Abstract
Abstract. The majority of human breast carcinomas exhibit luminal characteristics and as such, are most probably derived from progenitor cells within the luminal epithelial compartment. This has been subdivided recently into at least three luminal subtypes based on gene expression patterns. The value of knowing the cellular origin of individual tumours is clear and should aid in designing effective therapies. To do this, however, we need strategies aimed at defining the nature of stem and progenitor cell populations in the normal breast. In this review, we will discuss our technical approach for delineating the origin of the epithelial cell types. A major step forward was the purification of each cell type by the application of immunomagnetic cell sorting based on expression of lineage‐specific surface antigens. We then developed chemically defined media that could support either the luminal epithelial or the myoepithelial cell phenotype in primary cultures. Having succeeded in continuous propagation presumably without loss of markers, we could show that a subset of the luminal epithelial cells could convert to myoepithelial cells, signifying the possible existence of a progenitor cell population. By combining the information on marker expression and in situ localization with immunomagnetic sorting and subsequent immortalization, we have identifed and isolated a cytokeratin 19‐positive suprabasal putative precursor cell in the luminal epithelial compartment and established representative cell lines. This suprabasal‐derived epithelial cell line is able to generate both itself and differentiated luminal epithelial and myoepithelial cells, and in addition, is able to form elaborate terminal duct lobular unit (TDLU)‐like structures within a reconstituted basement membrane. As more than 90% of breast cancers arise in TDLUs and more than 90% are also cytokeratin 19‐positive, we suggest that this cell population contains a breast‐cancer progenitor.
INTRODUCTION
Early epidemiological studies indicated that the lifetime risk of developing breast cancer is related to the age at which a woman has her first full‐term pregnancy, and that nulliparous women have an increased breast cancer risk (MacMahon et al. 1970; Woods et al. 1980). One plausible explanation of this may be that pregnancy induces mammary gland differentiation and eliminates terminal end buds, resulting in refractoriness of the neoplastic potential of the gland (Sun et al. 1999). That stem cells or progenitor cells localized within the terminal duct lobular unit (TDLU) are indeed targets of transforming mutations is further supported by the fact that most neoplastic breast lesions arise at this particular site (Wellings et al. 1975). Until recently, however, information about stem‐cell characteristics in the human breast has been limited. In the mouse, there is evidence that any epithelial portion of a normal mammary gland can reproduce an entire functional gland when transplanted into an epithelium‐free mammary fat pad, which suggest that stem cells are spread throughout the mammary tree (Smith & Medina 1988). This is further supported by limited dilution studies with cell cultures derived from clonal outgrowths, which demonstrate the existence of single multipotent stem cells positioned throughout the mature gland with the capacity to recapitulate an entire functional gland (Kordon & Smith 1998). A clue as to how the stem cells organize the breast in humans was provided by clonal analyses. These studies suggest that entire lobules, large ducts and TDLUs are monoclonal in origin, i.e. each compartment, rather than the entire gland, is derived from the same stem cell (Tsai et al. 1996; Diallo et al. 2001). As a consequence, however, when assessed by the pattern of X‐chromosome inactivation, any lesion arising in a TDLU would be of monoclonal origin independently of its originating from one or more of stem cells (Diallo et al. 2001). Therefore, the identification and characterization of the individual stem cell within the TDLU is highly warranted. It is conceivable that the revelation of the stem‐cell populations within the epithelial compartment in general, and within the TDLU in particular, would provide insights into the cellular and molecular mechanisms of both normal breast morphogenesis and generation of breast tumours.
In order to understand how a cell becomes malignant, one must first define and understand how the normal cell is maintained (Bissell 1981). Our research over the years has been aimed at revealing the cellular basis of normal breast morphogenesis. Our search towards the identification of a possible stem cell of the human breast was aided by the identification of luminal and myoepithelial cells in primary culture.
SEPARATION AND CULTIVATION OF LUMINAL AND MYOEPITHELIAL CELLS
Easty et al. (1980) proposed originally that primary cultures of normal human breast tissue give rise to epithelial cell islands composed of a central zone of luminal‐like cells and a peripheral zone of myoepithelial‐like cells, based on morphological criteria and sialomucin expression. The direct evidence that the two cell types indeed represent luminal and myoepithelial cells was later provided (Petersen & van Deurs 1988) using a panel of immunocytochemical markers, including smooth muscle actins expressed by the peripheral zone of myoepithelial cells. The identification of the relevant cell types is of course prerequisite to any investigation, but another, no less important, issue is the purity of the cell population of interest.
