Abstract
The role of superoxide and its active byproduct peroxynitrite as mediators of nociceptive signaling is emerging. We have recently reported that nitration and inactivation of spinal mitochondrial superoxide dismutase (MnSOD) provides a critical source of these reactive oxygen and nitrogen species during central sensitization associated with the development of morphine-induced hyperalgesia and antinociceptive tolerance. In this study, we demonstrate that activation of spinal NADPH oxidase is another critical source for superoxide generation. Indeed, the development of morphine-induced hyperalgesia and antinociceptive tolerance was associated with increased activation of NADPH oxidase and superoxide release. Co-administration of morphine with systemic delivery of two structurally unrelated NADPH oxidase inhibitors namely apocynin or diphenyleneiodonium (DPI), blocked NADPH oxidase activation and the development of hyperalgesia and antinociceptive tolerance at doses devoid of behavioral side effects. These results suggest that activation of spinal NADPH oxidase contributes to the development of morphine-induced hyperalgesia and antinociceptive tolerance. The role of spinal NADPH oxidase was confirmed by showing that intrathecal delivery of apocynin blocked these events. Our results are the first to implicate the contribution of NADPH oxidase as an enzymatic source of superoxide and thus peroxynitrite in the development of central sensitization associated with morphine-induced hyperalgesia and antinociceptive tolerance. These results continue to support the critical role of these reactive oxygen and nitrogen species in pain while advancing our knowledge of their biomolecular sources.
Keywords: superoxide, peroxynitrite, morphine antinociceptive tolerance, NADPH oxidase
Superoxide (O2·−) and its downstream signaling mediator, peroxynitrite (ONOO−, PN), have emerged as powerful pronociceptive reactive oxygen and nitrogen species [37–38]. Indeed, their involvement in central sensitization has been reported during the development of thermal hyperalgesia associated with acute and chronic inflammation [7–8, 19, 30, 48, 52–53], in response to spinal activation of the N-methyl-D-aspartate receptor (NMDAR) [27], in the development of orofacial pain [53], and in the development of opiate-induced hyperalgesia and antinociceptive tolerance [4, 25, 31]. Furthermore, and as an extension to the above findings, a role for nitroxidative stress (herein defined as stress induced in the presence of O2 •−, PN and related species) was supported using a variety of non-selective agents [5, 26, 37] such as phenyl N-tert-butylnitrone (PBN) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL). Indeed, PBN or TEMPOL showed efficacy in inflammatory [19] and neurogenic pain [13, 21, 39–40], visceral pain [51], neuropathic pain [13, 33, 42, 47], and chemotherapy-induced pain [20]. These findings led us to put forth the hypothesis that targeting O2·− and PN should lead to development of novel analgesics for the management of pain [37–38]. Clearly unraveling the enzymatic sources in the production of O2·− and PN and understanding the signaling pathways engaged by these species in nociceptive processing is of paramount importance [37–38]. Inactivation of manganese superoxide dismutase (MnSOD), the enzyme that normally keeps [O2·−] under tight control, [24] is a central source for O2·−-derived PN in several diseases driven by overt production of PN [22]. Such enzymatic inactivation results from nitration of Tyr-34 by PN in a manganese-catalyzed process [23]. In a series of studies, our group revealed that spinal nitration and inactivation of MnSOD provides a critical “feed-forward” mechanism that allows for the accumulation of O2·− and PN during the development and maintenance of central sensitization [4, 25, 27, 31, 52], findings confirmed and subsequently extended by others [39–40]. Another important O2·−-generating enzyme system is nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [29] recently implicated in the development of central sensitization associated with inflammatory hyperalgesia [17]. This O2·−-generating enzyme, is dormant in resting cells and produces O2·− only upon activation. The principal regulation of NADPH oxidase is post-translational and depends on the assembly of several membrane-bound and cytosolic components to form an active enzyme complex [3]. In resting cells, the enzyme consists of two membrane-bound components, gp91phox and p22phox, and several cytosolic components, including p47phox, p40phox, p67phox, and rac1/2 [3]. Gp91phox is a flavocytochrome and the catalytic core of the enzyme. Upon activation, the cytosolic components translocate to the membrane and associate with membrane components to form an assembled, activated, and O2·−-producing enzyme complex [3]. Although this enzyme is best characterized in immune cells and leukocytes for its involvement in O2·−production, it is now known that various protein components of NADPH oxidase are expressed in neurons, astrocytes, and microglia [1, 14, 49]. Importantly, O2·− auto-augments its formation by up-regulating the expression of the Rac1 and gp91phox subunits of the holoenzyme creating a self-perpetuating cascade [28, 35]. Therefore, we hypothesize that post-translational nitration and inactivation of MnSOD and activation of NADPH oxidase represent two pathways that operate in synchrony to maintain central sensitization. To this end and in order to extend our previous studies and address our hypothesis, we investigated in this study whether the NADPH oxidase contributes to central sensitization associated with the development of morphine-induced hyperalgesia and antinociceptive tolerance by providing an additional source of O2·−.
