1 also acts as a cell-adhesive molecule by binding to integrins αvβ3 and α3β1 (Dedhar et al., 1992; Dong et al., 1995). Significantly, the cleavage of nidogen-1 by proteolysis during both normal involution and unscheduled apoptosis induced by overexpression of stromelysin-1 during pregnancy correlates with loss of tissue-specific epithelial function in the mammary gland (Alexander et al., 1996).
In a developmental model, Ekblom et al. (1994) used antibody-perturbing experiments to show that blocking the E3 fragment of the α1 chain of laminin-1, or the nidogen-1-binding site of γ1 chain of laminin-1 prevented BM nucleation and branching morphogenesis (Ekblom et al., 1994; Kadoya et al., 1995, 1997). In a different system using immune cells, ligation of nidogen-1 by the so-called ‘leukocyte-response integrin’ signaled for chemotaxis, while ligation of the G2-domain by α3β1 integrin mediated phagocytosis (Gresham et al., 1996). These two studies independently suggested that nidogen-1 plays a role in BM assembly and signal transduction through integrins.
In the present study, we explored which mammary cell types in vivo synthesized nidogen-1. We then took advantage of a number of functional cell lines, and a three-dimensional (3D) assay developed in our laboratory, to study the role of nidogen-1 in signalling for mammary-specific gene expression.
Materials and Methods
Primary cell cultures
Primary epithelial organoids were prepared from 10-week-old, female CD-1 mice. In brief, #4 glands were removed aseptically and minced with scalpel blades, incubated in a solution containing 0.2% trypsin, 0.2% collagenase A and 5% fetal calf serum in DMEM/F12 (Life Technologies, Gaithersburg, MD, USA) with agitation (100 rpm) for 30 minutes at 37°C. Following incubation, the resulting solution was centrifuged at 750 rpm for 10 minutes. After discarding the fat-containing supernatant, cells in the pellet were resuspended in growth medium supplemented with 1,000 units of DNAse I and incubated for 2 minutes. Cells were then washed once with growth medium. Separation of the single cell fibroblasts from the epithelial organoids was carried out by differential centrifugation in DMEM/F12. About 10 centrifugations at 50 seconds each at 750 rpm were needed to separate most fibroblasts, which were then pooled and cultured separately. The remaining organoids contained myoepithelial and epithelial cells.
Culture of cells in two-dimensional and three-dimensional microenvironments
Cell cultures used are summarized in Table 1. CID-9 cells (Schmidhauser et al., 1990), their clonal derivatives SCp2 and SCg6 (Desprez et al., 1993, 1995), NIH3T3, EPH4 (Reichmann et al., 1989) and TCL1 (Lochter et al., 1997) cell lines were all routinely grown in DMEM/F12, 5% fetal bovine serum, 5 μg/ml insulin (Sigma) (referred to as growth medium). Cells were induced to differentiate in DMEM/F12, by removal of serum and addition of 5 μg/ml insulin with 1 μg/ml hydrocortisone (Sigma) and/or 3 μg/ml prolactin (ID# AFP 10677C, NIDDK, NIH, Bethesda, USA) (referred to as differentiation medium), as previously described (Barcellos-Hoff et al., 1989). For 3D cultures, cells were maintained in suspension culture by coating the wells with 2 mg/ml poly(2-hydroxyethyl methacrylate) (polyHEMA) (Sigma) as described previously (Roskelley et al., 1994). Reconstituted BM (rBM) (Matrigel, Collaborative Biomedical Products, Bedford, MA, USA) was either given as a gel or as an overlay (Roskelley et al., 1994; Streuli et al., 1995). Conditioned medium was prepared in serum-free medium containing insulin (5 μg/ml) and was concentrated by ultrafiltration (Centricon 30, Millipore, Bedford, MA, USA). In some of the experiments, monensin (Calbiochem, San Diego, CA, USA) was added in the culture medium at 5 μM for 12 hours to block vesicular trafficking before harvesting or fixing of the cells (Mendez, 1995).
Table 1. Expression of various ECM molecules by cultured fibroblasts from various cell lines.
| Cell type | Vimentin | Cytokeratin | β-casein | Nidogen-1 | References |
|---|---|---|---|---|---|
| CID-9 | + | + | + (requires high density or exogenous laminin) |
+ | Schmidhauser et al. (1990) |
| SCp2 | − | + | + (requires exogenous laminin) |
− | Desprez et al. (1993, 1995) |
| EPH4 | − | + | + (requires exogenous laminin) |
− | Reichmann et al. (1989) |
| NIH3T3 | + | − | − | + | ATCC catalogue |
| SCg6 | + | − | − | + | Desprez et al. (1993, 1995) |
| TCL1 | + | − | − | + | Lochter et al. (1997) |
See Materials and Methods for details.
