Abstract
Background
If ‘bridging’ to allotransplantation is to be achieved by a pig liver xenograft, adequate hepatic function needs to be assured.
Methods
We have studied hepatic function in baboons after transplantation of livers from α1,3-galactosyltransferase gene-knockout (GTKO,n=1) or GTKO pigs transgenic for CD46 (GTKO/CD46,n=5). Monitoring was by liver function tests and coagulation parameters. Pig-specific proteins in the baboon serum/plasma were identified by Western blot. In 4 baboons, coagulation factors were measured. The results were compared with values from healthy humans, baboons, and pigs.
Results
Recipient baboons died or were euthanized after 4-7 days following internal bleeding associated with profound thrombocytopenia. However, parameters of liver function, including coagulation, remained in the near-normal range, except for some cholestasis. Western blot demonstrated that pig proteins (albumin, fibrinogen, haptoglobin, plasminogen) were produced by the liver from day 1. Production of several pig coagulation factors was confirmed.
Conclusions
After the transplantation of genetically-engineered pig livers into baboons (1) many parameters of hepatic function, including coagulation, were normal or near-normal; (2) there was evidence for production of pig proteins, including coagulation factors, and (3) these appeared to function adequately in baboons, though inter-species compatibility of such proteins remains to be confirmed.
Keywords: α1, 3-galactosylransferase gene-knockout, acute liver failure, baboon, coagulation, coagulation factors, liver, pig, genetically-modified, xenotransplantation
INTRODUCTION
Increasing discrepancy between the numbers of available organs and patients on the waiting list continues to be a major hurdle in organ transplantation (Tx). The lack of human donor livers has resulted in the deaths of approximately 30,000 patients on the waiting list during the past 13 years (1). The prompt availability of a donor liver is particularly important in patients with acute liver failure when clinical deterioration may develop rapidly (2-7). UNOS has an urgent listing for acute liver failure patients (Liver Status 1); its current median waiting time is 6 days (1,2). However, some patients need to undergo urgent liver Tx within 24-36h, before they become unacceptable candidates.
Pigs might provide livers for patients with fulminant liver failure (2,8,9). The ability to genetically-modify pigs (GM pigs), e.g., by introducing a transgene for a human complement-regulatory protein (10,11), or by deleting the α1,3-galactosyltransferase gene (GTKO) so that the pigs do not express the important Galα1,3Gal antigens against which primates have natural antibodies (12), has been a significant step toward the clinical application of xenoTx. Using GTKO pigs and novel immunosuppressive agents, survival of 2 to 6 months has been achieved after heterotopic heart Tx in baboons (13,14). In life-supporting kidney xenoTx, survival of close to 3 months has been reported (15). Pig organs expressing a human complement-regulatory protein, e.g., CD46 (11), in combination with GTKO are even better protected from antibody-mediated injury (16).
If pig livers are used to bridge a patient to alloTx, adequate hepatic function and coagulation will need to be guaranteed by the graft. We have investigated graft survival after the orthotopic Tx of GM pig livers in immunosuppressed baboons (17). The major aim of the present study was to determine whether a GM pig liver (largely protected from the baboon immune response) would function sufficiently well to support the recipient with good hepatic function until an allograft became available (e.g., 7-14 days).
Survival of the recipients (without rejection of the pig liver) was possible for 4-7 days, but was limited by the rapid development of a profound thrombocytopenia (17). We here report on parameters of hepatic function after Tx, and compare these data with measurements made in healthy baboons and pigs. We also measured pig-specific proteins and coagulation factors in the baboons’ blood.
MATERIALS AND METHODS
Animals
Blood was drawn from 7 healthy baboons (Papio species) (Oklahoma University Health Sciences Center, Oklahoma City, OK). Baboons were of either sex, with known AB blood type, aged 2.70-3.47 (mean 2.92) years, weighing 8-12 (mean 9.5) kg, that had not undergone any prior surgical or therapeutic procedures. Blood was also drawn from healthy GTKO (n=1) and GTKO pigs transgenic for the human complement-regulatory protein, CD46 (GTKO/CD46; n=6) (Revivicor, Blacksburg, VA). All pigs were female of blood type O (non-A), aged 10-32 (mean 17.7) days, weighing 3-12 (mean 6.7) kg.
Animal care procedures were described in Supplementary Methods-A (Supplemental Digital Content).
Pig-to-baboon liver transplantation
Six immunosuppressed baboons received orthotopic liver grafts from GTKO (n=1) or GTKO/CD46 (n=5) pigs. All transplants were carried out using the standard surgical technique for human orthotopic liver Tx with sequential end-to-end anastomoses after baboon liver hepatectomy (17). The immunosuppressive protocol predominantly consisted of induction with thymoglobulin and maintenance with tacrolimus, mycophenolate mofetil, and methylprednisolone (Table 1).
TABLE 1.
IMMUNOSUPPRESSIVE REGIMENS USED IN PIG-TO-BABOON LIVER TRANSPLANTATION
| Baboon # |
Donor Pig # |
Pig Type | Immunosuppressive Therapy | Survival (days) |
|---|---|---|---|---|
| B3108 | P8208 | GTKO | ATG + TAC + MMF + CS | 6 |
| B3208 | P14508 | GTKO/CD46 | ATG + TAC + MMF + CS | 4 |
| B7708 | P21708 | GTKO/CD46 | ATG + TAC + MMF + CS | 7 |
| B7808 | P22108 | GTKO/CD46 | ATG + TAC + MMF + CS | 6 |
| B18508 | P3909 | GTKO/CD46 | ATG + TAC + MMF + CS + CVF | 5 |
| B18908 | P4009 | GTKO/CD46 | CyP + TAC + MMF + CS | 6 |
ATG = thymoglobulin (5-10 mg/kg i.v. on days -3 and -1 according to T cell count); TAC = tacrolimus (0.05-0.1 mg/kg x2 daily i.m. to maintain 12h blood trough levels of 10-15 ng/mL); MMF = mycophenolate mofetil (110 mg/kg/day by continuous i.v. infusion to maintain blood levels of 3-5 μg/mL); CS = corticosteroids (10 mg/kg i.v., tapering from day of transplant); CVF = cobra venom factor (1-3 mg i.v. on days -1 to 1); CyP = cyclophosphamide (40 mg/kg and 20 mg/kg on days -2 and -1, respectively).