All experiments were performed in accordance with the International Association for the Study of Pain and the National Institutes of Health guidelines on laboratory animals welfare and the recommendations by Saint Louis University Institutional Animal Care and Use Committee. For all experiments animals were placed in a plastic restrainer for antinociception test and these were habituated to handling and testing equipment at least 20–30 minutes before experiments. All experiments were conducted with the experimenters blinded to treatment conditions. Male CD-1 mice (24–30g; Charles River Laboratory) were housed 4–5 per cage, maintained under identical conditions of temperature (21 ± 1°C) and humidity (65% ± 5%) with a 12-hour light/12-hour dark cycle, and allowed food ad libitum. The tail flick test, which measures withdrawal latencies of the tail from a noxious radiant heat source, was used to measure thermal nociceptive sensitivity [11]. The intensity of the heat stimulus was adjusted so that the mouse flicked its tail after 2–4 s. A cut-off time of 15 s was imposed to prevent tissue damage. Mice received a subcutaneous (sc, 0.2 ml) injection of morphine (20 mg/kg) or its vehicle sterile saline twice daily at 0800–0900 h and 1600–1700 h for 4 days. Apocynin (4-hydroxy-3-methoxy-acetophenone; 25, 50 and 100 mg/kg/day), DPI (diphenyleneiodonium, 0.25, 0.5 and 1 mg/kg/day) or their vehicle were given by intraperitoneal (i.p) injections (0.2 ml) twice a day and 15 minutes before each morphine injection over the 4 days for the drugs to be distributed more constantly. On day 5, and approximately 16 h after the last morphine injection, each mouse was tested twice on the tail flick and the latency (s) reaction times averaged to obtain a baseline. Mice were then challenged with acute subcutaneous (s.c) injection of morphine (10 mg/kg) to study the expression of tolerance. Data obtained were then converted to percentage maximal possible antinociceptive effect (%MPE) as equal to: (response latency−baseline latency)/(cut off latency−baseline latency) × 100. At least six mice per group were used. After the behavioral tests, animals were sacrificed with CO2 and rapidly decapitated to harvest spinal cord tissues from the lumbar enlargement segment of the spinal cord (L4–L6).
