Abstract
Attention deficits are considered to be fundamental in patients with schizophrenia. During attention tasks, patients with schizophrenia have been shown to display increased brain activity in some neuroimaging studies but reduced brain activity in others. These conflicting findings may be due to some study designs primarily eliciting transient engagement of attention and other study designs primarily eliciting sustained engagement of attention.
In the present study, ten males with schizophrenia and fourteen age-matched, male controls performed a visual selective attention task. A mixed block/event-related fMRI design was used, allowing for separate analysis of transient and sustained phases of attention.
Results revealed that the schizophrenia group made significantly fewer correct responses and displayed a significantly slower mean response time than the control group. Voxel-wise random effects analyses revealed that both groups displayed activation in regions considered to constitute a core attentional network including the anterior cingulate gyrus, dorsolateral prefrontal cortex, insula and inferior parietal sulcus. Region of Interest (ROI) analyses revealed that across the entire sequence of task and non-task blocks, the schizophrenia group displayed a greater percentage of active voxels than controls in many ROIs. However, during transient periods most pertinent to task performance, the schizophrenia group displayed a lower percentage of active voxels than controls.
These results help to explain contrasting findings across previous studies and suggest that attention deficits displayed by patients with schizophrenia are more likely to reflect deficits in modulating brain activity in response to variations in transient, attention demanding stimuli, rather than deficits in sustained attention.
Keywords: Schizophrenia, Attention, Transient, Sustained, Maintenance of Task Set, Functional Magnetic Resonance Imaging (fMRI), Mixed design
1. Introduction
Impaired attention is considered to be a fundamental cognitive deficit in patients with schizophrenia (Fioravanti, et al. 2005, Neuchterlein, et al. 1991). Brain imaging studies have consistently revealed that, during performance of attention tasks, the brain activity of patients with schizophrenia differs from that of controls, particularly in regions considered to constitute an attentional network including the dorsolateral prefrontal cortex (DLPFC), the insula, the anterior cingulate gyrus (ACG), the amygdala, hippocampus, ventral striatum, thalamus and cerebellum (Liddle, et al. 2006). While many of these neuroimaging studies have provided evidence that patients with schizophrenia display reduced brain activity as compared to controls (Barch, et al. 2001, Carter, et al. 1998, Karch, et al. 2009, Kerns, et al. 2005, Liddle, et al. 2006, Polli, et al. 2008, Quintana, et al. 2004, Schneider, et al. 2007, Weiss, et al. 2007), several studies have provided evidence of increased activity in patients with schizophrenia (Callicott, et al. 2000, Karch, et al. 2009, Manoach, et al. 2000, Manoach, et al. 1999, Weiss, et al. 2003).
There are a number of possible reasons for the contrasting findings across studies including: differences in the types of activation tasks used; differences in analysis techniques; differences in the degree to which task performance has become automated and task difficulty (Karch, et al. 2009, Manoach 2003). Regarding task difficulty, patients with schizophrenia have been shown to display greater cortical activity than controls on less demanding tasks of attention but less cortical activity than controls on more demanding tasks of attention (Karch, et al. 2009).
Such contrasting findings across studies are not surprising given that attention is a multi-faceted construct and given that different types of activation tasks are likely to engage to a different degree, the various aspects of attention (Dosenbach, et al. 2006, Huettel, et al. 2004). For instance, most activation tasks are likely to require some aspect of sustained attention that is maintained throughout performance of a task (i.e. maintenance of task set) as well as requiring more transient aspects of attention that are engaged during the most important moments of a task (Dosenbach, et al. 2006, Huettel, et al. 2004). Subsequently, study design is as important as the activation task used because study designs that utilize only a block design do not allow for the separate analysis of transient aspects of attention from the sustained aspects of maintenance of task set (Dosenbach, et al. 2006, Huettel, et al. 2004). Without making such a separation, it is not possible to determine whether activity in a given brain region is due to sustained attention that is continuously maintained throughout the task or whether it is due to more transient aspects of attention (Dosenbach, et al. 2006, Huettel, et al. 2004).
A mixed block/event-related design can be used across a wide range of conditions to extract and separately analyze transient signals that are related to the onset of a task-relevant block; transient signals that are related to the onset of a target; and sustained signals that endure across the entire task (Dosenbach, et al. 2006, Huettel, et al. 2004). Such different signals do appear to have overlapping but separate anatomical distributions that imply diverse functional roles (Dosenbach, et al. 2006).