Post-transplantation monitoring of hepatic function
All recipient baboons were followed for 4-7 days with daily measurements of (i) liver enzymes (aspartate transaminase [AST], alanine transaminase [ALT], gamma-glutamyltransferase [GGT], alkaline phosphatase [ALP], total and direct bilirubin), (ii) coagulation parameters (prothrombin time [PT], partial thromboplastin time [PTT], international normalized ratio [INR], fibrinogen, D-dimer), and (iii) protein production (total protein, albumin, complement activity) (all performed in the University of Pittsburgh Medical Center Central Laboratory, Presbyterian Hospital, Pittsburgh, PA). Western blot was used to determine pig-specific proteins in the baboon serum/plasma, and pig-specific coagulation factors were measured.
Determination of pig-specific proteins by Western blot
Western blot was performed on baboon blood to detect porcine albumin, fibrinogen, haptoglobin (reduced as well as denatured), and plasminogen (See Supplementary Methods-B) (Supplemental Digital Content).
Determination of pig-specific coagulation factors and antigens
In 4 baboons, selected coagulation factors (FI [fibrinogen], FII [prothrombin], FV, FVII, FVIII, FIX, FX, FXI, FXII), Protein C and S, antithrombin, and plasminogen activity, and Protein C, S and plasminogen antigens were measured pre- and post-Tx using human kits (See Supplementary Methods-C) (Supplemental Digital Content). The data were compared with values obtained from healthy baboons (n=7) and healthy GM pigs (n=7), as well as with values in humans, baboons, and pigs obtained from the literature. Some data from baboons have been reported previously (18). All assays were performed at the Institute for Transfusion Medicine Diagnostic Coagulation Reference Laboratory (Pittsburgh, PA).
Determination of total complement activity by CH50 assay
The CH50 assay (DiaMedix, Miami,FL) was used for determination of the classical pathway of complement activity in the fluid phase, as previously described (19).
Monitoring of histopathological changes in transplanted pig livers
Biopsies of the pig livers were taken 2h after reperfusion (before abdominal closure) and at necropsy (days 4-7). Standard techniques were used for microscopic examination (17).
RESULTS
Graft and recipient survival and outcome
Following GM pig-to-baboon liver Tx, the 6 baboons survived for 4, 5, 6, 6, 6 and 7 days, respectively. In one (B3208), very high levels of tacrolimus (>50ng/mL) were associated with the development of Gram-negative sepsis (Pseudomonas orzyhabitans) with death on day 4. In the remaining five, tacrolimus was discontinued for at least 24h after liver Tx until there was evidence of good hepatic function. These 5 baboons were euthanized or died from thromobocytopenic bleeding into the peritoneal, pleural, and/or pericardial cavities, small intestine, lungs, liver, and/or myocardium; details have been reported previously (17). (We do not believe the thrombocytopenia was related to the immunosuppressive regimen since we observed no decrease in platelet count after baboon liver alloTx using the same regimen [17].) There was minimal evidence of graft rejection in any transplanted liver either on the biopsies taken 2h after reperfusion or at necropsy (days 4-7) (17).
Clinical observations in baboons post-transplantation
After Tx, baboons were extubated immediately. Once awake, they remained active, alert, and began to eat and drink after 24-36h. There was no sign of hepatic encephalopathy. Ammonium levels were measured pre- and post-Tx (day 4) in only one baboon, remaining normal on day 4. All baboons passed urine and stools regularly, though melena stools were observed in 3 animals (presumably associated with spontaneous bleeding related to the profound thrombocytopenia). Renal function was monitored daily (creatinine, BUN, electrolytes) and remained normal in all cases, except in one baboon (B18508 – treated with CVF) in which the creatinine level was 2.5 mg/dL terminally. The high tacrolimus levels seen on day 1 may have been associated with decreased catabolic activity related to (i) the anhepatic phase, and (ii) ischemia-reperfusion injury. As no further reduction in tacrolimus dosage was required, this suggests that catabolic liver function recovered.
Pig hepatic function in baboons
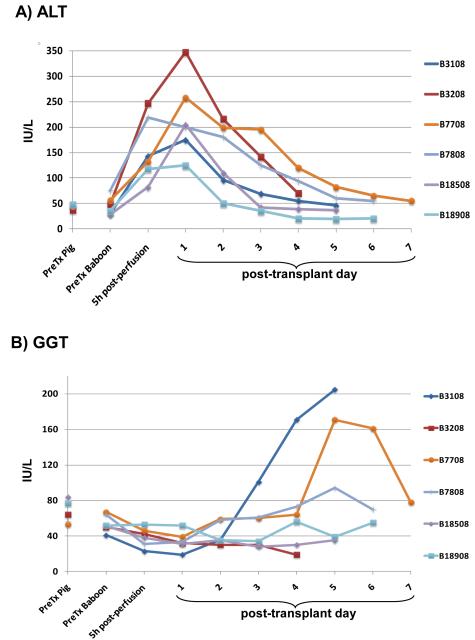
Normal values of selected parameters in healthy humans, baboons, and pigs are presented in Supplementary Table 1 (Supplemental Digital Content). After pig liver Tx, transaminases (AST, ALT) increased temporarily from ischemia/reperfusion injury, but returned to normal or near-normal values within 2 to 4 days (Figure 1A). Increases in AST, GGT, and ALP occurred in some baboons as a terminal event and were associated with hemorrhage in the liver and other sites (Figure 1B and 1C). Increases in total and direct bilirubin (Figure 1D) suggested an intrahepatic cholestatic injury, and was confirmed by histological examination of the liver at necropsy (not shown). This injury was due to abnormally viscous bile, but not to mechanical obstruction since the bile duct anastomoses were entirely patent at necropsy (17).