Antibodies and extracellular matrix molecules
We used the following antibodies: a murine monoclonal anti-rat β-casein immunoglobulin G (1:2,000 dilution) (Dr Kaetzel, Cleveland, OH, USA) to detect β-casein, a mouse-rat monoclonal anti-mouse nidogen-1 immunoglobulin G (1:600 dilution) (Upstate Biotechnology Inc., Lake Placid, NY, USA) to detect nidogen-1, a rabbit polyclonal anti-mouse laminin (1:2,000 dilution) (Sigma) to detect laminin, a mouse monoclonal anti-human vimentin immunoglobulin G (1:1,000 dilution) (Sigma) to detect vimentin (as a measure of mesenchymal cells), a rabbit polyclonal anti-bovine keratin (1:1,000 dilution) (Dako, Santa Barbara, CA, USA) to detect cytokeratin (as a measure of epithelial cells), a mouse monoclonal anti-human alpha-smooth muscle actin immunoglobulin G (1:1,000 dilution) (Sigma) to detect alpha-smooth muscle actin (as a measure of myoepithelial cells), and a mouse monoclonal anti-human E-cadherin immunoglobulin G (1:1,000 dilution) (Transduction Laboratories, Lexington, KY, USA) to detect E-cadherin. We used a rabbit polyclonal 1015 and its IgG fraction, and the rabbit affinity-purified antibodies N283 and N285, as reagents differentially blocking laminin-1/nidogen-1 binding (Kadoya et al., 1997). Matrigel, laminin-1/nidogen-1 and nidogen-1 free-laminin-1 were purchased from Collaborative, and fibronectin was from Sigma. Recombinant mouse nidogen-1 and recombinant fragment NdI and NdIII were prepared as described previously (Fox et al., 1991; Reinhardt et al., 1993).
Western blots
Conditioned medium and cell lysates extracted in RIPA buffer (1% NP-40, 0.5% deoxycholate, 0.2% SDS, 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, containing a cocktail of protease inhibitors from Calbiochem) were mixed in 10× reducing Laemmli buffer (Laemmli, 1970) and resolved by 8% SDS-PAGE. Proteins were transferred to Immobilon-P nitrocellulose filters, immunoprobed and detected by enhanced chemiluminescence (ECL, Amersham, UK). Blocking solution was from Pierce Chemical (Rockford, IL, USA) or bovine skimmed milk was used. Scanning and densitometric analysis of the E-cadherin were performed as a measure of equal loading (Lochter et al., 1997; Wang et al., 1998).
Immunofluorescence
Cells were cultured on eight-well glass slides (Becton Dickinson, Franklin Lakes, NJ, USA), washed with phosphate-buffered saline (PBS), fixed with methanol/acetone (1:1, v:v) for 5 minutes at −20°C, and washed with PBS. Primary and secondary antibodies were incubated for 1 hour each and followed by washing in PBS-containing 0.1% Tween 20. The coverslips were then stained in 4′,6-diamidino-2-phenylindole (DAPI) (Sigma), washed in water, mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA), and viewed by epifluorescence (Axiovert). Imaging was performed using Xphoto from Solaris™. Controls were stained with secondary antibodies only.
Reverse transcriptase-PCR
cDNA synthesis was performed at 37°C for 3 hours with 2.5 μg of total cellular RNA, prepared by TRIzol (Life Technologies) according to the manufacturer's instructions, and 20 units of reverse transcriptase (Boehringer Mannheim, Indianapolis, IN, USA). Nidogen-1 (GenBank accession number L17322) PCR amplifications were performed with primer 5′ (5′-GGC TGT AGT TGG GCG ATG TGG-3′) and primer 3′ (5′-CCC ACA CAC CCT GGT GCC CAG C-3′). As a control for total RNA integrity, actin RT-PCR experiments were performed with primer 5′ (5′-GCT GGT CGT CGA CAA CGG CT-3′) and primer 3′ (5′-ATG ACC TGG CCG TCA GGC-3′). Absence of genomic DNA was checked by actin PCR before cDNA synthesis. The resulting amplified fragments were analyzed on 1.5% ethidium bromide-stained agarose gels with an Eagle Eye II image analysis system (Stratagene, La Jolla, CA, USA).
Results
Nidogen-1 is expressed in mammary mesenchymal and myoepithelial cells
We determined the pattern of nidogen-1 expression in mouse mammary cells in primary cultures, and in epithelial and fibroblastic cell lines of mammary origin. Cells of mammary mesenchymal origin, but not mammary epithelial cells, expressed nidogen-1 in primary cultures (Fig. 1A,B). When nidogen-1 was detected by immunofluorescence in primary cultures of mammary epithelial cells, these cells also stained for α-smooth muscle actin (Fig. 1C-E). Thus, myoepithelial cells, which surround luminal epithelial cells in the acini, are also capable of expressing nidogen-1.
Fig. 1.

Expression of nidogen-1 by primary epithelial and mesenchymal cells in culture. (A,B) Cells were cultured for 4 days on tissue culture plastic and cell supernatants and lysates were harvested for nidogen-1 immunoblotting. Supernatants from mesenchymal cells (A) (see Materials and Methods) contained the intact 150 kDa and the cleaved 110 kDa forms of nidogen-1, whereas the epithelial-derived conditioned media (B) did not. There is little or no nidogen associated with cell lysates in either case (C-E). Nidogen-1 (red) could be visualized by immunofluorescence in mesenchymal and myoepithelial cells when monensin was used to block protein secretion. Cells were fixed for localization of nidogen-1 and cytoskeletal markers by immunofluorescence. Nidogen-1 staining was associated mostly with vimentin-positive and α-smooth muscle-positive cells (green, C,D), but not with cytokeratin-positive cells (green, E). Blue is the DAPI stain for the nuclei.
