Abstract
Emerging data indicate that traumatic injury to the brain or spinal cord activates B lymphocytes, culminating in the production of antibodies specific for antigens found within and outside the central nervous system (CNS). In this article, we summarize what is known about the effects of CNS injury on B cells. We outline the potential mechanisms for CNS trauma-induced B cell activation and discuss the potential consequences of these injury-induced B cell responses. Based on recent data, we hypothesize that a subset of autoimmune B cell responses initiated by CNS injury are pathogenic and that targeted inhibition of B cells could improve recovery in brain and spinal cord injured patients.
B cells as participants in central nervous system (CNS) injury immune responses
Historically, research efforts exploring interactions between the immune system and the diseased CNS have focused on neuroinflammation as well as immune regulation in multiple sclerosis (MS) and classical neurodegenerative diseases (e.g. Alzheimer’s and Parkinson’s disease). Less is known about how the immune system is affected by traumatic injury to the brain or spinal cord, but emerging data indicate that T and B cells play key roles in regulating CNS injury and repair1–3. In particular, recent studies implicate B cells, and the antibodies they produce, as pivotal players in the post-traumatic immune responses triggered by spinal cord injury (SCI). In vivo models show that B cells and SCI-induced antibodies exacerbate tissue damage and impair neurological recovery after SCI1,2. In this article, we summarize these data and discuss the implications of post-traumatic B cell activation, both in the context of host immunity and repair of the injured CNS. We also contemplate different mechanisms that may help to explain how trauma leads to dysregulation of B cell function and related mechanisms of neuroinflammation.
How and why does CNS injury activate B cells?
The canonical pathway for B cell activation involves recognition of a cognate antigen via mature B cell receptors and co-receptors with concomitant costimulation by T cells. These antigens, typically “non-self” pathogenic proteins, elicit a coordinated host immune response culminating in removal of antigen from the body. However, when the activating antigens are non-pathogenic host peptides, proteins, lipids or nucleic acids, autoimmune responses are elicited. Due to receptor editing and negative selection, most highly-autoreactive lymphocytes are deleted or inactivated in the thymus during development. However, during positive selection, “sub-threshold” stimulation of lymphocytes by self-peptides helps increase the sensitivity of lymphocytes to pathogenic proteins4. Thus, autoimmune recognition plays a physiological role in adjusting the strength of an immune response and only when a given threshold of activation is surpassed do autoreactive cells cause pathology. Current data suggest that after traumatic CNS injury, T-dependent- and perhaps T-independent self-antigens elicit adaptive immune responses with important functional consequences1,2,5–7. However, the nature and diversity of these autoantigens are presently unknown.
CNS antigens draining into peripheral lymphoid tissues after CNS injury might activate naïve neuroantigen-reactive lymphocytes (Figure 1). In support of this hypothesis, T cells in the spleen and lymph node become activated by spinal cord proteins including myelin basic protein (MBP)6. Indeed, after SCI, naïve T cells proliferate and when expanded ex vivo with MBP (or polyclonal stimuli), they can transfer a mild neuroinflammatory disease in naïve recipient animals6. The onset and progression of T cell-mediated autoimmune pathology is more striking when SCI is performed in CD4+ MBP T cell receptor transgenic mice8. T cells in these mice are naïve but are genetically predisposed to recognize and respond to the encephalitogenic epitope of MBP. After SCI, MBP-reactive T cells expand in the periphery then traffic to the traumatized CNS where they exacerbate pathology8. A similar expansion of MBP-reactive T cells occurs in SCI humans5.