Figure 1. Results of liver function tests.
(A) ALT, (B) GGT, (C) ALP, (D) total bilirubin, (E) albumin, (F) total complement activity (CH50). (In order to reduce complement activity, cobra venom factor was administered to one baboon [B18508] on days −1, 0, and 1. The CH50 remained between 11-13% for 4 days). Dashed lines indicate B3108, in which CH50 was measured only pre-Tx and 6 days post-Tx (as well as in the donor pig).
Healthy pigs have significantly lower levels of total protein and albumin than humans and baboons (Supplementary Table 1) (Supplemental Digital Content). Total protein and albumin levels fell within a few hours of pig liver reperfusion to levels that are normal for pigs, but could be maintained at levels normal for baboons by the continuous i.v. infusion of human albumin (Figure 1E).
Total complement activity (CH50) in healthy pigs is significantly less than in healthy baboons [Hara H et al, submitted]. After pig liver Tx, complement activity remained high, suggesting sustained activation (Figure 1F). Since the half-lives of complement fractions are generally <24h (20), the post-Tx complement activity was almost certainly through activation of complement produced by the pig liver, suggesting adequate pig hepatic function in this respect.
Pig-specific proteins identified by Western blot
The specificity of all antibodies (anti-porcine albumin, fibrinogen, haptoglobin, and plasminogen) was confirmed by positive testing in healthy pig plasma and sera, in comparison with negative results in healthy baboon plasma and sera (Figure 2). Porcine fibrinogen and haptoglobin were detected by strong bands immediately after Tx and throughout the post-Tx period of follow-up in all baboons. Porcine albumin and plasminogen were detected in all animals from day 1; in some, a weak band became stronger on day 2 and remained strong throughout follow-up. These data indicated that the pig liver synthesized these 4 proteins, and that they could be detected in baboon serum/plasma.
Figure 2. Results of Western blot to identify pig-specific proteins.
(A) albumin (molecular weight [MW] = 63 kDa), (B) plasminogen (MW= 82kDa), (C) fibrinogen (MW = 340kDa), (D) haptoglobin (MW = 100kDa). All antibodies were pig-specific, which was confirmed by testing in healthy pigs and baboons. In all cases molecular weight estimates are for the denaturated nonreduced form. (Western blot using anti-baboon antibodies was not performed, and therefore the disappearance of baboon proteins in the blood after native hepatectomy could not be confirmed.)
Coagulation factors in healthy baboons and genetically-engineered pigs
The sites of synthesis and half-lives of the coagulation factors measured are indicated in Supplementary Table 2 (Supplemental Digital Content). Values were determined in healthy baboons and GM pigs and compared with data from the literature (Table 2). The results are discussed below.
TABLE 2.
COAGULATION FACTORS OF HEALTHY BABOONS AND GENETICALLY-ENGINEERED PIGS, AND COMPARISON WITH VALUES FOR HUMANS, PIGS, AND BABOONS REPORTED IN THE LITERATURE
| W | F I | F II | F V | F VII | F VIII | F IX | F X | F XI | F XII | Pmgn | Pr C | Pr S | AT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HUMANS (RANGE) |
(kg) | 150-350 mg/dl |
0.7-1.3 U/mL |
0.7-1.5 U/mL |
0.6-1.6 U/mL |
0.6-1.5 U/mL |
0.6-1.5 U/mL |
0.7-1.5 U/mL |
0.6-1.4 U/mL |
0.5-1.7 U/mL |
75-150 % |
70-140 % |
58- 128 % |
80-120 % |