The functionally normal mammary epithelial cells, SCp2 and EPH4 (Table 1) did not express nidogen-1 when cultured on tissue culture plastic (Figs 2A,B, 3A-D). However, a fibroblast cell line (3T3), a mixed population of mammary epithelial and mesenchymal cells (CID-9), and mammary epithelial carcinoma cells that had undergone epithelial to mesenchymal transitions (SCg6 and TCL1), all expressed nidogen-1 and vimentin (Figs 2C-F, 3E-L). While the majority of nidogen-1 was soluble in the culture supernatant (Figs 1A, 2C-F) and detectable by immunofluorescence, nidogen-1 was visualized at the interface of the cell membrane and ECM (Fig. 3E,G,I,K). When secretion was blocked by monensin, a general inhibitor of secretion, nidogen-1 was found intracellularly in the mesenchymal cells but not in the epithelial cells (Fig. 3F,H,J,L). Interestingly, in the CID-9 cell line, which consists of both epithelial and mesenchymal cells, immunostaining for nidogen-1 localized to areas surrounding the epithelial compartment (Fig. 3I,K); however, this staining shifted to the mesenchymal compartment upon monensin treatment (Fig. 3J,L). This suggests that in mixed cell populations in culture, nidogen-1 is secreted by the mesenchymal cells, but is retained at the epithelial cell surface, presumably during assembly of BM. Thus, the origin of nidogen-1 is faithfully reproduced in these cell lines. Moreover, even in culture, mammary epithelial cells did not upregulate nidogen-1, and mammary stromal cells maintained their ability to synthesize and secrete it into the culture medium.
Fig. 2.

Expression of nidogen-1 by established epithelial, mesenchymal and carcinoma cell lines in culture. Cells were cultured for 4 days on tissue culture plastic and cell supernatants and lysates were harvested for nidogen-1 immunoblotting. The following cell lines were used: (A) Scp2 cells, (B) EPH4 cells, (C) 3T3 cells, (D) Scg6 cells, (E) TCL1 cells and (F) CID-9 cells (see Table 1 for characterization). Supernatants originating from mesenchymal and carcinoma cells contained the intact 150 kDa and the cleaved 110 kDa forms of nidogen-1, whereas none of the epithelial-derived supernatants contained nidogen-1. However, no nidogen-1 was found in the cell lysates of either the epithelial or mesenchymal cells. The positions of intact and cleaved nidogen-1 are shown on the right.
Fig. 3.
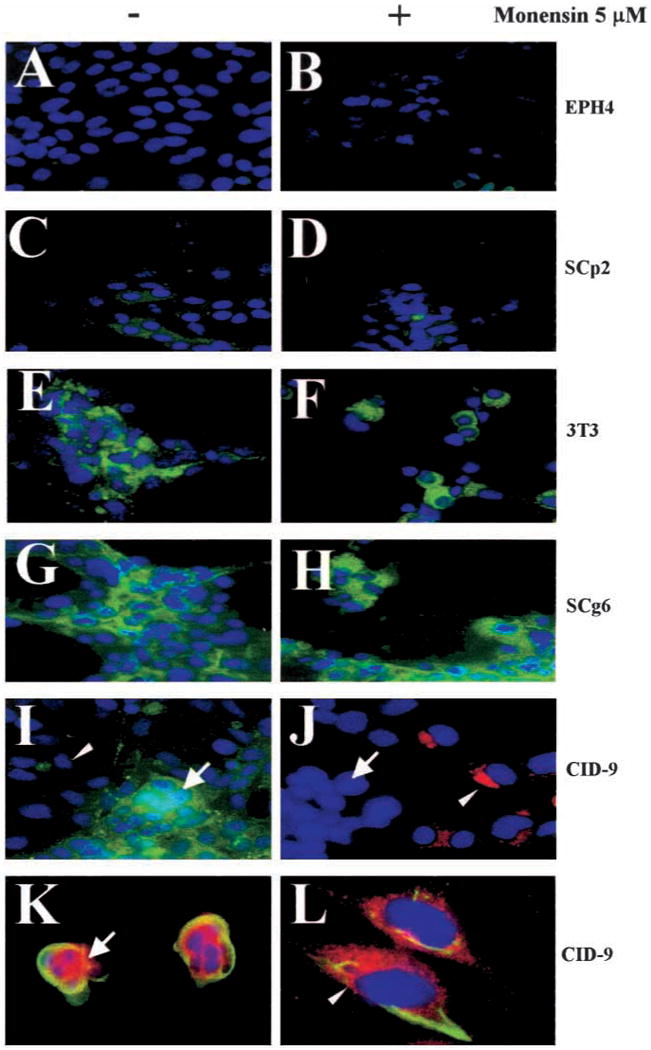
Localization of nidogen-1 in mammary epithelial and mesenchymal cell lines in culture. Cells were grown for 4 days on glass coverslips, then fixed and nidogen-1 visualized by immunofluorescence. The following cell lines were used: (A,B) EPH4 cells, (C,D) Scp2 cells, (E,F) 3T3 cells, (G,H) Scg6 cells and (I-L) CID-9 cells. Nidogen-1 was found in all the mesenchymal cell types (green in E,G,I; red in K), but not in the epithelial cell cultures (A,C). Blocking of the vesicular trafficking with monensin allowed visualization of an intracellular pool of nidogen-1 only in the mesenchymal cell types (green in F,H; red in J,L). Note that in the CID-9 cells treated with monensin (J,L), the nidogen-1 staining clearly was associated with the mesenchymal cells, which have the large nuclei (arrowheads), but not with the epithelial area, which has the small clustered nuclei (arrow). (K,L) Close-up dual immunostaining of nidogen-1 and cytokeratin (green, K) or vimentin (green, L). DAPI nuclear counterstaining is shown in blue.