The studies performed by Dosenbach et al. (2006) and Huettel et al. (2004) on normal participants and across numerous tasks have provided evidence of a core attentional network that is involved in the maintenance of task set (Dosenbach, et al. 2006, Huettel, et al. 2004).
A previous study from our lab utilized event related potentials (ERP) to explore the temporal dynamics of early sustained attention orienting and later “phasic” or transient aspects of attention deployment during a simple target detection in individuals with schizophrenia and “prodromal” individuals at imminent risk for schizophrenia (van der Stelt, 2006). This study found that the frontal selection positivity (SP) component of the ERP signal, which is thought to reflect “early” attention-related modulations of visual processing, is reliably elicited in healthy participants, but is significantly reduced, if not absent, in participants with schizophrenia as well as in prodromal individuals. This suggests that individuals both at risk for and suffering from schizophrenia show alterations in early attention orienting mechanisms, resulting in a failure of cortical feedback mechanisms to impart attentional influences on extrastriate visual cortices (van der Stelt, 2006). The neuroanatomical basis for this failure to deploy early attention to simple task-relevant color dimensions remains unclear. Furthermore, schizophrenia patients in this early ERP study, but not prodromal individuals, showed significant deficits in target-related P3 components. Taken together these findings raised the possibility that while certain neural pathways implicated in early attention deployment mechanisms may be altered in both patients and high-risk individuals, deficits in other cortical regions may not emerge until later in the disorder. Hence, the goal of the present study was twofold, (a) to investigate the neuroanatomical basis of the ERP findings by employing a mixed design fMRI task to dissociate activation during the two phases of attention deployment, and (b) to examine whether cortical activity in patients with schizophrenia differed during the “sustained” or “tonic” and “transient” or “phasic” phases of a selective attention task. To accomplish these aims, we designed a variant of the van der Stelt task implemented using a mixed-design fMRI paradigm, whereby an infrequent target event was embedded within blocks of task-relevant stimuli. Task-relevant stimuli were all colored blue whereas non-task stimuli were red. Targets were colored blue and were only embedded in a block of task-relevant stimuli (blue distractors) so that task-relevant blocks would heighten demand on attention processes whereas non-task blocks would place minimal demand on attention processes. Accordingly, the sustained phase of attention was defined as activation across the entire task, i.e. blue task-relevant blocks and the red non-task blocks. The transient phases of attention were defined as the onset of a task-relevant block and the onset of a target.
2. Materials and methods
2.1 Participants
Fourteen male neurotypical participants and ten male patients with schizophrenia participated in this study. Data from two control subjects was discarded due to equipment failure and excessive motion. Data from one patient was excluded due to failure to follow task instructions. Data from twelve control subjects and nine patients with schizophrenia were included in the data analysis. Summary demographic information is included below in Table 1. All participants were matched for age and handedness.
Table 1.
Demographic Characteristics
| Scz (n=9) mean (SD) | Ctr (n=12) mean (SD) | t (17) | |
|---|---|---|---|
| Age | 29.8±12.0 (20–59) | 25.5± 4.6 (20–34) | 0.299 |
| Full Scale IQ | 111.68±6.4 (98–121) | 113.63±6.01 (102–124) | 0.487 |
| Performance IQ | 110.7±3.4 (103–116) | 111.78±3.24 (106–117) | 0.487 |
| Verbal IQ | 110.3±7.3 (95–121) | 112.5 ±6.86 (99–124) | 0.487 |
| SANS Global Ratings | |||
| Affective Flattening or Blunting | 2.89 (1.45) | ||
| Alogia | 1.89 (1.54) | ||
| Avolition-Apathy | 3.00 (1.41) | ||
| Anhedonia-Asociality | 2.22 (1.92) | ||
| Attention | 1.33 (1.73) | ||
| SAPS Global Ratings | |||
| Hallucinations | 1.88 (1.9) | ||
| Delusions | 1.78 (1.72) | ||
| Bizarre Behavior | 0.33 (1.00) | ||
| Positive Formal Thought Disorder | 0.22 (0.67) |
p < 0.0001
Scz: Schizophrenia; Ctr: Control; SANS: Schedule for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms; IQ scores are estimated from the National Adult Reading Test (NART) (Nelson 1982).
Patients and controls were originally recruited from the University of North Carolina at Chapel Hill’s School of Medicine, with additional control subjects recruited through Duke University’s Brain Imaging and Analysis Center healthy subject registry. Each subject was paid $20 an hour for two hours of participation in this study.
