Abstract
Increased expression of matrix metalloproteinases (MMPs) is associated with nearly every tumor type. Although many studies have shown that MMPs can promote malignancy, recent evidence has revealed that MMPs can play a causative role also in the earliest stages of cancer development. A complex story is now emerging in which MMPs not only compromise cell–cell and cell–substratum adhesion processes that impact genomic surveillance mechanisms but also act directly on molecules at the cell surface to stimulate physiological processes that cause genetic alterations. Delineating the mechanisms involved in these processes and identifying how they are coordinated in vivo could aid identification of the crucial contribution of MMPs to tumorigenesis.
Introduction
Many mechanisms exist to maintain genomic integrity within cells: such systems recognize and correct damaged DNA, regulate the proper timing and accurate replication of the genetic material, and faithfully segregate the DNA into the daughter cells. It has long been known that extracellular factors have an impact on the function of these regulatory systems, but more-recent studies are revealing the specific mechanisms involved and providing an increased appreciation of how subtle alterations of the cellular microenvironment can dramatically influence cell fate and function. Matrix metalloproteinases (MMPs) have attracted particular attention as microenvironmental modulators, owing to their widespread expression and their ability to target molecules involved in cell–cell and cell–extracellular matrix (ECM) interactions, to activate soluble growth factors and cell surface receptors, and to facilitate paracrine signaling pathways [1,2]. Because all of these functions are involved also in tumor development and progression, and because MMPs are found to be upregulated in nearly all cancers and cancer cell lines, MMPs began to be viewed as potential therapeutic targets. However, clinical trials testing MMP inhibitors in late-stage cancer patients have been very disappointing [3], and attempts to find specific inhibitors were largely halted.
Nevertheless, MMPs are highly overexpressed in many forms of cancers. Furthermore, recent studies have shown that MMPs may be most important in tumorigenesis at the earliest stages and suggest that a role for MMPs in causing genomic instability may be a key component of this action. In this review, we discuss how MMPs might act as carcinogens by promoting epithelial–mesenchymal transition, often observed when tumors become invasive, and by affecting metabolic pathways and producing chemical agents that destabilize the genome.
MMPs and malignancy
MMPs were originally studied because of their ability to degrade nearly all structural components of the ECM, and, as a consequence, the common names of MMPs reflect their primary preferences for these substrates. As more members of the family have been discovered, a systematic renaming has been instituted according to structural homology, although the original names continue to be used [1]. Despite the distinctions suggested by the nomenclature, the MMPs show broad and overlapping substrate preferences, which might explain why transgenic animals lacking expression of particular MMPs can show quite subtle phenotypes [1]. Generally, MMPs are classified into two categories: those that are soluble, and those that are membrane-associated. Recent attention has focused on membrane-bound (membrane-type; MT) MMPs as modulators of the pericellular microenvironment, but there is considerable overlap here as well, because soluble MMPs can be localized at the cell surface through association with integrins or CD44 [4–7]. The actions of MMPs are balanced by endogenous inhibitors, of which the principal members are the tissue inhibitors of metalloproteinases (TIMPs). Four TIMPs have been identified, and these also show broad and overlapping effectiveness against different MMPs [8].
Many of the MMPs were initially cloned as cancer-specific genes [9], and a causal relationship between the upregulation of MMPs in tumors and progression to malignancy has long been suspected [3]. MMP-mediated degradation of ECM facilitates angiogenesis, tumor invasion and metastasis, and also releases molecules that have pro-tumorigenic properties [10]. By targeting cell surface molecules, MMPs can stimulate increased proliferation, resistance to apoptosis, and activation of growth factors and growth factor receptors (Figure 1). Exposure of mammary epithelial cells to MMPs can directly cause an epithelial–mesenchymal transition [11,12], a physiological process whereby cells become more invasive and mesenchymal-like [13], which can lead to metastasis in the pathological context of cancer. Promotion of tumors by MMPs has been demonstrated by several studies in transgenic mice; in two dramatic examples, MMP-9 was found to be vital in the development of tumor angiogenesis [14] and in the progression of squamous-cell carcinoma [15,16]. In addition to modifying the stroma, however, MMPs can promote malignancy by acting directly on tumor cells themselves. Studies in which MMPs were expressed in the epithelial cells of the mammary gland under control of mouse mammary tumor virus [17] or the whey acidic protein (WAP) [18] promoters have revealed that expression of MMPs can act also as tumor initiators. This implies that MMPs, much like tumor viruses, can be considered complete carcinogens — albeit acting more gradually, which, in fact, mimics human cancer more accurately.