| BABOONS | ||||||||||||||
| B7708 | 9.4 | 210 | 1.54 | 1.82 | 2.13 | 1.09 | 0.61 | 1.12 | 0.72 | 1.91 | 4.3 | 147.9 | 34.4 | 127 |
| B7808 | 10.1 | 201 | 1.32 | 1.66 | 1.62 | 1.21 | 0.56 | 0.88 | 0.71 | 2.78 | <0.1 | 111 | 37.7 | 108 |
| B18208 | 8.3 | 170 | 1.43 | 1 | 2.19 | 0.87 | 0.66 | 0.86 | 0.88 | 2.33 | <0.1 | 133.2 | 34.8 | 111 |
| B18408 | 8.8 | 157 | 1.31 | 0.75 | 1.65 | 1.06 | 0.7 | 0.85 | 0.83 | 2.3 | <0.1 | 110.9 | 44.7 | 115 |
| B18508 | 11.5 | 148 | 1.39 | 1.29 | 1.46 | 0.7 | 0.6 | 0.81 | 0.86 | 1.99 | <0.1 | 136.6 | 45.7 | 90 |
| B18608 | 8.9 | 350 | 1.38 | 1.04 | 2.84 | 0.87 | 0.64 | 0.81 | 1.01 | 1.63 | <0.1 | 124 | 27.5 | 91 |
| B18908 | 10 | 126 | 1.09 | 0.84 | 2.06 | 0.58 | 0.55 | 0.81 | 0.83 | 1.85 | <0.1 | 107.8 | 41 | 88 |
| mean±SD (n=7) | 9.6±1.1 | 195±75 | 1.35±0.14 | 1.20±0.41 | 1.99±0.47 | 0.91±0.22 | 0.62±0.05 | 0.88±0.11 | 0.83±0.10 | 2.11±0.38 | 0.7±1.6 | 124±15 | 38±6 | 104±15 |
| BABOONS [Ezzelarab et al, ref#18] | ||||||||||||||
| mean±SD (n=5) | 149±26 | 1.14±0.22 | 146±22 | 32±8 | 101±19 | |||||||||
| GENETICALLY-ENGINEERED PIGS (GTKO and GTKO/CD46) | ||||||||||||||
| P2109 | 11.5 | 352 | 0.94 | >6.02 | 1.27 | 6.02 | 3.8 | 1.45 | 3.01 | 1.57 | <0.1 | <10 | <10 | 80 |
| P3909 | 6 | 333 | 1.01 | >6.02 | 1.47 | >6.12 | 4.53 | 1.49 | 4.51 | 1.41 | <0.1 | <10 | <10 | 87 |
| P4009 | 5.8 | 330 | 1.28 | >6.02 | 1.44 | >6.12 | 3.55 | 1.26 | 3.42 | 1.2 | <0.1 | <10 | <10 | 76 |
| P14408 | 6.5 | 363 | 1.66 | >6.02 | 2.14 | >6.12 | 3.78 | 1.51 | 3.74 | 3 | 1.6 | <10 | <10 | 60 |
| P14508 | 6.7 | 535 | 1.8 | >6.02 | 2.17 | >6.12 | 3.55 | 1.26 | 3.38 | 2.95 | <0.1 | <10 | <10 | 73 |
| P21708 | 3.4 | 305 | 0.56 | >6.02 | 6.08 | 5.96 | 6.05 | 4.82 | 5.95 | 2.89 | 10 | <10 | <10 | 66 |
| P22108 | 6.7 | 493 | 0.61 | >6.02 | 1.93 | 5.96 | 3.97 | 2.01 | 4.49 | 1.33 | 8 | 20 | 20 | 55 |
| mean±SD (n=7) | 6.7±2.4 | 387±89 | 1.12±0.48 | 6.02±0.0 | 2.36±1.68 | 6.06±0.08 | 4.18±0.89 | 1.97±1.28 | 4.07±1.00 | 2.05±0.85 | 2.9±4.3 | 11±4 | 11±4 | 71±11 |
| WILD-TYPE PIGS [Lewis et al, ref#37] | ||||||||||||||
| mean (n=14) | 309 | 0.64 | 4.73 | 2.09 | 6.13 | 4.18 | 1.89 | 1.43 | 4.95 | 8 | 71 | 8 | 111 | |
| standard pig (n=6) | 60-180 | 348 ± 78 | 0.68 ± 0.3 | 2.80 ± 0.6 | 1.92 ± 0.4 | 6.42 ± 0.8 | 3.20 ± 0.3 | 2.54 ± 0.9 | 1.67 ± 0.3 | 5.65 ± 1.4 | 50 ± 10 | 70 ± 10 | 10 ± 0 | 109 ± 20 |
| mini pig (n=4) | 30-50 | 251 ± 104 | 0.43 ± 0.1 | 5.15 ± 0.8 | 1.67 ± 0.8 | 5.88 ± 1.0 | 4.10 ± 1.4 | 1.06 ± 0.3 | 1.16 ± 0.5 | 3.98 ± 0.7 | 8 ± 2 | 56 ± 20 | 7 ± 1 | 111 ± 5 |
| micro pig (n=4) | 20-40 | 310 ± 67 | 0.80 ± 0.6 | 7.20 ± 3.3 | 2.75 ± 1.7 | 5.95 ± 1.0 | 5.73 ± 1.5 | 1.75 ± 0.9 | 1.35 ± 0.1 | 4.95 ± 1.2 | 10 ± 1 | 94 ± 12 | 7 ± 1 | 118 ± 1 |
Pmgn = plasminogen; PrC = protein C; PrS = protein S; AT = antithrombin; SD = standard deviation; W = weigh.
Where a value is expressed as < or >, these values have been used to calculate mean and SD values.
Pig coagulation factors (F) in baboon plasma after pig liver transplantation
(See also Supplemental Digital Content – Supplementary Results).
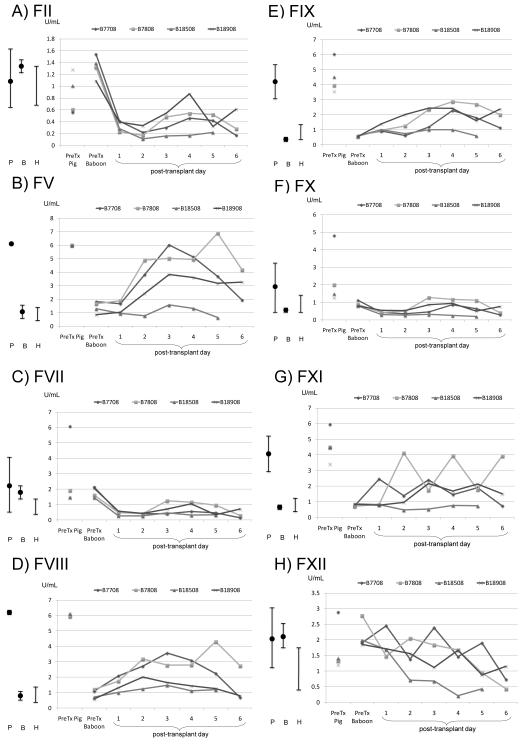
Factor II (prothrombin)
The level of FII produced by healthy GTKO and GTKO/CD46 pigs was similar to that in humans and baboons (Table 2 and Figure 3A). After pig liver Tx, considering the short half-life of FII of <70h (Supplementary Table 2), the prevention of a further decrease in FII level after day 3 suggests some porcine production, although the levels were low.