The control group did not meet criteria for any current or past Axis I disorder as assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (Spitzer, et al. 1992). All subjects had normal or corrected-to-normal vision and were screened for neurological illness, substance dependence, and substance abuse within the past month. Both groups also had no current or past history of migraines or major medical illness, including multiple sclerosis, stroke, insulin-dependent diabetes, significant head injury, and epilepsy within three years, had no history of treatment with ECT/rTMS within three months, were not pregnant at the time of scanning, and did not have a family history of neurologic or neurodegenerative disorder (e.g., Parkinson’s, Huntington’s chorea, Multiple Sclerosis). All procedures were approved by the institutional review boards at the University of North Carolina at Chapel Hill and at Duke University Medical Center, Durham, NC. After complete description of the study to the subjects, consent forms from both the University of North Carolina at Chapel Hill and at Duke University Medical Center were used to obtain informed consent. Negative results of urine toxicology were confirmed before study inclusion.
2.2 fMRI Task
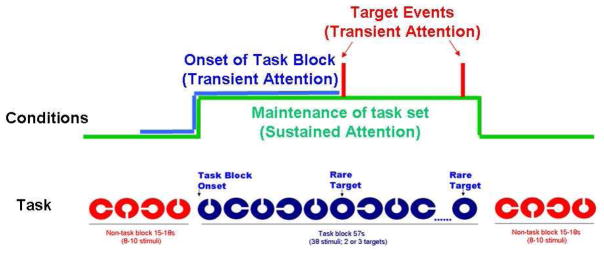
Participants performed a visual selective attention task that consisted of a mixed block and event-related design, as displayed in Figure 1. Alternating blocks of task-relevant stimuli and non-task stimuli were presented, with rare target events only presented during the task-relevant blocks. There were 2 categories of stimuli: Targets, which were identified as blue closed circles and non-target stimuli, which were incomplete blue, incomplete red, or complete red circles. The stimuli were presented in alternating blocks of red and blue stimuli with targets only presented in the blue blocks. This created task-relevant blocks (with all blue events, including the targets) and task-irrelevant blocks (with all red events). Participants were required to identify the rare target events (complete blue circles) and were informed that targets would not be presented during the blocks of red colored shapes. In both the task-relevant blocks and the non-task blocks, participants were required to press a particular button to non-targets and a unique button to targets. Targets appeared on 5% of task-relevant trials and 110 targets were presented in total. Stimuli were presented for 500ms with an ISI of 1000ms and a fixation cross was presented during the interval between trials. Each run contained 196 stimuli presented centrally against a white background. Participants performed 10 runs of the task, each of 4 min and 54 sec duration. Participants were trained on the task immediately prior to the scanning session. All stimuli were presented using CIGAL presentation software on a Windows-compatible computer and displayed to participants in the scanner through magnet-compatible goggles (Magnetic Resonance, Inc).
Figure 1.
Visual Selective Attention Task that served as the fMRI Activation Task and the associated analysis conditions.
2.3 Imaging
Scanning was performed on a General Electric 4T LX NVi MRI scanner system equipped with 41 mT/m gradients (General Electric, Waukesha, Wisconsin, USA). A quadrature birdcage radio frequency (RF) head coil was used for transmit and receive. A high resolution T1-weighted image with 68 slices was acquired using a 3D fast SPGR pulse sequence (TR = 500 ms; TE = 20 ms; FOV = 24 cm; image matrix = 256 × 256; voxel size = 0.9375 × 0.9375 × 1.9 mm) and used for coregistration with the functional data. This structural image was aligned in a near axial plane defined by the anterior and posterior commissures. Whole brain functional images were acquired using a gradient-recalled inward spiral pulse sequence (Glover and Law 2001, Guo and Song 2003) sensitive to blood oxygenation level dependent (BOLD) contrast (TR, 1500 ms; TE, 31 ms; FOV, 24 cm; image matrix, 64 × 64; α = 62°; voxel size, 3.75 × 3.75 × 3.8 mm; 34 axial slices). The functional images were aligned similarly to the T1-weighted structural image. A semi-automated high-order shimming program ensured global field homogeneity.
2.4 Imaging Data Analysis
Prior to statistical analysis, head motion was analyzed by center of mass measurements in three orthogonal planes. No participant had greater than a 3-mm deviation in the center of mass in any dimension. In addition, imaging epochs with mean intensities greater than three standard deviations of the average intensity in a run were excluded from analyses.
