Abstract
Although lizards have highly sensitive ears, it is difficult to condition them to sound, making standard psychophysical assays of hearing sensitivity impractical. This paper describes non-invasive measurements of the auditory brainstem response (ABR) in both Tokay geckos (Gekko gecko; nocturnal animals, known for their loud vocalizations) and the green anole (Anolis carolinensis, diurnal, non-vocal animals). Hearing sensitivity was measured in 5 geckos and 7 anoles. The lizards were sedated with isoflurane, and ABRs were measured at levels of 1 and 3% isoflurane. The typical ABR waveform in response to click stimulation showed one prominent and several smaller peaks occurring within 10 ms of the stimulus onset. ABRs to brief tone bursts revealed that geckos and anoles were most sensitive between 1.6–2 kHz and had similar hearing sensitivity up to about 5 kHz (thresholds typically 20–50 dB SPL). Above 5 kHz, however, anoles were more than 20 dB more sensitive than geckos and showed a wider range of sensitivity (1–7 kHz). Generally, thresholds from ABR audiograms were comparable to those of small birds. Best hearing sensitivity, however, extended over a larger frequency range in lizards than in most bird species.
INTRODUCTION
The tympanic ear probably originated independently in all major tetrapod groups (Clack, 1997). Lizards therefore represent an independent experiment in tympanic hearing and are highly interesting in comparative studies of ear evolution in tetrapods. Most lizards have delicate eardrums and sensitive ears (Manley, 1990), but the lack of behavioral assays of auditory sensitivity has made it difficult to compare lizard hearing to other vertebrate groups. Relatively non-invasive methods, such as cochlear microphonics (reviewed in Wever, 1978) and measurements of compound action potentials recorded from the round window (Werner et al., 2008) have been used successfully, but the thresholds and general frequency response of the ear are dependent on the method used and the surgical preparation needed (Manley, 1990; Werner et al., 2001. There is therefore a need for standardized, non-invasive measurements of auditory sensitivity.
The anatomy of the basilar papilla shows considerable variation (see reviews in Manley, 2000, 2002, 2004). The species chosen for this study, anoles and geckos, were selected to reflect this variation. Anoles belong to the Iguanidae and typically have short basilar papillae (range 300–500 μm) while the basilar papilla is both highly specialized and extended in Gekkonids (range 1800–2100 μm) (Manley, 2002, Manley et al., 1999). Furthermore, lizard papillae are divided into at least two distinct areas, one where most hair cells respond best to frequencies below 1 kHz and a second area (or two in some species) that responds to higher frequencies (see Fig. 4.3 in Manley, 2000). This high frequency region is particularly well developed in gekkonids (Manley et al., 1999; Manley, 2002).
Despite the diversity in their papillae, all lizard species share a similar hearing frequency range of about 0.1–5 kHz (see Wever, 1978 and reviews in Manley, 2000; 2004). In all lizards investigated thus far, the auditory nerve fibers have V-shaped tuning curves with lowest thresholds at 5 dB SPL and show phase locking to low-frequency stimuli (below approximately 1 kHz; Manley, 1981; Manley, 2000). The responses of most auditory nerve fibers are primary-like and characterized by robust onsets (for review, see Manley, 2000). Auditory nerve fibers project to the auditory brainstem (Szpir et al., 1995, for review see Grothe et al., 2005); further neural processing along the auditory pathway has not yet been investigated in detail (Manley, 1981).
The auditory brainstem response (ABR) is as an effective tool to study hearing sensitivity as well as the functionality of the auditory system, and is similar across most vertebrate classes (e.g., Corwin et al., 1982; Walsh et al., 1992). Most importantly, the ABR audiogram provides a good estimate of the shape of the behavioral audiogram (Borg and Engström, 1983; Brittan-Powell et al., 2002; Stapells and Oates, 1997; Wenstrup, 1984). For most animals tested, the ABR is unaffected by sleep and∕or sedation (Hall, 2007; Picton and Hillyard, 1974; but see Santarelli et al., 2003). It is manifested as a series of peaks occurring within the first 10 ms following stimulation and represents the progressive propagation of neural activity through the ascending auditory pathway.
This study compared hearing sensitivity of a vocal lizard (Tokay gecko, Gekko gecko) to a non-vocal lizard (anole, Anolis carolinensis). Tokay geckos are nocturnal Southeast Asian animals. Gekkonoids are noted among lizards, both for their ability to vocalize and for the complexity of their vocal apparatus (Blair, 1968; Bogert, 1960; Moore et al., 1991). Their vocalizations vary from quiet, insect-like chirrups to loud clucks, barks, and whistles (Marcellini, 1977; Seufer, 1991; Tang et al., 2001) and are distinguishable from those of most other reptiles in that the sound emitted often has tonal and harmonic qualities (Brown, 1985; Steck, 1908; Werner et al., 1978). In contrast, the green anole is a diurnal, non-vocal animal found in the southeastern United States. We have used the ABR to measure hearing sensitivity since it is a simple, non-invasive measurement that allows comparisons of sensitivity and frequency response not only within the group of lizards but also with other vertebrate groups. To our knowledge, this is the first such study in lizards.
METHODS
Animal preparation
Geckos were placed in a walk-in sound-attenuating chamber (IAC) for all measurements. Closed, custom-made sound systems were placed at the entrance of both ear canals, containing commercial miniature earphones and miniature microphones (Knowles EM 3068). After the sound systems were sealed into the ear canal using Gold Velvet II ear impression material (Earmold and Research Laboratories, Wichita, KS), the sound systems were calibrated individually at the start of each experiment, using built-in miniature microphones (Knowles EM3068, Itasca, IL).
Auditory brainstem response (ABR)
Hearing sensitivity was measured in 5 geckos and 7 anoles using ABRs. Anesthesia was induced in intubated animals by isoflurane inhalation via a chamber. Once the lizards were anesthetized, a constant gas flow of carbogen mixed with isoflurane at 1 ml∕min was connected via a loose fitting tube into the trachea (Bennett, 1998). Animals were sedated with isoflurane in concentrations of 1% and 3%. At levels below 1%, animals could be kept sedated but occasionally took deep breaths and would respond to toe-pinch. When changes to isoflurane levels were made, at least 30–60 min were allowed before the next data collection began. Body temperature was maintained at 27 °C by a heating blanket wrapped around the animal. To measure ABRs, platinum electrodes were inserted just under the skin at the vertex, behind the stimulated ear and grounded at the other side of the head. Responses to brief tone bursts, emitted through the coupler sealed over the eardrum, were evoked at frequencies between 0.2–10 kHz with intensity levels of 5 to 90 dB SPL.