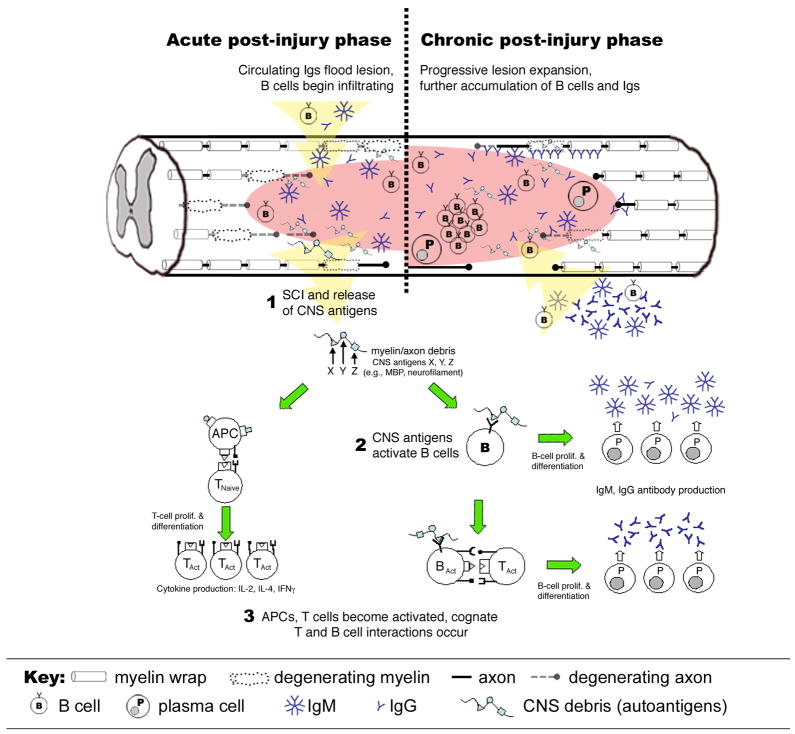
Figure 1.
Putative mechanisms of B cell activation after traumatic SCI. SCI causes cell death and blood-spinal cord barrier (BSCB) damage (1). At this time, circulating B cells and (pre-formed) immunoglobulins (Igs) cross the BSCB and accumulate at the injury site. BSCB also facilitates drainage of CNS antigens to peripheral lymphoid organs with subsequent B cell recognition of CNS antigens causing B cell proliferation and antibody production (2). Concurrently, APCs present CNS antigens to T cells (3). Cognate interactions between T and B cells amplifies the autoimmune response to CNS antigens such that during the chronic post-injury phase (weeks to months post-SCI), B cells and antibodies continue to accumulate at the injury site forming ectopic follicle-like structures with Igs decorating cells and tissues in and nearby the site of injury.
Given that titers of anti-MBP antibodies increase after SCI in humans9–11, it is likely that B cells are also affected by SCI. Indeed, an analysis of B cell responses in SCI mice revealed a marked increase in the number of CD45R/B220+CD19+ B cells and IgM- and IgG-antibody secreting cells in bone marrow and spleen2. Accompanying these cellular changes was an increase in total (polyclonal) and CNS-reactive serum antibodies2, suggesting that activated B cells release autoantibodies into the circulation after SCI. At later times post-injury, B cells accumulate at the site of injury where they form intraspinal structures that are reminiscent of the ectopic follicles that develop in chronic MS1,2. Intraspinal B cell accumulation following SCI was accompanied by upregulated expression of genes encoding autoreactive immunoglobulins, suggesting that B cell-mediated autoimmunity is initiated or maintained within the CNS after injury.
The above experimental data provide a mechanistic explanation for older clinical research studies. In the 1970s, studies revealed that supraphysiological levels of antibodies specific for gangliosides and other phospholipids were enriched in the sera of humans that had suffered a traumatic brain injury (TBI)12. It was shown that autoantibodies specific for MBP and galactocerebroside were elevated in the sera of individuals with a traumatic SCI11. More recent studies have shown that ~50–60% of people with TBI or SCI produce antibodies that target a range of CNS proteins and glycoproteins including GM1 gangliosides, myelin-associated glycoprotein, AMPA and NMDA glutamate receptors, β-III-tubulin and nuclear antigens9,13. These data suggest that post-traumatic autoimmunity is common but is not an inevitable consequence of TBI or SCI. More comprehensive proteomic and lipidomic analyses of clinical autoantibody samples are needed to establish the true prevalence and specificity of SCI (or TBI)-induced autoimmune reactions in humans.
Systemic polyclonal B cell activation after CNS injury
Naïve, CNS antigen-specific B cells might become activated in peripheral lymphoid tissues by injury as described above (also see Fig. 1). However, reactivation of memory T and B cells that had previously responded to bacterial-, viral- or non-CNS self-antigens could also be responsible for trauma-induced autoimmunity14. Indeed, lymphocytes with receptors that recognize pathogenic or systemic antigens (e.g., nucleic acids) can cross-react with CNS antigens. This concept of lymphocyte “polyspecificity”, previously referred to as molecular mimicry, could explain the increase in the prevalence of anti-CNS T and B cell responses after CNS injury. These lymphocytes could respond to CNS antigens directly or become re-activated by their cognate antigens or by polyclonal stimuli.