Figure 1.
MMPs impact cellular function. Exposure of epithelial cells to MMPs causes breakdown of (a) cell–ECM and (b) cell–cell interactions, compromising cell adhesion and potentially suppressing genomic surveillance mechanisms. Cleavage of E-cadherin results in (c) generation of bioactive cadherin fragments and (d) release of transcriptionally active junctional proteins into the cytoplasm; (e) activation of mitochondrial production of superoxide elevates cellular levels of ROS, which (f) directly damage DNA and (g) activate other transcriptional regulators.
MMP3 and mammary carcinogenesis
Analysis of the WAP–MMP3 transgenic mice revealed how the tumor-promoting effects of MMP3 are related to its physiological functions. Normally, MMP3 is produced primarily by mammary stromal cells, with the highest levels of expression occurring during the post-lactational developmental stage, a time of maximal ECM remodeling, and regression of glandular structures [19–21]. Directed expression of MMP3 in the milk-producing epithelial cells of the mammary gland causes increased proliferation and branching morphogenesis of ductal epithelial cells, and apoptosis of secretory alveolar cells [19,22]. Sustained expression of the transgenic MMP3 in mammary epithelial cells upregulates expression of endogenous MMP3 in the surrounding stromal cells to levels much greater than those produced by the transgene. This results in hyperplasia, dysplasia and other changes in the stromal components of the mammary gland [22–24], and eventual progression to full malignancy in some mouse backgrounds, such as CD-1 mice [18,25]. Analysis of the tumors that developed in MMP3 transgenic mice by comparative genomic hybridization revealed non-random chromosomal alterations [18], demonstrating that MMP3 was causing genomic instability as well as contributing to the progression and development of the resultant tumors.
Compromising genomic integrity
Genomic alterations ranging from single DNA base changes to gross chromosomal aneuploidy are found in nearly all tumors [26], and the high degree of genetic heterogeneity in tumors suggests that genomic instability is an ongoing process throughout tumor development. Because genetic damage is so detrimental to the cell and the organism, cellular surveillance mechanisms continuously monitor the genome for alterations or damage, directing either cell cycle arrest and DNA repair or apoptosis, depending upon the extent of the damage [27]. Attention to the connections between the microenvironment and the genome has been growing steadily, and several studies have investigated how alterations in cellular adhesion can attenuate key mechanisms of genomic surveillance [28•,29–31]. Researchers in the Tlsty laboratory [30,31] showed that loss of cell adhesion reduced expression of p53 in keratinocytes by 80%. Schwartz and colleagues [28•,29] extended these findings to additional cell types and, furthermore, showed that loss of adhesion also attenuated DNA damage responses mediated by c-Abl, and that adhesion-dependent loss of genomic surveillance mechanisms significantly increased DNA damage-induced genomic instability. Although the specific mechanisms by which loss of adhesion suppressed p53 and c-Abl genomic surveillance were not identified in these studies, it is relevant that MMPs target molecules involved in cell–ECM adhesion [32], as well as directly cleaving the cell–cell adhesion molecule E-cadherin [11,33].
Stimulating genomic alterations
Although loss or decreased function of genomic surveillance mechanisms facilitates the development of genomic instability, their absence is not sufficient to destabilize the genome [27]. One mechanism by which MMPs can stimulate genomic alterations in cells lacking p53 is by forcing cell cycle progression. Activation of cell cycle mediators in the absence of p53 is sufficient to induce genomic instability and malignancy [34], and expression of MT1–MMP in squamous carcinoma cells cultured in collagen gels was shown to cause expression of cyclins, cell cycle entry, and stimulation of proliferation [35•]. It should be emphasized that the causative agent is the combination of MT1–MMP and collagen I, and not the method of culturing cells in three dimensions, which, under more physiological conditions such as the use of laminin-containing ECM (or basement membrane substitutes), is known to preserve the differentiation function of epithelial cells.