The stimulus presentation, ABR acquisition, equipment control, and data management are similar to those used in previous bird and alligator studies (Brittan-Powell et al., 2002; Brittan-Powell et al., 2005; Higgs et al., 2002). Briefly, the system was coordinated using a Tucker-Davis Technologies (TDT; Gainesville, FL) System 3 modular rack-mount system USB linked to a Dell laptop running TDT ‘BIOSIG’ and ‘SIGGEN’ software. Sound stimuli were generated using SIGGEN and fed through a RP2.1. The output of the RP2.1 was connected to a programmable attenuator (PA5), which directly drove the speaker. Recording electrodes were connected to the low-impedance Medusa Digital Biological Amplifier System (RA4L Headstage and RA16PA PreAmp), which added an additional 10–20× gain, and then to the RA16BA (Medusa Base station) via fiber optic cables. All biological signals were notch filtered at 60 Hz during data collection. After data collection, the signals were bandpass filtered below 30 Hz and above 3000 Hz using BIOSIG.
As in previous studies, ABR thresholds were measured in response to multiple-intensity stimulus trains consisting of 9 single frequency tone-bursts of increasing intensity and presented at a rate of 2∕s. Tone burst stimuli were 10 ms in duration (1 ms cos2 rise∕fall) with 20 ms ISI. Each ABR represented the average of 200 alternating phase stimulus presentations, sampled at 25 kHz for 285 ms following onset of the stimulus. Each frequency-intensity combination was replicated. Latency to wave 1 was calculated as the time (ms) to the first positive peak while the amplitude was measured from baseline-to-peak for wave 1 [see inset Fig. 2B]. We defined ABR threshold as the intensity 2.5 dB (one-half step in intensity) below the lowest stimulus level at which a response could be visually detected on the trace between 1 and 6 ms, regardless of whether the peak in the waveform was positive or negative (Brittan-Powell and Dooling, 2004; Brittan-Powell et al., 2005).
Figure 2.
(A) I∕O functions (medians and ranges) for peak 1 latency and (B) amplitude (bottom) for click responses in 3 geckos (triangle) and 4 anoles (circle). Inset: Schematic showing how latency and amplitude measurements were taken.
The authors recognize that the brief stimuli and short rise∕fall times of the tone bursts used in this study were not ideal for accurately determining thresholds for low frequency sounds. However, increased rise∕fall times and longer stimulus durations affect the brainstem response morphology. In humans, ABR peak latency was directly and positively correlated with stimulus rise time (e.g., Hecox et al., 1976; Kodera et al., 1977; Kodera et al., 1979) although Beattie and Torre (1997) found that increasing rise∕fall time from 1 to 4 ms at 500 Hz had no effect on ABR threshold. Other studies showed that increasing the duration of the tone resulted in changes in waveform morphology, with decreased amplitudes and offset responses in stimuli as short as 10 ms (e.g., Kodera et al., 1977, see review in Hall, 2007). Given these constraints, we chose a similar methodology (i.e., 5 or 10 ms tone trains with 1 ms rise∕fall times) in order to compare ABR thresholds for lizards with ABR thresholds from small birds and alligators (collected by the first author).
RESULTS
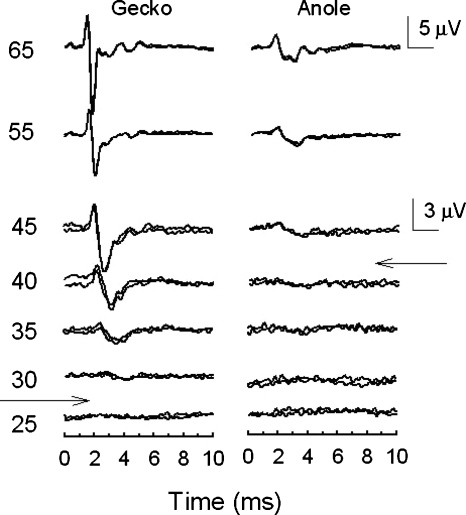
The typical ABR showed two to three prominent peaks occurring within 4–6 ms of the stimulus onset (Fig. 1). Peak 1 of the ABR is the far-field representation of the negatively-oriented peak of the compound action potential (CAP N1) of the auditory nerve, likely originating from along the tonotopic gradient within the basilar papilla (Jewett, 1970; Köppl and Gleich, 2007). Both geckos (n=3) and anoles (n=4) showed a similar sequence of peaks in response to a click, but geckos (median: 27.5 dB pSPL) exhibited lower click thresholds than anoles (median 42.5 dB pSPL).
Figure 1.
Representative ABR click waveforms for a single gecko and anole showing multiple waveform peaks within the first 4–5 ms following onset of the stimulus.
As with all animals tested to date, ABR latency decreased [Fig. 2A] and ABR amplitude increased [Fig. 2B] with increasing sound pressure (SPL) in both geckos and anoles. There were no species differences in the latency functions [F(1,33)=1.6, p>0.05]. There were noticeable species differences in the amplitude functions [species; F(1,33)=16.35, p<0.001]. In anoles, click-evoked ABRs gradually increased in amplitude with increasing SPL, while geckos showed large amplitudes and steeper slopes in response to clicks. The slopes of the amplitude functions did not differ [speciesx intensity F(6,33)=2.18, p>0.05], but geckos showed significantly larger amplitude responses in response to suprathreshold levels [55 dB and higher; F(2,33)=13.75, p<0.0001] compared to anoles at the same SPL. When intensity decreased below 55 dB pSPL, closer to threshold, there were no differences in amplitude between the two species [F(5,33)=0.71, p>0.05].