Recombinant nidogen-1 by itself is not able to trigger β-casein expression
Nidogen-1 may signal through direct interaction with its integrin receptors (Dedhar et al., 1992; Dong et al., 1995), or by crosslinking the BM components so that laminin-1 can signal more efficiently. Indeed, both rBM and laminin-1 preparations that contain nidogen-1 can signal through integrins to regulate mammary cell form and function (Roskelley et al., 1994; Streuli et al., 1995). We compared the production of β-casein in SCp2 cells in response to addition of recombinant nidogen-1 and other BM components. In an overlay assay, SCp2 cells were treated with different concentrations of rBM, recombinant nidogen-1 or fibronectin in culture medium containing lactogenic hormones (Streuli et al., 1995). After 4 days, β-casein levels increased with increasing concentrations of rBM added (Fig. 4). In addition, neither fibronectin nor recombinant nidogen-1 was able to induce β-casein production (Fig. 4). SCp2 cells remained well spread and appeared flat in the presence of recombinant nidogen-1. We have reported previously that the expression of the mammary epithelial differentiation marker β-casein is cell shape-dependent (Roskelley et al., 1994). Thus, the inability of nidogen-1 to induce differentiation in culture could be due to its inability to induce the cytoskeletal reorganization required for ECM molecules to signal in epithelial cells. To test this, we applied an overlay of recombinant nidogen-1 to SCp2 cells cultured on dishes coated with polyHEMA, so that the cells had been preclustered and rounded. Even under these conditions, β-casein was not expressed in SCp2 cells (Fig. 4).
Fig. 4.
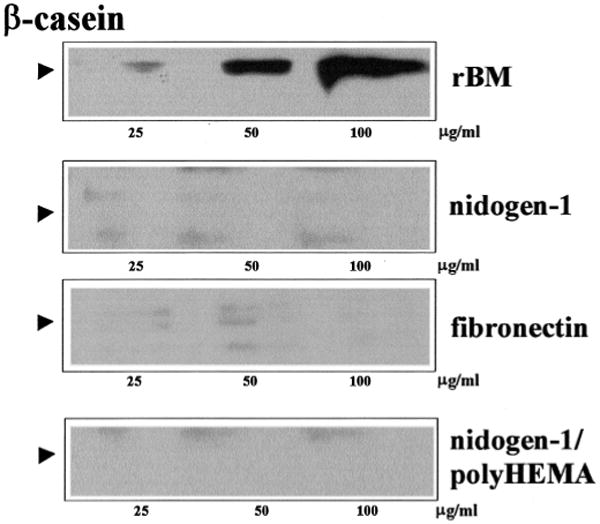
Nidogen-1 does not signal for β-casein expression by itself. SCp2 cells were cultured on tissue culture plastic and overlaid for 4 days with medium containing lactogenic hormones and the indicated amounts of different soluble ECM components marked on the right. Cell lysates were resolved by SDS-PAGE and immunoblotted for β-casein (position marked by arrowhead). rBM, but not nidogen-1 or fibronectin, induced β-casein expression. To determine whether the absence of nidogen-1 signaling was due to the inability of nidogen-1 to induce SCp2 cell rounding, cells were cultured on anti-adhesive polyHEMA-coated tissue culture plastic and overlaid for 4 days with medium containing lactogenic hormones and the indicated amounts of nidogen-1 (lane marked nidogen-1/polyHEMA). Even under these conditions, β-casein was still absent.