The most widely used method to isolate epithelial cells from human breast is to treat minced tissue with collagenase. It is then possible to separate epithelial organoids from the majority of stromal tissue, including interlobular fibroblasts and blood vessels, by differential centrifugation of the collagenase digest (Rønnov‐Jessen & Petersen 1993). The epithelial organoid preparation, however, still contains intralobular fibroblasts and the microvascular capillary network (Rønnov‐Jessen et al. 1990; Rønnov‐Jessen et al. 1996). One way of reducing this problem is to use serum‐free media in combination with an extracellular matrix coating for high‐density cultures, which favour the outgrowth and proliferation of epithelial cells (Petersen et al. 1985; Petersen & van Deurs 1987). Under these conditions, in a serum‐free medium that includes epidermal growth factor (EGF) and a cyclic‐AMP analogue (cholera toxin; CT), a balanced 1 : 1 ratio of luminal cells and myoepithelial cells can be maintained for up to 25 generations before senescence (Petersen & van Deurs 1987). Despite the fact that this approach was indeed advantageous for the characterization of the two cell types in culture, it was not sufficiently refined to elucidate the cellular origin of either cell type. To this end, a major leap forward was the technology of magnetic‐affinity cell sorting (MACS).
PURIFICATION OF EPITHELIAL CELL POPULATIONS
Originally, two different immunomagnetic techniques for separating normal human breast epithelial cells were compared (Clarke et al. 1994), based on the exclusive expression of epithelial membrane antigen (EMA) by luminal cells (Sloane et al. 1980; Taylor‐Papadimitriou et al. 1981) and CD10 (CALLA) by myoepithelial cells (Gusterson et al. 1986). A similar, well‐established approach to isolating mammary epithelial cells is the use of a fluorescence‐activated cell sorter (FACS) (Chang & Taylor‐Papadimitriou 1983; Stingl et al. 1998). Whereas both Dynabeads and the MACS system lead to purified cultures of luminal and myoepithelial cells, respectively, the Dynabeads could not be removed from the myoepithelial cells, which of course hampered the subsequent use of the cells. The degree of purity obtained by the MACS method was > 95% for the luminal cells and > 90% for the myoepithelial cell population (Clarke et al. 1994). In order to optimize the yield and purity, we subsequently modified the procedure as follows. (i) We used a MUC1 antibody instead of EMA to label and isolate luminal cells. (ii) The contribution from intralobular stromal cells was minimized by first isolating the epithelial organoids by centrifugation of the collagenase digest as previously described, and then further clearing the epithelial organoid preparation by pulling the suspension twice through a 0.4‐mm‐diameter needle (Péchoux et al. 1999). By doing this, the microvascular network, which is closely attached to the epithelium, is detached (Rønnov‐Jessen et al. 1996) and can be washed away (Péchoux et al. 1999). (iii) The cell suspensions were passed through the MACS column in a free flow without needles attached, prior to washing, elution and plating (Péchoux et al. 1999). In combination, these methods result in up to 100% and 99.7% pure populations of luminal and myoepithelial cells, respectively. The purified myoepithelial cells were cultured in CDM4 medium (Petersen & van Deurs 1988), in which the critical growth factors for survival and differentiation had earlier been shown to be insulin, EGF, and CT (Petersen & van Deurs 1988). The luminal epithelial cells were cultured in a modified CDM3, referred to as CDM6, which includes hepatocyte growth factor (HGF) and a reduced amount of EGF (Péchoux et al. 1999). It had been shown by others that HGF is mitogenic for luminal epithelial cells but not for myoepithelial cells (Niranjan et al. 1995), and in our study, inclusion of HGF in the culture medium was indispensable for the selective growth of luminal epithelial cells after purification.
CONVERSION OF LUMINAL CELLS TO MYOEPITHELIAL CELLS
Once purified, the cells could be cultured for up to seven passages, during which time the luminal cells fully retained their lineage‐specific characteristics, inlcuding a strong expression of cytokeratin 18 and 19, and the ability to form acinus‐like spheres inside a reconstituted basement membrane (Petersen et al. 1992; Péchoux et al. 1999). The myoepithelial cells, however, down‐regulated α‐smooth muscle actin (α‐sm actin) and CALLA, but retained all other myoepithelial characteristics measured, including a homogenous expression of vimentin, a strong expression of β4‐integrin, and the ability to form tight balls without a central lumen inside a reconstituted basement membrane. That they actually did retain their myoepithelial characteristics even after prolonged cultivation was further supported by the fact that they regained their lineage‐specific α‐sm actin expression at confluency in the presence of serum (Péchoux et al. 1999). Once we had isolated, purified, characterized and expanded the two epithelial cell populations, we could then address whether one cell type could give rise to the other. This was done by simply switching the culture medium. Upon addition of CDM6 (the optimal serum‐free medium for luminal cells), the myoepithelial cells remained unchanged for up to 2 weeks before they stopped growing and eventually senesced. In contrast, when purified luminal cells were cultured in CDM4 (the optimal serum‐free medium for myoepithelial cells), they exhibited focal conversion to myoepithelial cells (Péchoux et al. 1999). The converting cells showed a gradual loss of cytokeratins 18 and 19, and gain of vimentin, CALLA and serum‐inducible α‐sm actin (Péchoux et al. 1999). This allowed us to conclude that the luminal cells include precursors to myoepithelial cells, and it further indicated that the stem‐cell population of the human breast may reside within the luminal epithelial lineage. A number of other laboratories had arrived at the same conclusion, namely, that the purified epithelial lineages include progenitor cells of the human breast (Kang et al. 1997; Stingl et al. 1998; Chang et al. 2001). As for the mouse mammary gland, the data are somewhat more conflicting (compare Smalley et al. 1999 with 2002a, 2002b). The next task was to gain more information about the in situ localization of the progenitor cells, and we therefore sought to locate these cells in the breast.