Figure 3. Results of measurement of coagulation factors in healthy humans, baboons, and pigs, and after orthotopic genetically-engineered pig liver transplantation in baboons.
(A) Factor II (prothrombin), (B) Factor V (proaccelerin), (C) Factor VII (proconvertin), (D) Factor VIII (anti-hemophilic factor), (E) Factor IX (Christmas factor), (F) Factor X (Stuart-Prower factor), (G) Factor XI (plasma thromboplastin antecedent), and (H) Factor XII (Hageman factor). (P = mean genetically-engineered pig values with standard deviation; B = mean baboon values with standard deviation; H = human range. B18508 was treated with cobra venom factor).
Factor V (proaccelerin)
FV levels in healthy wild-type (WT) and GTKO or GTKO/CD46 pigs are significantly higher than in humans and baboons (Table 2 and Figure 3B). After pig liver Tx, porcine FV production increased in some cases from the low level seen in baboons towards the level seen in pigs. Part of this increase could be from release from platelets, although, because of the almost complete absence of platelets in the blood, this seems unlikely. Since a FV mutation predisposes to thrombosis, we tested for FV Leiden mutation in two baboons. However, only consensus FV genes were found.
Factor VII (proconvertin)
FVII levels in baboons and both WT and GTKO pigs tend to be slightly higher than in humans (Table 2 and Figure 3C). After pig liver Tx, although we observed a slight increase in porcine FVII after day 3 in some baboons, it did not reach baboon or pig pre-Tx values.
Factor VIII (anti-hemophilic factor)
Healthy baboon FVIII levels are similar to those in humans, but healthy WT and GTKO pig levels are much higher (Table 2 and Figure 3D). After pig liver Tx, an increase in porcine FVIII was documented from day 1, though it did not reach the level seen in healthy pigs.
Factor IX (Christmas factor)
Healthy baboon FIX levels fall into the lower range of levels seen in humans. Healthy WT and GTKO pig levels are three times higher than the upper limit in humans (Table 2 and Figure 3E). After pig liver Tx, porcine FIX was present in the baboon’s plasma from day 1, though it did not reach the high level seen in healthy pigs.
Factor X (Stuart-Prower factor)
Healthy human and baboon FX levels are similar. WT and GTKO pig FX levels are slightly higher (Table 2 and Figure 3F). After pig liver Tx, there was little change, the level remaining in the lower range seen in healthy pigs.
Factor XI (plasma thromboplastin antecedent)
Healthy GTKO pig FXI is at least 2-3-fold higher than in humans and baboons (Table 2 and Figure 3G). Although the half-life of FXI is longer than 2 days, some increase was seen after pig liver Tx, and the levels remained slightly higher than seen in baboons pre-Tx.
Factor XII (Hageman factor)
Healthy pig and baboon FXII levels are similar to, or possibly slightly higher than, those seen in humans (Table 2 and Figure 3H). After pig liver Tx, the levels trended downwards. However, given that the half-life is <48h, the presence of some FXII after day 3 may indicate that FXII was being produced by the pig liver.
Plasminogen activity
From the experience documented in the literature it would seem that the human plasminogen detection kit does not cross-react or adequately measure pig plasminogen activity [Table 2 and Supplementary Figure 1A, (Supplemental Digital Content)]. We could not detect plasminogen activity in either healthy baboons or pigs using this kit. However, Western blot confirmed that plasminogen was being produced by the pig liver (see above and Figure 2).
Protein C activity
The normal level of PrC in healthy baboons is similar to that in humans (Table 2). Data from the literature indicate that pig PrC is approximately 70% of that observed in baboons (in comparison with the human range of 70-140%) (Table 2 and Supplementary Figure 1B). We were not able to measure PrC activity in GTKO or GTKO/CD46 pigs with the available human protein detection kit. After pig liver Tx, we could not measure PrC activity in the baboon plasma.
Protein S activity
Although we were able to measure PrS activity in healthy baboons, the level was low when compared to the normal human range (Table 2). The literature suggests that the human kit does not measure PrS activity in pigs. We were not able to measure PrS activity in GTKO pigs or in baboons after pig liver Tx (Supplementary Figure 1C)
Antithrombin activity
The levels of antithrombin in humans and baboons are similar (Table 2 and Supplementary Figure 1D). After pig liver Tx, we observed an increase in antithrombin in some cases. Since the half-life of antithrombin is 3 days, the absence of a decrease after day 4 would suggest that some porcine antithrombin was present in the baboon’s plasma.
Measurement of protein C, S, and plasminogen antigens
Since we were not able to measure the activities of protein C, S, and plasminogen in pigs with human kits, we attempted to measure their antigen levels in GTKO and GTKO/CD46 pigs (n=6). Although we were able to measure these antigens in healthy baboon blood, we were unable to detect them in either healthy pigs or in baboons after pig liver Tx (even though we demonstrated the presence of porcine plasminogen by Western blot).