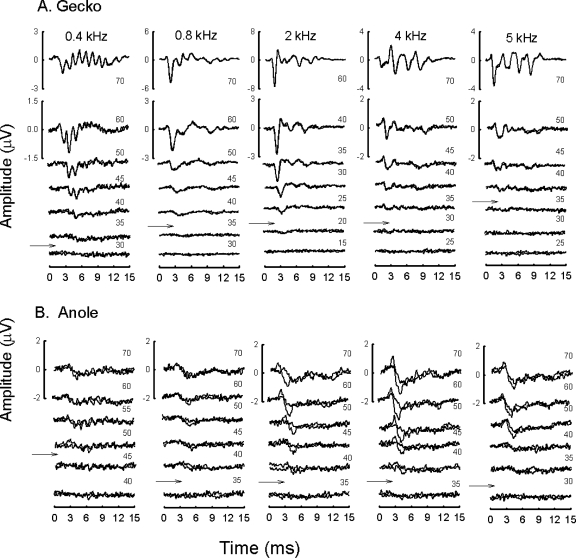
Exemplar tone evoked waveforms are shown for a single gecko and anole (Fig. 3). Gecko ABRs showed better synchrony, with more defined peaks, than anoles. Occasionally, a small, low amplitude shoulder was evident on the rising phase of wave 1; we assume this is a summating potential. Some of the responses to low frequency (e.g., 0.4 kHz) stimulation revealed well-defined stimulus-locked peaks for many of the geckos tested (seen at 400 Hz in gecko in Fig. 3 but not in anole). This frequency following response (FFR) was evoked at high intensity levels and masked later response peaks at these levels. When intensity levels decreased, however, the amplitude of the FFR decreased rapidly. Also, since we used alternating phases during data acquisition, note that the averaged FFR was about twice the frequency of the stimulation. When tone stimulation increased in frequency, sharper, more synchronized responses were evoked, especially in the gecko. Note also that the gecko response at 2 kHz was larger and better defined with respect to the other frequency responses shown. Similarly, enhanced responses were apparent in the anole ABR traces but to a lesser degree.
Figure 3.
Representative ABR waveforms for a single gecko and anole, evoked by tones of 0.4, 0.8, 2, 4 and 5 kHz. Different scales for the highest stimulus intensities are shown to the left of each averaged waveform. Arrows indicate estimated threshold. (A) Response waveforms for 0.4 kHz at 70 dB reveal well-defined frequency following response that masked later response peaks. Note that the use of alternating phases during data acquisition produces an averaged FFR of about 2F. (B) Response waveforms in the anole were smaller than those measured in the larger gecko. Note the large and sensitive response to increasing stimulus frequency.
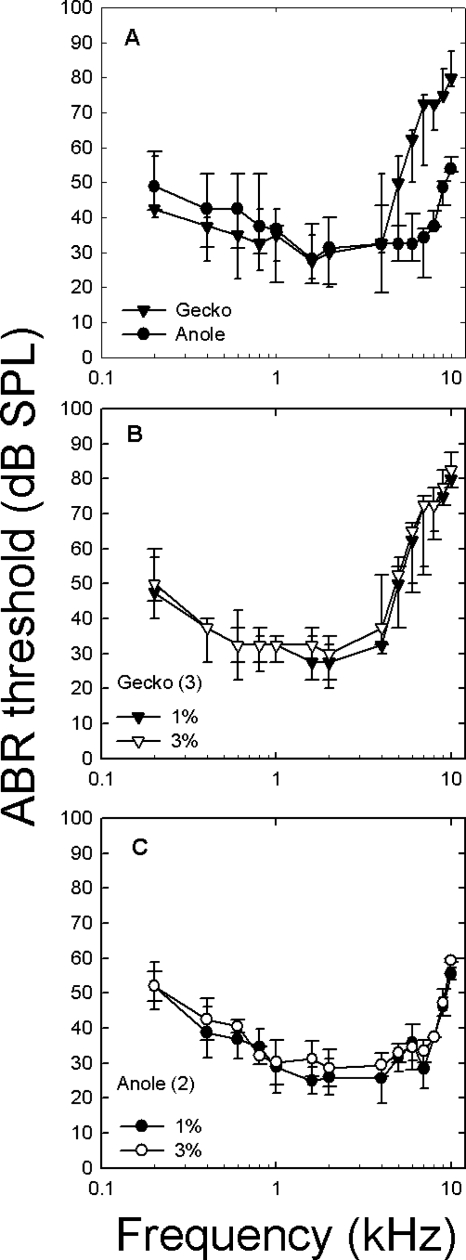
Both species had U-shaped ABR-derived audiograms and thresholds that were similar below 5 kHz [Fig. 4A]. However, there were significant species [F(1,105)=75.05 p<0.0001] and species by frequency [F(13,105)=11.91 p<0.0001] interaction differences. Although geckos appeared to have lower ABR thresholds than anoles, these were not significant below 3 kHz (with the exception of 1 kHz) [post hoc contrasts from species by frequency interaction showed no significant differences at frequencies from 0.2–4 kHz, F(8,105)=1.88, p>0.05]. Anoles were, however, about 20 dB more sensitive than geckos above 5 kHz [post hoc contrasts from species by frequency interaction showed significant differences from 5–10 kHz, F(6,105)=29.23, p<0.0001]. The gecko showed well defined peaks in sensitivity around 0.6 and 1.6 kHz (∼30 dB SPL) but a steeper high frequency cutoff; whereas, the anole ABR-derived audiogram showed extended sensitivity between 1–7 kHz.
Figure 4.
ABR audiograms for geckos and anoles and for two anesthetic levels. Median thresholds (and ranges) are shown for all data. (A) ABR-derived audiograms for anoles (circles) and geckos (triangles). Note the similar thresholds, except above 4 kHz, where anole audiograms were consistently more sensitive. (B) ABR audiograms for 3 geckos measured at 1% and 3% isoflurane. (C) ABR audiograms for 2 anoles measured at 1% and 3% isoflurane.
The effects of varying isoflurane levels are shown in Figs. 4B, 4C. Changing the level from 3% (producing full anesthesia) to 0.5%–1% (animal is lightly anesthetized or sedated), produced no significant changes in sensitivity [sedation level by frequency by individual F(52,84)=0.047 p>0.05].