Traumatic CNS injury in humans is often associated with concurrent polytrauma, including concomitant head injuries or major injuries to the chest, pelvis, skin or extremities. These injuries facilitate entry of environmental pathogens, including bacteria and viruses that can prime or reactivate resting or memory B cells. CNS trauma also can increase intestinal permeability, promoting movement of commensal bacteria from the intestines into the circulation (i.e., bacterial translocation)15,16. Although commensals should not reactivate pathogen-specific B cells as cognate antigens, these endogenous bacteria could trigger polyclonal activation of B cells via toll-like receptors (TLRs) including TLR4 and RP105. Ligation of these receptors on B cells has been shown to exacerbate inflammatory disease17. Through these interactions, exogenous or endogenous microorganisms could amplify B cell responses, leading to enhanced neuroinflammatory reactions after CNS injury. Commensal microbiota have also been implicated in regulating the balance between inflammatory (e.g., Th17) and regulatory T cells (Tregs)18. As discussed below, trauma-induced activation of Tregs could be important for regulating B cell activation and pathogenic neuroinflammation.
Other non-pathogenic TLR ligands are released or are upregulated at sites of tissue injury19. Necrotic cells expel nucleic acids which ligate TLRs and form immune complexes, leading to activation of autoreactive B cells20,21. As a consequence of cell stress and tissue remodeling, damage-associated molecular patterns (i.e., DAMPs) including heat shock proteins, fibronectin and hyaluronan are increased at sites of tissue damage. All are considered endogenous TLR ligands that can initiate or enhance B cell responses19,20,22. Apoptotic cells also produce polyclonal stimuli that appear to act independent of TLRs. Indeed, when present at high concentration, phosphatidyl serines, cardiolipin and other antigens found on apoptotic cells activate B cells resulting in autoantibody production23.
The site of CNS injury and time post-injury can influence B cell activation
Despite evidence that B cells are activated by injuries to the brain or spinal cord1,2, other data show that CNS injury potently suppresses cellular and humoral immunity, which could explain the high incidence of mortality caused by infection in neurotrauma patients24. How is it then that SCI and TBI cause immune suppression while activating T and B cells that can exacerbate tissue injury? The key to resolving this paradox is to recognize that all immune cells are not equally affected by injury and that effects on the immune system vary over time.
During the first few days or weeks after injury to the CNS, a subset of human immune cells are anergic or otherwise blunted in their responses to immune challenge25,26. For example, within 24 hours of TBI, the total number of circulating T cells is decreased, as is the ability of T cells to proliferate or produce cytokines in response to mitogens27. Similar studies in SCI patients revealed defects in T cell and NK cell responses between 2 weeks and 5 months post-injury28.
Despite these acute deficits in immune function, other clinical studies suggest that cognate T and B cell interactions are unaffected by SCI. Specifically, prophylactic pneumococcal and influenza vaccines were able to elicit immune responses with an expected increase in antibody synthesis29,30. Although the efficacy of these vaccinations was not affected by time post-injury, injury severity or injury location, the control groups were heterogeneous and group sizes small. Additional work is needed to fully characterize post-injury immune function, especially because newer data generated in controlled models of rat and mouse SCI show that post-injury B cell responses to specific, foreign antigens are blunted during the first few days to weeks after SCI31–33. Specifically, when antigen is injected into the spinal intrathecal space of rats with a high-thoracic (T4 level) SCI, the antibody response to that antigen is reduced relative to the response elicited in uninjured mice receiving an identical immunization protocol33. Subsequent studies in mice proved that the immune suppressive effects of SCI on B cell activation and antibody synthesis are injury level-dependent, i.e., antibody synthesis is suppressed after high thoracic (T3) SCI but not after injury to the mid-thoracic (T9) spinal cord31. This is likely because sympathetic innervation of peripheral lymphoid tissues is regulated in part via the thoracic spinal cord34. When SCI occurs at or above the T3 spinal level, supraspinal control of sympathetic preganglionic neurons in the spinal cord is abolished. In contrast, some sympathetic outflow is preserved when SCI occurs at lower levels (e.g., T9 spinal cord). A post-injury surge in serum corticosterone (CORT) and splenic norepinephrine (NE) was found to be one mechanism responsible for the acute suppression of B cell function after high-level SCI. Specifically, CORT upregulates beta-2 adrenergic receptors on lymphocytes which, when subsequently activated by NE, initiates apoptotic signaling in immune cells31,32,35. When considered together, these data indicate that impaired supraspinal control of spinal sympathetic outflow to the immune system causes significant immunological deficits, at least during the first few weeks post-injury. This could explain the increased incidence of infection in humans with TBI, stroke or high-level SCI. Whether dysregulation of the sympathetic-immune system axis also augments pathological autoimmune responses after CNS injury has not been determined. Indeed, profound leukocyte apoptosis with impaired clearance of dying cells, similar to that caused by high-level SCI, is a suspected mechanism of autoimmunity in rheumatoid arthritis (RA), systemic lupus erythramatosus (SLE) and other rheumatic diseases23,36.