Image preprocessing was performed with custom programs and SPM modules (Wellcome Department of Cognitive Neurology, UK). Images were time-adjusted to compensate for the interleaved slice acquisition and then motion-corrected to compensate for small head movements. The realigned and motion-corrected images were then normalized to the Montréal Neurological Institute (MNI) template found in SPM. These normalized functional data were then high-pass filtered and spatially smoothed with an 8 mm isotropic Gaussian kernel prior to statistical analysis. These normalized and smoothed data were used in the remaining analyses described below.
To identify voxels activated by events of interest, each subject’s data was analyzed using a random effects assessment of statistical maps generated from a voxel-based analysis. The first part of the analysis involved excising epochs of image volumes from the continuous time-series of volumes. For the target events, the epoch consisted of two images before (−3.0 s) and nine images after (13.5 s) the event onset. The onset of task blocks epoch was two images before (−3.0 s) and five images after (7.5 s) the onset of the task block, while the sustained activation epoch was longer, consisting of ten images before (−15 s) and forty-seven images after (70.5 s) of that same onset of the task block (Figure 1). The average intensity of all epochs of the three conditions (target events, task block onset and sustained activation) was computed over all trials. A t-statistic was calculated for each condition by calculating the correlation of the averaged epoch with a canonical hemodynamic response (HDR) reference template at each voxel. The theoretical HDR template for the target events was adapted from a typical event-related BOLD waveform (Huettel and McCarthy 2000). For the onset of task blocks condition and the sustained activation condition, a modified box-car waveform was used for the HDR template. The t-statistics were calculated separately for each subject. Finally, the individual t-maps from each subject were run through a random effects analysis that assessed the significance of differences across participants for each condition.
To reduce the number of statistical comparisons and limit the possibility of Type I error, the results of the random effects analyses above were limited to just those voxels that had a significant HDR evoked by that particular condition. For this analysis, we thresholded our activation at a false discovery rate (FDR) of 0.05 (Genovese, et al. 2002). We used the t-maps across all participants to produce an average t-map for each condition, and then calculated the FDR threshold for each averaged t-map. The random- effects analysis for each event type was then masked by the corresponding averaged t-map thresholded at the FDR threshold calculated above.
Region of interest (ROI) analyses were also performed to assess between-group differences. First, structural ROIs were manually drawn on the same MNI T1-weighted template brain used for the spatial normalization in specified regions using ROI tracing software ITK-SNAP (Yushkevich, et al. 2006). These regions included the anterior cingulate gyrus (ACG), the inferior frontal gyrus (IFG), the middle frontal gyrus (MFG), the intra-parietal sulcus (IPS), the basal ganglia (BG), the caudate nucleus (CN) and the thalamus (TH). Finally, for each trial type and ROI combination, the extent of activation for all participants was calculated as the number of voxels in that ROI that reached or exceeded a specific threshold in the spatially normalized T-map compared to the total number of voxels in that region. Group comparisons were made by calculating the average extent of activation for all neurotypical participants and for all schizophrenic participants for each region and condition.
3. Results
3.1 Behavioral Performance
As indicated in Table 2, the schizophrenia group made significantly fewer correct responses to targets than did the control group (F(1,17)=6.2, p<0.05) and displayed a significantly slower mean response time to targets than controls (F(1,17)=7.78, p<0.05).
Table 2.