IDENTIFICATION OF A SUPRABASAL CELL IN THE HUMAN BREAST AS THE PUTATIVE STEM CELL
Other laboratories had identified a cell type in culture with positive staining for the luminal epithelial marker ESA/Ep‐CAM (Litvinov et al. 1994) and a negative or weakly positive staining for sialomucin as a possible ‘stem’ cell of the human breast (Stingl et al. 1998). We further reasoned that as MUC1 (Taylor‐Papadimitriou et al. 1981) is the most prominent marker of luminal epithelial cells and is exclusively expressed on the apical surface of luminal epithelial cells (Petersen & van Deurs 1986), this would imply that some epithelial cells (not facing the lumen) are MUC1‐negative. In addition, in the mouse mammary gland, stem cells have been defined ultrastructurally as ‘basal cells’ (reviewed in Smith & Chepko 2001). Nevertheless, there is reason to believe that these cells are indeed full members of the luminal epithelial lineage. Thus, the cells on the basal side of multilayered breast ducts express several luminal epithelial markers, including simple epithelial keratins and ESA, but no markers of the myoepithelial lineage such as α‐sm actin or CALLA (Petersen & van Deurs 1988; Taylor‐Papadimitriou et al. 1989). With these considerations in mind, we double‐stained histological sections of human breast tissue for ESA and MUC1, and as expected, most luminal epithelial cells stained, i.e. MUC1 was expressed on the apical surface and ESA at the basolateral surface (Gudjonsson et al. 2002b). However, a subset of ESA‐positive cells in occasional acinar profiles was indeed abluminal in location, with no visible extensions reaching the lumen. That these cells were distinct from myoepithelial cells was confirmed by double‐staining for ESA and the myoepithelial marker α‐sm actin. Moreover, the average frequency of MUC1‐negative cells in smears of trypsinized, uncultured breast cells was 8 ± 3%, which agreed with previous published data for mice that showed the estimated frequency of mammary stem cells and first‐degree progenitor cells (defined ultrastructurally) to be about 8–12% (Smith & Chepko 2001). We therefore concluded that suprabasally positioned, abluminal cells within the luminal epithelial lineage also exist in the human breast. A consensus regarding the optimal combination of markers to reveal the stem‐cell population in the human breast has not yet been reached and the percentage of actual stem cells is probably less than 8%. Others have recently employed a method originally used to define the stem‐cell populations (referred to as ‘side population’) in haematopoietic and muscle systems, which is based on the observation that stems cells have the ability to efflux the dye Hoechst 33342 (Alvi et al. 2002). By using this approach on epithelial cells isolated from human breast, a fraction of only 0.18 ± 0.23% of the cells were defined as side‐population cells (Alvi et al. 2002). Other possible stem‐cell markers of the breast include cytokeratin 5 and a member of the p53 transcription‐factor family, p63. Cytokeratin 5 was originally described to label a subpopulation of luminal epithelial cells in addition to myoepithelial cells (Nagle et al. 1986). This staining pattern was used recently to label a population of putative stem cells, constituting approximately 5% of the total population (Bocker et al. 2002). p63 has been shown to positively identify stem cells in the epidermis (Pellegrini et al. 2001). The fact that p63 in the normal breast is expressed by cells in a cell layer that is one cell distal to the lumen, and that only some of the cells also express the myoepithelial cell marker α‐sm actin, further implies that the putative stem‐cell population is suprabasally positioned (Di Renzo et al. 2002). In accordance with these and our findings, the cytokeratin 5‐positive cells in normal breast acini also belong to the luminal lineage (see Fig. 1 in Bocker et al. 2002). Thus, in spite of the fact that these markers label seemingly separate populations of cells, collectively they apparently do cover the putative stem and progenitor cell populations.
Figure 1.