Clinical measurement of coagulation parameters
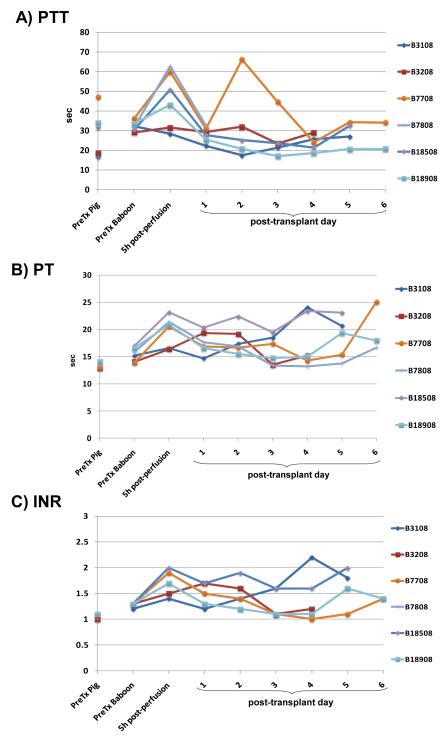
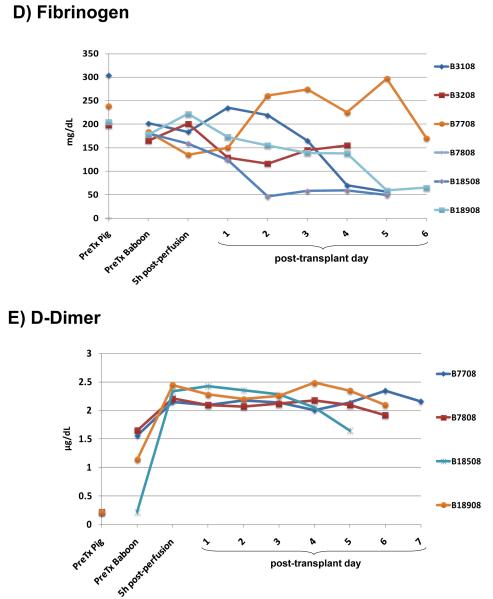
After pig liver Tx, PTT, PT, INR, fibrinogen, and D-dimer, measured by conventional techniques, remained largely within the normal ranges (Figure 4), suggesting that the porcine coagulation factors we had been able to measure were functioning in the baboon. There was some variability in PTT levels (Figure 4A), which might be related to variations in low-level heparinization (2-3units/kg/h) of the central intravascular catheters, e.g., after blood draws and flushing of the lines. The half-life of fibrinogen (FI) is 3-4 days, suggesting that the maintenance of the level after day 3 was at least in part through porcine hepatic production of fibrinogen (Figure 4D). Furthermore, the production of porcine fibrinogen was demonstrated by Western blot from day 1 (Figure 2). D-dimer levels in healthy baboons are significantly higher than in pigs, and were maintained or even increased after pig liver Tx (Figure 4E).
Figure 4. Results of measurement of parameters of coagulation.
(A) partial thromboplastin time (PTT), (B) prothrombin time (PT), (C) INR, (D) fibrinogen, (E) D-dimer. (Figures 5C and D reproduced from Ekser et al, Am J Transplant 2010; 10:273-285, with permission).
DISCUSSION
The liver is the main site of synthesis for the production of many proteins, including coagulation factors, and plays a major role in the maintenance of normal blood chemistry, detoxification, and coagulation (21). When liver failure occurs, patients suffer increasing encephalopathy and coagulopathy, which may be life-threatening (7). When conventional medical treatment proves insufficient, the optimum treatment for acute liver failure is urgent liver Tx.
However, there are insufficient human organs for Tx, especially in urgent situations. Several options have been employed to bridge the patient to liver alloTx, such as bioartificial liver devices or liver albumin dialysis systems (2,22). Although the available devices or systems provide some detoxification, they do not provide coagulation factors that lead to normal coagulation parameters (22-24). Indeed, there are reports suggesting that these devices can be detrimental to maintenance of coagulation (23-25).
Pig liver xenoTx is a potential option for ‘bridging’ to alloTx (2). However, adequate liver function and production of both pro- and anti-coagulants would need to be assured. There are known incompatibilities in coagulation factors between pigs and humans (26,27), and it is not known whether pig coagulation factors will function adequately in humans after native hepatectomy and pig liver Tx. The present study suggests that, after pig liver Tx, hepatic function will in many respects be adequate. In particular, pig coagulation factors may prove adequate to maintain hemostasis. Commercially-available coagulant/anticoagulant concentrates could possibly compensate for any clear deficiency associated with inadequate production. However, the development of a consumptive coagulopathy could not be definitively ruled out.
There are few reports that provide evidence that a liver xenograft will function adequately in humans. In 1993, Starzl et al performed baboon-to-human liver Tx in two patients (28). One recipient survived for 70 days, and normal liver function was documented, except for increases in bilirubin and ALP, suggesting bile stasis injury. Although there was no clear explanation for this phenomenon, two possible causes were considered – (i) cyclophosphamide-related bile duct injury, and (ii) bile duct-specific immunologic injury. We now can exclude the former possibility since we saw the same phenomenon when ATG induction, rather than cyclophosphamide, was administered. The absence of antibody-mediated or cellular rejection in the grafts in the present study suggests that immune injury may not be playing a major role, though this cannot be excluded completely; we are currently examining the bile ducts by electron microscopy. A third possible cause is inter-species incompatibility. Kobayashi et al (29) compared the chemistry of gallbladder and hepatic bile between humans, baboons, and pigs. They found no difference in gallbladder bile to account for sludging of bile. (In the present study, cholecystectomy was carried out in all pig grafts after reperfusion.) However, hepatic bile was significantly less viscous in baboons compared to humans and pigs, with pig and human hepatic bile viscosity being similar. Therefore, it is possible that bile stasis may not be a significant problem after pig liver Tx into humans.
Normal PT levels indicated that baboon coagulation factors functioned in the human recipient and were maintaining coagulation parameters. However, no information on individual coagulation factors was provided. The recipient suffered several infectious complications and eventually died.
Although nonhuman primates are phylogenetically closer than other species to humans, for a number of reasons they are not considered to be a suitable source of organs for clinical xenoTx (30,31). The pig is now the preferred source animal (8,30,31).