Future studies also should determine whether the cholinergic anti-inflammatory reflex is involved in regulating B cell activation and autoantibody synthesis. To date, this neural-immune reflex has been characterized as an important regulator of proinflammatory macrophage functions through reflex activation of the vagus nerve37. Vagal release of acetylcholine (ACh) with subsequent ligation of α7 nicotinic ACh receptors on macrophages results in decreased release of pro-inflammatory cytokines (e.g., TNF-α). Nicotinic ACh receptors may also influence B cell development and activation, suggesting that enhanced vagus nerve activation could limit B cell hyperactivity38. To date, this hypothesis has not been formally tested in models of CNS injury; however, a recent report suggests that the cholinergic anti-inflammatory pathway can suppress lymphocyte activation and/or migration in response to mild neurodegeneration but it cannot ameliorate pathogenic neuroinflammation caused by CNS autoimmune disease39.
Functional implications of B cell responses after spinal cord injury
Mice lacking B cells show improved locomotor function and reduced spinal pathology compared to wild-type mice after SCI, suggesting a pathogenic role for B cells1. The intraspinal pathology caused by B cells in wild-type mice is due in part to antibody-mediated ligation of Fc receptors and complement activation1,2. Even though intraspinal B cell clusters and autoantibodies are maintained indefinitely in injured mouse spinal cord and human cerebrospinal fluid, there is no proof that these immune responses cause protracted neurological deterioration. If they did, there would be a precipitous decline in function in both mice and humans as B cells became activated and autoantibodies were synthesized. Instead, delayed pathogenic autoimmune responses may target compensatory mechanisms of CNS repair. In human and animal models of SCI, spontaneous recovery of function stabilizes after a period of weeks or months post-injury, coincident with B cell activation and autoantibody synthesis. If B cells and autoantibodies are responding to proteins that are newly expressed in growing axons, remyelinating oligodendrocytes, stem cells or new endothelia, little or no additional gain of function beyond that achieved prior to the onset of the autoimmune response would be expected. In this way, chronic autoimmune reactions might be responsible for regenerative axon growth failure and permanent loss of function after CNS injury in mammals.
It is also likely that as pathogenic immune responses become activated, regulatory cascades are activated in parallel (or shortly thereafter) that limit chronic B cell/autoantibody toxicity. Indeed, clusterin and factor H, proteins that counteract complement-mediated tissue damage, are induced after CNS injury40. Myeloid suppressor cells, Tregs and B regulatory cells (Bregs) also have been shown to inhibit or titer the potentially injurious consequences of inflammation and autoimmunity. How these distinct cell populations might regulate pathogenic immune responses after CNS injury is comprehensively reviewed elsewhere18,41,42. Although these suppressor/regulatory cells are undoubtedly important for controlling neuroinflammation, none have been adequately studied in the context of SCI or TBI. There are limited data exploring the role of Tregs, but the data are not easily reconciled. On the one hand, Tregs were shown to inhibit a naturally-occurring T cell-mediated “protective autoimmune” response induced by injury to the optic nerve; in the absence of Tregs, injury-induced neuron loss was exacerbated43. Conversely, intraspinal inflammation and pathology were worse after traumatic SCI in mice lacking CD4+ Tregs44. How or if B cells or autoantibodies were influenced by Tregs was not considered in either study. A key variable important for the induction and differentiation of regulatory immune cells, whether they be monocyte/macrophages or lymphocytes, is the microenvironment in which these cells become active. As can be assumed from the two examples provided above, different types and locations of CNS injury will undoubtedly create unique lesion environments. Accordingly, the relative composition and participation of immune regulatory cells is expected to vary. In models of traumatic SCI, the lesion environment seems to favor the development and protracted activation of inflammatory macrophages45, T helper 1 lymphocytes8 and pathogenic B cells1,3. Future studies must attempt to reveal whether these potentially injurious neuroinflammatory cascades engage self-correcting regulatory or immune-suppressive networks. By understanding the breadth of regulatory networks that are involved, it might be possible to accelerate or augment their actions to limit any pathogenic effects caused by B cells or other destructive neuroinflammatory cascades.