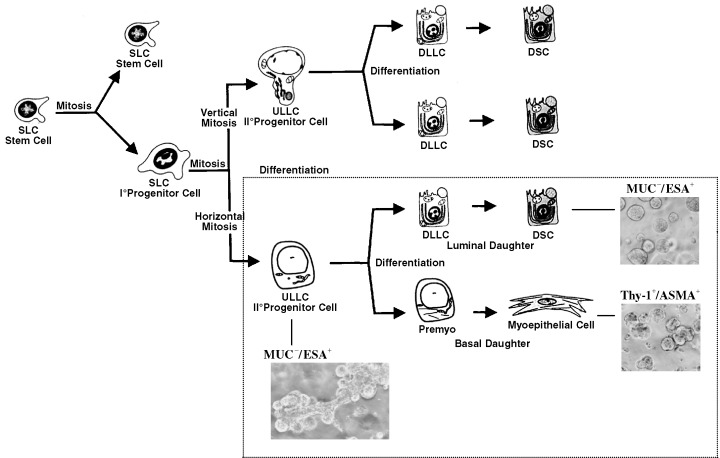
Patterns of maturation of the mammary gland. The frame indicates the part that best reflects the differentiation repertoire of MUC−/ESA+ cells and their progeny. Phase‐contrast figures illustrate the patterns of morphogenesis of these cells in a reconstituted basement membrane. SLC, small light cells; ULLC, undifferentiated large light cells; DLLC, differentiated large light cells; DSC, differentiated secretory cell; Premyo, precursor myoepithelial cell (Modified from Chepko & Smith 1997 and Gudjonsson et al. 2002b, with permission from Elsevier and Cold Spring Harbor Laboratory Press). An acinus is approximately 50 µm in diameter.
ESTABLISHMENT OF IMMORTALIZED PROGENITOR CELL LINES
The major advantage of using primary breast specimens is that the cells studied do indeed represent the tissue of origin. The drawbacks, however, include limited access to biopsy material and a finite lifespan of the explanted cells. In particular, with the aim of studying the putative stem‐cell population, which may comprise as little as 5% or less of the epithelial cell population, these obstacles become almost prohibitive. To accomplish the task, we therefore first isolated the relevant cell populations using immunomagnetic sorting and then immortalized them with retroviral constructs containing E6 and E7 oncogenes from human papilloma virus‐16 (Wazer et al. 1995; Band 1998; Gudjonsson et al. 2002b). It has been shown by others that luminal human breast epithelial cells (Type I HBEC) are more susceptible to immortalization by SV40 large T antigen than myoepithelial cells (Type II HBEC) (Chang et al. 2001). In our hands, the two cell types were equally susceptible to HPV immortalization. The luminal epithelial cells were purified from two consecutive MUC1 columns, and the suprabasal epithelial cells were purified on a MUC1 column, the flow‐through of which was later retained in an ESA column (Gudjonsson et al. 2002b). The purified cells were immortalized by transduction with HPV16‐E6/E7, resulting in two established cell lines: a luminal‐derived cell line, which was ESA+/MUC1+, and a suprabasal‐derived cell line, which was ESA+/MUC1− (Gudjonsson et al. 2002b). To date, we have established three suprabasal‐derived epithelial cell lines with comparable characteristics, and it appears that the order of events in terms of ESA purification and immortalization does not influence the results (unpublished observations).
The major concern with HPV16‐E6/E7 immortalization is that the inactivated p53 and retinoblastoma protein may not be the only affected molecules, and that other cellular functions may also be affected (Garbe et al. 1999; for review see Zwerschke & Jansen‐Durr 2000). Evidence suggests, however, that human cells derived from E6/E7 immortalization retain much of their original phenotype. Immortalization of normal human breast cells with either E6 or E7 did not lead to aberrant functional behaviour in luminal or myoepithelial cells tested (Wazer et al. 1995). While it has been reported that breast cells lose cytokeratin 19 expression as a consequence of E7 immortalization (Spancake et al. 1999), this is not necessarily the case, as we and others have now shown (Wazer et al. 1995; Gudjonsson et al. 2002b). It is likely that the result was due to immortalization of an already cytokeratin19‐negative subpopulation.
Both the luminal‐derived cell line and the suprabasal‐derived cell line we isolated are of the luminal epithelial lineage because they express the tight junctional proteins claudin and occludin, and exhibit a high transepithelial electrical resistance on transwell filters. However, whereas the luminal‐derived epithelial cell line remain homogenous, the suprabasal‐derived cell line keep generating minor subpopulations of MUC+/ESA+ and MUC−/ESA− cells (Gudjonsson et al. 2002b). Although this behaviour signals the multipotent nature of the cell line, it also emphasizes that to maintain the original cell line characteristics, repeated ESA purifications are needed. The MUC−/ESA− progeny of the suprabasal cell line share characteristics with myoepithelial cells in situ, which are also devoid of these proteins. To test whether this could be extended to other markers, clonal cultures were established and double‐stained for cytokeratin 18 (luminal marker) and cytokeratin 14 (myoepithelial marker). Whereas the luminal‐derived epithelial cell line did not generate any myoepithelial cells, the suprabasal‐derived cell line readily formed mixed clones of luminal epithelial and myoepithelial cells. From these cultures, we could further isolate a myoepithelial‐restricted subline, which can express α‐sm actin (Gudjonsson et al. 2002b). Taken together, these observations provide evidence that it is feasible to identify, isolate and immortalize suprabasal‐derived cell lines that give rise to both differentiated myoepithelial cells and luminal epithelial cells. Rather than inventing a new scheme for the maturation of human breast epithelial cells, we have adopted the scheme of Chepko & Smith (1997) for the mouse mammary gland (Fig. 1). Maturation of breast cells into different lineages has also been described for human cells (Li et al. 1998).