Performance of control and schizophrenia groups on selective attention task
| Control | Schizophrenia | F(17) | |
|---|---|---|---|
| * Correct Responses To Targets (Maximum of 110) | M=77.44; SD=16.54 | M=54.0; SD=23.0 | 6.2 |
| * Mean Response Time (Milliseconds) | M=441ms; SD=109ms | M=570ms; SD=85ms | 7.78 |
p < 0.05
3.2 Imaging Data
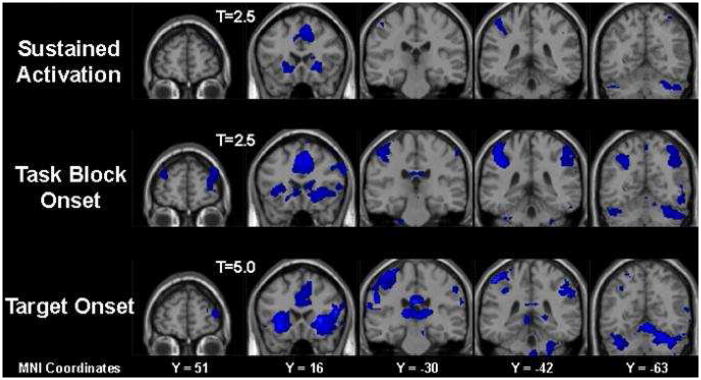
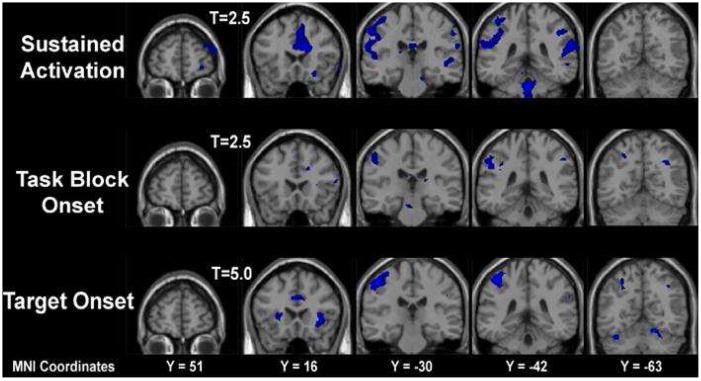
Brain imaging data are displayed in figure 2 for the control group and figure 3 for the schizophrenia group. Each figure shows activation during three aspects of the mixed design task: activation that was sustained across the entire sequence of task-relevant and non-task blocks; activation that occurred in response to the onset of task-relevant blocks; and activation that occurred in response to the target events.
Figure 2.
Brain Activation Maps for the Control Group as displayed during three conditions of interest: sustained throughout task performance, onset of a task block and onset of a target.
Figure 3.
Brain Activation Maps for the Schizophrenia Group as displayed during three conditions of interest: sustained throughout task performance, onset of a task block and onset of a target.
For the control group, the random effects differences displayed in figure 2 revealed that across the entire sequence of task-relevant and non-task blocks, sustained activation was apparent in the anterior cingulate gyrus (ACG), bilateral inferior frontal gyrus, bilateral insula, left inferior parietal sulcus and cerebellar regions. At the onset of task-relevant blocks, activation was apparent in all of these regions with additional activations apparent in the medial frontal gyrus, right inferior parietal sulcus, caudate and thalamus. A similar pattern of activation was displayed at the onset of target events, with thalamic and cerebellar activation being particularly apparent.
The pattern of random effects differences displayed by the schizophrenia group, as shown in figure 3, appeared to differ from the pattern displayed by the control group. In the sustained attention condition, the schizophrenia group displayed activation in the ACG, post-central gyrus, inferior parietal sulcus and cerebellar regions. At the onset of task-relevant blocks, the schizophrenia group predominantly displayed activation in the post-central gyrus and the inferior parietal sulcus but displayed little activation in the ACG. At the onset of target events the schizophrenia group displayed activation in the ACG, insula, post-central gyrus and inferior parietal sulcus.
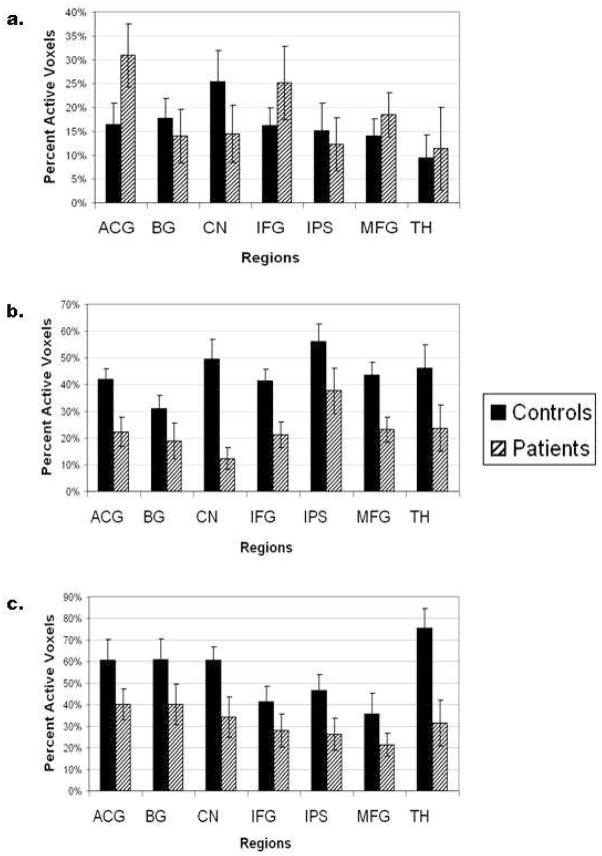
Region of interest (ROI) analyses for both the control group and the schizophrenia group are displayed in Figure 4. Activation associated with the sustained attention condition is displayed in Figure 4a; activation associated by the onset of a task block is displayed in Figure 4b and activation associated with the onset of a target is displayed in Figure 4c. For the control group, the ROI analyses revealed that numerous regions were activated to a greater extent at the onset of task blocks and to an even greater extent in response to target events than were activated during the sustained attention period. For instance, in the ACG only 16% of voxels were active during the sustained attention period, whereas 34% of voxels were active in response to the onset of task blocks and 60% of voxels were active in response to target events. In the thalamus, 9% of voxels were active during the sustained attention period, whereas approximately 40% of voxels were active at the onset of the task-relevant blocks and approximately 75% of voxels were active in response to the target events.