The only clinical attempt at pig liver xenoTx involved the liver from a WT pig transplanted heterotopically (without excision of the native liver) in an unsuccessful effort to bridge to alloTx in a patient with fulminant liver failure (32). After Tx, the PT fell from a very high pre-Tx level and stabilized, and reductions also occurred in AST and ALT. However, fresh frozen human plasma had been administered, potentially explaining the reversal of the coagulopathy. There was no report on measurement of coagulation factors. Within 34h, the liver xenograft showed thrombosis and ischemic necrosis, and the patient died from irreversible brain damage.
Following pig-to-human ex vivo liver perfusion for 19h, Adham et al studied PT and some coagulation factors, such as FV, FVII, FIX, FX, and FXII (33). Their data showed some benefit to the patient in total bilirubin levels, but they did not observe correction of coagulation. Furthermore, all of the measured coagulation factors, except FVII, have longer half-lives than the study period. In addition, as native hepatectomy was not performed, it is likely that there was still some production of human coagulation factors.
The relevant experimental literature is equally sparse. Using pigs transgenic for the human complement-regulatory protein, decay-accelerating factor, Ramirez et al carried out liver Tx in two baboons (34). One animal died on day 4 from a cardiac arrest related to aspiration, and the other was euthanized on day 8 with sepsis and coagulopathy. Both recipients demonstrated improvement in liver enzymes, but did not have normal liver function. Near-normal PT and PTT were documented, but both parameters increased 3-fold from pre-Tx levels by day 8 (probably associated with sepsis). Some coagulation factors were measured, but no (pre-Tx) control data from healthy pigs or baboons were documented. They also observed that albumin levels fell to normal pig albumin levels (34). In the present study, we corrected post-Tx albumin levels using human albumin (maximum dose 25g/day) to maintain pre-Tx albumin levels. Nevertheless, we obtained good evidence by Western blot that albumin was produced by the pig liver, though we were unable to quantify this.
Using complex in vitro laboratory methods, Chen et al (35) reported the characterization of WT pig FVII and FX and compared them with human FVII and FX. No significant difference in TF binding affinity was documented. Using 3-dimensional protein models, similarities in porcine and human FX in most important functional sites were demonstrated.
The same group (36) had previously documented in vitro compatibility between WT pig and human factors II, V, VII, X, and XII. Antithrombin and plasminogen activity were also measured. Their studies indicated that the porcine coagulation factors measured were 2.9 to 4.7-fold higher than in humans. (Their data do not always correlate with our own findings.) They also concluded that WT pig coagulation factors XII, VII, and X triggered the human intrinsic, extrinsic, and common pathways, respectively, which functioned normally. Porcine plasminogen was activated by human tissue plasminogen activator.
In the present study, GTKO and GTKO/CD46 pigs were utilized as donors for liver xenoTx for the first time. We measured coagulation factors in healthy GTKO pigs, and compared them with data from WT pigs, humans, and baboons available in the literature or from our own observations. Values in baboons are similar to those in humans, but there are some differences between pig and human/baboon values. Pig plasminogen, protein C, and protein S activities could not be measured by the human enzyme detection kits, and measurements of the respective antigens were also negative.
The present study provides new data, and suggests that at least some parameters of hepatic function will be adequate after pig liver Tx into primates. However, both a longer period of follow-up and the efficacy of porcine coagulation factors to maintain an adequate coagulation status require further study.
In summary, although follow-up was necessarily short, after the orthotopic Tx of GM pig livers into baboons, (i) many hepatic functions, including coagulation, were maintained normal or near-normal; (ii) there was evidence for pig protein and coagulation factor production, and (iii) these appeared to function adequately in baboons, suggesting that they could function adequately in humans, though this needs to be confirmed in future experimental studies. Of particular importance, Western blot demonstrated selected pig proteins in the recipient baboon, providing evidence of pig hepatic function. However, the almost immediate development of a profound thrombocytopenia (discussed in [17]), requires investigation and must be prevented if GM pigs are to become sources of livers to bridge patients successfully to alloTx.
Supplementary Material
ACKNOWLEDGEMENTS
Burcin Ekser, MD, is a recipient of an American Society of Transplantation/European Society for Organ Transplantation Exchange Grant, of a Young Investigator Award from the American Transplant Congress, 2009, and of a Travel Award from the International Xenotransplantation Association Congress, 2009. The authors thank Diann Flunk-Flavin, Stacey Cashman, and Michael Nakon for excellent technical help with the operative procedures. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants # U01 AI068642 and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboons were provided by the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported in part by NIH P40 sponsored grant RR012317-09.
FINANCIAL SUPPORT Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants # U01 AI068642 and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboons were provided by the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported in part by NIH P40 sponsored grant RR012317-09.
ABBREVIATIONS
- F
Factor
- GM
genetically-modified
- GTKO
α1,3-galactosyltransferase gene-knockout
- PrC
protein C
- PrS
protein S
- TF
tissue factor
- Tx
transplantation
- WT
wild-type
- xenoTx
xenotransplantation
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.UNOS - United Network for Organ Sharing (UNOS) [accessed March 29th, 2009]; http://www.unos.org.