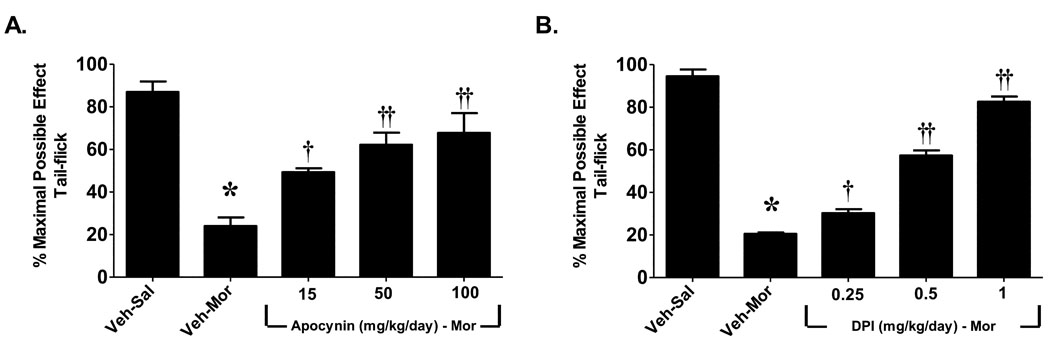
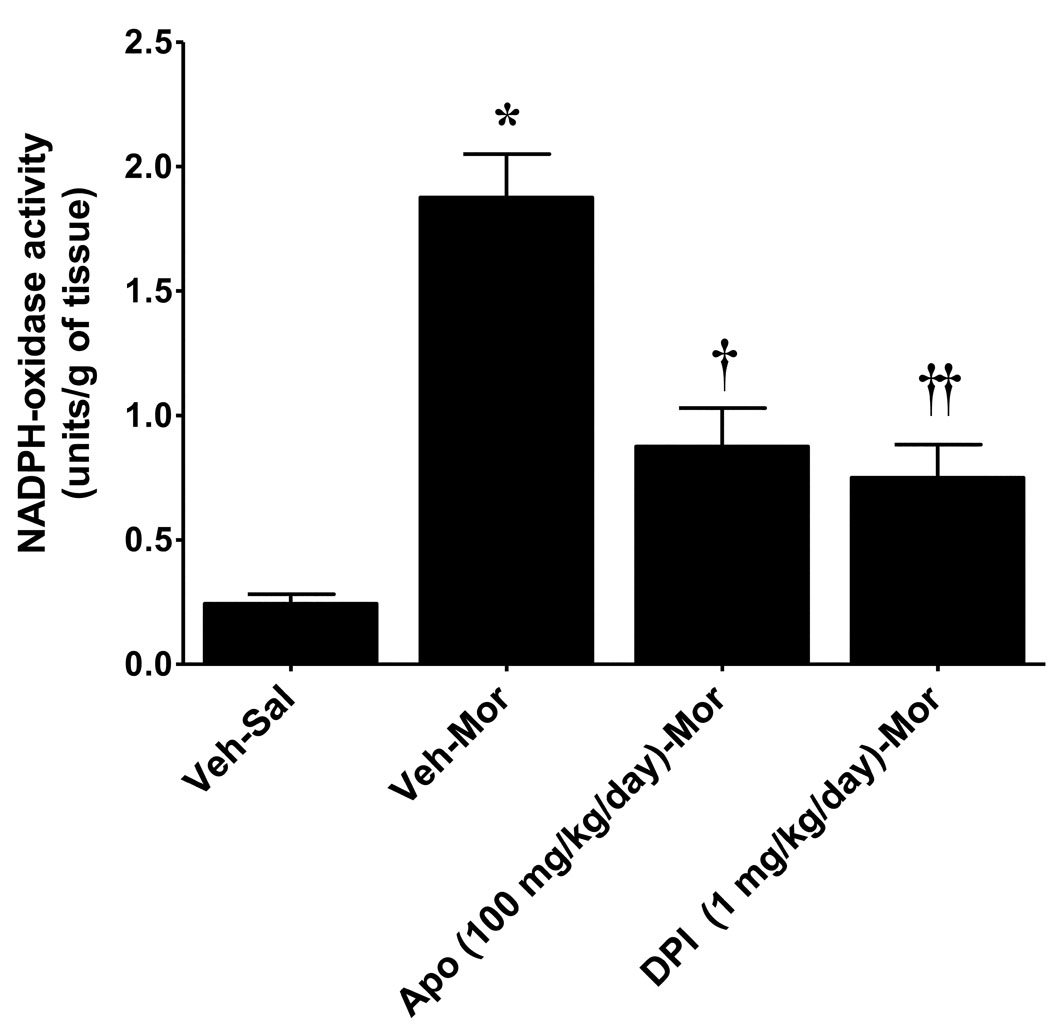
When compared to mice that received a subcutaneous injection of saline (Veh-Sal, n=6) over 5 days, injection of morphine over the same time frame (Veh-Mor, n=6) led to the development of antinociceptive tolerance (Fig. 1). This was indicated by a significant (P<0.001) reduction in tail flick latency over time (0–120 min) after an acute dose of morphine challenge (10 mg/kg) in rats receiving repeated morphine administration over 5 days compared to mice receiving repeated saline injection over the same interval (Fig. 1). The role of the NADPH oxidase was assessed by the use of apocynin or DPI, well-characterized inhibitors of the NADPH-oxidase [9, 41, 43–44]. Apocynin prevents serine phosphorylation of p47phox and blocks its association with gp91phox, thus blunting NADPH oxidase activation [41, 44], whereas DPI forms adducts with FAD interrupting oxygen reduction through gp91phox [32]. These inhibitors exert beneficial effects in several animal models of nitroxidative stress including rheumatoid arthritis, diabetes, atherosclerosis, neurodegeneration, stroke and ischemia-reperfusion injuries [2, 6, 9–10, 16, 18, 34, 36, 43, 46]. We now show, for the first time, that activation of spinal NADPH oxidase plays a critical role in the development of morphine-induced antinociceptive tolerance. Indeed and as can be seen in Fig. 2, the development of morphine-induced antinociceptive tolerance was associated with increased activation of NADPH-oxidase (P<0.001) and superoxide formation in spinal cord tissues as measured by increased spectrophotometric absorbtion at 550nm by reduced cytochrome c using a commercially available kit (CY0100; Sigma, St. Louis, MO). Co-administration of morphine with daily (5 days) injections of apocynin (100 mg/kg/day, n=4) or DPI (1 mg/kg/day, n=4) blocked spinal NADPH oxidase activation (P<0.01) (Fig. 2) and blocked in a