Figure 4.
Region of Interest (ROI) Percentage of Active Voxels for the Control Group and the Schizophrenia Group. Figure 4a displays the percentage of active voxels sustained across the task; Figure 4b displays the percentage of active voxels at the onset of a task block; and Figure 4c displays the percentage of active voxels at the onset of a target.
Comparing the control group to the schizophrenia group, the ROI analysis indicated that in the sustained attention phase of the task, the schizophrenia group displayed a greater percentage of active voxels than the control group in many of the ROIs including the ACG, inferior frontal gyrus, medial frontal gyrus and thalamus, though the differences were not significant. However, during the transient phase of attention, as measured at the onset of task-relevant blocks and at the onset of target events, the schizophrenia group displayed a lower percentage of active voxels in all ROIs, than did the control group. These between group differences at the onset of task-relevant blocks were significant in the caudate nucleus (F(1,19)=8.85, p<0.01); the inferior parietal sulcus (F(1,19)=5.07, p<0.05); the medial frontal gyrus (F(1,19)=4.37, p<0.05) and the thalamus (F(1,19)=5.28, p<0.05). At the onset of target events, the between group difference was significant in the thalamus (F(1,19)=10.39, p<0.01). Interestingly, the thalamus of individuals with schizophrenia also displays a significant decrease in percent signal change in response to target events (F(1,19)=6.538, p<0.05).
Given that the two groups showed significantly different accuracy and response time on the task, we decided to explore the nature of the association between task performance and extent of activation (percent voxels in range) to sustained attention and to target events. To achieve this, we computed Pearson’s r correlations between the extent of activation for each ROI in all three conditions (i.e. sustained attention, transient attention, and target events). We then performed a Fisher’s r to z transformation to identify regions where the correlation between brain activation and the behavioral performance measures significantly differed between groups. This analysis revealed a significant positive correlation between accuracy to target events and extent of activation to the sustained attention condition in the caudate nucleus in the healthy controls (r = 0.700; p < 0.05), and this correlation was significantly different from that of the patient group (control r = 0.700; patient r = −0.339; z = 2.26; p <0.05). Response time was not associated with activation to the sustained attention condition in any of the regions of interest. Additionally, a significant negative correlation was found between response time to target events and extent of activation to the target events in the basal ganglia in the participants with schizophrenia (r = −0.712; p < 0.05) and this was significantly different from that of the healthy group (control r = 0.231; patient r = −0.712; z = 2.09; p<0.05). No other significant correlations were found between behavioral measures and activation to target events.
4. Discussion
The behavioral findings of the current study indicate that the schizophrenia group displayed reduced accuracy on a selective attention task compared to the control group, consistent with the well-established findings that attention deficits are fundamental cognitive deficits in schizophrenia (Fioravanti, et al. 2005, Neuchterlein, et al. 1991).
The brain imaging data for the control group was consistent with the previous findings of Dosenbach et al (2006) and Liddle et al. (2006), revealing that sustained activation was apparent in a core attentional network that included the ACG, inferior frontal gyrus, insula, inferior parietal sulcus and cerebellar regions (Dosenbach, et al. 2006, Liddle, et al. 2006). Such a finding has been expanded upon in the present study by using a mixed block/event-related design to differentiate brain activity during sustained and transient phases of attention. While the relatively small sample size of our study requires a cautious interpretation of our preliminary findings, the observation that activation in these same regions was apparent during the transient phases of task-block onset and target onset suggests that these regions are commonly activated during both transient and sustained phases of attention. Activation that appeared to be more specific to the transient aspects of target selection occurred in the medial frontal gyrus, caudate, thalamus and inferior parietal sulcus. ROI analysis of the control group data revealed that in many regions in which activation was apparent during the sustained attention phase of the task, a greater percentage of voxels was activated at the onset of task-relevant blocks and an even greater percentage of voxels was activated at the onset of target events. This was apparent in the ACG, inferior frontal and thalamic regions and may suggest that, for the control group, activation within these regions of the attentional network is modulated according to the demands of the task and is further enhanced during the selection of task-relevant responses associated with target events.