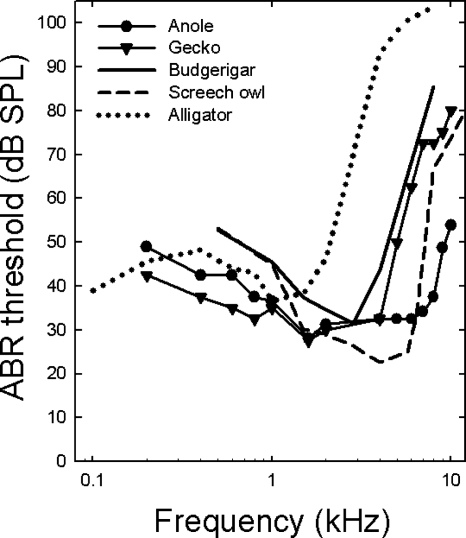
ABR-derived audiograms for budgerigars, screech owls, alligators and lizards show striking similarities but also differences (Fig. 5). At low frequencies, both lizards and alligators appeared to show increased sensitivity at and below 1 kHz when compared to the budgerigar and the screech owl tested using the same stimuli, although sounds were delivered in free field for the birds. The differences between birds and alligators were significant at 1 kHz [F(4,43)=7.73, p<0.0001]. Post-hoc Turkey’s HSD tests showed that birds had significantly higher ABR thresholds at 1 kHz than anoles or geckos at the 0.05 level of significance. All other comparisons were not significant. The opposite held for the mid-frequency range (between 2–4 kHz), where geckos, anoles and birds had similar thresholds, with alligators showing a steep increase in ABR thresholds (these were derived in air, not water). There were significant differences in thresholds at 2 kHz [F(4,43)=6.47, p<0.0001] and 4 kHz [F(4,43)=91.78, p<0.0001]. While post-hoc Tukey’s HSD showed that the alligators had significantly higher thresholds than the lizards and the birds at 2 kHz and 4 kHz, budgerigars and geckos had similar thresholds, as did anoles and screech owls, at 4 kHz. As frequency increased above 4 kHz, gecko and budgerigar thresholds showed similar trajectories (steeper increases in threshold). ABR thresholds for anoles were more similar to, but not as low as, the screech owl, an auditory specialist. In both the anoles and screech owls, ABR thresholds began to increase dramatically around 7 kHz. At 8 kHz, thresholds were significantly different [F(4,24)=44.58, p<0.0001], with post-hoc Turkey’s HSD tests showing significantly lower thresholds in anoles compared to all other species.
Figure 5.
ABR-derived audiograms for anoles (closed circles) and geckos (closed triangles) in comparison to budgerigars (solid line, Brittan-Powell et al., 2002), screech owls (dashed line, Brittan-Powell et al., 2005) and alligators (dotted gray line, Higgs et al., 2002). Note all ABRs were measured using the same equipment, except that the sound source for the bird and alligator ABRs was free field while lizard sound stimuli were delivered through earphones. Error bars for these average data are not shown for the sake of clarity. Averaged data are shown for other studies, but median data are shown for the anole and gecko for the sake of consistency.
DISCUSSION
Lizard hearing
The ABR proved a robust and useful method to evaluate lizard hearing. We found no evidence that level of anesthesia affected ABR thresholds in either species (but see Dodd and Capranica, 1992; Santarelli et al., 2003; Stronks et al., 2010). Overall, signals were relatively large in the two lizards, with geckos showing better neural synchrony (sharper peaks in waveform) than anoles. The increased synchrony may be due to geckos having three times as many auditory nerve fibers as the anole (Manley, 2000; Miller, 1985). Interestingly, the increased synchrony and larger amplitudes at higher sound intensities did not translate to lower thresholds. As intensity neared threshold levels, amplitude was similar in both species. The audiograms of the two lizard species were similar in frequency range and show similar peak sensitivity at 30 dB SPL. However, anoles were sensitive to a larger range of frequencies (thresholds ∼30–35 dB SPL from 1–7 kHz) and had significantly lower thresholds than geckos between 5–10 kHz. The increased high-frequency sensitivity in anoles was consistent with experiments showing high frequency spontaneous otoacoustic emissions among anolid lizards (7.7 kHz, Manley and Gallo, 1997) and laser vibrometry measurements showing extended high-frequency sensitivity of the Anolis eardrum (Christensen-Dalsgaard and Manley, 2008). Unlike the anole, geckos showed distinct peaks in sensitivity, one of which corresponds to the fundamental frequency of their advertisement calls. The rattle (mononote) and the ge ko (binote) call both show fundamental frequencies around 500 Hz (Tang et al., 2001). Geckos also showed increased sensitivity around 1.6 kHz which may correspond to the second frequency peak at 1.3 kHz (Brillet and Paillette, 1991). Lastly, low frequencies evoked FFR in high intensity signals, especially in geckos, These responses were twice the stimulation frequency, which is typical in studies when opposite phases are added together (see Aiken and Picton, 2008; Sohmer et al., 1977).
We occasionally saw summating potentials that are generated by summed inner-ear receptor potentials. Such summating potentials have been reported in round window recordings in several species of lizards (Johnstone and Johnstone, 1969) and should be small in far field ABR recordings. Although small, we consider summating potentials here because they could contribute differentially to the ABR and result in differences in thresholds in lizards with different papilla organization. We think this unlikely because the summating potentials, when observed, were much smaller than wave 1 of the ABR. Furthermore, we defined ABR threshold as the intensity 2.5 dB (one-half step in intensity) below the lowest stimulus level at which a response could be visually detected on the trace between 1 and 6 ms, regardless of waveform (Brittan-Powell and Dooling, 2004; Brittan-Powell et al., 2005). Thus, although is possible that the waveforms contain far field components of the summating potential, given our definition of threshold, it is unlikely that their small contribution affected the threshold estimations.
The comparable low best frequency ABR thresholds in lizards, alligators and birds may reflect the similar organization of their auditory systems (Carr, 1992; Carr and Code, 2000; Gleich and Manley, 2000; Manley, 1970). Birds, alligators and lizards have unidirectional (hair bundles oriented in the same direction) populations of hair cells covered by a tectorial membrane and similar auditory nerve projections (Gleich and Manley, 2000; Leake, 1974). Manley has proposed that the primitive lepidosaur papilla supported unidirectional hair cells covered with a tectorial membrane. Such a papilla would only have responded to low sound frequencies (below about 1 kHz). The evolution of additional unique populations of high frequency hair cells in most lizards may have increased lizard high frequency sensitivity (Köppl and Manley, 1990; Manley, 1990; Miller, 1980).