dose-dependent manner (25–100 mg/kg/day, n=6 for apocynin and 0.25–1 mg/kg/day, n=6 for DPI) the development of antinociceptive tolerance (Fig. 1) at doses devoid of motor function impairment (as tested on the Rotarod; n=4, not shown). Baseline values for tail flick latency from all groups on day 5 before injection of acute morphine, were statistically insignificant from each other and ranged between 2–3 sec. Also, inhibiting O2·− production with an acute injection of apocynin did not reverse established tolerance (n=4, not shown); thus confirming results obtained with other O2·−-targeted approaches, which established that O2·− and PN contribute to events in the development, but not expression, of tolerance [4, 25]. These results indicate that spinal activation of NADPH oxidase is a critical source of O2· in the development of morphine-induced antinociceptive tolerance.
Fig. 1. The development of morphine antinociceptive tolerance is attenuated by inhibitors of the NADPH oxidase.
On day 5, acute injection of morphine (10 mg/kg) in animals that received saline over 4 days (vehicle group, Veh-Sal) produced a significant antinociceptive response. On the other hand, a significant loss to the antinociceptive effect of the acute injection of morphine was observed in animals that received morphine over the same time-period indicative of antinociceptive tolerance (Veh-Mor). Co-administration of morphine over 4 days with (A) apocynin (25–100 mg/kg/day) or (B) DPI (0.25–1 mg/kg/day) inhibited the development of antinociceptive tolerance in a dose-dependent manner. Results are expressed as percent maximal possible antinociceptive effect (%MPE) with mean ± SEM for 6 animals. Data were analyzed by ANOVA with Dunnett’s post hoc test where *P<0.001 for morphine alone vs. vehicle and † P<0.05 or †† P<0.001 for morphine plus apocynin or DPI vs. morphine alone.
Fig. 2. Chronic morphine administration activate NADPH-oxidase superoxide production.