ROI analysis also revealed that during the sustained phase of the task, the schizophrenia group displayed a greater percentage of active voxels than the control group, in many regions of the attentional network including the ACG, inferior frontal gyrus, medial frontal gyrus and thalamus. However, during transient phases that were most pertinent to task performance – i.e. the onset of task-relevant blocks and the onset of target events – the schizophrenia group displayed a lower percentage of active voxels in all ROIs, relative to the control group.
While still preliminary, this suggests that the schizophrenia group may have been less effective at modulating brain activity and attention processes in response to the varying demands of the target selection task. For the control group, attention processes appeared to be heightened at the most task-relevant moments and reduced during task-irrelevant periods. By contrast, the schizophrenia group may not modulate cortical activity as effectively in response to the changing demands of the task.
This suggestion is consistent with those made in several previous studies. Barbalat et al. (2009) suggested that patients with schizophrenia display evidence of inefficient use of temporal episodic information (Barbalat, et al. 2009). Polli et al. (2008) concluded that reduced anterior cingulate activation in response to errors by patients with schizophrenia may reflect deficient modification of prepotent stimulus-response mappings in response to errors (Polli, et al. 2008). Finally, Liddle et al. (2006) suggested that patients with schizophrenia display a deficit in cerebral activation in the brain regions that are typically activated by attention captivating stimuli (Liddle, et al. 2006).
Exploratory analysis of correlations between behavioral results and fMRI activations revealed that while the response accuracy to target events were significantly associated with the extent of activation to the sustained attention condition in the caudate nucleus in the healthy controls, such an association was absent in the patients. This pattern may reflect an underlying abnormal fronto-striatal projection deficit in schizophrenia patients, which may contribute to their poor behavioral regulation and task-appropriate response generation. The association between the viability of fronto-striate projections and task-appropriate response generation in schizophrenia will need to be further explored with multimodal imaging, namely the integration of white matter tractography and functional activation measures with behavioral performance speed and accuracy during selective attention tasks.
In discussing the results of the present study, it is important to consider the value of the mixed block/event-related design and to consider what the results may have revealed had only a block design been used. The mixed design allows for the differentiation of brain activity that occurs during the sustained and transient phases of attention (Dosenbach, et al. 2006, Huettel, et al. 2004). Had only a block design been employed in the present study, the potentially misleading conclusion may have been drawn that the schizophrenia group displayed greater brain activity than the control group during performance of a selective attention task. Instead, the mixed design has revealed a richer picture – that the schizophrenia group displayed greater activity than controls during non-task periods, but displayed less activity than controls during task-relevant periods.
One limitation of the current study is the slightly elevated IQ of our schizophrenia sample relative to IQ measures reported in recent metaanalysis studies (Woodberry et al, 2008). While there was no effect of IQ on the results of this study, the current sample of individuals with schizophrenia may not be representative of the schizophrenic population as a whole. We propose, however, that individuals with schizophrenia with lower IQs than those from the current sample would exhibit greater behavioral deficits on the current task and this would be associated with greater differences in brain activity modulation in response to variations in attentional modulation. Future studies will be needed to address the validity of this hypothesis.
In conclusion, the findings of the present study help to explain contrasting findings across previous studies and suggest that attention deficits displayed by patients with schizophrenia may reflect deficits in modulating brain activity in response to variations in transient, attention demanding stimuli, rather than deficits in sustained attention.
Acknowledgments
This research was supported by grants from NIMH (MH58251), The UNC-Schizophrenia Research Center – an NIMH Silvio O. Conte Center for the Neuroscience of Mental Disorders (MH64065), UL1RR025747 from the NIH Clinical and Translational Science Award Program of the Division of Research Resources, and The Foundation of Hope of Raleigh, N.C. Assistance for this study was provided by the Neuroimaging Core of the UNC Neurodevelopmental Disorders Research Center Gabriel Dichter was supported by Postdoctoral Research in Neurodevelopmental Disorders, NICHD T32-HD40127, and a career development award from UNC-Chapel Hill, NIH/NCRR K12 RR023248.