In Fig. 1 and with our current level of knowledge, MUC−/ESA+ cells most likely correspond to the second‐degree progenitor of undifferentiated large light cells. This is because, although the cells are able to differentiate towards both the luminal epithelial lineage and the myoepithelial lineage, we have not as yet been able to detect any oestrogen receptor in these cells, which normally reside in a subpopulation of luminal epithelial cells. Similarly, we have no evidence yet for the ability of MUC−/ESA+ cells to make interlobular ducts in three‐dimensional cultures (see below), which for the mouse mammary gland has been attributed to a separate progenitor cell (Kordon et al. 1995). If this placement of the MUC−/ESA+ cell line is correct and its phenotype were to penetrate fully upon malignant transformation, then the phenotype of the tumour that could be derived from these cells would correspond to that of the highly malignant bimodal or basal, estrogen receptor‐negative breast cancers that constitute about 18% of all breast cancers (see below; Moll 1998; Sorlie et al. 2001).
RECAPITULATION OF TDLU‐LIKE STRUCTURES
In the mouse and rat, the traditional assay for the presence of stem cells is the ability to regenerate the entire structure of the mammary gland upon reimplantation of cells in syngeneic gland‐free fat pads (Smith & Medina 1988; Alvi et al. 2002; Deugnier et al. 2002a). We have previously shown that mammary gland morphogenesis in a reconstituted basement membrane may be a physiologically relevant and viable alternative (Barcellos‐Hoff et al. 1989; Aggeler et al. 1991; Petersen et al. 1992; Petersen et al. 1995). Thus, we have shown that primary human luminal cells form acinus‐like, polarized structures with a central lumen, and myoepithelial cells form solid structures (Petersen et al. 1992). The mixture of the two purified cell types, however, may lead to formation of double layered acini rather than the reformation of TDLU‐like structures (Gudjonsson et al. 2002a). Embedding the luminal‐derived epithelial cell line alone yielded a morphology very similar to what has already been described for primary breast cells and other luminal cell lines (Petersen et al. 1992). In contrast, embedding the suprabasal‐derived epithelial cells gave rise to TDLU‐like structures as assessed by both morphology and marker expression (Gudjonsson et al. 2002b). Subcutaneous inoculation in nude mice confirmed that the ability to generate the DLU‐like structures resided in the suprabasal‐derived cell line. In order to rule out that the HPV16‐E6/E7 immortalization might somehow trigger this phenotypic response pattern, the morphological behaviour of suprabasal cells freshly prepared from primary cultures was analysed in reconstituted basement membrane, and the ability to form the same pattern was confirmed (Fig. 2).
Figure 2.
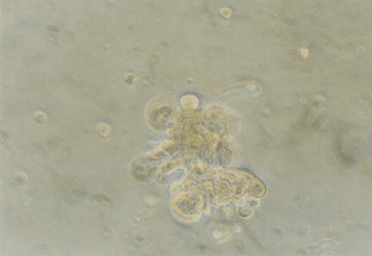
Primary cultures of MUC−/ESA+ cells formed elaborate TDLU‐like structures inside a reconstituted basement membrane. 100 µm = 10 mm.
THE FATE OF STEM CELL PROGENY IN CANCER
Increasing evidence is emerging that characterization of stem cells could provide insights into cancer biology because tumours might contain cancer stem cells that drive cancer cell proliferation (reviewed in Reya et al. 2001). The similarity in the mechanisms that regulate self‐renewal of normal stem cells and cancer cells point to a possible link between stem cells and tumour cells. Therefore, after having observed that the isolated suprabasal cells exhibited stem‐cell‐like characteristics in culture, we sought to establish whether this cell population contained any characteristics that might link normal stem cells and breast cancer cells. In the majority of breast carcinomas, the neoplastic epithelial cells stain positive for cytokeratin 19 (Bartek et al. 1985). In the normal gland, however, cytokeratin 19 is known to be expressed in only a subpopulation of luminal epithelial cells within the TDLU (Bartek et al. 1985). As such, cytokeratin 19 expression may cover the candidate stem‐cell population. Interestingly, we found that cytokeratin 19 was a distinctive trait expressed by the suprabasal‐derived epithelial cells, and moreover, that the cell line showed evidence of multipotency with regard to cytokeratin 19 expression (Fig. 3).