Additional experiments also are needed to reveal the origin of autoantibody synthesis (i.e., within vs. outside the CNS) and the breadth of CNS and non-CNS antigens that elicit antibody production after injury. Moreover, the functional significance of natural autoantibodies, i.e., self-reactive antibodies that are found in all individuals in the absence of inflammation or injury46, have not been explored in the context of CNS injury and repair. Natural autoantibodies are produced by a primordial subset of lymphocytes called B-1 cells and bind a range of highly conserved (T-independent) self-antigens, including carbohydrates, phospholipids and oxidized lipoproteins. Natural autoantibodies have been implicated as effectors of pathology after ischemia/reperfusion injury in muscle and gut47. However, they also might help coordinate neuroprotective functions in microglia and macrophages, including the induction of recycling endocytosis and TNF-α release, ligation of anti-inflammatory FcRs and induction of remyelination via stimulation of oligodendrocyte progenitor cells48,49. The induction of these protective mechanisms during an immune response might explain the therapeutic benefit of intravenous immunoglobulin (IVIG) therapy for CNS and non-CNS diseases49,50.
Do B cells and autoantibodies cause systemic pathology after CNS injury?
After CNS injury, B cells and autoantibodies might cause or influence systemic pathology, in addition to mediating adverse CNS effects. Serum rheumatoid factor (both IgM- and IgG-RF) is increased in SCI subjects51, leading to the conclusion that circulating levels of RF could serve as a biomarker capable of predicting injury severity. There is a growing interest in identifying acute serum or cerebrospinal fluid biomarkers as prognostic indicators of chronic outcome after TBI, SCI and stroke but it is not known if measurement of RF or other autoantibodies will be useful for this purpose. Autoantibodies produced in mice after SCI bind double stranded DNA (ds-DNA), chromatin and other nuclear antigens2 and autoantibodies with similar binding specificities to those antibodies found in the blood of patients with systemic autoimmune diseases including RA and SLE36,52. In both RA and SLE, immune complex formation and the coordinated activation of innate and adaptive immune responses are implicated in disease pathogenesis1,2. A similar cause-effect relationship in post-CNS injury systemic complications has not been proven but remains an intriguing possibility, especially because loss of neurological function cannot explain many of the co-morbidities that accompany CNS injuries. For example, male sexual infertility that is unrelated to erectile dysfunction, kidney disease/failure and aberrant skin inflammation (e.g., seborrheic dermatitis) are chronic problems after SCI, but dennervation has not proven to be a universally compelling argument to explain these problems.
Recently, it was shown that SCI primes oxidative metabolism, inflammatory signaling and trafficking of neutrophils and monocytes to lung and kidney after traumatic SCI in humans and rodents53,54. The enhanced production of autoantibodies specific for spermatozoa antigens, DNA or other nuclear antigens might help explain some of the systemic comorbidities associated with SCI and other forms of CNS injury2,55–57.