Assistance for this study was provided by the Neuroimaging Core of the UNC Neurodevelopmental Disorders Research Center. We thank Susan Music, Garett Rosania, and Justin Woodlief for technical assistance.
Role of Funding Source
This research was supported by grants from NIMH (MH58251), The UNC-Schizophrenia Research Center – an NIMH Silvio O. Conte Center for the Neuroscience of Mental Disorders (MH64065), UL1RR025747 from the NIH Clinical and Translational Science Award Program of the Division of Research Resources, and The Foundation of Hope of Raleigh, N.C. Gabriel Dichter was supported by Postdoctoral Research in Neurodevelopmental Disorders, NICHD T32-HD40127, and a career development award from UNC-Chapel Hill, NIH/NCRR K12 RR023248. The NIMH, the NIH, The Foundation of Hope, and the NICHD had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
All authors declare that they do not have any conflicts of interest.
Role of Contributors
Aysenil Belger and James Carter conceptualized and designed the study and wrote the protocol. James Carter, Carolyn Bellion, and Kimberly Carpenter managed the literature searches and analyses of clinical data. Dr. Gabriel Dichter assisted in the acquisition of the imaging and clinical demographics data. Dr. James Carter, Mr. Joshua Bizzell, and Mr. Cy Kim undertook the image processing and statistical analysis. Dr. James Carter wrote the first draft of the manuscript. All authors participated in the interpretation and discussion of the results, and subsequent drafts and review of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbalat G, Chambon V, Franck N, Koechlin E, Farrer C. Organization of cognitive control within the lateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 2009;66(4):377–386. doi: 10.1001/archgenpsychiatry.2009.10. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58(3):280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155(9):1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15(2):73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Guo H, Song AW. Single-shot spiral image acquisition with embedded z-shimming for susceptibility signal recovery. J Magn Reson Imaging. 2003;18(3):389–395. doi: 10.1002/jmri.10355. [DOI] [PubMed] [Google Scholar]
- Huettel SA, McCarthy G. Evidence for a refractory period in the hemodynamic response to visual stimuli as measured by MRI. Neuroimage. 2000;11(5 Pt 1):547–553. doi: 10.1006/nimg.2000.0553. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Misiurek J, Jurkowski AJ, McCarthy G. Dynamic and strategic aspects of executive processing. Brain Res. 2004;1000(1–2):78–84. doi: 10.1016/j.brainres.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Karch S, Leicht G, Giegling I, Lutz J, Kunz J, Buselmeier M, Hey P, Sporl A, Jager L, Meindl T, Pogarell O, Moller HJ, Hegerl U, Rujescu D, Mulert C. Inefficient neural activity in patients with schizophrenia and nonpsychotic relatives of schizophrenic patients: Evidence from a working memory task. J Psychiatr Res. 2009 doi: 10.1016/j.jpsychires.2009.04.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Laurens KR, Kiehl KA, Ngan ET. Abnormal function of the brain system supporting motivated attention in medicated patients with schizophrenia: an fMRI study. Psychol Med. 2006;36(8):1097–1108. doi: 10.1017/S0033291706007677. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45(9):1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- Neuchterlein KH, Dawson ME, Ventura J, Miklowitz D, Konishi G. Information-processing anomalies in the early course of schizophrenia and bipolar disorder. Schizophr Res. 1991;5(3):195–196. doi: 10.1016/0920-9964(91)90069-4. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Anterior cingulate dysfunction during choice anticipation in schizophrenia. Psychiatry Res. 2004;132(2):117–130. doi: 10.1016/j.pscychresns.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Kellermann T, Stocker T, Shah NJ, Zilles K, Braus DF, Schmitt A, Schlosser R, Wagner M, Frommann I, Kircher T, Rapp A, Meisenzahl E, Ufer S, Ruhrmann S, Thienel R, Sauer H, Henn FA, Gaebel W. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophr Res. 2007;89(1–3):198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Lieberman JA, Belger A. Attentional modulation of early-stage visual processing in schizophrenia. Brain Res. 2006;1125(1):194–198. doi: 10.1016/j.brainres.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, Kremser C, Brinkhoff C, Felber SR, Fleischhacker WW. Brain activation patterns during a selective attention test-a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res. 2003;123(1):1–15. doi: 10.1016/s0925-4927(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Siedentopf C, Golaszewski S, Mottaghy FM, Hofer A, Kremser C, Felber S, Fleischhacker WW. Brain activation patterns during a selective attention test--a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res. 2007;154(1):31–40. doi: 10.1016/j.pscychresns.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]