That MMPs can cause genomic alterations by activating genotoxic cellular metabolic pathways was the finding of a recent study that dissected the mechanism by which MMP3 caused measurable genomic instability in p53-deficient mouse mammary epithelial cells [36••]. Exposure to MMP3 stimulated production of Rac1b, an alternatively spliced and highly activated isoform of Rac1 [37]. Rac1b was originally identified in breast and colorectal tumors [38,39] and has been shown to stimulate accumulation of cyclin D1, cell cycle progression, apoptosis resistance, and cellular transformation [40•,41•]. The finding that MMP3 could influence splicing processes was reminiscent of another recent study in which conditioned medium from cultured mouse mammary fibro-blasts stimulated production of an alternatively spliced isoform of fibronectin [42•], because conditioned medium from these cells is enriched with MMPs (DC Radisky and MJ Bissell, unpublished).
Production of Rac1b in cells treated with MMP3 led to marked elevations in levels of reactive oxygen species (ROS) through production of mitochondrial superoxide. ROS have long been linked with cancer because they can directly react with genomic DNA to cause oxidative lesions and inactivate proteins responsible for DNA repair [43]. ROS can also act as signaling molecules and have been shown to stimulate cell proliferation by activation of mitogenic signal transduction pathways and by inhibition of cell cycle checkpoint controls; moreover, increased levels of cellular ROS also stimulates expression of MMPs, further enhancing the malignant potential of transformed cells [44]. It is important to note that there are many different species of ROS within cells, and that each has distinct biochemical and signaling properties; however, due to facile interconversion of ROS in the cytoplasm [44], sustained production of a single oxygen species can cause elevation of the levels of the other species.
Another mechanism by which MMPs can stimulate genomic instability is by compromising normal cytokinesis, which can directly stimulate aneuploidy and malignant transformation. In a recent study, overexpression of MT1–MMP in MDCK (Madin–Darby canine kidney) cells caused significant increases in DNA content and chromosome number, and multi-polar and misaligned mitotic spindle formation [45•]. Yet another recent study suggests the possibility of a reciprocal relationship between MMP expression and genomic instability, because induction of cytokinetic failure in p53-null primary mouse mammary epithelial cells caused immediate genetic instability, and tumors derived from these cells showed selective, 8- to 30-fold gene amplification of a chromosomal locus containing a cluster of MMP genes [46••].
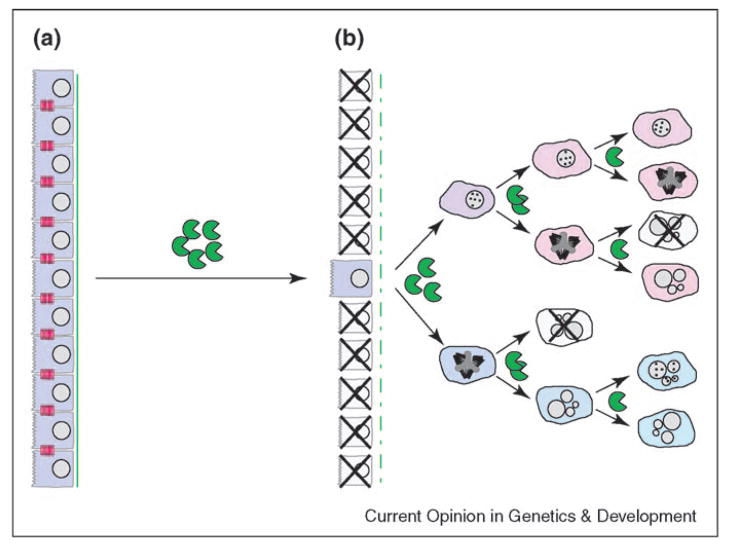
These observations suggest a model by which MMPs act to initiate tumors (Figure 2). First, exposure to MMPs alters cell–ECM or cell–cell adhesion by cleaving ECM molecules or E-cadherin, respectively, thereby inhibiting the activity of genomic surveillance mechanisms, such as p53 signaling, through alteration of cell adhesion. Although the majority of cells exposed to MMPs will undergo detachment-dependent apoptosis [32], a small minority survive MMP3 treatment and show persistent resistance to MMPs as well as to other genotoxic reagents (DC Radisky and MJ Bissell, unpublished). Cells in which p53 function is suppressed will then be sensitive to genetic alterations caused by ROS [36••] as well as by MMP-induced centrosome defects [45•]. Further upregulation of MMP expression in the surrounding stromal cells [24] or directly within the tumor cells [12,46••] would create a feedback loop, producing additional genomic alterations while promoting the tumor progression.