Manipulating B cell function as therapeutic approach for CNS injury
To date, most data indicate that B cell activation after a traumatic CNS injury, especially SCI, causes pathology leading to impaired recovery of neurological function1,2; therefore, it is logical to explore inhibition of B cell function as a therapeutic option. A number of B cell-directed therapies (e.g., B cell-depleting monoclonal antibodies including Rituxan® or Ocrelizumab®) already exist or are in clinical trials for treating SLE, RA and most recently, MS58. However, to date, there have been no attempts to use similar approaches for treating individuals with traumatic CNS injuries. The use of alternative therapies, including IVIG therapy, plasmapheresis or biologics that target endogenous factors that regulate B cell growth/differentiation (e.g., BAFF or APRIL) should also be considered for limiting the accumulation of potentially pathogenic antibodies in the circulation. In mice, intralesional B cell follicles form, and gene expression arrays indicate that B cell growth and differentiation occurs within the chronically injured CNS1,2,59. Therefore, the intraspinal or intracranial delivery of antibodies or pharmacological agents that block BAFF and APRIL is feasible and would presumably limit the effects of B cells and antibodies within the CNS parenchyma. However, as with other strategies that interfere with T cell or macrophage function, B cell inhibition strategies can also be associated with severe immunological complications, [such as the development of progressive multifocal leukoencephalopathy (PML)], which must be a strong consideration when contemplating immune-based interventions in patient populations60.
Future research might also show that even though pathogenic B cell responses are activated after injury, safe and efficient vaccine therapies could be exploited for CNS repair. Pre-clinical vaccination protocols have been developed with the goal of enhancing anti-myelin antibody synthesis for accelerating the removal of axon growth inhibitory myelin debris61. Clinical trials are underway to test whether exogenous monoclonal antibodies specific for Nogo-A and other repair-inhibiting myelin antigens can be used to safely treat patients with CNS injuries (clinicaltrials.gov). The administration of exogenous antibodies has the benefit of potentially inducing repair without directly activating (or re-activating) B cell clones that are set in motion by CNS injury.
Conclusions
It is clear that B cells are activated by traumatic CNS injury and that these cells participate in ongoing cascades of CNS injury. After SCI in mice, B cells produce pathogenic antibodies that cause neuroinflammation, cell death and sustained neurological dysfunction. Accordingly, strategies that inhibit B cell function after SCI could be therapeutic. Based on the overlapping specificities of autoantibodies produced after SCI and in autoimmune diseases like RA and SLE, future studies should determine if SCI autoantibodies also cause immune complex disease, cognitive deficits and kidney or sexual dysfunction in SCI subjects. Indeed, RA and SLE autoantibodies are known to cause systemic and cognitive impairments that mimic those described in people with SCI. As with any new and emerging area of biomedical research, there are many fundamental questions that remain unanswered (see Box 1). Hopefully, the answers to these questions can be used to develop more effective therapies for individuals who sustain a CNS injury.
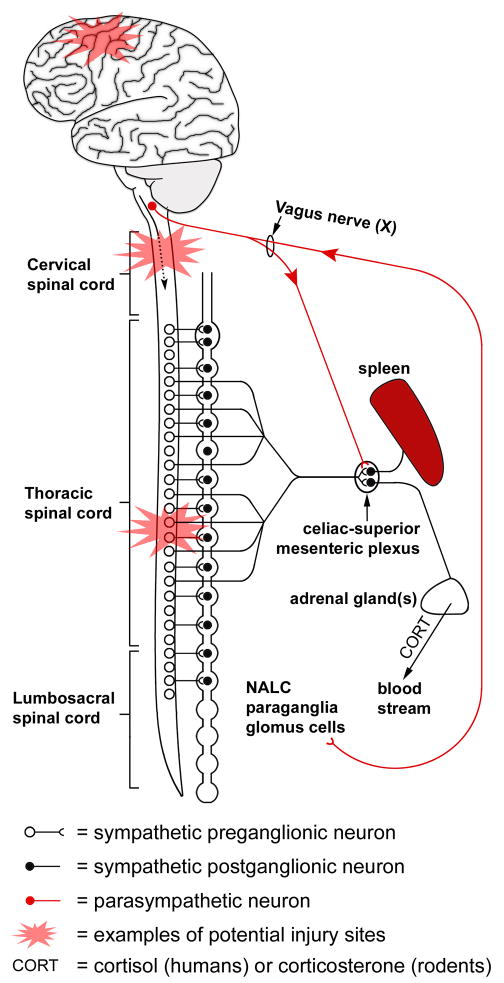
Figure 2.