Comparisons with earlier Tokay data
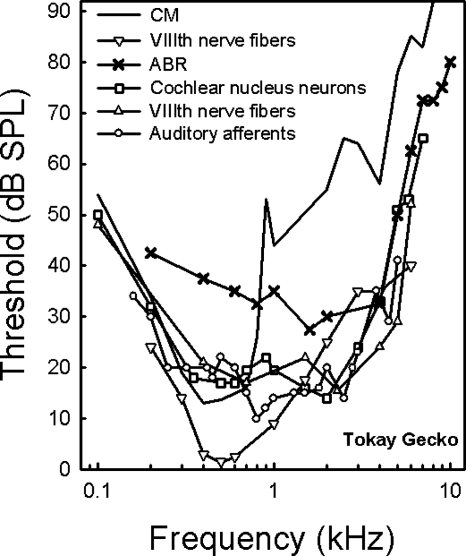
Comparison of previous data on audiograms in the Tokay gecko obtained from single unit nerve recordings (Eatock et al., 1981; Manley, 1972; Manley et al., 1999; Sams-Dodd and Capranica, 1996), cochlear microphonics (CM) (Werner and Wever, 1972), and our ABRs, reveal differences in audiogram shape (Fig. 6). The data fall into two groups: one which shows a distinct low best frequency peak and another which is more bimodal. The first group (gray lines) includes CM studies (Werner and Wever, 1972) and a single-unit study (Sams-Dodd and Capranica, 1996) and shows best sensitivity around 4–500 Hz and a relatively monotonic decline of sensitivity from 600 Hz to 8 kHz. The second group (black lines) includes audiograms based on the lowest thresholds of cochlear nucleus neurons (Manley, 1972), primary nerve fibers (Eatock et al., 1981; Manley et al., 1999) and our ABR data, and shows bimodal audiograms with best sensitivities at 400 Hz and 1–2 kHz. Each method comes with its own set of limitations. CM measurements may be biased toward low-frequency responses, where the synchronization is greatest. Furthermore, Manley (1990) has pointed out that CMs from the bidirectional higher best frequency population may largely cancel each other out because there are equally large populations with both orientations (Manley, 1990; Köppl and Authier, 1995).
Figure 6.
Data showing the median gecko ABR audiogram (black X’s) in relation to cochlear microphonic data (CM gray solid line; Werner and Wever, 1972, Fig. 6), lowest thresholds of cochlear nucleus cells (black open square: Manley, 1972, Fig. 2), and VIIIth nerve data [gray inverted triangle: Sams-Dodd and Capranica, 1996, Fig. 2A; black triangle: Eatock et al., 1981, Fig. 2] and lowest thresholds of auditory afferents [black open circle: Manley et al., 1999, Fig. 2A].
Experimental data will further be influenced by the surgical method used to access the auditory nerve, and both the ventral surgical approach used by Sams-Dodd and Capranica (1996) and the approaches used to record CM and compound action potentials (CAP) from the round window are considerably more invasive than the present method. CAP and laser interferometry measurements carried out in the same species of geckos (Eublepharis macularius and Oedura marmorata) showed that the shape of the CAP audiograms (very similar to the CM audiogram in the Tokay) was not predicted well by the tympanic membrane response. For example, the peak response of the adult E. macularius velocity function was approximately 2.2 kHz while the most sensitive CAP threshold was at 0.7 kHz (Werner et al., 2002). It seems reasonable, as suggested by Werner et al. (2001), that these differences are caused by the ventral surgical approach; surgical fenestration of the ventral throat wall may cause artificial enhancement of sensitivity at low frequencies and erratic responses at high frequencies. The role of the intact middle ear cavity in high frequency hearing is also supported by recent laser vibrometry studies and mathematical modeling of the gecko middle ear that show a pronounced resonance of the middle ear cavities (Christensen-Dalsgaard and Manley, 2008).
The bimodal audiogram is most consistent with laser vibrometry studies of the Tokay gecko since the ABR peak sensitivity at 2 kHz also reflects the maximal sensitivity of the eardrum with laser measurements (Christensen-Dalsgaard and Manley, 2005; Werner et al., 2002). At low frequencies, the slope of the present ABR audiogram is slightly shallower than for the previous physiological measurements (see Fig. 6). Below 400 Hz, however, the very brief tone pulse (10 ms) is more broad-band than the nominal frequency which may lead to frequency splatter and recruitment of additional auditory responses from adjacent regions of the papilla.
The 20 dB general difference in sensitivity between the ABR audiogram and the single-unit audiograms is easier to explain. As previously stated, ABR studies have shown that frequency-dependent ABRs are good predictors of audiogram shape but not of absolute auditory sensitivity. ABR thresholds are generally about 10–30 dB greater than behavioral thresholds (e.g., Brittan-Powell et al., 2002; Gorga et al., 1988; but see Bullock et al., 1968; Popov and Supin, 1990). The synchrony required for an ABR response may be well above the threshold for behavioral sensation (Hecox et al., 1976; Szymanski et al., 1999). In the barn owl, where there is a large nerve data set, Köppl and Gleich (2007) compared measures of behavioral sensitivity with CAP, CM, and single units and found very similar shapes of the CM and CAP audiograms and relative thresholds of single auditory-nerve fibers, although the CAP and CM thresholds were much larger than the single unit or behavioral measures.
It should be noted that behavioral thresholds have not been measured in most lizards (or in other reptiles except birds), and although non-avian reptiles produce a variety of sounds ranging from the rattling of snake tails to the bellows of crocodilians (see review Gans and Maderson, 1973), in no case has communication by sound been well documented except in the gekkonid lizards. In the Tokay gecko, the dominant frequencies of the call match the region of best sensitivity in the ABR. Peak energy of vocalizations and maximum sensitivity of the audiogram usually match in birds and frogs (Ryan and Wilzcynski, 1988, Dooling et al., 2000), and the same relationship may hold for gekkonid lizards. However, the present results show that a non-vocal lizard (anole) has a more extended high frequency range and is as sensitive as or more sensitive than the vocal gecko. This would suggest that sound communication is not the prime function of the exquisite auditory sensitivity in lizards.