- 2.Ekser B, Gridelli B, Tector AJ, Cooper DKC. Pig liver xenotransplantation as a bridge to allotransplantation: which patients might benefit? Transplantation. 2009;88:1041–1049. doi: 10.1097/TP.0b013e3181ba0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Grady JG, Portmann B, Wiliamms R. Fulminant hepatic failure. In: Schiff L, Schiff R, editors. Diseases of the liver. JB Lippincott; Philadelphia: 1993. [Google Scholar]
- 4.Bernuau J, Rueff B, Benhamou J. Fulminant and subfulminant hepatic failure: definition and causes. Semin Liver Dis. 1986;6:97–106. doi: 10.1055/s-2008-1040593. [DOI] [PubMed] [Google Scholar]
- 5.Schiodt FV, Atillasoy E, Shakil AO, et al. Etiology and outcome for 295 patients with acute liver in the United States. Liver Transpl Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 6.Shakil AO, Kramer D, Mazariegos GV, et al. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 7.Lee WM, Squires RH, Jr, Nyberg SL, et al. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper DKC, Ye Y, Rolf LL, Zuhdi N. The pig as potential organ donor for man. In: Cooper DKC, Kemp E, Reemtsma K, White DJG, editors. Xenotransplantation. Springer; Heidelberg: 1991. pp. 481–500. [Google Scholar]
- 9.Cooper DKC, Dorling A, Pierson RN, III, et al. α1,3-galactosyltransferase gene knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84:1–7. doi: 10.1097/01.tp.0000260427.75804.f2. [DOI] [PubMed] [Google Scholar]
- 10.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 11.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 12.Phelps CJ, Koike C, Vaught TD, et al. Production of 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 14.Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 16.Azimzadeh A, Kelishadi S, Ezzelarab M, et al. Early graft failure of GTKO pigs organs in baboons is reduced by hCPRP expression. Xenotransplantation. 2009;16(5):356. doi: 10.1111/xen.12176. (Abstract IXA-O-2.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekser B, Long C, Echeverri GJ, Hara H, Ezzelarab M, Lin CC, et al. Impact of thrombocytopenia on survival of baboons with genetically-modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 18.Ezzelarab M, Cortese-Hassett A, Cooper DK, Yazer MH. Extended coagulation profiles of healthy baboons and of baboons rejecting GT-KO pig heart grafts. Xenotransplantation. 2006;13:522–528. doi: 10.1111/j.1399-3089.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 19.Rood PPM, Bottino R, Balamurugan AN, Smetenka C, Ayares D, Groth CG, et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;83:202–210. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 20.Kolln J, Bredehorst R, Spillner E. Engineering of human complement component C3 for catalytic inhibition of complement. Immunol Lett. 2005;98:49–56. doi: 10.1016/j.imlet.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Castalino DJ, Salem HH. Natural anticoagulants and the liver. J Gastorenterol Hepatol. 1997;12:77–83. doi: 10.1111/j.1440-1746.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 22.van de Kerkhove MP, Hoekstra R, Chamuleau RAFM, et al. Clinical application of bioartificialliver support systems. Ann Surg. 2004;240:216–23. doi: 10.1097/01.sla.0000132986.75257.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachli EB, Schuepbach RA, Maggiorini M, Stocker R, Müllhaupt B, Renner EL. Artificial liver support with the molecular adsorbent recirculating system: activation of coagulation and bleeding complications. Liver Int. 2007;27:475–484. doi: 10.1111/j.1478-3231.2006.01398.x. [DOI] [PubMed] [Google Scholar]
- 24.Sgroi A, Serre Beinier V, Morel P, Bühler L. What clinical alternatives to whole liver transplantation? Current status of artificial devices and hepatocyte transplantation. Transplantation. 2009;87:457–466. doi: 10.1097/TP.0b013e3181963ad3. [DOI] [PubMed] [Google Scholar]
- 25.Sosef MN, Van De Kerkhove MP, Abrahamse SL, Levi MM, Chamuleau RA, Van Gulik TM. Blood coagulation in anhepatic pigs: effects of treatment with the AMC-bioartificial liver. J Thromb Haemost. 2003;1:511–515. doi: 10.1046/j.1538-7836.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 26.Robson SC, Cooper DKC, d’Apice AJF. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7:166–176. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 27.Cowan PJ, d’Apice AJ. The coagulation in xenotransplantation: incompabilities and strategies to overcome them. Curr Opin Organ Transplant. 2008;13:178–183. doi: 10.1097/MOT.0b013e3282f63c74. [DOI] [PubMed] [Google Scholar]
- 28.Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, et al. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi T, Taniguchi S, Ye Y, Niekrasz M, Nour B, Cooper DKC. Comparison of bile chemistry between humans, baboons, and pigs: implications for clinical and experimental liver xenotransplantation. Lab Anim Sci. 1998;48:197–200. [PubMed] [Google Scholar]
- 30.Cooper DKC, Lanza RP. Xeno - The Promise of Transplanting Animal Organs into Humans. Oxford University Press; New York: 2000. pp. 1–274. [Google Scholar]
- 31.Cooper DKC, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 32.Makowka L, Cramer DV, Hoffman A, et al. The use of pig liver xenograft for temporary support of a patient with fulminant hepatic failure. Transplantation. 1995;59:1654–1659. doi: 10.1097/00007890-199506270-00002. [DOI] [PubMed] [Google Scholar]
- 33.Adham M, Sab JM, Ducerf C, Tassaux D, Vianey-Saban C, Chevallier M, et al. Correction of acute liver failure disorders through liver xenoperfusion: experimental study. Transplant Proc. 1997;29:3013–3014. doi: 10.1016/s0041-1345(97)00764-1. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez P, Chavez R, Majado M, Munitiz V, Muñoz A, Hernandez Q, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. doi: 10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Qiao J, Tan W, Lu Y, Qin S, Zhang J, et al. Characterization of porcine factor VII, X and comparison with human factor VII, X. Blood Cells Mol Dis. 2009;43:111–118. doi: 10.1016/j.bcmd.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Li Y, Jiang H, Liu J, Zeng Y, Cheng J. Comparison of hepatic coagulant, fibrinolytic, and anticoagulant functions between banna minipig inbred line and humans. Transplantation. 2005;79:1128–1131. doi: 10.1097/00007890-200505150-00031. [DOI] [PubMed] [Google Scholar]
- 37.Lewis JH. Comparative hemostasis in vertebrates. Springer; 1996. Chapter 24; pp. 285–298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.