Figure 3.
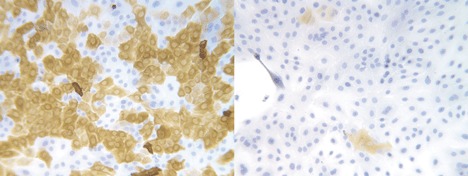
Early passage MUC−/ESA+ cell line (TH69) stained with BA17 against keratin K19 (left) and 115D8 against MUC1 (right). The keratin staining is heterogeneous and the MUC staining is almost negative. 50 µm = 5 mm.
Thus, in clonal cultures double‐stained for cytokeratin 19 and the myoepithelial cytokeratin 14, clones that diversified into both CK14‐ and CK19‐positive cells could be identified (Fig. 4) (Gudjonsson et al. 2002b).
Figure 4.
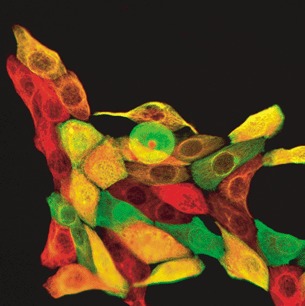
Cloned MUC−/ESA+ cells doublestained with keratin K19 (red) and keratin K14 (green). Note the heterogenous staining pattern (from Gudjonsson et al. 2002b, with permission from Cold Spring Harbor Laboratory Press). 20 µm = 8 mm.
We propose that CK19‐positive cells are indeed the stem/progenitor cells of the human breast. In other organs, including liver, pancreas, skin, testes, and prostate, the ‘stem‐cell compartment’ also express cytokeratin 19 (Stosiek et al. 1990; Fridmacher et al. 1995; Michel et al. 1996; Bouwens 1998; Hudson et al. 2001).
In summary, our research so far suggests that candidate stem cells of the breast belong to the luminal lineage and that some progenitors are suprabasally positioned. We further suspect that this population may give rise to some carcinomas or at least be involved in their genesis.
ACKNOWLEDGEMENTS
We thank Tove Marianne Lund for expert technical assistance The he Aasted Clinic, the Private Clinic, and the Søllerød Plastic Surgery Clinic are gratefully acknowledged for providing the biopsy material. We also wish to thank the following sources of funding: The Icelandic Research Council (TG), The Novo Nordisk Foundation, Friis Fonden, The Meyer Foundation, The Danish Cancer Society, The Danish Research Council (OWP and LR‐J), Weimanns Legat (LR‐J) and The US National Cancer Institute (grant CA‐64786‐02 to MJB and OWP) and United States Department of Energy Office of Biological and Environmental Research (contract DE‐AC03‐76SF00098 to MJB).
REFERENCES
- Aggeler J, Ward J, Blackie LM, Barcellos‐Hoff MH, Streuli CH, Bissell MJ (1991) Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo . J. Cell Sci. 99, 407. [DOI] [PubMed] [Google Scholar]
- Alvi AJ, Clayton H, Joshi C, Enver T, Asworth A, Vivanco MDM, Dale TC, Smalley MJ (2002) Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 5, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V (1998) The role of retinoblastoma and p53 tumor suppressor pathways in human mammary epithelial cell immortalization. Int. J. Oncol. 12, 499. [DOI] [PubMed] [Google Scholar]
- Barcellos‐Hoff MH, Aggeler J, Ram TG, Bissell MJ (1989) Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Durban EM, Hallowes RC, Taylor‐Papadimitriou J (1985) A subclass of luminal epithelial cells in the human mammary gland, defined by antibodies to cytokeratins. J. Cell Sci. 75, 17. [DOI] [PubMed] [Google Scholar]
- Bissell MJ (1981) The differentiated state of normal and malignant cells or how to define a ‘normal’ cell in culture. Int. Rev. Cytol. 70, 27. [DOI] [PubMed] [Google Scholar]
- Bocker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, Burger H, Wai D, Ina Diallo R, Brandt B, Herbst H, Schmidt A, Lerch MM, Buchwallow IB (2002) Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab. Invest. 82, 737. [DOI] [PubMed] [Google Scholar]
- Bouwens L (1998) Cytokeratins and cell differentiation in the pancreas. J. Pathol. 184, 234. [DOI] [PubMed] [Google Scholar]
- Chang CC, Sun W, Cruz A, Saitoh M, Tai MH, Trosko JE (2001) A human breast epithelial cell type with stem cell characteristics as target cells for carcinogenesis. Radiat. Res. 155, 201. [DOI] [PubMed] [Google Scholar]
- Chang SE, Taylor‐Papadimitriou J (1983) Modulation of phenotype in cultures of human milk epithelial cells and its relation to the expression of a membrane antigen. Cell Differ. 12, 143. [DOI] [PubMed] [Google Scholar]
- Chepko G, Smith GH (1997) Three division‐competent, structurally‐distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29, 239. [DOI] [PubMed] [Google Scholar]
- Clarke C, Titley J, Davies S, O'Hare MJ (1994) An immunomagnetic separation method using superparamagnetic (MACS) beads for large‐scale purification of human mammary luminal and myoepithelial cells. Epithelial Cell Biol. 3, 38. [PubMed] [Google Scholar]