ACKNOWLEDGMENTS
This work was supported in part by Grant No. P30 DC004664 to the University of Maryland Center for the Evolutionary Biology of Hearing, by Grant No. DC00436 to CEC from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health, and by the Chinese Academy of Sciences Bairenjihua Grant No. KSCX2-YW-R-077 to Y.T., and by grants from the Danish Natural Science Research Council to J.C.-D.
References
- Aiken, S. J., and Picton, T. W. (2008). “Envelope and spectral frequency-following responses to vowel sounds,” Hear. Res. 245, 35–47. 10.1016/j.heares.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Beattie, R. C., and Torre, P. (1997). “Effects of rise-fall time and repetition rate on the auditory brainstem response to 0.5 and 1 kHz tone bursts using normal-hearing and hearing-impaired subjects,” Scand. Audiol. 26, 23–32. [DOI] [PubMed] [Google Scholar]
- Bennett, A. (1998). “Reptile anesthesia,” Semin Avian Exotic Pet Med 7, 30–40. 10.1016/S1055-937X(98)80055-8 [DOI] [Google Scholar]
- Blair, W. F. (1968). “Communication in selected groups: Amphibians and reptiles,” in Animal Communication, edited by Sebeok T. A. (Indiana University Press, Bloomington: ), pp. 289–310. [Google Scholar]
- Bogert, C. M. (1960). “The influence of sound on the behavior of amphibians and reptiles,” in Animal Sounds and Communication, edited by Lanyon W. E. and Tavolga W. N. (AIBS, Washington DC: ), pp. 137–320. [Google Scholar]
- Borg, E., and Engström, B. (1983). “Hearing thresholds in the rabbit,” Acta Oto-Laryngol. 95, 19–26. 10.3109/00016488309130911 [DOI] [PubMed] [Google Scholar]
- Brillet, C., and Paillette, M. (1991). “Acoustics signals of the nocturnal lizard Gekko gecko: analysis of the “long complex sequence”,” Bioacoustics 3, 33–44. [Google Scholar]
- Brittan-Powell, E. F., and Dooling, R. J. (2004). “Development of auditory sensitivity in budgerigars (Melopsittacus undulatus),” J. Acoust. Soc. Am. 115, 3092–3102. 10.1121/1.1739479 [DOI] [PubMed] [Google Scholar]
- Brittan-Powell, E. F., Dooling, R. J., and Gleich, O. (2002). “Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus),” J. Acoust. Soc. Am. 112, 999–1008. 10.1121/1.1494807 [DOI] [PubMed] [Google Scholar]
- Brittan-Powell, E. F., Lohr, B., Hahn, D. C., and Dooling, R. J. (2005). “Auditory brainstem responses in the Eastern Screech Owl: An estimate of auditory thresholds,” J. Acoust. Soc. Am. 118, 314–321. 10.1121/1.1928767 [DOI] [PubMed] [Google Scholar]
- Brown, A. M. (1985). “Ultrasound in gecko distress calls (Reptilia: Gekkonidae),” Isr. J. Zool. 33, 95–101. [Google Scholar]
- Bullock, T. H., Grinnell, A. D., Ikezono, E., Kameda, K., Katsuki, Y., Nomoto, M., Sato, O., Suga, N., and Yanagisawa, K. (1968). “Electrophysiological studies of the central auditory mechanisms in cetaceans,” Z. Vgl. Physiol. 59, 117–156. [Google Scholar]
- Carr, C. E. (1992). “The evolution of the central auditory system in reptiles and birds,” in The Evolutionary Biology of Hearing, edited by Webster D. B., Fay R. R., and Popper A. N. (Springer-Verlag, New York: ), pp. 511–544. [Google Scholar]
- Carr, C. E., and Code, R. A. (2000). “The central auditory system of reptiles and birds,” in Comparative Hearing: Birds and Reptiles, edited by Dooling R. J., Fay R. R., and Popper A. N. (Springer-Verlag, New York: ), pp. 197–248. [Google Scholar]
- Christensen-Dalsgaard, J., and Manley, G. A. (2005). “Directionality of the lizard ear,” J. Exp. Biol. 208, 1209–1217. 10.1242/jeb.01511 [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard, J., and Manley, G. A. (2008). “Acoustical coupling of lizard eardrums,” J. Assoc. Res. Otolaryngol. 9, 407–416. 10.1007/s10162-008-0130-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack, J. A. (1997). “The evolution of tetrapod ears and the fossil record,” Brain Behav. Evol. 50, 198–212. 10.1159/000113334 [DOI] [PubMed] [Google Scholar]
- Corwin, J. T., Bullock, T. H., and Schweitzer, J. (1982). “The auditory brain stem response in five vertebrate classes,” Electroencephalogr. Clin. Neurophysiol. 54, 629–641. 10.1016/0013-4694(82)90117-1 [DOI] [PubMed] [Google Scholar]
- Dodd, F., and Capranica, R. R. (1992). “A comparison of anesthetic agents and their effects on the response properties of the peripheral auditory system,” Hear. Res. 62, 173–180. 10.1016/0378-5955(92)90183-N [DOI] [PubMed] [Google Scholar]
- Dooling, R. J., Lohr, B., and Dent, M. L. (2000). “Hearing in birds and reptiles,” in Comparative Hearing: Birds and Reptiles, edited by Dooling R. J., Popper A. N., and Fay R. R. (Springer-Verlag, New York: ), pp. 308–359. [Google Scholar]
- Eatock, R., Manley, G., and Pawson, L. (1981). “Auditory nerve fibre activity in the tokay gecko. I. Implications for cochlear processing,” J. Comp. Physiol., A 142, 203–218. 10.1007/BF00605739 [DOI] [Google Scholar]
- Gans, C., and Maderson, P. F. A. (1973). “Sound producing mechanisms in recent reptiles: Review and comment,” Am. Zool. 13, 1195–1203. [Google Scholar]