When compared to animals receiving vehicle over 4 days, repeated administration of morphine over the same time frame led to (A) activation of the NADPH oxidase in spinal cord tissues from the lumbar enlargement (L4–L6). Co-administration of morphine over 4 days with apocynin (100 mg/kg/day) or DPI (1 mg/kg/day) attenuated the activation of NADPH oxidase. Results are expressed as NADPH-oxidase activity (units/g of tissue) with mean ± SEM for 4 animals. Data were analyzed by ANOVA with Dunnett’s post hoc test where *P<0.001 for morphine alone vs. vehicle and † P<0.01 for morphine plus apocynin or DPI vs. morphine alone.
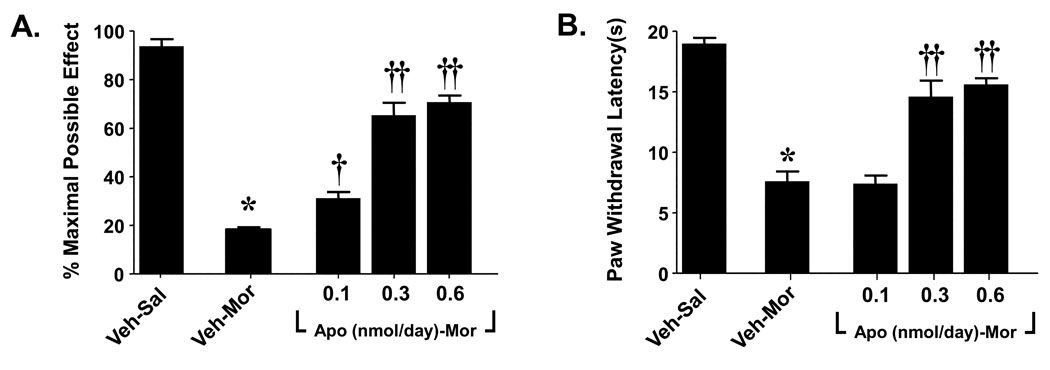
The role for spinal NADPH oxidase was confirmed in subsequent studies using intrathecal (i.th) delivery of apocynin. To this end, rats, under light isoflurane anesthesia, were subcutaneously implanted (in the interscapular region) with osmotic pumps (Alzet 2001; Alza, Mountain View CA), to deliver saline at 0.5 µl/h or morphine at 7.5 µg µl−1 h−1 for 7 days over 7 days as previously described [50]. Drugs or isovolumetric vehicle (10 µl followed by a 10 µl flush with sterile physiological saline) were given once a day for 6 days by i.t.h injections in rats with chronically implanted i.t.h catheter as described previously [45]. The method of Hargreaves and colleagues was used to assess changes in baseline nociceptive responses to a thermal nociceptive stimulus [15] with baseline latencies of 18–20 sec and a maximal cut-off time of 20 sec to prevent tissue damage. The tail flick test was used to measure thermal nociceptive sensitivity with baseline latencies of 4–5 sec and a cutoff time of 10 sec [11]. When compared to rats that received a chronic subcutaneous (s.c) infusion of saline (Veh-Sal, n=6) over 7 days, morphine infusions over the same time frame (Veh-Mor, n=6) led to 1) the development of thermal hyperalgesia [15] as evidenced by a significant (P<0.001) reduction in paw-withdrawal latency on day 6 compared to paw-withdrawal latency from before implantation of the osmotic minipump (baseline) (Fig. 3A) and 2) the development of antinociceptive tolerance (Fig. 3B). The latter was indicated by a significant (P<0.001) reduction in tail flick latency 30 min after challenge with an acute dose of morphine (6 mg/kg) given intraperitoneally (i.p) on day 6 in rats receiving chronic morphine infusion over 7 days compared to rats receiving an infusion of saline over the same interval (Fig. 3A). Co-administration of morphine with daily (6 days) i.th injections of apocynin (Apo-Mor; 0.1–0.6 nmol/day, n=6) blocked the development of hyperalgesia and antinociceptive tolerance (Fig. 3A, B) thus establishing the critical role of superoxide derived from spinal activation of the NADPH oxidase. When given alone daily and over 6 days to rats that received saline infusion (Veh-Sal), apocynin (0.6 nmol/day) had no effect (n=3, not shown).