Nidogen-1 facilitates laminin-1 signaling in mammary epithelial cells
From the studies described above, it is clear that nidogen-1 does not directly signal for β-casein production. This observation led us to test the hypothesis that nidogen-1 may facilitate laminin-1 signalling by allowing the formation of a more efficient BM. In an overlay assay, rBM, laminin-1 containing nidogen-1 or laminin-1 free of nidogen-1 (Fig. 5A) were added to SCp2 cells cultured on polyHEMA-coated microplates in culture medium containing lactogenic hormones. The expression of β-casein measured after 4 days was dependent on the nature of the ECM molecules and their concentrations. Whereas rBM was the most potent ECM activator of β-casein (Fig. 5B), nidogen-1-containing laminin-1 was more effective at inducing β-casein production than nidogen-1-free laminin-1 (Fig. 5B). Also, addition of recombinant nidogen-1 at the indicated concentrations, to nidogen-1-free laminin-1, was found to increase laminin-1 activity (Fig. 5C). We found that neither exogenous nor endogenous nidogen-1 is required for β-casein production, since treatment with nidogen-1-free laminin-1 could signal for β-casein expression in epithelial cells (Fig. 5B). Lack of induction of endogenous nidogen-1 synthesis was determined by RT-PCR (data not shown). Mesenchymal cells were used as a positive control for nidogen-1 expression. These results suggest that nidogen-1 and laminin-1 collaborate to signal for β-casein gene expression, but that laminin-1 is the primary inducer.
Fig. 5.

Nidogen-1-containing laminin-1 is a better inducer of β-casein than laminin-1 alone. (A) Two different preparations of laminin-1 were immunoblotted for the presence of nidogen-1. Lane 1, laminin-1 alone; lane 2, nidogen-1-containing laminin-1. Note also that the anti-nidogen-1 antibody does not cross-react with laminin-1. (B) SCp2 cells were cultured on polyHEMA-coated tissue culture plastic and overlaid for 4 days with medium containing lactogenic hormones and the indicated amounts of soluble ECM components marked on the right. Cell lysates were resolved by SDS-PAGE and immunoblotted for β-casein. Laminin-1 containing nidogen-1 is a better inducer of β-casein production compared to nidogen-1-free laminin-1. (C) β-casein expression was increased when recombinant nidogen-1 was added to a low amount of nidogen-1-free laminin-1.
A laminin-binding nidogen-1 fragment inhibits β-casein production in cocultures
If the mechanism of action of nidogen-1 is to regulate BM assembly by bridging laminin-1 and type IV collagen, then recombinant separation of the binding domains on nidogen-1 should abrogate its function, and result in decreased β-casein production by epithelial cells. We used a 70 kDa recombinant nidogen-1 fragment (NdIII, Reinhardt et al., 1993) containing the laminin-1, but not the type IV collagen binding domain, as a negative effector of BM assembly (Fig. 6A). We used the mixed population of CID-9 cells, which produce their own nidogen-1 (Figs 2F, 3I-L) and express β-casein in the presence of lactogenic hormones without addition of laminin-1 (Fig. 6B,C). When blocking antibodies to laminin-1/nidogen-1 binding (0-60 μg/ml) were added to the CID-9 clusters, β-casein production was not inhibited (data not shown). However, addition of the NdIII nidogen-1 fragment inhibited β-casein production (Fig. 6C). The full-length nidogen-1 had no effect on β-casein production (Fig. 6C). Also, an NdI-truncated nidogen-1 recombinant fragment containing G1 and G2 domains, but lacking the G3 laminin-1 binding site, did not inhibit β-casein (Fig. 6B). Addition of the recombinant NdIII fragment to a coculture of SCp2 and SCg6 also inhibited β-casein production (not shown). Immunofluorescence of the CID-9 clusters indicated that the majority of the cells were cytokeratin-positive epithelial cells (Fig. 6D), and that few were vimentin-positive mesenchymal cells (Fig. 6D).
Fig. 6.

Disrupting nidogen-1 ligand affinity extinguishes cell-specific gene expression in a mammary epithelial-mesenchymal coculture. (A) Recombinant mouse full-length nidogen-1 (lane 1) and an NdIII nidogen-1 fragment (lane 2) were assessed for purity by SDS-PAGE and silver staining. (B) CID-9 cells, plated for 4 days as clusters on a non-adhesive polyHEMA substratum, were maintained in the presence of prolactin and in the absence or in the presence of a control NdI fragment of nidogen-1. Under these culture conditions, CID-9 cells expressed β-casein without addition of exogenous laminin-1 (lane 0), and NdI did not interfere with this expression. Immunoblotting of E-cadherin was used as a loading control. (C) Under these same conditions, the presence of the laminin-1 binding recombinant NdIII fragment, at the indicated doses, inhibited β-casein, whereas the presence of the full-length nidogen-1 did not. Immunofluorescence of the CID-9 clusters indicated that the majority of the cells were cytokeratin-positive epithelial cells (D), and only a few were vimentin-positive mesenchymal cells (E). Iodine propidium red staining indicates the nuclei.
Discussion
We know more about the biochemistry and the immunolocalization of nidogen-1 than its physiological role as a signaling molecule. During mammary gland involution, the BM is remodeled and mammary cells lose their ability to produce milk-specific genes. This process is mediated by proteinases, such as stromelysin-1 (Talhouk et al., 1991). Involution can be mimicked in vivo in transgenic mice overexpressing stromelysin-1 in the mammary gland (Sympson et al., 1994; Thomasset et al., 1998) or in culture after transfection of stromelysin-1 in mammary epithelial cells (Boudreau et al., 1995, 1996). Nidogen-1 fragments remain cross-linked to laminin-1 and collagen, and persist during BM degradation, possibly implicating them in the loss-of-tissue-specific function (Alexander et al., 1996). The establishment of cell culture models in which cell-BM interactions can be studied under controlled conditions has allowed us to decipher whether nidogen-1 has a signaling role in mammary-specific gene expression. Our data suggest that nidogen-1 plays a critical role in deposition of a functional BM, allowing laminin-1 to signal more efficiently for β-casein expression.