- Gleich, O., and Manley, G. A. (2000). “The hearing organ of birds and crocodilia,” in Comparative Hearing: Birds and Reptiles, edited by Dooling R. J., Popper A. N., and Fay R. R., (Springer-Verlag, New York: ), pp. 70–138. [Google Scholar]
- Gorga, M., Kaminski, J., Beauchaine, K., and Jesteadt, W. (1988). “Auditory brain response to tone burst in normal hearing subjects,” J. Speech Hear. Res. 31, 89–97. [DOI] [PubMed] [Google Scholar]
- Grothe, B., Carr, C. E., Casseday, J. H., Fritzsch, B., and Köppl, C. (2005). “The evolution of central pathways and their neural processing patterns,” in Evolution of the Vertebrate Auditory System, edited by Manley G. A. (Springer, New York: ), pp. 289–359. [Google Scholar]
- Hall, J. W. (2007). New Handbook of Auditory Evoked Responses (Allyn and Bacon, Boston: ). [Google Scholar]
- Hecox, K., Squires, N., and Galambos, R. (1976). “Brain stem evoked responses in man: I. Effects of stimulus rise∕fall time and duration,” J. Acoust. Soc. Am. 60, 1187–1192. 10.1121/1.381194 [DOI] [PubMed] [Google Scholar]
- Higgs, D. M., Brittan-Powell, E. F., Soares, D., Souza, M. J., Carr, C. E., Dooling, R. J., and Popper, A. N. (2002). “Amphibious auditory responses of the American alligator (Alligator mississipiensis),” J. Comp. Physiol. 188, 217–223. 10.1007/s00359-002-0296-8 [DOI] [PubMed] [Google Scholar]
- Jewett, D. (1970). “Volume-conducted potentials in response to auditory stimuli as detected by averaging in the cat,” Electroencephalogr. Clin. Neurophysiol. 28, 609–618. 10.1016/0013-4694(70)90203-8 [DOI] [PubMed] [Google Scholar]
- Johnstone, J. R., and Johnstone, B. M. (1969). “Electrophysiology of the lizard cochlea,” Exp. Neurol. 24, 99–109. 10.1016/0014-4886(69)90008-9 [DOI] [PubMed] [Google Scholar]
- Kodera, K., Hink, R. F., Yamada, O., and Suzuki, J. I. (1979). “Effects of rise time on simultaneously recorded auditory-evoked potentials from the early, middle, and late ranges,” Audiology 18, 395–402. 10.3109/00206097909070065 [DOI] [PubMed] [Google Scholar]
- Kodera, K., Yamane, H., Yamada, O., and Suzuki, J. I. (1977). “The effects of onset, offset, and rise-decay times of tone bursts on brain stem responses,” Scand. Audiol. 6, 205–210. [DOI] [PubMed] [Google Scholar]
- Köppl, C., and Authier, S. (1995). “Quantitative anatomical basis for a model of micromechanical frequency tuning in the Tokay gecko, Gekko gecko,” Hear. Res. 82, 14–25. 10.1016/0378-5955(94)00139-H [DOI] [PubMed] [Google Scholar]
- Köppl, C., and Gleich, O. (2007). “Evoked cochlear potentials in the barn owl,” J. Comp. Physiol. [A] 193, 601–612. 10.1007/s00359-007-0215-0 [DOI] [PubMed] [Google Scholar]
- Köppl, C., and Manley, G. A. (1990). “Peripheral auditory processing in the Bobtail Lizard Tiliqua rugosa. 2. Tonotopic organization and innervation pattern of the basilar papilla,” J. Comp. Physiol. [A] 167, 101–112. 10.1007/BF00192410 [DOI] [Google Scholar]
- Leake, P. A. (1974). “Central projections of the statoacoustic nerve in Caiman crocodilus,” Brain Behav. Evol. 10, 170–196. 10.1159/000124311 [DOI] [PubMed] [Google Scholar]
- Manley, G. A. (1970). “Comparative studies of auditory physiology in reptiles,” Z. Vgl. Physiol. 67, 363–381. 10.1007/BF00297906 [DOI] [Google Scholar]
- Manley, G. A. (1972). “Frequency response of the ear of the Tokay gecko,” J. Exp. Zool. 181, 159–168. 10.1002/jez.1401810203 [DOI] [PubMed] [Google Scholar]
- Manley, G. A. (1981). “A review of the auditory physiology of reptiles,” in Progress in Sensory Physiology, edited by Autrum H., Perl E., and Schmidt R. F. (Springer, Berlin: ), pp. 49–134. [Google Scholar]
- Manley, G. A. (1990). Peripheral Hearing Mechanisms in Reptiles and Birds (Springer-Verlag, Heidelberg: ). [Google Scholar]
- Manley, G. A. (2000). “The hearing organs of lizards,” in Comparative Hearing: Birds and Reptiles, edited by Dooling R. J., Fay R. R., and Popper A. N. (Springer-Verlag, New York: ), pp. 139–196. [Google Scholar]
- Manley, G. A. (2002). “Evolution of structure and function of the hearing organ of lizards,” J. Neurobiol. 53, 202–211. 10.1002/neu.10115 [DOI] [PubMed] [Google Scholar]
- Manley, G. A. (2004). “The lizard basilar papilla and its evolution,” in Evolution of the Vertebrate Auditory System, edited by Manley G. A., Popper A., and Fay R. R. (Springer-Verlag, New York: ), pp. 200–223. [Google Scholar]
- Manley, G. A., and Gallo, L. (1997). “Otoacoustic emissions, hair cells, and myosin motors,” J. Acoust. Soc. Am. 102, 1049–1055. 10.1121/1.419858 [DOI] [PubMed] [Google Scholar]
- Manley, G. A., Köppl, C., and Sneary, M. (1999). “Reversed tonotopic map of the basilar papilla in Gekko gecko,” Hear. Res. 131, 107–116. 10.1016/S0378-5955(99)00021-0 [DOI] [PubMed] [Google Scholar]
- Marcellini, D. L. (1977). “The acoustic behavior of lizards,” in Behavior and Neurology of Lizards, edited by Greenberg N. and MacLean P. D. (U.S. Department of Health, Education and Welfare, Rockville, MD: ), pp. 287–300. [Google Scholar]
- Miller, M. R. (1980). “The reptilian cochlear duct,” in Comparative Studies of Hearing in Vertebrates, edited by Popper A. N. and Fay R. R. (Springer, Berlin: ), pp. 169–204. [Google Scholar]