Fig. 3. Intrathecal delivery of apocynin attenuates the development of morphine-induced hyperalgesia and antinociceptive tolerance.
When compared to rats that received a chronic s.c infusion of saline (Veh-Sal, n=6) over 7 days, infusion of morphine over the same time frame (Veh-Mor, n=6) led to the development of (A) thermal hyperalgesia as evidenced by a significant reduction in paw-withdrawal latency(s) on day 6 when compared to paw-withdrawal latency from before implantation of the osmotic minipump (baseline) and (B) antinociceptive tolerance. The development of morphine induced thermal hyperalgesia (A) and antinociceptive tolerance (B) were attenuated in a dose-dependent fashion by intrathecal delivery of apocynin (0.1–0.6 nmol/day). Results are expressed as mean ± SEM for n = 6 animals. Data were analyzed by ANOVA with Dunnett’s post hoc test where *P<0.001 for morphine alone vs. vehicle and † P<0.05 or †† P<0.001 for morphine plus apocynin vs. morphine alone.
In summary, results derived from our studies have defined for the first time the importance of spinal NADPH oxidase as a source of O2·− and thus of its downstream signaling mediator PN in the development of antinociceptive tolerance. Since both neurons and glial cells possess functional NADPH oxidase [1, 14, 49], we expect both cell populations to contribute to the production of superoxide derived from the activation of this enzyme; we are currently testing this in our laboratories. Whereas in this paper we focused on the role of spinal NADPH oxidase, we are not excluding the likely possibility that this O2·−-generating enzyme may, in addition, contribute to the development of morphine-induced hyperalgesia and antinociceptive tolerance at supraspinal sites. In support, we have recently reported that O2·− and PN contribute to the development of morphine-induced hyperalgesia and antinociceptive tolerance by acting both at spinal [4, 25, 31] and supraspinal sites [12].
Collectively these results continue to support our general hypothesis that targeting O2·− and PN is an evidence-based approach to develop novel therapeutics for managing pain of several etiologies [37–38].
Research Highlights.
-
▶
Spinal NADPH-oxidase superoxide increases with morphine antinociceptive tolerance.
-
▶
Inhibition of NADPH-oxidase activity blocks morphine antinociceptive tolerance.
-
▶
Targeting spinal NADPH-oxidase may be a therapeutic pain management strategy.
Acknowledgements
Supported by R01 DA024074 and 1RC1AR058231 (DS). The authors declare no conflicts of interest. We would like to thank Dr Bill Neumann (Southern Illinois University Edwardsville) for helpful discussions and editing of this manuscript.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 4.Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009;46:212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batinic-Haberle I, Reboucas JS, Spasojevich I. Superoxide Dismutase Mimics: Chemistry, Pharmacology and Therapeutic Potential. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- 7.Bezerra MM, Brain SD, Girao VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Muscoli C, Doyle T, Bryant L, Cuzzocrea S, Mollace V, Mastroianni R, Masini E, Salvemini D. NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain. 2010;149:100–106. doi: 10.1016/j.pain.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffey MJ, Serezani CH, Phare SM, Flamand N, Peters-Golden M. NADPH oxidase deficiency results in reduced alveolar macrophage 5-lipoxygenase expression and decreased leukotriene synthesis. J Leukoc Biol. 2007;82:1585–1591. doi: 10.1189/jlb.0107019. [DOI] [PubMed] [Google Scholar]
- 10.Cotter MA, Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73:1813–1824. doi: 10.1016/s0024-3205(03)00508-3. [DOI] [PubMed] [Google Scholar]
- 11.D'Amour F. A method for determining loss of pain sensation. J Pharmacol xp Ther. 1941;72:74–79. [Google Scholar]
- 12.Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D. Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 16.Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, van den Berg WB, van Beuningen HM, Smit HF. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur J Pharmacol. 2006;531:264–269. doi: 10.1016/j.ejphar.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango K, Shirai Y, Yokoyama T, Kaneko S, Saito N, Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai J, Ando K, Tojo A, Shimosawa T, Takahashi K, Onozato ML, Yamasaki M, Ogita T, Nakaoka T, Fujita T. Endogenous adrenomedullin protects against vascular response to injury in mice. Circulation. 2004;109:1147–1153. doi: 10.1161/01.CIR.0000117231.40057.6D. [DOI] [PubMed] [Google Scholar]