Figure 2.
Model of MMP-induced genomic instability. (a) Compromising genomic surveillance. Degradation of cell–cell and cell–ECM adhesions by MMPs can cause apoptosis, but may select for cells with compromised apoptosis response pathways. Although pre-existing mutations in genomic surveillance pathways could be selected by this process, loss of adhesion can directly suppress p53 and Abl function. (b) Inducing genetic alterations. Cells selected for apoptosis resistance can continue to proliferate despite MMP-induced genetic alterations from oxidative DNA damage and altered cytokinesis; although some of the resultant progeny will not be viable, the remainder can manifest progressive genomic alterations and eventual malignancy.
Conclusions
The increased understanding of the molecular mechanisms involved in MMP-induced genomic instability has come almost entirely from cell culture and animal studies. It is unclear how well these processes reflect the development of human tumors, although the presence of fields of genomic instability in histologically normal tissues adjacent to breast tumors has prompted the proposal that soluble elements of the tumor microenvironment can directly stimulate genomic alterations in neighboring tissues [47]. Given that MMPs are ubiquitous elements of the tumor microenvironment and have been shown to induce genomic instability under experimental conditions, they are an obvious candidate as one of the mediators of this phenomenon in human cancers.
A role for MMPs in causing genomic instability in human tumors is also consistent with the results from the clinical trials of MMP inhibitors [3]. That these inhibitors were largely unsuccessful with late-stage disease cancer patients [3] is not surprising given that one of the key functions of MMPs in tumor development may be the induction of genomic instability, because genetic alterations play a role in the earliest stages of tumor development [48,49]. Another commonly cited reason for the failure of the MMP inhibitor clinical trials is the lack of specificity of the tested inhibitors [2], which would have effectively inhibited the normal physiological functions of MMPs. This possibility highlights the importance of studying how MMPs cause genomic instability, because although general inhibitors of MMPs may block normal as well as tumor-promoting activities, targeting the genome-destabilizing effects of MMPs will probably only block their carcinogenic effects. The discovery that MMPs stimulate production of Rac1b, a splice variant of Rac1 that has transforming properties, provides an example of such a target; further investigations will doubtless reveal more.
Acknowledgments
We would like to thank members of the Bissell laboratory for editorial comments and helpful suggestions. This work was supported by grants from the Office of Biological and Environmental Research of the Department of Energy (DE-AC03-76SF00098, MJB), the National Cancer Institute (CA64786, MJB; CA57621, MJB with Zena Werb), an Innovator award of the Breast Cancer Research Program (BCRP) of the Department of Defense (DOD; DAMD17-02-1-438) to MJB, and research support from the Mayo Foundation and the Mayo Comprehensive Cancer Center to DCR.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 4.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 5.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori H, Tomari T, Koshikawa N, Kajita M, Itoh Y, Sato H, Tojo H, Yana I, Seiki M. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J. 2002;21:3949–3959. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 9.McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6:149–156. doi: 10.1016/s1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 10.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 11.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem. 1997;272:5007–5015. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- 13.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 14.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha HY, Moon HB, Nam MS, Lee JW, Ryoo ZY, Lee TH, Lee KK, So BJ, Sato H, Seiki M, et al. Overexpression of membrane-type matrix metalloproteinase-1 gene induces mammary gland abnormalities and adenocarcinoma in transgenic mice. Cancer Res. 2001;61:984–990. [PubMed] [Google Scholar]
- 18.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Dano K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, Werb Z, Bissell MJ. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 27.Duensing A, Duensing S. Guilt by association? p53 and the development of aneuploidy in cancer. Biochem Biophys Res Commun. 2005;331:694–700. doi: 10.1016/j.bbrc.2005.03.157. [DOI] [PubMed] [Google Scholar]