Autonomic nervous system innervation to the spleen, nerve-associated lymphoid cells (NALC) and adrenal glands. Injuries sustained at different levels of the neuraxis differentially influence immune function. Injuries to the brain or brainstem will block supraspinal control of (dashed arrow) sympathetic preganglionic neurons which in turn regulate noradrenergic postganglionic neurons in the sympathetic chain ganglia. The latter innervate the spleen and adrenal glands. Dysregulation of this “hard-wiring” will adversely affect immune function. Immune function in the periphery and the CNS may also be adversely affected by CNS injury if reflex activation via the vagus nerve is damaged. Normally, this “cholinergic anti-inflammatory vagal reflex” helps limit inflammatory signaling cascades generated in the periphery (see 37 for review).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ankeny DP, et al. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119 (10):2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankeny DP, et al. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99 (4):1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 3.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9 (6):481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 4.Stefanova I, et al. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420 (6914):429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 5.Kil K, et al. T cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98 (2):201–207. doi: 10.1016/s0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 6.Popovich PG, et al. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45 (4):349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz M, Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med. 2001;7 (6):252–258. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- 8.Jones TB, et al. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22 (7):2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies AL, et al. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88 (11):1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Hayes KC, et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19 (6):753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- 11.Mizrachi Y, et al. Systemic humoral factors participating in the course of spinal cord injury. Paraplegia. 1983;21 (5):287–293. doi: 10.1038/sc.1983.48. [DOI] [PubMed] [Google Scholar]
- 12.Prochazka M, et al. Studies of immunologic reactions after brain injury. II. Antibodies against brain tissue lipids after blunt head injury in man. Int Surg. 1971;55 (5):322–326. [PubMed] [Google Scholar]
- 13.Skoda D, et al. Antibody formation against beta-tubulin class III in response to brain trauma. Brain Res Bull. 2006;68 (4):213–216. doi: 10.1016/j.brainresbull.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Kivity S, et al. Infections and autoimmunity--friends or foes? Trends Immunol. 2009;30 (8):409–414. doi: 10.1016/j.it.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, et al. Study of bacterial translocation from gut after paraplegia caused by spinal cord injury in rats. Spine (Phila Pa 1976) 2004;29 (2):164–169. doi: 10.1097/01.BRS.0000107234.74249.CD. [DOI] [PubMed] [Google Scholar]
- 16.Bansal V, et al. Traumatic Brain Injury and Intestinal Dysfunction: Uncovering the Neuro-Enteric Axis. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T, et al. Tonic B cell activation by Radioprotective105/MD-1 promotes disease progression in MRL/lpr mice. Int Immunol. 2008;20 (7):881–891. doi: 10.1093/intimm/dxn049. [DOI] [PubMed] [Google Scholar]
- 18.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140 (6):845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Paterson HM, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171 (3):1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 20.Fields ML, et al. Exogenous and endogenous TLR ligands activate anti-chromatin and polyreactive B cells. J Immunol. 2006;176 (11):6491–6502. doi: 10.4049/jimmunol.176.11.6491. [DOI] [PubMed] [Google Scholar]
- 21.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416 (6881):603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 22.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23 (11):509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 23.Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304 (5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 24.Hagen EM, et al. Mortality after traumatic spinal cord injury: 50 years of follow-up. J Neurol Neurosurg Psychiatry. 2009 doi: 10.1136/jnnp.2009.178798. [DOI] [PubMed] [Google Scholar]
- 25.Campagnolo DI, et al. Influence of neurological level on immune function following spinal cord injury: a review. J Spinal Cord Med. 2000;23 (2):121–128. doi: 10.1080/10790268.2000.11753519. [DOI] [PubMed] [Google Scholar]
- 26.Riegger T, et al. Immune depression syndrome following human spinal cord injury (SCI): a pilot study. Neuroscience. 2009;158 (3):1194–1199. doi: 10.1016/j.neuroscience.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Quattrocchi KB, et al. Impairment of helper T-cell function following severe head injury. J Neurotrauma. 1992;9 (1):1–9. doi: 10.1089/neu.1992.9.1. [DOI] [PubMed] [Google Scholar]
- 28.Cruse JM, et al. Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunol Res. 1992;11 (2):104–116. doi: 10.1007/BF02918615. [DOI] [PubMed] [Google Scholar]