- Deugnier MA, Faraldo MM, Janji B, Rousselle P, Thiery JP, Glukhova MA (2002a) EGF controls the in vivo developmental potential of a mammary epithelial cell line possessing progenitor properties. J. Cell Biol. 159, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deugnier MA, Teuliére J, Faraldo MM, Thiery JP, Glukhova MA (2002b) The importance of being a myoepithelial cell. Breast Cancer Res. 4, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo R, Schaefer KL, Poremba C, Shivazi N, Willmann V, Buerger H, Dockhorn‐Dworniczak B, Boecker W (2001) Monoclonality in normal epithelium and in hyperplastic and neoplastic lesions of the breast. J. Pathol. 193, 27. [DOI] [PubMed] [Google Scholar]
- DiRenzo J, Signoretti S, Nakamura N, Rivera‐Gonzalez R, Sellers W, Loda M, Brown M (2002) Growth factor requirements and basal phenotype of an immortalized mammary epithelial cell line. Cancer Res. 62, 89. [PubMed] [Google Scholar]
- Easty GC, Easty DM, Monaghan P, Ormerod MG, Neville AM (1980) Preparation and identification of human breast epithelial cells in culture. Int. J. Cancer 26, 577. [DOI] [PubMed] [Google Scholar]
- Fridmacher V, Le Bert M, Guillou F, Magre S (1995) Switch in the expression of the K19/K18 keratin genes as a very early evidence of testicular differentiation in the rat. Mech. Dev. 52, 199. [DOI] [PubMed] [Google Scholar]
- Garbe J, Wong M, Wigington D, Yaswen P, Stampfer MR (1999) Viral oncogenes accelerate conversion to immortality of cultured conditionally immortal human mammary epithelial cells. Oncogene 18, 2169. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Rønnov‐Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW (2002a) Normal and tumor‐derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J. Cell Sci. 115, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Rønnov‐Jessen L, Bissell MJ, Petersen OW (2002b) Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 16, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson BA, Monaghan P, Mahendran R, Ellis J, O'Hare MJ (1986) Identification of myoepithelial cells in human and rat breasts by anti‐common acute lymphoblastic leukemia antigen antibody A12. J. Natl. Cancer Inst. 77, 343. [PubMed] [Google Scholar]
- Hudson DL, Guy AT, Fry P, O'Hare MJ, Watt FM, Masters JR (2001) Epithelial cell differentiation pathways in the human prostate: identification of intermediate phenotypes by keratin expression. J. Histochem. Cytochem. 49, 271. [DOI] [PubMed] [Google Scholar]
- Kang KS, Morita I, Cruz A, Jeon YJ, Trosko JE, Chang CC (1997) Expression of estrogen receptors in a normal human breast epithelial cell type with luminal and stem cell characteristics and its neoplastically transformed cell lines. Carcinogenesis 18, 251. [DOI] [PubMed] [Google Scholar]
- Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH (1995) Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev. Biol. 168, 47. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921. [DOI] [PubMed] [Google Scholar]
- Li P, Barraclough R, Fernig DG, Smith JA, Rudland PS (1998) Stem cells in breast epithelia. Int. J. Exp. Pathol. 79, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO (1994) Ep‐CAM: a human epithelial antigen is a homophilic cell‐cell adhesion molecule. J. Cell Biol. 125, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S (1970) Age at first birth and breast cancer risk. Bull. World Health Organ 43, 209. [PMC free article] [PubMed] [Google Scholar]
- Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L (1996) Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J. Cell Sci. 109, 1017. [DOI] [PubMed] [Google Scholar]
- Moll R (1998) Cytokeratins as markers of differentiation in the diagnosis of epithelial tumors. Subcell. Biochem. 31, 205. [PubMed] [Google Scholar]
- Nagle RB, Bocker W, Davis JR, Heid HW, Kaufmann M, Lucas DO, Jarasch ED (1986) Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J. Histochem. Cytochem. 34, 869. [DOI] [PubMed] [Google Scholar]
- Niranjan B, Buluwela L, Yant J, Perusinghe N, Atherton A, Phippard D, Dale T, Gusterson B, Kamalati T (1995) HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development 121, 2897. [DOI] [PubMed] [Google Scholar]
- Péchoux C, Gudjonsson T, Rønnov‐Jessen L, Bissell MJ, Petersen OW (1999) Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev. Biol. 206, 88. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, De McKeon F, Luca M (2001) p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 98, 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Hoyer PE, Hilgers J, Briand P, Van Deurs B (1985) Characterization of epithelial cell islets in primary monolayer cultures of human breast carcinomas by the tetrazolium reaction for glucose 6‐phosphate dehydrogenase. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 50, 27. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Rønnov‐Jessen L, Bissell MJ (1995) The microenvironment of the breast: three‐dimensional models to study the roles of the stroma and the extracellular matrix in function and dysfunction. Breast J. 1, 22. [Google Scholar]