- 19.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Zhang YP, Gwak YS, Abdi S. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology. 2010;112:432–439. doi: 10.1097/ALN.0b013e3181ca31bd. [DOI] [PubMed] [Google Scholar]
- 21.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 23.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 24.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 25.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Superoxide auto-augments superoxide formation and upregulates gp91(phox) expression in porcine pulmonary artery endothelial cells: inhibition by iloprost. Eur J Pharmacol. 2006;538:108–114. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Nauseef WM. The NADPH-dependent oxidase of phagocytes. Proc Assoc Am Physicians. 1999;111:373–382. doi: 10.1111/paa.1999.111.5.373. [DOI] [PubMed] [Google Scholar]
- 30.Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. Faseb J. 2008;22:3154–3164. doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- 31.Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, Petrusca DN, Mollace V, Mazzon E, Li D, Petrache I, Matuschak GM, Salvemini D. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. J Pharmacol Exp Ther. 2009;329:64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290(Pt 1):41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 34.Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun. 2004;313:812–817. doi: 10.1016/j.bbrc.2003.11.173. [DOI] [PubMed] [Google Scholar]
- 35.Puntambekar P, Mukherjea D, Jajoo S, Ramkumar V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem. 2005;95:1689–1703. doi: 10.1111/j.1471-4159.2005.03518.x. [DOI] [PubMed] [Google Scholar]
- 36.Rachmilewitz D, Okon E, Karmeli F. Sulphydryl blocker induced small intestinal inflammation in rats: a new model mimicking Crohn's disease. Gut. 1997;41:358–365. doi: 10.1136/gut.41.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salvemini D, Neumann W. Targeting peroxynitrite driven nitroxidative stress with synzymes: A novel therapeutic approach in chronic pain management. Life Sci. 2010;86:604–614. doi: 10.1016/j.lfs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Salvemini D, Neumann WL. Peroxynitrite: a strategic linchpin of opioid analgesic tolerance. Trends Pharmacol Sci. 2009;30:194–202. doi: 10.1016/j.tips.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009;29:159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–258. doi: 10.1016/0891-5849(90)90070-y. [DOI] [PubMed] [Google Scholar]
- 42.Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55:158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Siragy HM, Huang J. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol. 2008;93:709–714. doi: 10.1113/expphysiol.2007.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 45.Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 46.Supinski G, Stofan D, Nethery D, Szweda L, DiMarco A. Apocynin improves diaphragmatic function after endotoxin administration. J Appl Physiol. 1999;87:776–782. doi: 10.1152/jappl.1999.87.2.776. [DOI] [PubMed] [Google Scholar]
- 47.Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport. 1996;7:1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- 48.Tang N, Ong WY, Yeo JF, Farooqui AA. Anti-allodynic effect of intracerebroventricularly administered antioxidant and free radical scavenger in a mouse model of orofacial pain. J Orofac Pain. 2009;23:167–173. [PubMed] [Google Scholar]
- 49.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vera-Portocarrero LP, Zhang ET, King T, Ossipov MH, Vanderah TW, Lai J, Porreca F. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2006 doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Cochran V, Abdi S, Chung JM, Chung K, Kim HK. Phenyl N-t-butylnitrone, a reactive oxygen species scavenger, reduces zymosan-induced visceral pain in rats. Neurosci Lett. 2008;439:216–219. doi: 10.1016/j.neulet.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 53.Yeo JF, Ling SF, Tang N, Ong WY. Antinociceptive effect of CNS peroxynitrite scavenger in a mouse model of orofacial pain. Exp Brain Res. 2008;184:435–438. doi: 10.1007/s00221-007-1211-x. [DOI] [PubMed] [Google Scholar]