- Miller, M. R. (1985). “Quantitative studies of auditory hair cells and nerves in lizards,” J. Comp. Neurol. 232, 1–24. 10.1002/cne.902320102 [DOI] [PubMed] [Google Scholar]
- Moore, M. C., Thompson, C. W., and Marler, C. A. (1991). “Reciprocal changes in corticosterone and testosterone levels following acute and chronic handling stress in the tree lizard, Urosaurus ornatus,” Gen. Comp. Endocrinol. 81, 217–226. 10.1016/0016-6480(91)90006-R [DOI] [PubMed] [Google Scholar]
- Picton, T., and Hillyard, S. (1974). “Human auditory evoked potentials: II. Effects of attention,” Electroencephalogr. Clin. Neurophysiol. 36, 191–200. 10.1016/0013-4694(74)90156-4 [DOI] [PubMed] [Google Scholar]
- Popov, V., and Supin, A. (1990). “Electrophysiological study of hearing in some cetaceans and manatee,” in Sensory Abilities of Cetaceans, edited by Kastalien J. T. R. (Plenum, New York: ), pp. 405–415. [Google Scholar]
- Ryan, M. J., and Wilzcynski, W. (1988). “Coevolution of sender and receiver: Effects on local mate preference in cricket frogs,” Science 240, 1786–1788. 10.1126/science.240.4860.1786 [DOI] [PubMed] [Google Scholar]
- Sams-Dodd, F., and Capranica, R. R. (1996). “Representation of acoustic signals in the eighth nerve of the Tokay gecko. II. Masking of pure tones with noise,” Hear. Res. 100, 131–142. 10.1016/0378-5955(96)00104-9 [DOI] [PubMed] [Google Scholar]
- Santarelli, R., Arslan, E., Carraro, L., Conti, G., Capello, M., and Plourde, G. (2003). “Effects of isoflurane on the auditory brainstem responses and middle latency responses of rats,” Acta Oto-Laryngol. 123, 176–181. 10.1080/0036554021000028108 [DOI] [PubMed] [Google Scholar]
- Seufer, H. (1991). Keeping and Breeding Geckos (TFH, Neptune, NJ: ). [Google Scholar]
- Sohmer, H., Pratt, H., and Kinarti, R. (1977). “Sources of frequency following responses (FFR) in man,” Electroencephalogr. Clin. Neurophysiol. 42, 656–664. 10.1016/0013-4694(77)90282-6 [DOI] [PubMed] [Google Scholar]
- Stapells, D. R., and Oates, P. (1997). “Estimation of pure-tone audiogram by the auditory brainstem response: A review,” Audiol. Neuro-Otol. 2, 257–280. 10.1159/000259252 [DOI] [PubMed] [Google Scholar]
- Steck, L. (1908). “The vocal apparatus of Hemidactylus garnoti and a contribution to the anatomy of the gekkotids,” Zoologische Jahrbuecher. Abteilung fuer Anatomie 25, 611–635. [Google Scholar]
- Stronks, H. C., Aarts, M. C. J., and Klis, S. F. (2010). “Effects of isoflourane on auditory evoked potentials in the cochlea and brainstem of guinea pigs,” Hear. Res. 260, 20–29. 10.1016/j.heares.2009.10.015 [DOI] [PubMed] [Google Scholar]
- Szpir, M. R., Wright, D. D., and Ryugo, D. K. (1995). “Neuronal organization of the cochlear nuclei in alligator lizards: A light and electron microscopic investigation,” J. Comp. Neurol. 357, 217–241. 10.1002/cne.903570204 [DOI] [PubMed] [Google Scholar]
- Szymanski, M., Bain, D., Kiehl, K., Pennington, S., Wong, S., and Henry, K. (1999). “Killer whale hearing: Auditory brainstem responses and behavioral audiograms,” J. Acoust. Soc. Am. 106, 1134–1141. 10.1121/1.427121 [DOI] [PubMed] [Google Scholar]
- Tang, Y., Zhuang, L., and Wang, Z. (2001). “Advertisement calls and their relation to reproductive cycles in the Gekko gecko,” Copeia 2001, 248–253. 10.1643/0045-8511(2001)001[0248:ACATRT]2.0.CO;2 [DOI] [Google Scholar]
- Walsh, E. J., Gorga, M., and McGee, J. (1992). “Comparisons of the development of auditory brainstem response latencies between cats and humans,” Hear. Res. 60, 53–63. 10.1016/0378-5955(92)90058-U [DOI] [PubMed] [Google Scholar]
- Wenstrup, J. J. (1984). “Auditory sensitivity in the fish-catching bat, Noctilio leporinus,” J. Comp. Physiol. 155, 91–101. 10.1007/BF00610934 [DOI] [Google Scholar]
- Werner, L. A., and Wever, E. G. (1972). “The function of the middle ear in lizards: Gekko gecko and Eublepharis macularius (Gekkonoidea),” J. Exp. Zool. 179, 1–16. 10.1002/jez.1401790102 [DOI] [Google Scholar]
- Werner, Y. L., Frankenberg, E., and Adar, O. (1978). “Further observations on the distinctive vocal repertoire of Ptyodactylus hasselquistii cf. hesselquistii (Reptilia: Gekkonidae),” Isr. J. Zool. 27, 176–188. [Google Scholar]
- Werner, Y. L., Igic, P. G., and Saunders, J. C. (2001). “Effects of surgery and other experimental factors on the evaluation of middle ear function in gekkonoid lizards,” Hear. Res. 160, 22–30. 10.1016/S0378-5955(01)00331-8 [DOI] [PubMed] [Google Scholar]
- Werner, Y. L., Igic, P. G., Seifan, M., and Saunders, J. C. (2002). “Effects of age and size in the ears of gekkonomorph lizards: Middle-ear sensitivity,” J. Exp. Biol. 205, 3215–3223. [DOI] [PubMed] [Google Scholar]
- Werner, Y. L., Montgomery, L. G., Seifan, M., and Saunders, J. C. (2008). “Effects of age and size in the ears of gekkotan lizards: Auditory sensitivity, its determinants, and new insights into tetrapod middle-ear function,” Pfluegers Arch. Gesamte Physiol. Menschen Tiere 456, 951–967. 10.1007/s00424-008-0462-0 [DOI] [PubMed] [Google Scholar]
- Wever, E. G. (1978). The Reptile Ear (Princeton University Press, Princeton. [Google Scholar]