To study nidogen-1 function in a system where gain- and loss-of-function is the outcome of ECM signaling, we first needed to characterize which cell types of the mammary gland express the protein. In vivo, nidogen-1 is synthesized primarily by mesenchymal cell types; however, some epithelial cells have also been shown to synthesize nidogen-1 (Ekblom et al., 1994; Dziadek et al., 1995; Senior et al., 1996). In rat mammary glands, nidogen-1 was reported to be produced by mammary fibroblasts, but not by luminal epithelial or myoepithelial cells (Warburton et al., 1984). We found that while luminal epithelial cells do not produce nidogen-1, it is produced both by vimentin-expressing mesenchymal cells and α-smooth muscle actin-expressing myoepithelial cells. Therefore mammary BM, analogous to skin BM (Fleischmajer et al., 1995; Smola et al., 1998) and intestinal BM (Simon-Assmann et al., 1993; Pujuguet et al., 1994,1996), is a result of epithelial-mesenchymal collaboration. In cocultures of cells from the intestine, the formation of a BM appears to be mandatory for the enterocytic differentiation. Inhibition of the assembly of this BM by abolishing the synthesis of α1 chain of laminin-1, impaired differentiation (De Arcangelis et al., 1996). The presence of laminin-1 was shown to correlate with β-casein production by cocultures of mammary epithelial and mesenchymal cells (Reichmann et al., 1989; Chammas et al., 1994). We had shown previously that a laminin-containing BM is required for mammary-specific gene expression (Barcellos-Hoff et al., 1989; Streuli et al., 1991). The present study has now extended the paradigm by showing that the deposition of a subepithelial BM in the mammary gland is the result of a cooperation between the mesenchymal, the myoepithelial and the epithelial cells. Interestingly, nidogen-1, as well as stromelysin-1, are both produced by mesenchymal cells, which emphasizes the importance of epithelial-mesenchymal interactions in regulating mammary epithelial cell phenotype.
Cell adhesion to nidogen-1 is mediated through two integrins, α3β1 and αvβ3 (Dedhar et al., 1992; Dong et al., 1995). Despite mammary cells' possession of these four integrin subunits (Delcommenne et al., 1995), recombinant nidogen-1 was not sufficient to mediate mammary-specific gene expression. This was true even when the cells were primed by preclustering in order to induce the cell shape changes required for β-casein expression (Roskelley et al., 1994). Because α3β1 integrins may also participate in ECM deposition (Wu et al., 1995), and because we have shown that β1 integrin signaling is necessary for β-casein expression (Streuli et al., 1991), it is possible that this integrin facilitates correct localization of nidogen-1 in relation to laminin-1 to allow laminin-1 to signal more efficiently. The nidogen-1/laminin-1 was more effective in inducing β-casein production than the laminin-1 alone, indicating a functional cooperative process by these two BM molecules in mammary epithelial cells.
Nidogen-1 is believed to be crucial in the formation of BM because it can bridge laminin-1 and type IV collagen (Fox et al., 1991; Dziadek et al., 1995). The high affinity of the laminin-1 γ1 chain binding site for the G3 domain of nidogen-1 has been precisely mapped to a single LE module, γ1III4, by site-directed mutagenesis (Pöschl et al., 1996). The nidogen-1 NdIII fragment used in this study contains the G3 laminin-1 binding domain and the rod-like part, but lacks the N-terminal part encompassing the G1 and G2 domains able to bind type IV collagen. Thus, this fragment binds to laminin-1, but does not support its ternary bridging function (Reinhardt et al., 1993). Introduction of the nidogen-1 NdIII recombinant fragment in the epithelial-mesenchymal mammary cocultures had serious repercussions for β-casein production. Its inhibitory activity in our assay most probably results from its ability to compete with endogenous nidogen-1 for laminin-1 binding. Disruption of laminin/nidogen binding by antibodies, but not by recombinant interfering laminin fragments, was reported to have an inhibitory effect on branching morphogenesis in development of submandibular glands (Kadoya et al., 1997). In our case, where tissue-specific gene expression in mammary epithelial cells was measured, we observed an inhibition when we used recombinant truncated nidogen-1, but not blocking antibodies. These results suggest that the perturbation of the laminin-1/nidogen-1 interaction by targeting different epitopes interferes with more than one function in different cell types. Absence of LAMC1 gene, encoding the laminin γ1-chain, which was recently shown to be an embryonic lethal due to failure of endoderm differentiation, is necessary for BM and nidogen-1 assembly (Smyth et al., 1999). Tissue-specific knockout of the laminin-1 γ1 chain, or of the nidogen-1 binding part of it, would probably result in the absence of function of nidogen-1 and a loss of tissue-specific gene expression. Nidogen-1 knockout mice are also early embryonic lethals, ruling out the possibility of using embryonic nidogen-1−/− fibroblasts, or nidogen-1−/− epithelial cells, in coculture models. The recent identification of the new nidogen isoform nidogen-2 adds complexity to the previous view that nidogen-1 was the only nidogen binding to laminin (Kohfeldt et al., 1998) and raises the possibility of functional compensatory mechanisms.