- •28.Truong T, Sun G, Doorly M, Wang JY, Schwartz MA. Modulation of DNA damage-induced apoptosis by cell adhesion is independently mediated by p53 and c-Abl. Proc Natl Acad Sci USA. 2003;100:10281–10286. doi: 10.1073/pnas.1635435100. This study shows that compromised cell-adhesion can suppress genomic surveillance by the p53 and Abl response pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis JM, Truong TN, Schwartz MA. Integrins regulate the apoptotic response to DNA damage through modulation of p53. Proc Natl Acad Sci USA. 2002;99:3627–3632. doi: 10.1073/pnas.062698499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tlsty TD. Cell-adhesion-dependent influences on genomic instability and carcinogenesis. Curr Opin Cell Biol. 1998;10:647–653. doi: 10.1016/s0955-0674(98)80041-0. [DOI] [PubMed] [Google Scholar]
- 31.Nigro JM, Aldape KD, Hess SM, Tlsty TD. Cellular adhesion regulates p53 protein levels in primary human keratinocytes. Cancer Res. 1997;57:3635–3639. [PubMed] [Google Scholar]
- 32.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 34.Felsher DW, Bishop JM. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3940–3944. doi: 10.1073/pnas.96.7.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •35.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. The authors define the molecular mechanism by which MT1–MMP confers growth advantage on cells cultured within three-dimensional collagen matrices. [DOI] [PubMed] [Google Scholar]
- ••36.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. This study describes the molecular mechanisms by which exposure to MMP3 stimulates genomic instability and phenotypic alterations in mammary epithelial cells through Rac1b-induced elevation of reactive oxygen species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matos P, Collard JG, Jordan P. Tumor-related alternatively spliced Rac1b is not regulated by Rho-GDP dissociation inhibitors and exhibits selective downstream signaling. J Biol Chem. 2003;278:50442–50448. doi: 10.1074/jbc.M308215200. [DOI] [PubMed] [Google Scholar]
- 38.Jordan P, Brazao R, Boavida MG, Gespach C, Chastre E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene. 1999;18:6835–6839. doi: 10.1038/sj.onc.1203233. [DOI] [PubMed] [Google Scholar]
- 39.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 40•.Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene. 2004;23:9369–9380. doi: 10.1038/sj.onc.1208182. See annotation [41•] [DOI] [PubMed] [Google Scholar]
- •41.Matos P, Jordan P. Expression of Rac1b stimulates NF-κB-mediated cell survival and G1/S progression. Exp Cell Res. 2005;305:292–299. doi: 10.1016/j.yexcr.2004.12.029. This study, together with that by Singh et al. [40•], shows that the newly discovered Rac1b is highly activated and has oncogenic activities distinct from those of Rac1. [DOI] [PubMed] [Google Scholar]
- •42.Blaustein M, Pelisch F, Coso OA, Bissell MJ, Kornblihtt AR, Srebrow A. Mammary epithelial-mesenchymal interaction regulates fibronectin alternative splicing via phosphatidylinositol 3-kinase. J Biol Chem. 2004;279:21029–21037. doi: 10.1074/jbc.M314260200. This study provides insight into the molecular pathways by which a fibroblast-conditioned medium known to be rich in MMPs controls alternative RNA-splicing. [DOI] [PubMed] [Google Scholar]
- 43.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 44.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- •45.Golubkov VS, Boyd S, Savinov AY, Chekanov AV, Osterman AL, Remacle A, Rozanov DV, Doxsey SJ, Strongin AY. Membrane type-1 matrix metalloproteinase (MT1-MMP) exhibits an important intracellular cleavage function and causes chromosome instability. J Biol Chem. 2005;280:25079–25086. doi: 10.1074/jbc.M502779200. This study shows that expression of MT1–MMP stimulates chromosomal aberrations and cytokinetic failure, and presents evidence suggesting an intracellular role for MT1–MMP in these effects. [DOI] [PubMed] [Google Scholar]
- ••46.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. This study shows that transiently blocking cytokinesis in mouse mammary epithelial cells stimulates aneuploidy and the development of malignancy, and that tumors derived from these cells show selective amplification of an MMP gene cluster. [DOI] [PubMed] [Google Scholar]
- 47.Ellsworth DL, Ellsworth RE, Liebman MN, Hooke JA, Shriver CD. Genomic instability in histologically normal breast tissues: implications for carcinogenesis. Lancet Oncol. 2004;5:753–758. doi: 10.1016/S1470-2045(04)01653-5. [DOI] [PubMed] [Google Scholar]
- 48.Pihan GA, Wallace J, Zhou Y, Doxsey SJ. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003;63:1398–1404. [PubMed] [Google Scholar]
- 49.Shih IM, Zhou W, Goodman SN, Lengauer C, Kinzler KW, Vogelstein B. Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res. 2001;61:818–822. [PubMed] [Google Scholar]