- 29.Waites KB, et al. Immunogenicity of pneumococcal vaccine in persons with spinal cord injury. Arch Phys Med Rehabil. 1998;79 (12):1504–1509. doi: 10.1016/s0003-9993(98)90410-5. [DOI] [PubMed] [Google Scholar]
- 30.Trautner BW, et al. Inactivated influenza vaccination for people with spinal cord injury. Arch Phys Med Rehabil. 2004;85 (11):1886–1889. doi: 10.1016/j.apmr.2004.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucin KM, et al. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207 (1):75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucin KM, et al. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. J Neurochem. 2009;110 (5):1409–1421. doi: 10.1111/j.1471-4159.2009.06232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vega JL, et al. Acute down-regulation of antibody production following spinal cord injury: role of systemic catecholamines. J Neuropathol Exp Neurol. 2003;62 (8):848–854. doi: 10.1093/jnen/62.8.848. [DOI] [PubMed] [Google Scholar]
- 34.Cano G, et al. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439 (1):1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 35.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21 (6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mevorach D. Systemic lupus erythematosus and apoptosis: a question of balance. Clin Rev Allergy Immunol. 2003;25 (1):49–60. doi: 10.1385/CRIAI:25:1:49. [DOI] [PubMed] [Google Scholar]
- 37.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9 (6):418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skok MV, et al. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007;80 (24–25):2334–2336. doi: 10.1016/j.lfs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Nicolussi EM, et al. The cholinergic anti-inflammatory system limits T cell infiltration into the neurodegenerative CNS, but cannot counteract complex CNS inflammation. Neurobiol Dis. 2009;35 (1):24–31. doi: 10.1016/j.nbd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Tornqvist E, et al. Complement and clusterin in the injured nervous system. Neurobiol Aging. 1996;17 (5):695–705. doi: 10.1016/0197-4580(96)00120-0. [DOI] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9 (3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10 (4):236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kipnis J, et al. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99 (24):15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcondes MC, et al. Immune regulatory mechanisms influence early pathology in spinal cord injury and in spontaneous autoimmune encephalomyelitis. Am J Pathol. 2005;166 (6):1749–1760. doi: 10.1016/S0002-9440(10)62485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29 (43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutz HU, et al. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol. 2009;30 (1):43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Fleming SD, Tsokos GC. Complement, natural antibodies, autoantibodies and tissue injury. Autoimmun Rev. 2006;5 (2):89–92. doi: 10.1016/j.autrev.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Wright BR, et al. Cellular mechanisms of central nervous system repair by natural autoreactive monoclonal antibodies. Arch Neurol. 2009;66 (12):1456–1459. doi: 10.1001/archneurol.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuelsson A, et al. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291 (5503):484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 50.Tha-In T, et al. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29 (12):608–615. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Petrova NV, et al. Serum rheumatoid factors in spinal cord injury patients. Paraplegia. 1993;31 (4):265–268. doi: 10.1038/sc.1993.47. [DOI] [PubMed] [Google Scholar]
- 52.Nagy G, et al. T- and B-cell abnormalities in systemic lupus erythematosus. Crit Rev Immunol. 2005;25 (2):123–140. doi: 10.1615/critrevimmunol.v25.i2.30. [DOI] [PubMed] [Google Scholar]
- 53.Bao F, et al. Increased oxidative activity in human blood neutrophils and monocytes after spinal cord injury. Exp Neurol. 2009;215 (2):308–316. doi: 10.1016/j.expneurol.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 54.Gris D, et al. The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp Neurol. 2008;211 (1):259–270. doi: 10.1016/j.expneurol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 55.Siosteen A, et al. Auto-immunity to spermatozoa and quality of semen in men with spinal cord injury. Int J Fertil. 1993;38 (2):117–122. [PubMed] [Google Scholar]
- 56.DeGiorgio LA, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7 (11):1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, et al. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun. 2009;33 (3–4):270–274. doi: 10.1016/j.jaut.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dorner T, et al. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5 (8):433–441. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- 59.Ankeny DP, Popovich PG. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 2009;158 (3):1112–1121. doi: 10.1016/j.neuroscience.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carson KR, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10 (8):816–824. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 61.Huang DW, et al. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24 (3):639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]