- Petersen OW, Rønnov‐Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl Acad. Sci. USA 89, 9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Van Deurs B (1986) Characterization of epithelial membrane antigen expression in human mammary epithelium by ultrastructural immunoperoxidase cytochemistry. J. Histochem. Cytochem. 34, 801. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Van Deurs B (1987) Preservation of defined phenotypic traits in short‐term cultured human breast carcinoma derived epithelial cells. Cancer Res. 47, 856. [PubMed] [Google Scholar]
- Petersen OW, Van Deurs B (1988) Growth factor control of myoepithelial‐cell differentiation in cultures of human mammary gland. Differentiation 39, 197. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105. [DOI] [PubMed] [Google Scholar]
- Rønnov‐Jessen L, Petersen OW (1993) Induction of alpha‐smooth muscle actin by transforming growth factor‐beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab. Invest. 68, 696. [PubMed] [Google Scholar]
- Rønnov‐Jessen L, Petersen OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol. Rev. 76, 69. [DOI] [PubMed] [Google Scholar]
- Rønnov‐Jessen L, Van Deurs B, Celis JE, Petersen OW (1990) Smooth muscle differentiation in cultured human breast gland stromal cells. Lab. Invest. 63, 532. [PubMed] [Google Scholar]
- Sloane JP, Ormerod MG, Imrie SF, Coombes RC (1980) The use of antisera to epithelial membrane antigen in detecting micrometastases in histological sections. Br. J. Cancer 42, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley MJ, Titley J, Paterson H, Perusinghe N, Clarke C, O'Hare MJ (1999) Differentiation of separated mouse mammary luminal epithelial and myoepithelial cells cultured on EHS matrix analyzed by indirect immunofluorescence of cytoskeletal antigens. J. Histochem. Cytochem. 47, 1513. [DOI] [PubMed] [Google Scholar]
- Smith GH, Chepko G (2001) Mammary epithelial stem cells. Microsc Res. Tech. 52, 190. [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell Sci. 90, 173. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, Van De Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen‐Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spancake KM, Anderson CB, Weaver VM, Matsunami N, Bissell MJ, White RL (1999) E7‐transduced human breast epithelial cells show partial differentiation in three‐dimensional culture. Cancer Res. 59, 6042. [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U, Emerman JT (1998) Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63, 201. [DOI] [PubMed] [Google Scholar]
- Stosiek P, Kasper M, Karsten U (1990) Expression of cytokeratin 19 during human liver organogenesis. Liver 10, 59. [DOI] [PubMed] [Google Scholar]
- Sun W, Kang KS, Morita I, Trosko JE, Chang CC (1999) High susceptibility of a human breast epithelial cell type with stem cell characteristics to telomerase activation and immortalization. Cancer Res. 59, 6118. [PubMed] [Google Scholar]
- Taylor‐Papadimitriou J, Peterson JA, Arklie J, Burchell J, Ceriani RL, Bodmer WF (1981) Monoclonal antibodies to epithelium‐specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int. J. Cancer 28, 17. [DOI] [PubMed] [Google Scholar]
- Taylor‐Papadimitriou J, Stampfer M, Bartek J, Lewis A, Boshell M, Lane EB, Leigh IM (1989) Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J. Cell Sci. 94, 403. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Lu Y, Nichols PW, Zlotnikov G, Jones PA, Smith HS (1996) Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res. 56, 402. [PubMed] [Google Scholar]
- Wazer DE, Liu XL, Chu Q, Gao Q, Band V (1995) Immortalization of distinct human mammary epithelial cell types by human papilloma virus 16, E6 or E7. Proc. Natl Acad. Sci. USA 92, 3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J. Natl. Cancer Inst. 55, 231. [PubMed] [Google Scholar]
- Woods KL, Smith SR, Morrison JM (1980) Parity and breast cancer: evidence of a dual effect. Br. Med. J. 281, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerschke W, Jansen‐Durr P (2000) Cell transformation by the E7 oncoprotein of human papillomavirus type 16: interactions with nuclear and cytoplasmic target proteins. Adv. Cancer Res. 78, 1. [DOI] [PubMed] [Google Scholar]