The gain or loss of mammary-specific gene expression after nidogen-1 manipulation in culture is reminiscent of the in vivo situation in transgenic mice, where proteolysis of the mammary gland BM was selectively perturbed (Sympson et al., 1994; Boudreau et al., 1995). Nidogen-1 was shown to be a BM target of stromelysin-1 in these mice (Alexander et al., 1996). It is possible that, in vivo, the cleavage of nidogen-1 leads to laminin-1 disassembly. Under in vivo conditions of proteolysis, disassembly may proceed by cleavage of a number of different sites in the proteolytically sensitive nidogen-1 molecule (Mayer et al., 1993). While cleavage of nidogen-1 may be involved in disruption of BM function, our study shows that nidogen-1 also has a positive contribution to mammary-specific gene expression by facilitating laminin-1 signaling. Whether the termination of the lactation signals sent by the BM in epithelial cells occurs primarily through a disruption of the laminin-1/ nidogen-1/ type IV collagen ternary complex is an open question.
In conclusion, our report provides a cellular basis for the signaling activity of nidogen-1. This signaling is indirect. Nidogen-1 secreted by mesenchymal and myoepithelial cells is incorporated into BM-like structures in epithelial and mesenchymal cocultures to ensure optimal laminin-1-mediated signals for mammary-specific gene expression. Thus, the ability of nidogen-1 to allow BM ternary complexes, as shown previously in cell-free systems, has physiological consequences for mammary function.
Acknowledgments
We thank Drs C. Hagios, Y. Hirai and A. Lochter, as well as Dinah Levy and Ali Ravanpay, for helpful suggestions and discussion, Dr C. Park for help with image analysis and Ali Ravanpay for additional help with the figures. This work was supported by the US Department of Energy, Office of Biological and Environmental Research (contract DE-AC03-76SF00098) to M.J.B. and by the National Institutes of Health (grant CA-57621) to Z.W. and M.J.B. P.P. was supported by a fellowship from the French League Against Cancer and the Breast Cancer Research Program of California.
References
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chammas R, Taverna D, Cella N, Santos C, Hynes NE. Laminin and tenascin assembly and expression regulate HC11 mouse mammary cell differentiation. J Cell Sci. 1994;107:1031–1040. doi: 10.1242/jcs.107.4.1031. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Neuville P, Boukamel R, Lefebvre O, Kedinger M, Simon-Assmann P. Inhibition of laminin alpha 1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J Cell Biol. 1996;133:417–430. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S, Jewell K, Rojiani M, Gray V. The receptor for the basement membrane glycoprotein entactin is the integrin alpha 3/beta 1. J Biol Chem. 1992;267:18908–18914. [PubMed] [Google Scholar]
- Delcommenne M, Streuli CH. Control of integrin expression by extracellular matrix. J Biol Chem. 1995;270:26794–26801. doi: 10.1074/jbc.270.45.26794. [DOI] [PubMed] [Google Scholar]
- Desprez PY, Hara E, Bissell MJ, Campisi J. Suppression of mammary epithelial cell differentiation by the helix-loop-helix protein Id-1. Mol Cell Biol. 1995;15:3398–3404. doi: 10.1128/mcb.15.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez PY, Roskelley C, Campisi J, Bissell MJ. Isolation of functional cell lines from a mouse mammary epithelial strain: the importance of basement membrane and cell-cell interaction. Mol Cell Diff. 1993;1:99–110. [Google Scholar]
- Dong LJ, Hsieh JC, Chung AE. Two distinct cell attachment sites in entactin are revealed by amino acid substitutions and deletion of the RGD sequence in the cysteine- rich epidermal growth factor repeat 2. J Biol Chem. 1995;270:15838–15843. doi: 10.1074/jbc.270.26.15838. [DOI] [PubMed] [Google Scholar]
- Durkin ME, Chakravarti S, Bartos BB, Liu SH, Friedman RL, Chung AE. Amino acid sequence and domain structure of entactin. Homology with epidermal growth factor precursor and low density lipoprotein receptor. J Cell Biol. 1988;107:2749–2756. doi: 10.1083/jcb.107.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadek M. Role of laminin-nidogen complexes in basement membrane formation during embryonic development. Experientia. 1995;51:901–913. doi: 10.1007/BF01921740. [DOI] [PubMed] [Google Scholar]
- Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoya Y, Chu ML, Mayer U, Timpl R. Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development. 1994;120:2003–2014. doi: 10.1242/dev.120.7.2003. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Schechter A, Bruns M, Perlish JS, Macdonald ED, Pan TC, Timpl R, Chu ML. Skin fibroblasts are the only source of nidogen during early basal lamina formation in vitro. J Invest Dermatol. 1995;105:597–601. doi: 10.1111/1523-1747.ep12323604. [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham HD, Graham IL, Griffin GL, Hsieh JC, Dong LJ, Chung AE, Senior RM. Domain-specific interactions between entactin and neutrophil integrins. G2 domain ligation of integrin alpha3beta1 and E domain ligation of the leukocyte response integrin signal for different responses. J Biol Chem. 1996;271:30587–30594. doi: 10.1074/jbc.271.48.30587. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya Y, Salmivirta K, Talts JF, Kadoya K, Mayer U, Timpl R, Ekblom P. Importance of nidogen binding to laminin gamma1 for branching epithelial morphogenesis of the submandibular gland. Development. 1997;124:683–691. doi: 10.1242/dev.124.3.683. [DOI] [PubMed] [Google Scholar]
- Kohfeldt E, Sasaki T, Gohring W, Timpl R. Nidogen-2: a new basement membrane protein with diverse binding properties. J Mol Biol. 1998;282:99–109. doi: 10.1006/jmbi.1998.2004. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;15:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. see comments. [DOI] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Mann K, Timpl R, Murphy G. Sites of nidogen cleavage by proteases involved in tissue homeostasis and remodelling. Eur J Biochem. 1993;217:877–884. doi: 10.1111/j.1432-1033.1993.tb18316.x. [DOI] [PubMed] [Google Scholar]
- Mendez AJ. Monensin and brefeldin A inhibit high density lipoprotein-mediated cholesterol efflux from cholesterol-enriched cells. Implications for intracellular cholesterol transport. J Biol Chem. 1995;270:5891–5900. doi: 10.1074/jbc.270.11.5891. [DOI] [PubMed] [Google Scholar]
- Poschl E, Mayer U, Stetefeld J, Baumgartner R, Holak TA, Huber R, Timpl R. Site-directed mutagenesis and structural interpretation of the nidogen binding site of the laminin gamma1 chain. EMBO J. 1996;15:5154–5159. [PMC free article] [PubMed] [Google Scholar]
- Pujuguet P, Hammann A, Martin F, Martin M. Abnormal basement membrane in tumors induced by rat colon cancer cells. Gastroenterology. 1994;107:701–711. doi: 10.1016/0016-5085(94)90117-1. see comments. [DOI] [PubMed] [Google Scholar]
- Pujuguet P, Hammann A, Moutet M, Samuel JL, Martin F, Martin M. Expression of fibronectin ED-A+ and ED-B+ isoforms by human and experimental colorectal cancer. Contribution of cancer cells and tumor-associated myofibroblasts. Am J Pathol. 1996;148:579–592. [PMC free article] [PubMed] [Google Scholar]
- Reichmann E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1989;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Mann K, Nischt R, Fox JW, Chu ML, Krieg T, Timpl R. Mapping of nidogen binding sites for collagen type IV, heparan sulfate proteoglycan, and zinc. J Biol Chem. 1993;268:10881–10887. [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5′ sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA. 1990;87:9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuger L, Skubitz AP, Zhang J, Sorokin L, He L. Laminin alpha1 chain synthesis in the mouse developing lung: requirement for epithelial-mesenchymal contact and possible role in bronchial smooth muscle development. J Cell Biol. 1997;139:553–562. doi: 10.1083/jcb.139.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Pierce RA. Entactin expression by rat lung and rat alveolar epithelial cells. Am J Resp Cell Mol Biol. 1996;14:239–247. doi: 10.1165/ajrcmb.14.3.8845174. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P, Kedinger M. Heterotypic cellular cooperation in gut morphogenesis and differentiation. Semin Cell Biol. 1993;4:221–230. doi: 10.1006/scel.1993.1026. [DOI] [PubMed] [Google Scholar]
- Smola H, Stark HJ, Thiekotter G, Mirancea N, Krieg T, Fusenig NE. Dynamics of basement membrane formation by keratinocyte-fibroblast interactions in organotypic skin culture. Exp Cell Res. 1998;239:399–410. doi: 10.1006/excr.1997.3910. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression [published erratum appears in J. Cell Biol. 1996 Feb;132(4):following 752] J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, Werb Z, Bissell MJ. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. Supramolecular assembly of basement membranes. BioEssays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–1486. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton MJ, Monaghan P, Ferns SA, Rudland PS, Perusinghe N, Chung AE. Distribution of entactin in the basement membrane of the rat mammary gland. Evidence for a non-epithelial origin. Exp Cell Res. 1984;152:240–254. doi: 10.1016/0014-4827(84)90249-0. [DOI] [PubMed] [Google Scholar]
- Werb Z, Sympson CJ, Alexander CM, Thomasset N, Lund LR, MacAuley A, Ashkenas J, Bissell MJ. Extracellular matrix remodeling and the regulation of epithelial-stromal interactions during differentiation and involution. Kidney Int Suppl. 1996;54:S68–S74. [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chung AE, McDonald JA. A novel role for alpha 3 beta 1 integrins in extracellular matrix assembly. J Cell Sci. 1995;108:2511–2523. doi: 10.1242/jcs.108.6.2511. [DOI] [PubMed] [Google Scholar]


